Abstract
Certain dermatoses that present during pregnancy have a predilection for populations with skin of color (SOC). Additionally, certain systemic diseases such as systemic lupus erythematosus tend to be more aggressive during pregnancy and confer worse prognoses in women with SOC. The purpose of this review is to highlight the unique implications of selected diseases during pregnancy as it relates to SOC. Dermatologists should be vigilant for the unique clinical variations of dermatological conditions in patients of color who are pregnant to ensure correct diagnoses and optimize treatment outcomes.
Keywords: skin of color, dermatoses, pregnancy
Introduction
Certain dermatoses that present during pregnancy have a predilection for populations with skin of color (SOC). Additionally, certain systemic diseases such as systemic lupus erythematosus (SLE) tend to be more aggressive during pregnancy and confer worse prognoses in women with SOC. This review highlights the unique implications of selected diseases during pregnancy as it relates to SOC.
Connective tissue changes
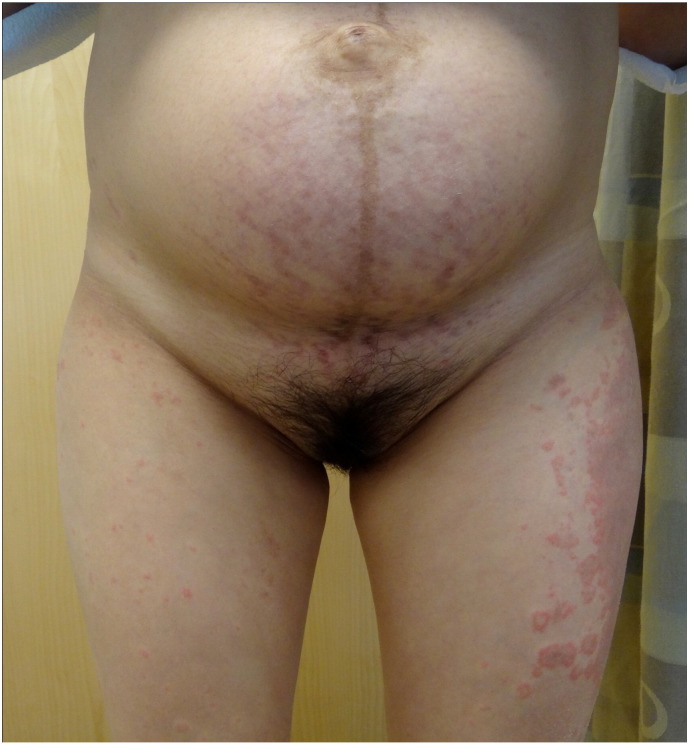
Striae gravidarum (SG) is the most common connective tissue change during pregnancy and is more common in women with black, Hispanic, or Asian ethnicities (Chang et al. 2004). The clinical evolution of SG starts with immature, red striae (striae rubra [SR]) that progress to mature, white striae (striae alba [SA]). In the SOC population, the hue of the striae can be more darkly pigmented (striae nigra [SN]) and there may be absence of SR, or the color can be a combination of hyperpigmentation and erythema (Fig. 1). Dermoscopy shows hypermelanosis of the epidermal rete ridges that transversally crosses lesions in a ladder-like fashion in SN but minimal melanosis is observed in SA (Piérard-Franchimont et al. 2005).
Fig. 1.
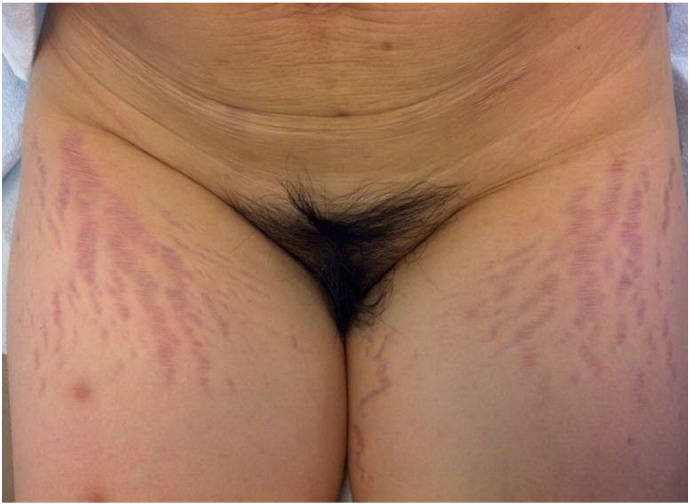
A 35-year-old Chinese female who experienced polymorphic eruption of pregnancy that presented with striae on her thighs postpartum, which was likely the result of the pregnancy and the use of topical steroid medications.
There is no strong evidence that topical treatments are effective to prevent SG (Brennan et al. 2012) although there is limited evidence on the use of over-the-counter remedies such as centella and bitter almond oil (Korgavkar and Wang 2015). The treatment approach should be based on the stage of striae (SR vs SA). Postpartum, topical tretinoin (pregnancy category C) can lead to improvement of SR (Kang et al. 1996) and SG (Rangel et al. 2001) but no studies have been done exclusively in a SOC population. Products that contain glycolic acid improve SA in patients with skin types I through V (Ash et al. 1998). Microdermabrasion may also benefit patients (Hexsel et al. 2014).
Various lasers have become popular as a therapeutic alternative but all have a risk of causing hyperpigmentation. A 585-nm pulsed dye laser (PDL) can be beneficial but should be avoided or used with great caution in patients with skin types IV through VI (Jiménez et al., 2003, Nouri et al., 1999). Non-ablative fractional lasers (1540-nm, 1550-nm, and 1560-nm) can be beneficial, but whether they are more beneficial to treat SR versus SA is unclear (Graber et al., 2008, Malekzad et al., 2014, Stotland et al., 2008, Tretti Clementoni and Lavagno, 2015). Ablative lasers have a greater risk of causing complications when compared with nonablative lasers and treatment in patients with skin types IV and higher are associated with scarring and hyperpigmentation (Metelitsa and Alster, 2010, Savas et al., 2014). Overall, due to the higher risk of hyperpigmentation in the SOC population, patients should be adequately counseled on the risks and benefits of treatment with laser therapy.
Beneficial nonlaser treatments include intense pulsed light (IPL; Hernández-Pérez et al. 2002) and radiation frequency (Manuskiatti et al. 2009). The safety and efficacy of other potential laser and nonlaser treatments require further research (Alexiades-Armenakas et al., 2004, Goldman et al., 2008, Park et al., 2012, Sadick et al., 2007).
Hypertrophic scars and keloids
Hypertrophic scars and keloids are particularly common in patients of black, Hispanic, or Asian descent (Shridharani et al. 2010). There may be a genetic predisposition in these populations that leads to excessive fibroblast proliferation and collagen synthesis during wound healing (Fujiwara et al., 2005, Nakaoka et al., 1995, Wolfram et al., 2009). During pregnancy, keloid formation is a concern in surgical sites and particularly in caesarean sections (Tulandi et al. 2011). There are no reliable preventive measures but risks can be reduced with careful surgical techniques and wound care. For example, when performing surgical procedures on women who are pregnant, careful surgical techniques should be used to achieve atraumatic and precise hemostasis and skin edge eversion closure.
Caesarean sections that use an absorbable subcuticular stitch closure lead to better cosmetic results than those with surgical staples (Alderdice et al. 2003). Bilayered closures of trunk and extremities with subcuticular running polyglactin 910 suture left in place also has a better appearance than simple running epidermal closures (Alam et al. 2006). Proper wound care and infection prevention are important. Silicone gel sheets or pressure therapy can be used for surgical sites as well as intralesional corticosteroid medications (pregnancy category C). Triamcinolone acetonide (10-40 mg/mL) is the most commonly used among intralesional corticosteroid medications and injections are repeated several times at 4- to 6-week intervals (Lumenta et al. 2014). There is limited evidence to support the use of lasers for the treatment of hypertrophic scars and keloids (Gauglitz 2013). Surgical resection is associated with a high recurrence rate up to 100% (Berman and Bieley 1996).
Pigmentary changes
Melasma
Ninety percent of women experience some form of hyperpigmentation during pregnancy (Kroumpouzos and Cohen 2001). The pigmentary change may be more obvious in the SOC population (Nakama et al. 2009). Melasma (chloasma) is the most cosmetically disturbing form of hyperpigmentation and occurs during up to 75% of pregnancies (McHugh and Laurent 1989). It is most common in women of black, Hispanic, or Asian descent (Grimes 1995).
Prevention includes counseling on sun protection. Topical treatment options are summarized in Table 1. Azelaic acid is a pregnancy category B drug (Intendis 2005) that is generally considered safe to take during pregnancy. Less is known about hydroquinone (pregnancy category C) in terms of safety and it is generally not recommended to be taken during pregnancy (Nussbaum and Benedetto 2006). Topical steroid medications (pregnancy category C) are often mixed with tretinoin and hydroquinone and need to be used with caution in order to not result in steroid-induced acne (Plewig and Kligman 1973).
Table 1.
Most commonly used postpartum topical agents for melasma.
| Agent | Pregnancy Category | Recommended Prescribing Method | Adverse Event(s) |
|---|---|---|---|
| Azelaic acida | B | Azelaic acid 20% cream or 15% gel (20% concentration of azelaic acid equivalent to 4% hydroquinone in some studies but with fewer side effects) | Erythema, burning, scaling, and pruritus |
| Hydroquinoneb | C | Most effective as combination therapy: triple-combination cream contains hydroquinone 4%, tretinoin 0.05%, and mid-potency topical corticosteroid (fluocinolone acetonide 0.01%) | Most commonly erythema, stinging, and desquamation. Dose and duration dependent effects – irritant contact dermatitis, hypopigmentation of surrounding skin, and, rarely, exogenous ochronosis |
| Topical corticosteroid medicationsc | C | Most effective as combination therapy, as noted above. | Irritation, rosacea-like dermatosis, atrophy, telangiectasia, hypertrichosis, and steroid-induced acne |
| Tretinoind | C | Most effective as combination therapy, as noted above. | Most commonly erythema, stinging, and desquamation, postinflammatory hyper- and hypopigmentation |
Postpartum, topical therapy can include hydroquinone, tretinoin, azelaic acid, or topical corticosteroid combinations. Both hyper- and hypopigmentation may be a side effect with combination use (Kligman and Willis 1975) and long-term use of hydroquinone can lead to ochronosis (Katsambas and Antoniou 1995). Chemical peels and laser therapies should be administered with caution in the SOC population due to the risk for postinflammatory hyperpigmentation (PIH; Graber et al., 2008, Ingber, 2009, Kroumpouzos and Cohen, 2001, Malekzad et al., 2014, Metelitsa and Alster, 2010, Nouri et al., 1999, Stotland et al., 2008, Tretti Clementoni and Lavagno, 2015). Botanicals such as Chinese herbs and various plant extracts have also become increasingly popular (Fisk et al. 2014).
Dermatoses of pregnancy
Intrahepatic cholestasis of pregnancy
Higher incidence of intrahepatic cholestasis of pregnancy (ICP) has been noted in certain groups with SOC including American Indian (Reyes et al. 1978), Indian, and Pakistani populations (Abedin et al. 1999). Although ICP resolves with delivery, untreated ICP can lead to fatal fetal outcomes (Rioseco et al. 1994); thus, a timely diagnosis is important. Pruritus without rash is common and characteristic but clinical jaundice only occurs in approximately 10% to 15% of patients (Lunzer 1989). Jaundice may not be apparent or reliable on the basis of an examination of the skin in patients with SOC; thus, an assessment should focus on the sclera of eyes, hard palate, palms of the hand, and soles of the feet to identify yellow discoloration. It is important to note that the diagnosis of ICP is not made on the basis of clinical findings but by confirmation of elevated serum bile acid and/or elevated aminotransferase levels (Pusl and Beuers 2007). Thus, laboratory tests should be considered for any woman who is pregnant and suspected of having ICP such as those patients with the symptomology of pruritus but without evidence of primary lesions.
Polymorphic eruption of pregnancy
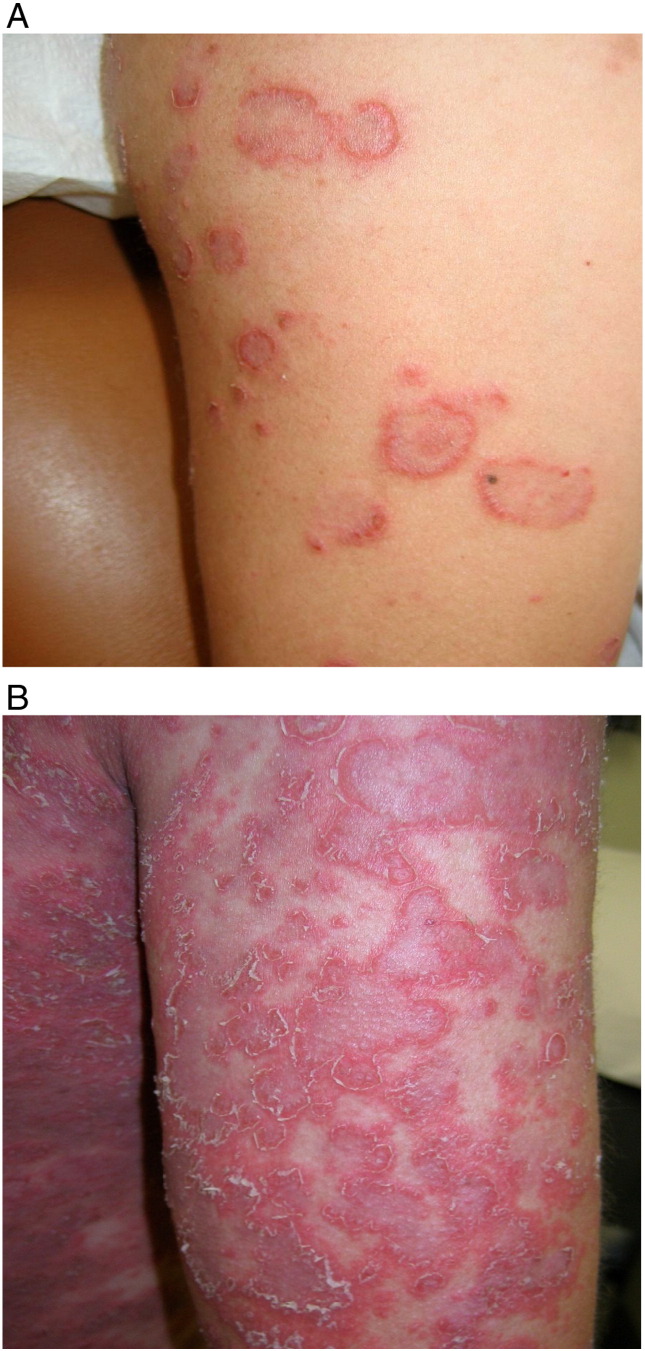
Polymorphic eruption of pregnancy (PEP), formerly known as pruritic urticarial papules and plaques of pregnancy, is the most common dermatosis during pregnancy in the Indian population (Kumari et al. 2007). PEP poses no risk to maternal and fetal outcomes during pregnancy (Yancey et al. 1984) and rarely recurs (Ambros-Rudolph and Black 2008). Erythematous papules within abdominal striae with periumbilical sparing are an early finding (Fig. 2; Aronson et al. 1998). White halos that typically surround these papules are less common in populations with a darker skin (Roger et al. 1994). Topical corticosteroid medications with mild-to-moderate potency and oral antihistamines to control symptoms of pruritus may benefit patients during pregnancy (Ambros-Rudolph, 2011, Ambros-Rudolph and Shornick, 2008). Patients with more severe disease and significant pruritus can be safely treated with a short course of systemic corticosteroid medications (Ambros-Rudolph and Shornick 2008).
Fig. 2.
A 36-year-old Korean female who presented with polymorphic eruption of pregnancy in the third trimester with lesions that were focused on the abdominal striae and the lateral thighs.
Special considerations
Acne vulgaris
Acne vulgaris is the most common dermatologic condition in patients with SOC (Vaughan Jones et al. 2014). Acne often worsens during the third trimester of pregnancy due to increased maternal androgen concentrations and the resultant effects on sebum production (Vaughan Jones et al. 2014). Pregnancy-associated immunologic processes may also contribute to the course of disease (Pugashetti and Shinkai 2013). Although there is no unique presentation of acne during pregnancy, in patients with SOC the sequelae of PIH, keloids, and severe scarring are common (Coley and Alexis, 2009, Davis and Callender, 2010, Taylor et al., 2002). Thus, early and aggressive control of the disease process is imperative. To treat patients during pregnancy, topical azelaic acid can be recommended for mild acne with noninflammatory lesions. For inflammatory lesions, a combination with topical erythromycin or clindamycin is recommended. Moderate-to severe inflammatory acne can be managed with oral amoxicillin or medications with cephalosporin such as cefadroxil or cephalexin. With regard to fulminant nodular cystic acne, a course of oral prednisolone that lasts no longer than a month may be appropriate after the first trimester (Chien et al. 2016). Patient education is important to prevent the excoriation of acne lesions, harsh skin regimen, and cultural skin and hair care practices by the patient that can either worsen acne or cause PIH or scarring (Taylor et al. 2002).
Vitiligo, psoriasis, and atopic dermatitis
The etiology of vitiligo is thought to be multifactorial and no increase in incidence during pregnancy has been reported (Njoo and Westerhof, 2001, Papadopoulos et al., 1998). Although not associated with adverse outcomes during pregnancy (Horev et al. 2011), vitiligo can be particularly distressing in patients with SOC due to its obvious presentation in contrast to darker skin tones and hyperpigmentation during pregnancy may exaggerate this contrast.
Psoriasis, a common diagnosis in non-white populations (Davis et al. 2012), improves in approximately half and worsens in approximately one fourth of patients. In patients whose condition improves, the reduction of lesions is marked and approximately 80% to 90% of lesions clear from the first to the third trimester (Boyd et al., 1996, Murase et al., 2005). In the population with SOC, the presentation may be subtler with lesions that appear more violaceous than red (Fig. 3A) and less prominent scaling (Fig. 3B).
Fig. 3.
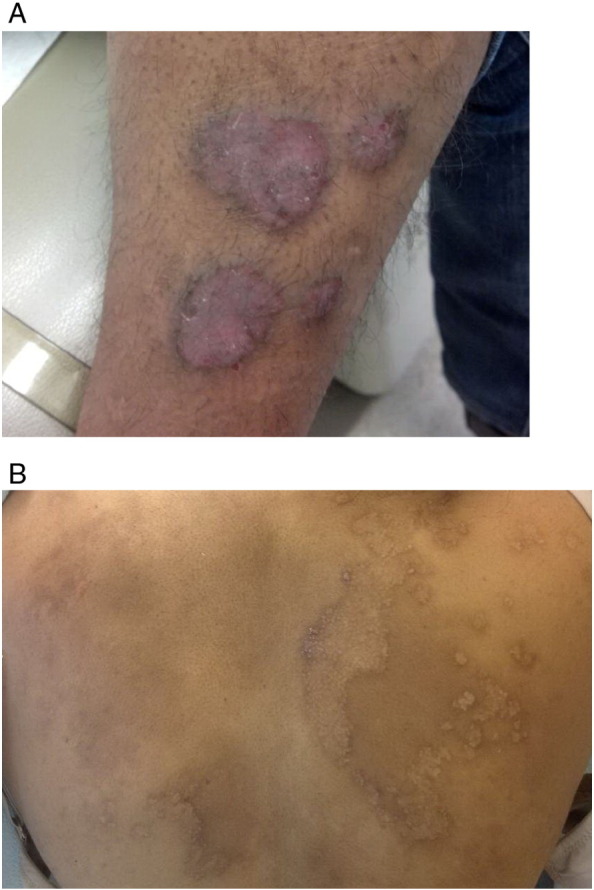
A 34-year-old Indian woman with chronic plaque psoriasis who begins to experience a flare of her psoriasis after the birth of her child. (A) Plaques that were clear during the pregnancy have recurred on her leg. (B) Thin psoriasis form plaques that begin to form on the right upper back after her back had been clear for the majority of the pregnancy. The areas that were clear during the pregnancy are present as faint patches of postinflammatory pigmentation that cover the back.
An atopic dermatitis (AD) flare during pregnancy, which is also known as atopic eruption of pregnancy, is the most common dermatosis during pregnancy and accounts for 36% to 50% of cases. It is the second most common skin disease in patients of black race (Halder et al. 1983). Furthermore, in women with SOC who are pregnant, AD is more likely to present with hyperpigmented lichenified patches and plaques and the erythema may be more difficult to visualize (Fig. 4).
Fig. 4.
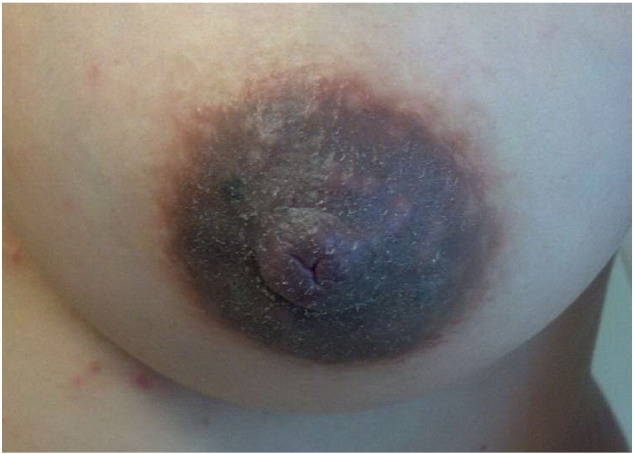
A 30-year-old Chinese patient who presented with atopic eruption of pregnancy (AEP) during the first trimester of her pregnancy.
Note that the dark hyperpigmentation masks the hue of erythema that is present on the nipples, which are some of the most vulnerable areas of the body to AEP.
Ultraviolet B phototherapy may be considered as a management option for the above conditions. However, photodegradation of folic acid is associated with light therapy; thus, to mitigate the risk of folate deficiency during the first trimester, it is important for the mother to supplement with 0.4 mg to 1 mg of folic acid per day (Park and Murase 2012). Currently, there is no consensus on the appropriate folate supplementation during phototherapy (Murase et al. 2010) and patients who are at high risk may require up to 5 mg daily (Wilson et al. 2003). Of important note, vitamin B12 deficiency must be ruled out if a patient supplements with more than 1 mg per day because vitamin B12 deficiency and high serum folate are associated with an increased risk of cognitive impairment and anemia in adults (Selhub et al. 2007). Also, patients with SOC are at a higher risk of melasma during pregnancy so photoprotection of the face is important during light therapy (Sharquie et al. 2008).
Systemic lupus erythematosus, sarcoidosis, and scleroderma
SLE is more prevalent in women of black race compared with other populations (Clowse et al. 2008). Disease flares increase during pregnancy and usually present with dermatologic manifestations (Figs. 5A and B; Clowse 2007). Specifically, the discoid lupus erythematosus (DLE) subtype presents with erythematous plaques and scaling that becomes atrophied with excessive scarring with borders of PIH in patients of SOC but Caucasian patients rarely present with PIH (McCauliffe, 2001, Nozile et al., 2015, Rothfield et al., 2006). This leads to a significant contrast between normal and lesional skin particularly in populations of SOC. In addition, individuals of African descent may show dark, blue-black nail dyschromia (Vaughan Jones et al. 2014). Although DLE increases the risk for squamous cell carcinoma (Hordinsky 2008), SLE carries a higher risk of spontaneous abortion, fetal death, preterm delivery, and intrauterine growth restriction that is secondary to antiphospholipid syndrome, lupus nephritis, or hypertension (Chakravarty et al. 2006).
Fig. 5.
A 32-year-old Hispanic female at 9 weeks of gestation (A) and again at 18 weeks of gestation (B) with systemic cutaneous lupus erythematosus. The pregnancy resulted in a prominent flaring of her disease.
Sarcoidosis is also seen more commonly in women of black race (Rybicki et al. 1997). Both specific and nonspecific cutaneous lesions are associated with sarcoidosis during pregnancy and women of black race most commonly present with papular lesions (Minus and Grimes 1983). In patients with SOC, plaques can develop hypopigmentation (Elgart 1986). Plaques are more likely to cause permanent scarring compared with papular lesions (Hanno and Callen 1980). Other clinical findings that primarily occur in women of black race include lupus pernio and ulcerative lesions (Albertini et al., 1997, Hunninghake et al., 1999, Mañá et al., 1997). Although rare, cicatricial or noncicatricial alopecia also occurs primarily in patients of black race (Katta et al. 2000). Erythema nodosum is significantly less common in Black and Asian populations compared with Caucasian populations (Davis and Callender, 2010, Edmondstone and Wilson, 1985, James et al., 1976). Sarcoidosis carries a higher risk of preeclampsia, eclampsia, thromboembolic events, and premature delivery. There is also a higher risk of cesarean deliveries and postpartum hemorrhages (Hadid et al. 2015). In women who are pregnant and have advanced pulmonary disease or extrapulmonary lesions, exacerbation of the disease can manifest with hypercalcemia as well as respiratory and/or heart failure that is secondary to restrictive disease (Ellafi and Valeyre, 1999, Subramanian et al., 2004).
Scleroderma also has increased incidence and severity in women of black race compared with Caucasian patients (Steen et al. 1997). Additionally, patients with SOC are more likely to develop multi-organ disease, be diagnosed at a younger age, have a higher incidence of inflammatory features, and have a worse age-adjusted prognosis (Laing et al. 1997). In patients with SOC, involved skin is noted for focal, macular, perifollicular hypopigmentation and most commonly in the areas of the hands, arms, and trunk (Adelowo and Oguntona, 2009, Ee and Tan, 2005, Halder et al., 2003). Diffuse hyperpigmentation may also be present (Steen 1998). Pigmentary changes are one of the most common cutaneous manifestation in patients of black race (Kuwana et al., 1999, Reveille et al., 2001). Diffuse or active disease can lead to maternal renal crisis as well as prematurity and small full-term infants (Rabhi et al. 2002).
There are no treatment options available exclusively for patients with SOC for these conditions. Early detection of disease activity as well as patient education on disease activity and risk of complications with pregnancy are paramount. Clinicians and particularly dermatologists should ask general screening questions during the early phase of pregnancy with regard to common rheumatologic disease skin manifestations, especially in the case of patients with SOC (Spinillo et al. 2012).
Conclusion
Certain dermatologic conditions during pregnancy are more common in women with SOC and often present with unique clinical manifestations. Treatment options for these conditions are limited and management can be difficult, particularly for women in these populations. Thus, dermatologists should be vigilant for variations in clinical findings for patients with SOC to ensure correct and timely diagnoses and optimize treatment outcomes for these patients.
Footnotes
Conflicts of interest: None.
References
- Abedin P., Weaver J.B., Egginton E. Intrahepatic cholestasis of pregnancy: Prevalence and ethnic distribution. Ethn Health. 1999;4:35–37. doi: 10.1080/13557859998173. [DOI] [PubMed] [Google Scholar]
- Adelowo O.O., Oguntona S. Scleroderma (systemic sclerosis) among Nigerians. Clin Rheumatol. 2009;28:1121–1125. doi: 10.1007/s10067-009-1191-2. [DOI] [PubMed] [Google Scholar]
- Alam M., Posten W., Martini M.C., Wrone D.A., Rademaker A.W. Aesthetic and functional efficacy of subcuticular running epidermal closures of the trunk and extremity: A rater-blinded randomized control trial. Arch Dermatol. 2006;142:1272–1278. doi: 10.1001/archderm.142.10.1272. [DOI] [PubMed] [Google Scholar]
- Albertini J.G., Tyler W., Miller OF Ulcerative sarcoidosis. Case report and review of the literature. Arch Dermatol. 1997;133:215–219. doi: 10.1001/archderm.133.2.215. [DOI] [PubMed] [Google Scholar]
- Alderdice F., McKenna D., Dornan J. Techniques and materials for skin closure in caesarean section. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003577. [DOI] [PubMed] [Google Scholar]
- Alexiades-Armenakas M.R., Bernstein L.J., Friedman P.M., Geronemus R.G. The safety and efficacy of the 308-nm excimer laser for pigment correction of hypopigmented scars and striae alba. Arch Dermatol. 2004;140:955–960. doi: 10.1001/archderm.140.8.955. [DOI] [PubMed] [Google Scholar]
- Ambros-Rudolph C., Black M. Obstetric and Gynecologic Dermatology. 3rd ed. Elsevier Health Sciences; London: 2008. Polymorphic eruption of pregnancy; pp. 49–55. [Google Scholar]
- Ambros-Rudolph C., Shornick J. Dermatology. 2nd ed. Elsevier; London: 2008. Pregnancy dermatoses; pp. 1119–1120. [Google Scholar]
- Ambros-Rudolph C.M. Dermatoses of pregnancy - clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265–275. doi: 10.5021/ad.2011.23.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson I.K., Bond S., Fiedler V.C., Vomvouras S., Gruber D., Ruiz C. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933–939. doi: 10.1016/s0190-9622(98)70265-8. [DOI] [PubMed] [Google Scholar]
- Ash K., Lord J., Zukowski M., McDaniel D.H. Comparison of topical therapy for striae alba (20% glycolic acid/0.05% tretinoin versus 20% glycolic acid/10% L-ascorbic acid) Dermatol Surg. 1998;24:849–856. doi: 10.1111/j.1524-4725.1998.tb04262.x. [DOI] [PubMed] [Google Scholar]
- Balina L.M., Graupe K. The treatment of melasma. 20% azelaic acid versus 4% hydroquinone cream. Int J Dermatol. 1991;30:893–895. doi: 10.1111/j.1365-4362.1991.tb04362.x. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D. Topical treatment of melasma. Indian J Dermatol. 2009;54:303–309. doi: 10.4103/0019-5154.57602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman B., Bieley H.C. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996;22:126–130. doi: 10.1111/j.1524-4725.1996.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Boyd A.S., Morris L.F., Phillips C.M., Menter M.A. Psoriasis and pregnancy: Hormone and immune system interaction. Int J Dermatol. 1996;35:169–172. doi: 10.1111/j.1365-4362.1996.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Brennan M., Young G., Devane D. Topical preparations for preventing stretch marks in pregnancy. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD000066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty E.F., Nelson L., Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:899–907. doi: 10.1002/art.21663. [DOI] [PubMed] [Google Scholar]
- Chan R., Park K.C., Lee M.H., Lee E.-S., Chang S.E., Leow Y.H. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br J Dermatol. 2008;159:697–703. doi: 10.1111/j.1365-2133.2008.08717.x. [DOI] [PubMed] [Google Scholar]
- Chang A.L., Agredano Y.Z., Kimball A.B. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881–885. doi: 10.1016/j.jaad.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Chien A.L., Qi J., Rainer B., Sachs D.L., Helfrich Y.R. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254–262. doi: 10.3122/jabfm.2016.02.150165. [DOI] [PubMed] [Google Scholar]
- Clowse M.E.B. Lupus activity in pregnancy. Rheum Dis Clin North Am. 2007;33:237–252. doi: 10.1016/j.rdc.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowse M.E.B., Jamison M., Myers E., James A.H. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199:127.e1–127.e6. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley M.K., Alexis A.F. Managing common dermatoses in skin of color. Semin Cutan Med Surg. 2009;28:63–70. doi: 10.1016/j.sder.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Davis E.C., Callender V.D. A review of acne in ethnic skin: Pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24–38. [PMC free article] [PubMed] [Google Scholar]
- Davis S.A., Narahari S., Feldman S.R., Huang W., Pichardo-Geisinger R.O., McMichael A.J. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466–473. [PubMed] [Google Scholar]
- Edmondstone W.M., Wilson A.G. Sarcoidosis in Caucasians, Blacks and Asians in London. Br J Dis Chest. 1985;79:27–36. doi: 10.1016/0007-0971(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Ee H.L., Tan S.H. Reticulate hyperpigmented scleroderma: A new pigmentary manifestation. Clin Exp Dermatol. 2005;30:131–133. doi: 10.1111/j.1365-2230.2004.01683.x. [DOI] [PubMed] [Google Scholar]
- Elgart M.L. Cutaneous sarcoidosis: Definitions and types of lesions. Clin Dermatol. 1986;4:35–45. doi: 10.1016/0738-081x(86)90032-5. [DOI] [PubMed] [Google Scholar]
- Ellafi M., Valeyre D. Sarcoidosis and pregnancy. Rev Pneumol Clin. 1999;55:335–337. [PubMed] [Google Scholar]
- Fisk W.A., Agbai O., Lev-Tov H.A., Sivamani R.K. The use of botanically derived agents for hyperpigmentation: a systematic review. J Am Acad Dermatol. 2014;70:352–365. doi: 10.1016/j.jaad.2013.09.048. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Muragaki Y., Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153:295–300. doi: 10.1111/j.1365-2133.2005.06698.x. [DOI] [PubMed] [Google Scholar]
- Gauglitz G.G. Management of keloids and hypertrophic scars: current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103–114. doi: 10.2147/CCID.S35252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A., Rossato F., Prati C. Stretch marks: treatment using the 1,064-nm Nd:YAG laser. Dermatol Surg. 2008;34:686–691. doi: 10.1111/j.1524-4725.2008.34129.x. discussion 691–2. [DOI] [PubMed] [Google Scholar]
- Graber E.M., Tanzi E.L., Alster T.S. Side effects and complications of fractional laser photothermolysis: Experience with 961 treatments. Dermatol Surg. 2008;34:301–305. doi: 10.1111/j.1524-4725.2007.34062.x. discussion 305–7. [DOI] [PubMed] [Google Scholar]
- Grimes P.E. Melasma. Etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453–1457. doi: 10.1001/archderm.131.12.1453. [DOI] [PubMed] [Google Scholar]
- Hadid V., Patenaude V., Oddy L., Abenhaim H.A. Sarcoidosis and pregnancy: Obstetrical and neonatal outcomes in a population-based cohort of 7 million births. J Perinat Med. 2015;43:201–207. doi: 10.1515/jpm-2014-0017. [DOI] [PubMed] [Google Scholar]
- Halder R.M., Grimes P.E., McLaurin C.I., Kress M.A., Kenney J.A. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388–390. [PubMed] [Google Scholar]
- Halder R.M., Roberts C.I., Nootheti P.K. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679–687. doi: 10.1016/s0733-8635(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Hanno R., Callen J.P. Sarcoidosis: A disorder with prominent cutaneous features and their interrelationship with systemic disease. Med Clin North Am. 1980;64:847–866. doi: 10.1016/s0025-7125(16)31570-x. [DOI] [PubMed] [Google Scholar]
- Hernández-Pérez E., Colombo-Charrier E., Valencia-Ibiett E. Intense pulsed light in the treatment of striae distensae. Dermatol Surg. 2002;28:1124–1130. doi: 10.1046/j.1524-4725.2002.02111.x. [DOI] [PubMed] [Google Scholar]
- Hexsel D., Soirefmann M., Porto M.D., Schilling-Souza J., Siega C., Dal’Forno T. Superficial dermabrasion versus topical tretinoin on early striae distensae: A randomized, pilot study. Dermatol Surg. 2014;40:537–544. doi: 10.1111/dsu.12460. [DOI] [PubMed] [Google Scholar]
- Hordinsky M. Cicatricial alopecia: discoid lupus erythematosus. Dermatol Ther. 2008;21:245–248. doi: 10.1111/j.1529-8019.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- Horev A., Weintraub A.Y., Sergienko R., Wiznitzer A., Halevy S., Sheiner E. Pregnancy outcome in women with vitiligo. Int J Dermatol. 2011;50:1083–1085. doi: 10.1111/j.1365-4632.2010.04839.x. [DOI] [PubMed] [Google Scholar]
- Hunninghake G.W., Costabel U., Ando M., Baughman R., Cordier J.F., du Bois R. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- Ingber A. Springer-Verlag; Berlin: 2009. Obstetric dermatology: A practical guide. [Google Scholar]
- Intendis Inc. 2005. Finacea® (azelaic acid) Gel, 15% (package insert) [Google Scholar]
- James D.G., Neville E., Siltzbach L.E. A worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321–334. doi: 10.1111/j.1749-6632.1976.tb47043.x. [DOI] [PubMed] [Google Scholar]
- Jiménez G.P., Flores F., Berman B., Gunja-Smith Z. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362–365. doi: 10.1046/j.1524-4725.2003.29086.x. [DOI] [PubMed] [Google Scholar]
- Kang S., Kim K.J., Griffiths C.E., Wong T.Y., Talwar H.S., Fisher G.J. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519–526. [PubMed] [Google Scholar]
- Katsambas A., Antoniou C. Melasma. Classification and treatment. J Eur Acad Dermatol Venereol. 1995;4:217–223. [Google Scholar]
- Katta R., Nelson B., Chen D., Roenigk H. Sarcoidosis of the scalp: A case series and review of the literature. J Am Acad Dermatol. 2000;42:690–692. [PubMed] [Google Scholar]
- Kligman A.M., Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40–48. [PubMed] [Google Scholar]
- Korgavkar K., Wang F. Stretch marks during pregnancy: A review of topical prevention. Br J Dermatol. 2015;172:606–615. doi: 10.1111/bjd.13426. [DOI] [PubMed] [Google Scholar]
- Kroumpouzos G., Cohen L.M. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1–19. doi: 10.1067/mjd.2001.114595. [DOI] [PubMed] [Google Scholar]
- Kumari R., Jaisankar T.J., Thappa D.M. A clinical study of skin changes in pregnancy. Indian J Dermatol Venereol Leprol. 2007;73:141. doi: 10.4103/0378-6323.31910. [DOI] [PubMed] [Google Scholar]
- Kuwana M., Kaburaki J., Arnett F.C., Howard R.F., Medsger T.A., Wright T.M. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheum. 1999;42:465–474. doi: 10.1002/1529-0131(199904)42:3<465::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Laing T.J., Gillespie B.W., Toth M.B., Mayes M.D., Gallavan R.H., Burns C.J. Racial differences in scleroderma among women in Michigan. Arthritis Rheum. 1997;40:734–742. doi: 10.1002/art.1780400421. [DOI] [PubMed] [Google Scholar]
- Lumenta D.B., Siepmann E., Kamolz L.P. Internet-based survey on current practice for evaluation, prevention, and treatment of scars, hypertrophic scars, and keloids. Wound Repair Regen. 2014;22:483–491. doi: 10.1111/wrr.12185. [DOI] [PubMed] [Google Scholar]
- Lunzer M.R. Jaundice in pregnancy. Baillieres Clin Gastroenterol. 1989;3:467–483. doi: 10.1016/0950-3528(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Lynde C.B., Kraft J.N., Lynde C.W. Topical treatments for melasma and postinflammatory hyperpigmentation. Skin Therapy Lett. 2006;11:1–6. [PubMed] [Google Scholar]
- Malekzad F., Shakoei S., Ayatollahi A., Hejazi S. The safety and efficacy of the 1540nm non-ablative fractional XD probe of Star Lux 500 device in the treatment of striae alba: Before-after study. J Lasers Med Sci. 2014;5:194–198. [PMC free article] [PubMed] [Google Scholar]
- Mañá J., Marcoval J., Graells J., Salazar A., Peyrí J., Pujol R. Cutaneous involvement in sarcoidosis. Relationship to systemic disease. Arch Dermatol. 1997;133:882–888. doi: 10.1001/archderm.1997.03890430098013. [DOI] [PubMed] [Google Scholar]
- Manuskiatti W., Boonthaweeyuwat E., Varothai S. Treatment of striae distensae with a TriPollar radiofrequency device: a pilot study. J Dermatolog Treat. 2009;20:359–364. doi: 10.3109/09546630903085278. [DOI] [PubMed] [Google Scholar]
- McCauliffe D.P. Cutaneous lupus erythematosus. Semin Cutan Med Surg. 2001;20:14–26. doi: 10.1053/sder.2001.23091. [DOI] [PubMed] [Google Scholar]
- McHugh N.J., Laurent M.R. The effect of pregnancy on the onset of psoriatic arthritis. Br J Rheumatol. 1989;28:50–52. doi: 10.1093/rheumatology/28.1.50. [DOI] [PubMed] [Google Scholar]
- Metelitsa A.I., Alster T.S. Fractionated laser skin resurfacing treatment complications: A review. Dermatol Surg. 2010;36:299–306. doi: 10.1111/j.1524-4725.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- Minus H.R., Grimes P.E. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361–363. 372. [PubMed] [Google Scholar]
- Murase J.E., Chan K.K., Garite T.J., Cooper D.M., Weinstein G.D. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;141:601–606. doi: 10.1001/archderm.141.5.601. [DOI] [PubMed] [Google Scholar]
- Murase J.E., Koo J.Y.M., Berger T.G. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710–711. doi: 10.1016/j.jaad.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Nakama T., Hashikawa K., Higuchi M., Ishii N., Miyasato M., Hamada T. Pigmentary demarcation lines associated with pregnancy. Clin Exp Dermatol. 2009;34:e573–e576. doi: 10.1111/j.1365-2230.2009.03247.x. [DOI] [PubMed] [Google Scholar]
- Nakaoka H., Miyauchi S., Miki Y. Proliferating activity of dermal fibroblasts in keloids and hypertrophic scars. Acta Derm Venereol. 1995;75:102–104. doi: 10.2340/0001555575102104. [DOI] [PubMed] [Google Scholar]
- Njoo M.D., Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol. 2001;2:167–181. doi: 10.2165/00128071-200102030-00006. [DOI] [PubMed] [Google Scholar]
- Nouri K., Romagosa R., Chartier T., Bowes L., Spencer J.M. Comparison of the 585 nm pulse dye laser and the short pulsed CO2 laser in the treatment of striae distensae in skin types IV and VI. Dermatol Surg. 1999;25:368–370. doi: 10.1046/j.1524-4725.1999.07320.x. [DOI] [PubMed] [Google Scholar]
- Nozile W., Adgerson C.N., Cohen G.F. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343–349. [PubMed] [Google Scholar]
- Nussbaum R., Benedetto A.V. Cosmetic aspects of pregnancy. Clin Dermatol. 2006;24:133–141. doi: 10.1016/j.clindermatol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Papadopoulos L., Bor R., Legg C., Hawk J.L. Impact of life events on the onset of vitiligo in adults: Preliminary evidence for a psychological dimension in aetiology. Clin Exp Dermatol. 1998;23:243–248. doi: 10.1046/j.1365-2230.1998.00384.x. [DOI] [PubMed] [Google Scholar]
- Park K.K., Murase J.E. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132–133. doi: 10.1001/archdermatol.2011.1614. [DOI] [PubMed] [Google Scholar]
- Park K.Y., Kim H.K., Kim S.E., Kim B.J., Kim M.N. Treatment of striae distensae using needling therapy: A pilot study. Dermatol Surg. 2012;38:1823–1828. doi: 10.1111/j.1524-4725.2012.02552.x. [DOI] [PubMed] [Google Scholar]
- Piérard-Franchimont C., Hermanns J.F., Hermanns-Lê T., Piérard G.E. Striae distensae in darker skin types: The influence of melanocyte mechanobiology. J Cosmet Dermatol. 2005;4:174–178. doi: 10.1111/j.1473-2165.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- Plewig G., Kligman A.M. Induction of acne by topical steroids. Arch Dermatol Forsch. 1973;247:29–52. doi: 10.1007/BF00595699. [DOI] [PubMed] [Google Scholar]
- Pugashetti R., Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302–311. doi: 10.1111/dth.12077. [DOI] [PubMed] [Google Scholar]
- Pusl T., Beuers U. Intrahepatic cholestasis of pregnancy. Orphanet J Rare Dis. 2007;2:26. doi: 10.1186/1750-1172-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabhi M., Tiev K.P., Genereau T., Cabane J. Scleroderma and pregnancy. Ann Med Interne (Paris) 2002;153:193–200. [PubMed] [Google Scholar]
- Rangel O., Arias I., García E., Lopez-Padilla S. Topical tretinoin 0.1% for pregnancy-related abdominal striae: An open-label, multicenter, prospective study. Adv Ther. 2001;18:181–186. doi: 10.1007/BF02850112. [DOI] [PubMed] [Google Scholar]
- Reveille J.D., Fischbach M., McNearney T., Friedman A.W., Aguilar M.B., Lisse J. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–346. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- Reyes H., Gonzalez M.C., Ribalta J., Aburto H., Matus C., Schramm G. Prevalence of intrahepatic cholestasis of pregnancy in Chile. Ann Intern Med. 1978;88:487–493. doi: 10.7326/0003-4819-88-4-487. [DOI] [PubMed] [Google Scholar]
- Rioseco A.J., Ivankovic M.B., Manzur A., Hamed F., Kato S.R., Parer J.T. Intrahepatic cholestasis of pregnancy: A retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890–895. doi: 10.1016/s0002-9378(94)70304-3. [DOI] [PubMed] [Google Scholar]
- Roger D., Vaillant L., Fignon A., Pierre F., Bacq Y., Brechot J.F. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130:734–739. [PubMed] [Google Scholar]
- Rothfield N., Sontheimer R.D., Bernstein M. Lupus erythematosus: Systemic and cutaneous manifestations. Clinics in Dermatol. 2006;24:348–362. doi: 10.1016/j.clindermatol.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Rybicki B.A., Major M., Popovich J., Maliarik M.J., Iannuzzi M.C. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- Sadick N.S., Magro C., Hoenig A. Prospective clinical and histological study to evaluate the efficacy and safety of a targeted high-intensity narrow band UVB/UVA1 therapy for striae alba. J Cosmet Laser Ther. 2007;9:79–83. doi: 10.1080/14764170701313767. [DOI] [PubMed] [Google Scholar]
- Savas J.A., Ledon J.A., Franca K., Nouri K. Lasers and lights for the treatment of striae distensae. Lasers Med Sci. 2014;29:1735–1743. doi: 10.1007/s10103-013-1342-1. [DOI] [PubMed] [Google Scholar]
- Selhub J., Morris M.S., Jacques P.F. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A. 2007;104:19995–20000. doi: 10.1073/pnas.0709487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Pastuszak A., Curto G., Koren G. Safety of first-trimester exposure to topical tretinoin: Prospective cohort study. Lancet. 1997;350:1143–1144. doi: 10.1016/S0140-6736(05)63790-7. [DOI] [PubMed] [Google Scholar]
- Sharquie K.E., Al-Mashhadani S.A., Salman H.A. Topical 10% zinc sulfate solution for treatment of melasma. Dermatol Surg. 2008;34:1346–1349. doi: 10.1111/j.1524-4725.2008.34287.x. [DOI] [PubMed] [Google Scholar]
- Shridharani S.M., Magarakis M., Manson P.N., Singh N.K., Basdag B., Rosson G.D. The emerging role of antineoplastic agents in the treatment of keloids and hypertrophic scars: A review. Ann Plast Surg. 2010;64:355–361. doi: 10.1097/SAP.0b013e3181afaab0. [DOI] [PubMed] [Google Scholar]
- Spinillo A., Beneventi F., Ramoni V., Caporali R., Locatelli E., Simonetta M. Prevalence and significance of previously undiagnosed rheumatic diseases in pregnancy. Ann Rheum Dis. 2012;71:918–923. doi: 10.1136/annrheumdis-2011-154146. [DOI] [PubMed] [Google Scholar]
- Steen V.D. Clinical manifestations of systemic sclerosis. Semin Cutan Med Surg. 1998;17:48–54. doi: 10.1016/s1085-5629(98)80062-x. [DOI] [PubMed] [Google Scholar]
- Steen V.D., Oddis C.V., Conte C.G., Janoski J., Casterline G.Z., Medsger T.A. Incidence of systemic sclerosis in Allegheny County, Pennsylvania. A twenty-year study of hospital-diagnosed cases, 1963-1982. Arthritis Rheum. 1997;40:441–445. doi: 10.1002/art.1780400309. [DOI] [PubMed] [Google Scholar]
- Stotland M., Chapas A.M., Brightman L., Sukal S., Hale E., Karen J. The safety and efficacy of fractional photothermolysis for the correction of striae distensae. J Drugs Dermatol. 2008;7:857–861. [PubMed] [Google Scholar]
- Subramanian P., Chinthalapalli H., Krishnan M., Tarlo S.M., Lobbedez T., Pineda M.E. Pregnancy and sarcoidosis: An insight into the pathogenesis of hypercalciuria. Chest. 2004;126:995–998. doi: 10.1378/chest.126.3.995. [DOI] [PubMed] [Google Scholar]
- Taylor S.C., Cook-Bolden F., Rahman Z., Strachan D. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46:S98–106. doi: 10.1067/mjd.2002.120791. [DOI] [PubMed] [Google Scholar]
- Taylor S.C., Torok H., Jones T., Lowe N., Rich P., Tschen E. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67–72. [PubMed] [Google Scholar]
- Tretti Clementoni M., Lavagno R. A novel 1565 nm non-ablative fractional device for stretch marks: A preliminary report. J Cosmet Laser Ther. 2015;17:148–155. doi: 10.3109/14764172.2015.1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulandi T., Al-Sannan B., Akbar G., Ziegler C., Miner L. Prospective study of intraabdominal adhesions among women of different races with or without keloids. Am J Obstet Gynecol. 2011;204:132.e1–132.e4. doi: 10.1016/j.ajog.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Vaughan Jones S., Ambros-Rudolph C., Nelson-Piercy C. Skin disease in pregnancy. BMJ. 2014;348:g3489. doi: 10.1136/bmj.g3489. [DOI] [PubMed] [Google Scholar]
- Wilson R.D., Davies G., Desilets V., Reid G.J., Summers A., Wyatt P. The use of folic acid for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. 2003;25:959–973. doi: 10.1016/s1701-2163(16)30248-1. [DOI] [PubMed] [Google Scholar]
- Wolfram D., Tzankov A., Pülzl P., Piza-Katzer H. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- Yancey K.B., Hall R.P., Lawley T.J. Pruritic urticarial papules and plaques of pregnancy. Clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473–480. doi: 10.1016/s0190-9622(84)80097-3. [DOI] [PubMed] [Google Scholar]