Abstract
Background and Objectives:
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been established as a safe and accurate method for diagnosing and staging intra-abdominal mass. However, few studies investigated its feasibility, efficacy, and safety for targeting liver mass. We evaluated the efficacy and safety of EUS-FNA in patients with liver masses including the right lobe.
Patients and Methods:
The technical feasibility, safety, and diagnostic yield were determined in 47 patients (30 in the left lobe and 17 in the right lobe) presenting with liver masses between September 2010 and February 2016.
Results:
Thirty-eight patients (80.9%) had malignancies whereas nine patients (19.1%) had benign liver masses. Technical success rate was 97.9% (46/47). EUS-FNA was diagnostic in 38 of 42 patients (90.5%). When the outcomes of EUS-FNA between right liver mass and left mass were accessed, the technical success rates were similar in both lobes (100% vs. 94.1%, P = 0.2). The median tumor size on EUS (25.5 mm, interquartile range [IQR] 13.8–30.3 vs. 28 mm, IQR 18.5–43.5, P = 0.24) and number of needle passes (3, IQR 3–4 vs. 3, IQR 3–3, P = 0.24) were not significantly different. Adequate specimen obtained was statistically higher in the left lobe (28/30, 93.3% vs. 14/17, 82.4%, P = 0.04). However, diagnostic accuracy for liver masses was not different (25/28, 89.3% vs. 13/14, 92.9%, P = 0.86). No complications developed after procedure.
Conclusions:
EUS-FNA can be a safe and efficient method for the diagnosis of liver mass and it is technically feasible even for those in the right lobe.
Keywords: Endoscopic ultrasound, liver mass, fine-needle aspiration
INTRODUCTION
The diagnosis of hepatic masses is important for clinical decision-making and it is typically achieved by percutaneous or transjugular biopsy.[1] Small liver masses (<2 cm) and those that are difficult to differentiate from the background parenchyma are not easily accessible by ultrasound (US)- or computed tomography (CT)-guided percutaneous biopsy. Recently, endoscopic ultrasound (EUS) has been widely used for the diagnosis and management of gastrointestinal and pancreatobiliary diseases.[2] Further, EUS can detect small liver lesions undetected by CT.[3] However, the role of EUS-guided fine-needle aspiration (EUS-FNA) in the diagnosis of liver lesions has not been well described.[4] To date, few studies have demonstrated the feasibility and safety of EUS-guided liver biopsy; the results indicated that the specimen adequacy with EUS-FNA was variable (19%–100%).[5,6,7] Furthermore, there was no study to investigate the role of EUS-FNA in the right hepatic lobe. The aim of this study is to evaluate the feasibility and safety of EUS-FNA in patients with liver masses including the right lobe.
PATIENTS AND METHODS
Patients
Forty-seven consecutive patients who underwent EUS-FNA for liver lesions between September 2010 and February 2016 at Asan Medical Center were included in this single-arm observational study. All patients were aged >20 years and had hepatic masses detected by US or CT. The indications of EUS-FNA included a pancreatic lesion with liver mass, failure of percutaneous liver biopsy, contraindication of percutaneous liver biopsy, and liver mass to assess primary lesion and establish tissue diagnosis. No patient used antiplatelet agents within the last 5 days before the procedure. Written informed consent was obtained from all patients before the procedure. This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB number: 2016-0417).
Endoscopic ultrasound-guided fine-needle aspiration procedure
EUS-FNA was performed by an experienced endosonographer (D.W.S). All procedures were performed under conscious sedation with intravenous midazolam and meperidine using a linear array echoendoscope (GF-UCT 240; Olympus Optical, Tokyo, Japan). Tissue acquisition was performed with a 22-gauge or a 25-gauge needle (Expect™, Boston Scientific, Natick, Mass). After careful evaluation of the target lesion and regional vasculature with EUS, including real-time Doppler, FNA was performed either from the stomach or duodenum. The needle was advanced into the target lesion under EUS guidance. Once the lesion was penetrated, the stylet was removed. Specimen was obtained by moving the needle to-and-fro within the lesion while applying negative pressure by using a 10 mL syringe. Suction was released by closing the syringe lock, and the needle was finally removed. The aspiration was repeated until enough specimen was obtained, as determined by gross inspection. Aspiration specimens were expelled onto glass slides by reinserting the stylet. The material on the slide was carefully inspected by the endosonographer after each pass to determine successful acquisition of specimen, as is done for percutaneous liver biopsy, with additional passes performed if necessary.[8] Any large blood clot, if present, was removed from the slide. If a specimen obtained by the first needle pass was bloody, EUS-FNA was performed without applying negative pressure. The specimen samples were smeared on glass slides for cytological examination, fixed in 95% ethanol, and stained with hematoxylin and eosin stain. No on-site cytopathological evaluation was available during the procedure. Patients were observed for immediate complications in the recovery room for 1 h and were followed up for 1 month to monitor for potential late adverse events. Patients with hepatic masses for whom EUS-FNA was not diagnostic were clinically followed up for a minimum of 6 months.
Cytopathological examination
Cytological examination was performed by experienced pathologists. Cytological diagnoses were classified as positive for malignancy, suspicious for malignancy, atypical, negative, and inadequate.[9] Cytological diagnoses classified as positive for malignancy and suspicious for malignancy were considered malignant.[10] Cytological examination that was diagnostic for malignancy was considered the definitive diagnosis. For patients in whom EUS-FNA was not diagnostic for liver masses, the definite diagnosis was made on the basis of additional tissue specimens obtained by other methods such as surgical resection and US-guided liver biopsy or on clinical follow-up with imaging studies >6 months.
Statistical analysis
Statistical analysis was performed using SPSS® Statistics 22.0 (SPSS Inc., Chicago, IL, USA). Sample size calculation was not planned because of the nature of the feasibility trial in the first pilot study. Continuous parameters were presented as median and interquartile range (IQR). Mann–Whitney U-test and Fisher's exact test were used to test for differences in comparisons between continuous and dichotomous variables, respectively. P < 0.05 was considered statistically significant.
RESULTS
A total of 47 patients were enrolled in this study. The baseline characteristics of patients are summarized in Table 1. The results are summarized in Tables 2 and 3. The median patient age was 63 years (IQR: 59–73), and 27 patients (57.4%) were male. Thirty-eight (80.9%) of 47 patients were diagnosed with malignancy. The indications for EUS-FNA are shown in Table 2. The most common indication was a pancreatic lesion with liver mass and concurrent EUS-FNA of pancreatic and liver masses underwent in 16 of 47 patients (34%) Figure 1.
Table 1.
The baseline characteristics of patients who underwent endoscopic ultrasound-guided fine-needle aspiration
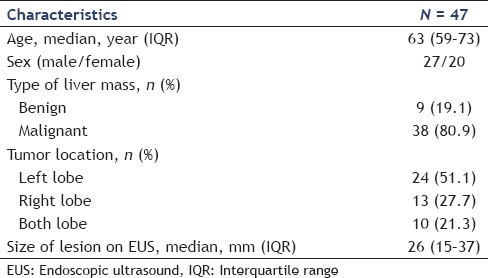
Table 2.
Clinical characteristics and cytological results of endoscopic ultrasound-guided fine-needle aspiration
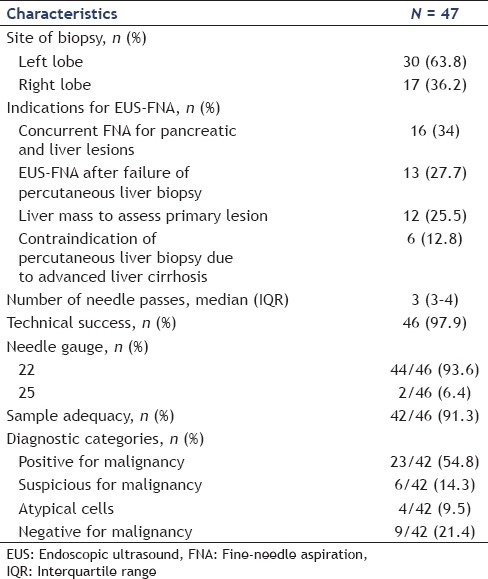
Table 3.
Final diagnosis achieved with endoscopic ultrasound-guided fine needle aspiration
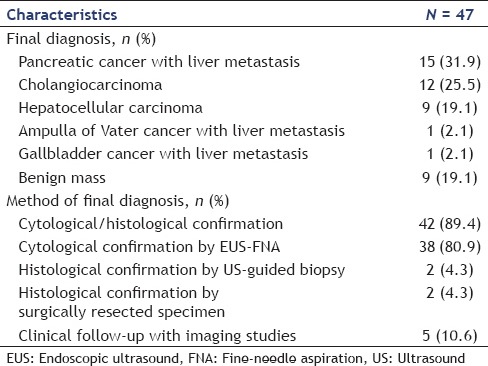
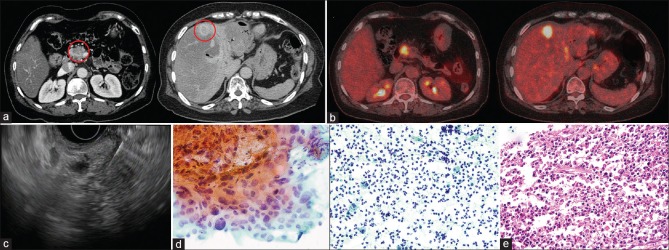
Figure 1.
(a) Computed tomography showing a pancreatic mass accompanied with a liver mass at segment 4. (b) Positron emission tomography original magnification ×40 showing hypermetabolic lesions in the pancreas and liver. (c) Endoscopic ultrasound-guided fine-needle aspiration concurrently performed in pancreas and liver. (d) Cytological examination showing adenocarcinoma of the pancreas (left). However, liver fine-needle aspiration showed only inflammatory cells (right). (e) The patient underwent pancreaticoduodenectomy. Surgical specimen of liver acquired during pancreaticoduodenectomy shows no malignant cells in liver
Tissue acquisition was successful in 46 of 47 (97.9%) patients. EUS-FNA failed in one patient because the liver lesion was not detected on EUS. Specimens were acquired from the left lobe in 30 patients (63.8%) and the right lobe in 17 patients (36.2%). The mean tumor size on EUS was 26 mm (IQR: 15–37). The median number of needle passes was 3 (IQR: 3–4). The median distance of the liver mass from the transducer was 1.9 cm (IQR: 1.5–2.7). On microscopic examination, tissue specimens obtained by EUS-FNA were determined as adequate in 42 of 46 patients (91.3%). The pathological diagnosis was malignancy in 23 of 46 patients (50%), suspicious for malignancy in 6 patients (13%), atypical in 4 patients (8.7%), and negative for malignancy in 9 patients (19.6%).
The final diagnosis was based on cytological/histological findings in 42 patients (89.4%) and clinical follow-up with imaging studies in five patients (10.6%). Among patients who were diagnosed by cytology, diagnosis was achieved with EUS-FNA in 38 patients (80.9%). Two patients (4.3%) were diagnosed by US-guided liver biopsy, and the surgical specimens in the remaining two patients showed inconclusive histological findings on EUS-FNA.
We compared the outcomes of EUS-FNA between the right lobe and left lobe mass. Median size of lesions on EUS and median number of needle passes did not differ significantly between the left and right lobes [Table 4]. The median distance of the liver mass from the transducer in the right lobe and in the left lobe was 2.3 cm (IQR: 1.9–2.9) and 1.6 cm (IQR: 1.4–2.3), respectively. The distance of the liver mass from the transducer was significantly longer in the right lobe (P = 0.01). Technical success rate was also similar in both lobes (30/30, 100% vs. 16/17, 94.1%, P = 0.2).
Table 4.
Comparison of characteristics and outcomes of endoscopic ultrasound-guided fine needle aspiration between left and right lobes
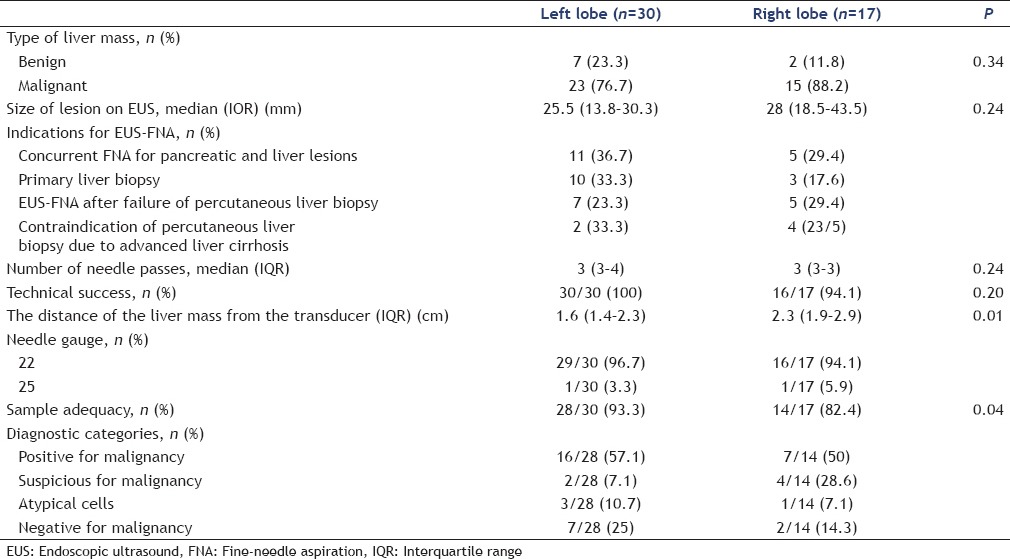
However, adequate specimen obtained was significantly higher in the left lobe (28/30, 93.3% vs. 14/17, 82.4%, P = 0.04). Diagnostic accuracy for liver masses was not statistically different (25/28, 89.3% vs. 13/14, 92.9%, P = 0.86). None of the patients experienced procedure-related adverse events.
DISCUSSION
Our study demonstrated that EUS-FNA is a technically feasible and safe procedure, even the mass located in the right lobe. It can provide a cytopathological diagnosis of liver masses, and the study further showed that EUS could identify liver lesions that could not be visualized by US or CT. In addition, our results suggested that EUS-FNA might be utilized as a rescue procedure in cases of failure to obtain specimen by a percutaneous approach. EUS-FNA for liver lesions could be performed concurrently in the setting of intra-abdominal tumor metastases, where confirmation of malignancy and staging could be accomplished in the same session. Finally, EUS-FNA for evaluation of liver lesions showed excellent safety in this study.
Liver metastasis can significantly affect the management and prognosis of patients; thus, liver biopsy is critical for diagnosis, staging, and clinical management of patients with liver lesions.[1] Nonoperative liver tissue acquisition is usually obtained by a percutaneous approach. Although trans-abdominal US and contrast-enhanced CT are the most commonly used imaging studies to evaluate liver masses, detection rates of these modalities for small lesions are relatively low.[11,12] US- or CT-guided percutaneous liver biopsy has technical limitations in the assessment of small focal lesions whereas EUS and EUS-FNA can detect small liver lesions.[12] Furthermore, EUS provides an excellent resolution of liver lesions without interference of the rib cage or pleural deflection.
In the current study, EUS-FNA was performed for lesions that were located in the right lobe in 17 patients (36.2%). To the best of our knowledge, this is the largest study of its kind assessing the diagnostic accuracy of EUS-FNA for right hepatic lesions. Diagnostic yield for liver lesions is similar in both lobes (25/28, 89.3% vs. 13/14, 92.9%, P = 0.86). In one patient, the liver lesion that was located in the right posterior segment was not well visualized; therefore, EUS-FNA was not possible. Although the diagnostic accuracy of lesions in the right lobe is generally limited, our results demonstrated that EUS-FNA is possible for the acquisition of specimens from the right lobe. Evaluation of lesions in the right lobe is usually difficult because endosonographic examination is performed from the duodenum, which has a small endosonographic window.[12] In the setting of higher frequencies, the depth of examination is limited to 5–6 cm; hence, lesions in the right anteriosuperior and right posterior segments may not be visualized well.[13] However, the inherent disadvantage with decreased depth of penetration may be partially overcome by reducing the frequency of echoendoscope down to 5 MHz. Therefore, the development of wide frequency of echoendoscope to assess the right lobe lesions by EUS-FNA may be warranted.
In the current study, 12 patients (25.5%) underwent EUS-FNA after failure with the percutaneous approach. EUS-FNA was diagnostically helpful in 9 of 12 patients (75%), in agreement with a study by tenBerge et al. who also reported that EUS-FNA was able to diagnose malignancy in 23 of 26 patients following a nondiagnostic FNA under transabdominal US guidance.[13] The higher success rate achieved with EUS-FNA than with trans-abdominal US approach appears to be because of the higher resolution of images which improved access to lesions.[13] EUS examination is not dependent on the body habitus.[12] In our experience, EUS-FNA led to a diagnosis of malignancy in cases where percutaneous liver FNA failed to obtain specimen for cytological diagnosis. The utility of EUS-FNA as a complementary method will be more significant although it cannot completely replace percutaneous biopsy.
As one of its major advantages, EUS-FNA can be performed simultaneously for lesions of the pancreas and liver. In patients with pancreatic cancer, the probability that small liver lesions are metastatic is higher; thus, their evaluation is critical for clinical management.[12] The presence of liver metastasis is generally an indication of an inoperable and incurable malignancy. EUS has been used to evaluate pancreatic cancer with high sensitivity, ranging from 88% to 99%.[14,15] Chang et al. reported that EUS could detect small, focal liver lesions that were missed by CT scan in 11 of 574 patients (2.4%).[14,15] In our study, 16 of 47 patients (34%) underwent EUS-FNA for pancreatic and liver lesions in the same session. Cytopathological diagnosis was confirmed by EUS-FNA in 14 of 16 patients (87.5%). Thirteen of these 14 patients were diagnosed with metastases and avoided unnecessary surgery. In our experience, EUS-FNA might be considered for the evaluation of liver metastases in the setting of intra-abdominal tumors.
tenBerge et al. reported that 4% of the patients developed EUS-FNA-related adverse events and that EUS-FNA was more sensitive than percutaneous biopsy in the diagnosis of liver masses in the same patient cohort.[13] In our study, there were no procedure-related adverse events among a total of 47 patients who underwent EUS-FNA. Our results provide further evidence that EUS-FNA for liver lesions is a safe procedure. In contrast, percutaneous liver biopsy may cause significant pain that is not relieved by preprocedural local anesthesia in most patients and may further cause serious adverse events such as severe bleeding and bile peritonitis.[10,16,17,18] Under EUS guidance, FNA can be safely performed as intervening blood vessels can be avoided by the simultaneous use of color flow and Doppler, thus minimizing the risk of bleeding in the setting of advanced liver cirrhosis and coagulopathy.[4] In addition, albeit rare, percutaneous FNA for malignancy was associated with tumor implantation.[19] To date, no occurrences of tumor seeding after EUS-FNA for liver metastases were reported.[2,20] Moreover, EUS-FNA can assess caudate lobe mass which is challenging by percutaneous biopsy due to its anatomic location.[21] Therefore, EUS-FNA may be an alternative, safe, and reproducible method of liver biopsy that can yield satisfactory specimens. It will be more powerful tool as a complementary method for percutaneous biopsy.
There are several limitations in this study. First, tissue analysis was performed by cytological assessment alone. On-site cytopathological evaluation was not available during the procedure. Optimal diagnostic results may be achieved by combining cytological and histological analyses, especially when assessment of architectural features is essential (e.g., malignant lymphomas or well-differentiated tumors) or when a large number of cells are required to determine the malignancy potential (e.g., gastrointestinal stromal tumors).[22,23] In addition, on-site evaluation of cytological specimens for adequacy can improve diagnostic accuracy.[24] Second, this study was a single-arm observational study and did not compare the outcomes of EUS-FNA with those of percutaneous biopsy. Therefore, further comparative studies with a large number of patients are necessary to confirm the efficacy and safety of EUS-FNA for liver lesions.
CONCLUSIONS
EUS-FNA for liver masses was helpful and safe for the diagnosis of malignant liver masses, especially in cases where the percutaneous approach failed. EUS-FNA may be considered as the next step in patients for whom the percutaneous approach fails. Furthermore, cytological diagnosis of liver metastases in the setting of intra-abdominal tumors, which may change the clinical management of patients, is possible within the same session by EUS-FNA. Finally, the right lobe lesions can be assessed and targeted by EUS-FNA although there is a limitation of penetration depth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dewitt J, McGreevy K, Cummings O, et al. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest Endosc. 2009;69(3 Pt 1):535–42. doi: 10.1016/j.gie.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 2.Parekh PJ, Majithia R, Diehl DL, et al. Endoscopic ultrasound-guided liver biopsy. Endosc Ultrasound. 2015;4:85–91. doi: 10.4103/2303-9027.156711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad SS, Fagan S, Abudayyeh S, et al. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg. 2002;184:601–4. doi: 10.1016/s0002-9610(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–61. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 5.Mathew A. EUS-guided routine liver biopsy in selected patients. Am J Gastroenterol. 2007;102:2354–5. doi: 10.1111/j.1572-0241.2007.01353_7.x. [DOI] [PubMed] [Google Scholar]
- 6.Gleeson FC, Clayton AC, Zhang L, et al. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437–40. doi: 10.1016/j.cgh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Stavropoulos SN, Im GY, Jlayer Z, et al. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc. 2012;75:310–8. doi: 10.1016/j.gie.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Sporea I, Popescu A, Sirli R. Why, who and how should perform liver biopsy in chronic liver diseases. World J Gastroenterol. 2008;14:3396–402. doi: 10.3748/wjg.14.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YN, Moon JH, Kim HK, et al. Usefulness of endoscopic ultrasound-guided sampling using core biopsy needle as a percutaneous biopsy rescue for diagnosis of solid liver mass: Combined histological-cytological analysis. J Gastroenterol Hepatol. 2015;30:1161–6. doi: 10.1111/jgh.12922. [DOI] [PubMed] [Google Scholar]
- 10.Kuo FY, Chen WJ, Lu SN, et al. Fine needle aspiration cytodiagnosis of liver tumors. Acta Cytol. 2004;48:142–8. doi: 10.1159/000326307. [DOI] [PubMed] [Google Scholar]
- 11.Wernecke K, Rummeny E, Bongartz G, et al. Detection of hepatic masses in patients with carcinoma: Comparative sensitivities of sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;157:731–9. doi: 10.2214/ajr.157.4.1892027. [DOI] [PubMed] [Google Scholar]
- 12.Singh P, Mukhopadhyay P, Bhatt B, et al. Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: Results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367–73. doi: 10.1097/MCG.0b013e318167b8cc. [DOI] [PubMed] [Google Scholar]
- 13.tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859–62. doi: 10.1067/mge.2002.124557. [DOI] [PubMed] [Google Scholar]
- 14.Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–52. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 15.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 16.Terry R. Risks of needle biopsy of the liver. Br Med J. 1952;1:1102–5. doi: 10.1136/bmj.1.4768.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg E, Konopniki M, Veitsman E, et al. Prevalence and characteristics of pain induced by percutaneous liver biopsy. Anesth Analg. 2003;96:1392–6. doi: 10.1213/01.ANE.0000060453.74744.17. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877–83. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goletti O, Chiarugi M, Buccianti P, et al. Subcutaneous implantation of liver metastasis after fine needle biopsy. Eur J Surg Oncol. 1992;18:636–7. [PubMed] [Google Scholar]
- 20.Diehl DL, Johal AS, Khara HS, et al. Endoscopic ultrasound-guided liver biopsy: A multicenter experience. Endosc Int Open. 2015;3:E210–5. doi: 10.1055/s-0034-1391412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keohane J, Dimaio CJ, Schattner MA, et al. EUS-guided transgastric drainage of caudate lobe liver abscesses. J Interv Gastroenterol. 2011;1:139–41. doi: 10.4161/jig.1.3.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollerbach S, Willert J, Topalidis T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: Histological and cytological assessment. Endoscopy. 2003;35:743–9. doi: 10.1055/s-2003-41593. [DOI] [PubMed] [Google Scholar]
- 23.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 24.Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration: sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol. 2007;5:1248–54. doi: 10.1016/j.cgh.2007.09.011. [DOI] [PubMed] [Google Scholar]