Abstract
Background and Objectives:
Pancreatic cysts are evaluated by endoscopic ultrasound and fine needle aspiration (EUS). The only accepted treatment is pancreatectomy, which is associated with morbidity and mortality. This study evaluated the optimal thermal dosimetry of a novel radiofrequency ablation device using a standard electrosurgical unit in ex vivo cyst models.
Methods:
A modified EUS 22-gauge monopolar needle prototype with a tip electrode connected to a standard electrosurgical unit (Erbe USA, Marietta, GA, USA) was used to induce a subboiling point temperature. A cyst model was created using 2-cm sections of porcine small intestine ligated and filled with saline. After ablation, the cyst models were prepared for pathological evaluation. The epithelial layers were measured in at least two different sites with a micrometer and compared with the corresponding control sample.
Results:
Thirty-two cyst models were ablated with maximum temperatures of 50°C, 60°C, 90°C, and 97°C in 8, 11, 11, and 2 cysts, respectively. Longer ablation times were required to induce higher temperatures. A trend in the reduction in thickness of the measured layers was observed after exposure to higher temperatures. A temperature over 50°C was required for the ablation of the muscularis, submucosa, and villi, and over 60°C was required to ablate the mucosal crypts.
Conclusions:
In a preclinical model, a novel radiofrequency EUS-capable needle connected to a standard electrosurgical unit using standard low-voltage coagulation provided ablation in a temperature-dependent fashion with a threshold of at least 60°C and a safe cyst margin below 97°C. This potentially will allow low-cost, convenient cyst ablation.
Keywords: Ablation, cyst, needle, pancreas, prototype
INTRODUCTION
Pancreatic cysts (PCs) have been increasingly diagnosed[1,2] due to the technical improvements in cross-sectional imaging techniques.[3,4,5] PCs may become malignant,[6] and the only accepted method of treatment is pancreatectomy, which may carry severe morbidity.[7,8]
Among the new treatment strategies that are being developed to manage these PCs, the endoscopic ultrasound (EUS)-guided injection of ethanol/paclitaxel had initial promising results.[9,10,11] However, recent studies have shown insufficient outcomes in long-term follow-up.[12]
The aim of this study is to evaluate the feasibility and efficacy of radiofrequency ablation (RFA) in ex vivo cyst models with a new EUS-needle prototype using a standard, commercially available, electrosurgical unit.
MATERIALS AND METHODS
Because this study did not use human tissue, it did not require any Ethical Board Reviews.
Two centers were involved in this project: institution 1 and institution 2. The same methodology was strictly followed at both centers to assure homogenous and comparable results project.
Cyst model
In the ex vivo phase of the study, the cyst models were created from fresh tissue from pig small intestines. The model was chosen due to the presence of villi that mimic the papillary projections of intraductal papillary mucinous neoplasms (IPMNs). All cysts had a 2-cm diameter and contained 2-mL of saline. To create the cyst, two ligatures were made with a surgical suture at each end of a 2-cm segment of the pig intestine. Saline solution was injected with a subcutaneous needle (25–30 gauge) through the ligature at one end to avoid damaging the surface of the cyst.
Ablation
A new EUS RFA needle prototype was used for each ablation. This device consisted of a prototype 22-gauge monopolar EUS needle with a 1-cm electrode at the tip [Figure 1]. The device was connected to a standard electrosurgical unit (ERBE VIO 300, Erbe USA, Marietta, GA, USA) through a standard active cord to the needle hub on the proximal end of the device. Fresh chicken tissue was placed between the grounding pad and the cyst model to simulate the soft tissue interface between the target lesion and the return electrode. Once positioned, fixed generator settings consisting of a 10-watt soft coagulation mode and a hemostatic effect of 4 was used for all the samples. The engineering team at the needle's manufacturer indicated these settings. The settings were tested to obtain the maximum ablation effect, decreasing at the same time the risk of achieving the boiling point. Soft coagulation current implies a low-(<200) voltage energy, which avoids electrical arc formation and minimizes the risk of exceeding the boiling point. A scientific thermometer with an external sensor located at the tip was placed in direct contact with the cyst surface throughout the ablation, measuring the cyst temperature changes in real time [Figure 2]. Each ablation was performed until a specific, predetermined maximum temperature (50°C, 60°C, 90°C, and 97°C, respectively) was reached in each of the experiments. To select the temperature parameters, we performed several prestudy ablations using a broad spectrum of temperatures (20°C–100°C). The first macroscopic changes were observed at 60°C, but we decided to include 50°C to avoid selection bias should there be any microscopic changes at this lower threshold. The temperatures of 90°C and 97°C were selected after a consensus meeting between the two institutions. The aim was to have ablations performed under a high, but still clinically feasible, temperature that was close to the boiling point. The number of cysts ablated per preselected temperature depended on the availability of fresh tissue with the intent of having at least 5 cysts ablated per “clinically feasible” temperature (50°C–90°C) and one ablated cyst at the closest boiling point (97°C). Immediately after ablation, all samples were preserved in formaldehyde solution and processed for hematoxylin and eosin staining.
Figure 1.
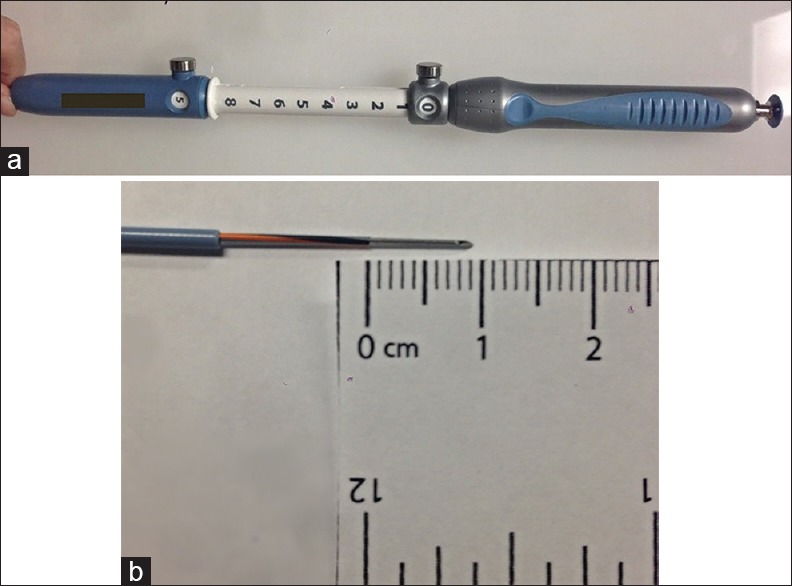
Prototype needle: (a) Endoscopic ultrasound needle prototype, (b) detail of the 1 cm electrode tip
Figure 2.
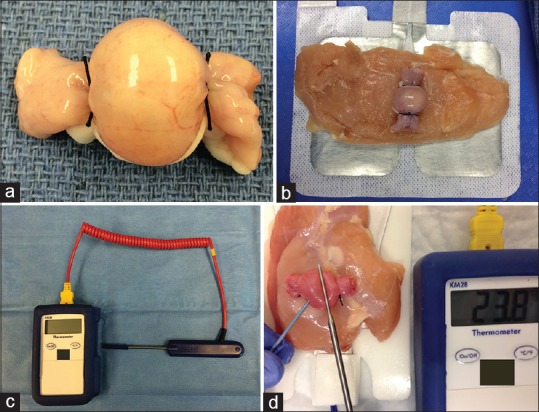
Cyst ablation: (a) Cystic model, (b) chicken placed between the cystic model and the grounding pad, (c) Scientific thermometer, (d) Ablation performed with the needle punctured inside the cyst and the thermometer tip placed on the cyst surface
Measurements
A specimen of fresh pig intestine was processed at the beginning of each experiment to act as the control sample. This control slide was used to assess the presence of autolysis of the tissue before any ablation. Moreover, to minimize the effect of autolysis, the pig was euthanized within the previous 12 h of the experiment at both institutions.
Because the model involves nonvital tissues, standard histological measures of necrosis were not feasible. In pilot phases of the study (data not shown), we observed progressive thinning of the tissue layers with thermal injury. Thus, the thickness of the muscularis propria, submucosa, and mucosa (differentiating among the crypts and the villi layers) were measured with a micrometer in at least two different locations on each slide. A predetermined magnification of ×10 for the ablated slides and ×4 for the control samples was used in both centers. Measurements were made using an ocular microscope lens with a built-in micrometer.
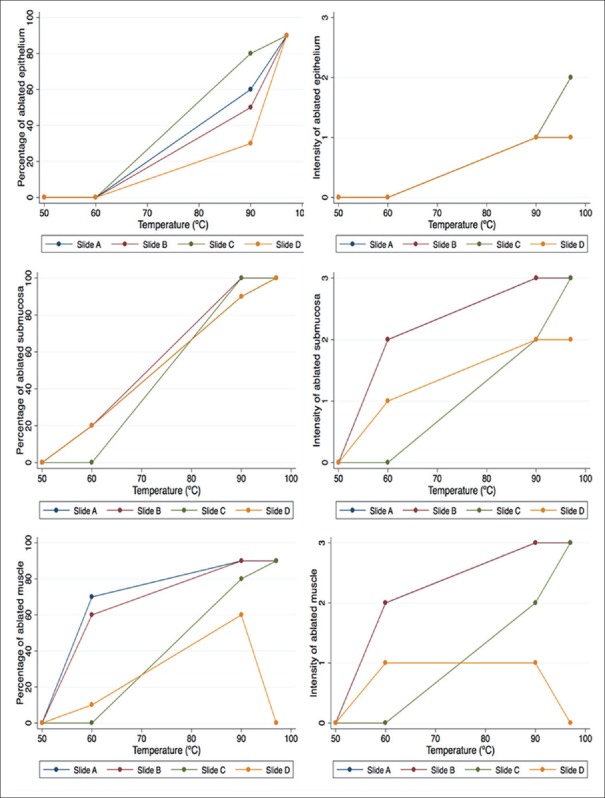
For the cysts created and processed at institution 2, a subanalysis was also performed to determine the intracystic homogeneity of each ablation by cutting each cyst into four different specimens following a predetermined order immediately after ablation. Specimen A was the area of the tissue where the needle was previously introduced, specimen C was located across from A, and specimens B and D were the cystic tissue surrounding the A section [Figure 3]. We measured the extension of the ablated area (scored from 0% to 100%) and the intensity (depth) of the ablation (classified from 0 to 3). For the latter, if a heterogeneous pattern was observed, a higher score was noted. Both measurements were performed for the muscularis propria, submucosa, and mucosa. For the extension and intensity of the ablation, a consensus between a pathologist and a gastroenterologist was needed to minimize any observer-dependent bias.
Figure 3.
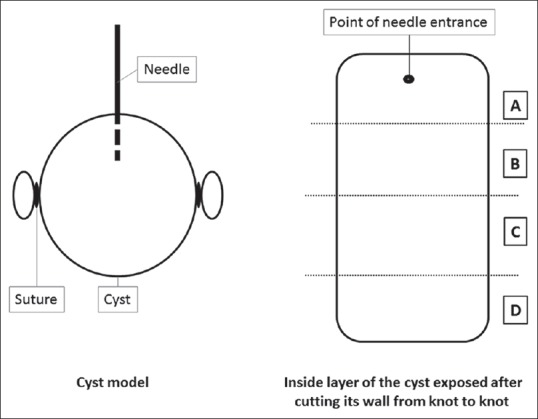
Diagram of the pathology specimens
Statistical analysis
Stata 13 software (Stata Inc., College Station, TX, USA) was used to manage the data. Cysts from both centers were grouped based on the temperature reached during ablation and the cyst layer. The thickness of each layer was measured, and the mean and standard deviation values were calculated per group. The thickness decrement with respect to the control slide was expressed in percentiles. For the institution 2 subgroup, the median value of the extension and intensity was calculated for each slide (A, B, C, or D) and further classified by the maximum temperature reached during ablation.
RESULTS
Thirty-two cyst segments were ablated, 19 from institution 2 and 13 from institution 1. The maximum temperatures reached were 50°C, 60°C, 90°C, and 97°C in 8, 11, 11, and 2 cysts, respectively.
The total ablation time per cyst increased progressively to achieve higher temperatures, with an overall range of 102–440 s (50°C–97°C). The macroscopic cyst changes also correlated with the temperature increases. However, these changes were only noticeable at a minimum temperature of 60°C. The spectrum ranged from minimal color changes to visible color and texture changes and total dehydration/thinning of the tissue [Figure 4]. In addition, regarding the fresh chicken tissue, a mild color change was noticed once a minimum temperature of 90°C was reached. Otherwise, no clear macroscopic changes were observed at this level.
Figure 4.
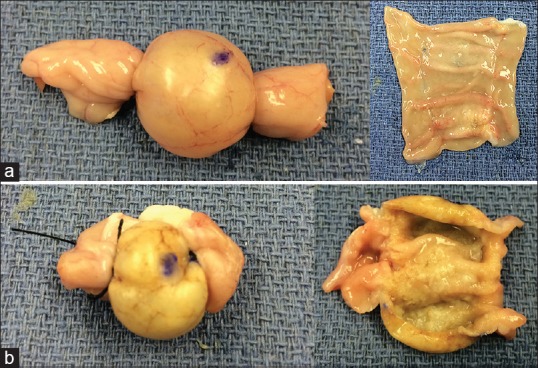
Ablation-induced macroscopic changes in the external (left panels) and internal (right panels) layers of 60°C (a) and 90°C (b) ablated cyst models showing color changes from pink to yellow as well as progressive dehydration of the tissue
Overall, there was a reduction in the thickness of all the measured layers as greater temperatures were achieved [Table 1]. However, not all the layers responded equally to the same amount of energy. A temperature over 50°C was required for obtaining any measurable effect in the muscularis propria, submucosa, and villi of the mucosa [Figure 5]. In the case of the crypts of the mucosa, a higher threshold of 60°C was necessary to observe any changes.
Table 1.
Measurements postablation
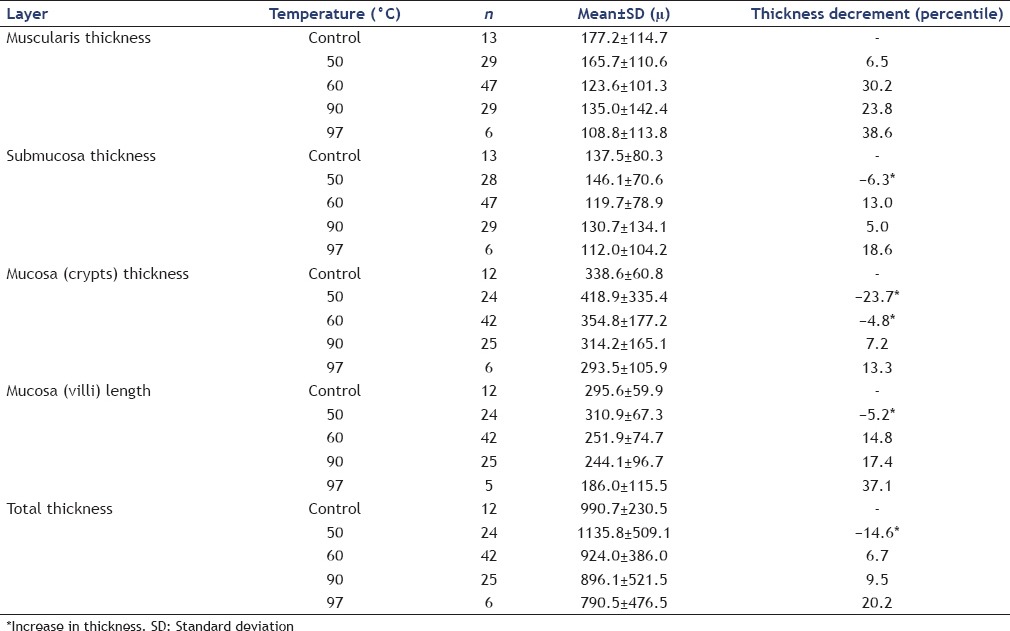
Figure 5.
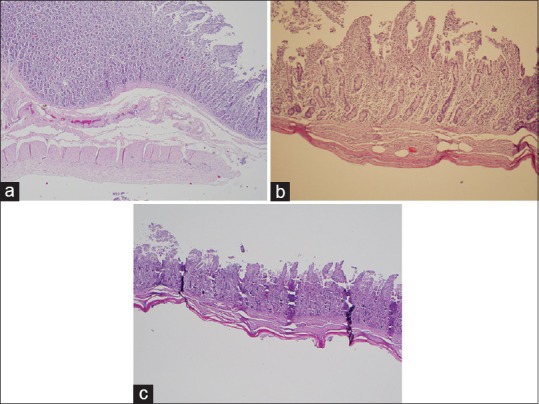
H and E staining of ablated samples. Microscopic changes showing a progressive decrease in the total thickness of the ablated samples comparing the control tissue (×40) (a) with the ablated samples at 60°C (×100) (b) and 90°C (×40) (c)
For the institution 2 subgroup analysis, an increased trend in the overall grade of ablation that was proportional to increases in temperature was seen in both the epithelium and the submucosa layers of each cyst. Despite this, some intracystic heterogeneity was observed in the ablation patterns among the different areas of the same cyst [Figure 6]. In the case of the muscularis propria, an asymmetric ablation pattern was observed in both the extension and the intensity of ablation in the cysts exposed to 90°C and 97°C.
Figure 6.
Representation of the intracystic variability percentage (extension of the ablation) and intensity (depth of the ablation) classified by temperature (range: 50°C–97°C) and distance from the incision of the needle (slide A, B, C, and D)
DISCUSSION
In this preclinical study, a novel EUS RFA needle using commonly available electrosurgical generators and settings produced a dose-dependent thermal injury to a villi form cyst model of pancreatic cystic lesions. A model of a PC (ligated segments of small intestine) seemed to provide a simple experimental model for thermal cyst ablation. Several studies have reported optimal results in in vivo EUS-guided RFA in porcine models with different probe and needle prototypes.[13,14,15,16,17] However, these experiments have all targeted healthy pancreatic parenchyma. Our study is based on the premise that energy propagates differently in solid mediums than in enclosed thinned-wall cavities filled with liquid/mucus. Therefore, the determination of the optimal generator settings in ex vivo cyst models seemed necessary before the in vivo studies. The novel needle RFA catheter delivered energy to the fluid-filled segments, resulting in time-dependent increases in temperature. The ablated area appeared to be temperature dependent, with a temperature threshold of at least 60°C and a safe cyst margin below 97°C.
Among new minimally invasive strategies, EUS-guided ethanol and chemotherapy lavage seem to be the most promising techniques, with several studies reporting optimal outcomes in in vivo patients.[9,10,11] However, a recent publication by Gómez et al. showed unsatisfactory results with ethanol alone, with only 9% of the participants achieving complete cyst resolution on follow-up imaging.[12] This group concluded that ethanol lavage did not appear to be a useful technique for preventing malignancy in PCs. One of the main limitations of this technique is the heterogeneous fluid distribution within the cyst when mucinous content or septations are present. Because mucinous cysts with septa pose a higher risk of malignancy, achieving homogenous epithelium ablation is necessary.
RFA generally refers to electrosurgical energy in the 350–500 kHz range. It is a well-established technique for the treatment of malignant lesions.[18] Recently, it has been used to treat pancreatic cancer in nonsurgical candidates with promising initial results;[19,20] however, the high rate of postprocedural morbidities limits its widespread application.[21] These undesirable effects are mainly due to the high susceptibility of the pancreatic tissue to heat. If the energy spreads outside the targeted tissue, parenchymal inflammation is highly likely to occur and result in clinical consequences. To address this matter, EUS RFA needle prototypes have been developed. As opposed to RFA during laparotomy, EUS-guided ablation allows a less invasive approach with real-time monitoring of tissue changes.
Fresh pig intestine was selected as an optimal cyst model due to the presence of villi that mimicked the papillary projections of IPMNs. With a simulated epithelium, we could evaluate the ablated epithelium and villi behavior, whereas the behavior of the external layers was described for the sake of the study and to represent and extrapolate how the energy propagates extrinsically to the cyst.
Overall, the decreasing thickness of the three layers correlated with exposure to higher temperatures. Because the tip of the thermometer was in direct contact with the cyst surface, we could obtain real-time monitoring of temperature changes. Initially, we evaluated a slightly different prototype needle with a temperature sensor in the needle tip. However, we found the temperature to vary widely due to the proximity of the sensor to the heating unit, and it distributed the heat unevenly within the cyst cavity. Therefore, we modified the design to include the external sensor to obtain accurate temperature readings for validation purposes. The maximum predetermined temperature of 97°C (safety margin) was used to avoid boiling point (100°C) and the consequent risk of cyst rupture. The results showed that 60°C was the lowest temperature required to observe any microscopic changes in the mucosa.
Although there was a clear correlation between the achieved maximum cyst temperature and the ablated area, there was some heterogeneity in the ablated pattern within the same cysts. The extension and the intensity ablation scores of the same cyst slightly varied depending on the slide reviewed. However, this pattern changed from one cyst to another, suggesting that there was no clear correlation between tissue ablation and the distance from the needle. This finding suggests an operator-dependent variability; the needle could have been displaced toward any of the cystic walls during the ablation, resulting in higher energy delivered to that specific area. An asymmetrical propagation of the thermal energy through the liquid seems improbable, as the cyst models were filled with a saline solution, which is an optimal energy conductor. On the other hand, the heterogeneous pattern was mainly seen under higher temperatures, which should be avoided in the clinical application to prevent ebullition. We postulate that this decrease in the ablation pattern, mostly in the muscularis propria, was secondary to dehydration of the tissue and was the result of a decrease in the conduction of energy. However, the muscularis propria was not the target of the ablation, and an asymmetrical ablation pattern should not be of concern with respect to the treatment of epithelial lesions.
This study is novel in that we used an RFA EUS needle prototype that used commercial and widely available electrosurgical units and settings in a cyst model. Moreover, it correlates preprocedure settings to histological outcomes. There is one study reporting RFA in human cystic neoplasms,[22] but the outcomes were monitored using cross-sectional imaging and could not be histologically corroborated. The optimal radio frequency power settings have not been determined to achieve cyst ablation without inducing tissue injury.
The main advantage of this new EUS needle prototype is that it connects with a standard electrosurgical unit that can be found in many endoscopy laboratories, avoiding the cost, and training of a dedicated RFA generator. Previously reported RFA prototypes[13,14,15,16,17] required specifically designed generator devices, which made their availability and widespread use more difficult. In our study, the prototype uses a soft-coagulation mode, which is already predetermined in any of the conventional electrosurgical units. Due to this novel use in experimental cyst ablation, a preliminary ex vivo phase of the study was necessary to determine the optimal generator patterns for a posterior application in an in vivo phase.
We believe that our results substantiate the feasibility of this technique and allow us to proceed to an in vivo animal phase of the study. Moreover, we believe that RFA could be applied to any type of cyst independently of its size because of the theoretical equal propagation of energy within an enclosed cavity. However, there are also some limitations in this study. The cyst models were filled with saline, which is an optimal electricity conductor. We are aware that mucinous cysts may not behave identically to these models. However, because heat transfer within fluid is determined by the heat transfer coefficient (h) and fluid thermodynamic laws and because current available data suggests similar or even overlapping “h” measures for both simple liquid and medium viscosity solutions, we believe these data can be safely extrapolated in these initial phases of the study. Because of the ex vivo nature of the project, the tissue characteristics are different from in vivo tissues. Other variables, such as the resistance caused by the surrounding cystic tissue or the flow from the vessels may influence how the energy propagates in in vivo tissues. It is likely that internal temperature probes attached to the needle with standard temperature targets as were found here may facilitate reproducibility in an in vivo setting. Furthermore, the fact that no pathological changes were seen in the epithelium at lower temperatures does not mean that this layer was still functional or that it did not denudate with time. In addition, the temperature changes were measured directly from the cyst surface. Therefore, correlation with the corresponding internal measurements may not have been completely accurate, but we believe this did not compromise the aim of the study. Last, the experimental models used here were all unilocular cysts, which may not represent some of the cysts seen in daily practice. However, despite this limitation, we believe that using thermal conductive ablation offers advantages over current chemical (ethanol, paclitaxel) ablation techniques with regard to multicystic lesions where fluid (e.g., ethanol) may not propagate into each cavity. The in vivo secondary stage of this project will hopefully clarify these unanswered questions and contribute to the ultimate application of EUS-guided RFA in the daily care of patients.
The management strategy for PCs has significantly changed to a more conservative and expectant approach.[23,24] Some subtypes of PCs without high-risk features or those that are less than 3 cm can be regularly followed with imaging studies. However, this continued surveillance strategy carries increased costs for each institution and significant anxiety for patients. Despite this, the morbidity related to surgical pancreatectomy outweighs the benefits of treating these incipient lesions. Further, PCs are typically detected in a population where surgery may not be a suitable option due to comorbidities and age-related conditions. Thus, the development of effective and less-invasive novel approaches for these PCs is warranted.
CONCLUSIONS
RFA of ex vivo cystic models with this new EUS needle prototype appeared to be a safe temperature-dependent technique that can be easily implemented in regular endoscopic labs.
Financial support and sponsorship
This study was partly funded by the Joyce E. Baker Foundation for Research at Mayo Clinic in Jacksonville, Florida.
Conflicts of interest
Devices for this study were provided by Cook Medical.
REFERENCES
- 1.Fernández-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. (713e1-2).Gastroenterology. 2010;139:708–13. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: Changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg. 2011;212:590–600. doi: 10.1016/j.jamcollsurg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–84. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 5.Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol. 2016;14:585–93e3. doi: 10.1016/j.cgh.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Maitra A, Fukushima N, Takaori K, et al. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 7.Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: A systematic review and meta-analysis. Ann Surg. 2012;255:1048–59. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 8.Swanson RS, Pezzi CM, Mallin K, et al. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: More than 20,000 resections from the national cancer data base. Ann Surg Oncol. 2014;21:4059–67. doi: 10.1245/s10434-014-4036-4. [DOI] [PubMed] [Google Scholar]
- 9.Swanson RS, Pezzi CM, Mallin K, et al. Ethanol lavage of pancreatic cystic lesions: Initial pilot study. Gastrointest Endosc. 2005;61:746–52. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–6. doi: 10.1016/j.gie.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–9. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Gómez V, Takahashi N, Levy MJ, et al. EUS-guided ethanol lavage does not reliably ablate pancreatic cystic neoplasms (with video) Gastrointest Endosc. 2016;83:914–20. doi: 10.1016/j.gie.2015.08.069. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: Results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 14.Carrara S, Arcidiacono PG, Albarello L, et al. Endoscopic ultrasound-guided application of a new internally gas-cooled radiofrequency ablation probe in the liver and spleen of an animal model: A preliminary study. Endoscopy. 2008;40:759–63. doi: 10.1055/s-2008-1077520. [DOI] [PubMed] [Google Scholar]
- 15.Di Matteo F, Martino M, Rea R, et al. EUS-guided Nd: YAG laser ablation of normal pancreatic tissue: A pilot study in a pig model. Gastrointest Endosc. 2010;72:358–63. doi: 10.1016/j.gie.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Seo DW, Hassanuddin A, et al. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039–43. doi: 10.1016/j.gie.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Gaidhane M, Smith I, Ellen K, et al. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) of the pancreas in a porcine model. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/431451. 431451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MH, Yang W, Yan K, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005;11:6395–401. doi: 10.3748/wjg.v11.i40.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui Y, Nakagawa A, Kamiyama Y, et al. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14–20. doi: 10.1097/00006676-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220–5. doi: 10.1002/bjs.6800. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Tang Z, Fang H, et al. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392–5. doi: 10.1002/jso.20580. [DOI] [PubMed] [Google Scholar]
- 22.Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–9. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]