Abstract
Clinical cytology was originally used by clinicians to provide rapid diagnosis. However, with advancing medical subspecialization, few clinicians interpret cytology themselves these days, for example, gynecologists, hematologists, urologists, and occasional gastroenterologist (mainly in Asian countries). Cytological assessment enjoyed a renaissance with the development of endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA). Subsequently, pathologists, most of them more experienced in histology, had to take over. Recently, it has been shown that in-room cytology can be easily performed by the endoscopist themselves for initial evaluation of the quality of the EUS-FNA specimen and an initial diagnosis distinguishing benign or malignant cells. Bringing cytology back to the clinician has some advantages but does not substitute the professional cytopathologist. This report has written to lower the threshold for the clinician to find his way back to the microscope, which may improve both their diagnostic yield and assessment of EUS-FNA sample quality.
Keywords: Endoscopy, guidelines, histology, intervention, oncology
INTRODUCTION
With the introduction of endoscopic ultrasound (EUS) fine-needle aspiration (FNA) tissue, acquisition from previously inaccessible areas in the body became routinely possible.[1] According to a German survey, EUS-FNA is mainly used to obtain specimens from enlarged mediastinal and abdominal lymph nodes and from the pancreas.[2] Furthermore, endoscopists can now take specimens from all areas in proximity of the upper gastrointestinal tract, including liver lesions, left adrenal gland, and spleen.[3] The EUS-FNA technique has increased the diagnostic potential of EUS enormously, decreasing the number of surgical interventions for diagnostic sampling. With increasing availability of this new sampling method, endoscopists had to train their skills in the preparation of specimens and pathologists had to deal with FNA samples of formerly rarely targeted areas using only cytological preparation and staining methods. Endoscopists as well as pathologists strive to improve the diagnostic processes in obtaining very small samples to increase accuracy for clinical decision-making.[4,5,6] Some studies demonstrate that the presence of a cytopathologist in the endoscopy suite might further improve the results.[7] Rapid on-site evaluation (ROSE) evolved and is still performed in various centers though its cost-effectiveness is highly disputed.[8] In view of limited resources, the question arises as to whether the endoscopist themselves could be trained to acquire basic skills in cytology.[9] This report will guide the reader in initial on-site self-assessment of EUS-FNA specimens to further improve their own diagnostic abilities.
FINE-NEEDLE ASPIRATION CYTOLOGY
Needle sizes in relation to quality of cytologic specimen
In the early days of EUS-guided FNA, only the 22-gauge Vilman type aspiration needle was available for diagnostic cytology.[10] Since then, a number of technical improvements have been made; however, the basic initial system remains in principle unchanged. According to the EFSUMB guidelines of interventional ultrasound, 22-gauge is still the mostly used size for endoscopic FNA.[8] Larger caliber needles were designed to obtain specimens large enough for histological methods, including development of the 19-gauge and 19-gauge truecut needle.[11] However, in practice, 19-gauge needles did not produce adequate histological specimens any more often than 22-gauge needles but added technical challenges as the larger needle is hard to handle in difficult positions with angulation of the endoscope tip. Increasing diameter of the needle also increases blood contamination of the specimen and the cytological quality of the specimen worsens due to bigger cell blocks which cannot be analyzed in conventional smears.[12] Therefore, 22-gauge needles are not widely used in clinical practice.[13]
Again, new needle designs such as the Cook Procore® system with reverse bevel technology, available in 22- and 25-gauge, have the same problems for cytological assessment because cytology is best performed by single cells spread over the slide and not cell blocks.[14] For this reason, 25-gauge needles, initially developed for puncturing hard or freely movable lesions, perform very well as cytology needles; blood contamination is limited, and obtained cells spread nicely over slides.[15]
Different methods of fine-needle aspiration and their impact on cytology
To improve results, many studies determining puncture technique have been performed. There are studies regarding the optimal number of passes, and the impact of negative pressure applied to the needle (no suction, little suction, high suction, or suction by gradually removing the stylet).[16,17,18] In general, both blood contamination and diagnostic yield improve with increasing number of passes through the lesion, so generally five or more passes are recommended.[19,20] The suction studies tend to show better results with gradual removal of the stylet; however, in clinical practice, negative pressure using the provided syringe performs well. We generally use high negative pressure for the first puncture; however, if the lesion is highly vascular and blood rapidly appears in the syringe, the second puncture is performed with little or no negative pressure. In general, a fan-like puncturing of a lesion should be immediately stopped as soon as blood appears in the syringe because the obtained material cannot be as easily removed from the syringe as from the needle. In this instance, a single use brush can be used to transfer the blood containing the diagnostic cells to glass slides.
Removing the material from the needle
The best method to remove the material from the needle is by slowly reinserting the stylet while holding the needle tip on a slide.[21] The use of water or saline should be avoided due to cause of “osmotic artefacts” which make diagnosis difficult. Flushing the needle with saline should be avoided because it results in uncontrolled splashing of the specimen as well as drying artefacts because of the wet surrounding. Blowing through the needle with air could be performed after removing the stylet again to harvest remaining material. However, normally, clearance of material from the needle is sufficient using the stylet insertion method alone. Approximately, a half drop of water should be placed on one slide, and then, a second slide should be immediately placed on top of the first slide and pulled apart in a continuously and steady matter.[22] The immediate preparation of the smear avoids drying artefacts which start as soon as the specimen is out of the needle. The principal aim is to gain thin smears with a single layer of cells on the slide.
Handling of the specimen after performing smears
The requirements for specimen fixation depend on the staining method. At present, two major staining methods are used for performing basic cytology. The original staining technique developed, and still used in gynecology, is the Papanicolaou staining with wet fixation of the slides with alcohol.[23] The Papanicolaou staining does improve the visibility of the chromatin structure of the cell nucleus but does not improve the visibility of the cytoplasma. Another disadvantage is that alcohol fixated smears are harder to use for further immunocytological staining methods.[24] The easier method to apply is May-Gruenwald-Giemsa (MGG) staining. This staining method is currently used as a standard staining method in hematology for blood – and bone marrow – smears and is routinely available in every laboratory. The advantage of the method is that no fixation of the specimen is necessary. The MGG staining gives excellent differential morphology of all cell structures and the ability to perform cytochemistry for further differentiation of cells. Immunochemistry and immunostaining methods are turning out to be very reliable because of the missing denaturation effect of proteins due to a fixation method.[25] The air-dried, nonfixated material is also the optimal material for molecular diagnosis/mutation analysis, which can be easily performed on EUS-FNA material.
To apply ROSE in the endoscopy suite, a quick stain method is required. There are commercially available sets such as Häma Quick stain on-hand, which allow staining of single slides within 2–3 min and include simple instructions.[26] In the normal staining protocol for rapid stain solutions (e.g. Hämacolor™ or Diff-Quick™ – Stain), air-dried slides are immersed 3–5 times (about 5–10 s) in a fixation solution and then in a red and a blue staining solution before excess stain is washed off with water. After air-drying again (which can be hastened with a hair drier), the slides are ready for microscopic examination.
The quick staining method allows an initial assessment of smear adequacy and an initial evaluation regarding the presence of benign or malignant cells. Increasing experience enables an immediate diagnosis in the endoscopic room. The same slides can be reviewed by formal cytopathologists.
MICROSCOPY OF THE SPECIMEN
The dried slides can be covered with a protecting slide or used as they are. For the initial evaluation, a 20 times magnification objective is most suitable. After identifying a promising area, the cells should be evaluated with 400 times magnification or by oil immersion objective with 1000 times magnification. The slide should be searched in a meandering manner, to find the best cells for evaluation. The first goal is to prove that the material is representative of the organ or target lesion. The second purpose is to perform an immediate diagnosis. Blood clots are not suitable for cytological evaluation. The best areas are cells lying in a single layer either on their own or in single cell connections. If there are at least 10–20 good preserved and representative cells available in the first stained slides, a final morphological diagnosis can be made by an experienced cytologist and the material can be concluded “good.”
Typical examples for initial evaluation
Knowledge of the typical cell appearance from pancreatic and lymph node tissue is crucial for the endoscopist as they are most frequently targeted by EUS-guided FNA. Based on air-dried material and staining methods as, for example, Diff-Quick-Stain, a quick learning of the basics of morphology is possible. For initial evaluation, the rules to distinguish benign from malignant cells are quite simple. Knowing the typical cells of the target organ/lesion and variety of benign cells enables one to make a correct diagnosis immediately. Benign cell groups are mostly still connected to each other and do not show a large variety of nucleoli. The nuclei are positioned mostly in the middle of the cell, the chromatin is homogenous, and the core is well defined. Cells with a strong anisokaryosis, hyperchromasy, and irregularity of the nuclei are usually malignant. Malignant nucleoli of an adenocarcinoma or squamous cell carcinoma are inhomogenous, the size of the core is often bigger than 2.5 times in diameter of an erythrocyte (<7 μm), the chromatin is inhomogenous and the nucleoli are often not in the center. To detect neuroendocrine tumors and small cell carcinomas is harder because the size of the nuclei is similar to the size of nuclei of benign cells. Whereas the chromatin of neuroendocrine cells is basically homogenous, the main discrimination from a small cell carcinoma is based on identifying inclusion core bodies which are typically in small cell carcinoma and can only be visualized using an oil immersion objective. The most important feature, however, is the pleomorphy of the nuclei. Those four entities can diagnose the majority of the pancreatic diseases requiring EUS-FNAC [Table 1 and Figures 1–5].
Table 1.
Cytological criteria for malignancy
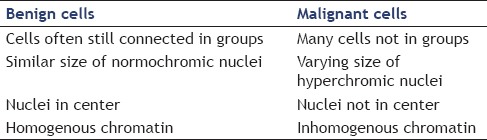
Figure 1.
Normal pancreatic tissue (May Grünwald Giemsa staining, ×400), note that the cells are connected to each other and the nuclei are similar to each other. The nuclei are roughly the size of an erythrocyte (not visible in this picture)
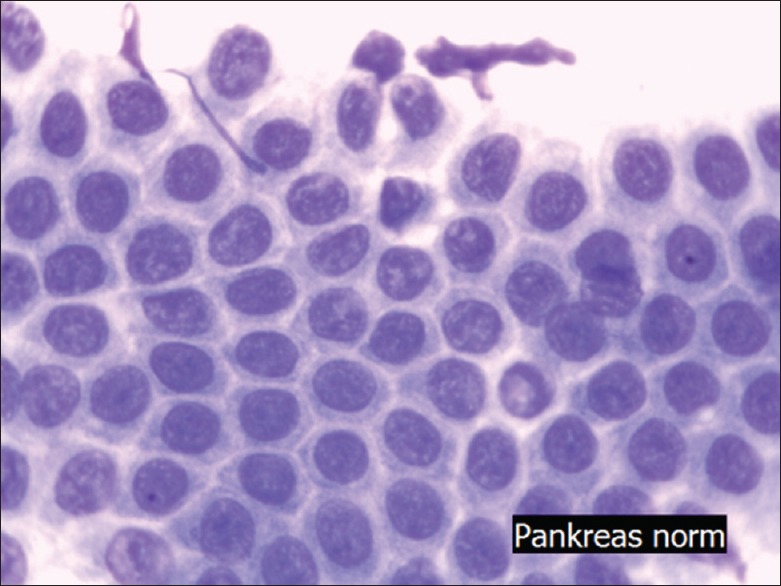
Figure 5.
Epitheloid granuloma (May Grünwald Giemsa, ×400) cells are connected to each other as a granuloma. Nuclei are bean shaped, hypochromatic, and have a big cytoplasmatic rim (squamous cell like)
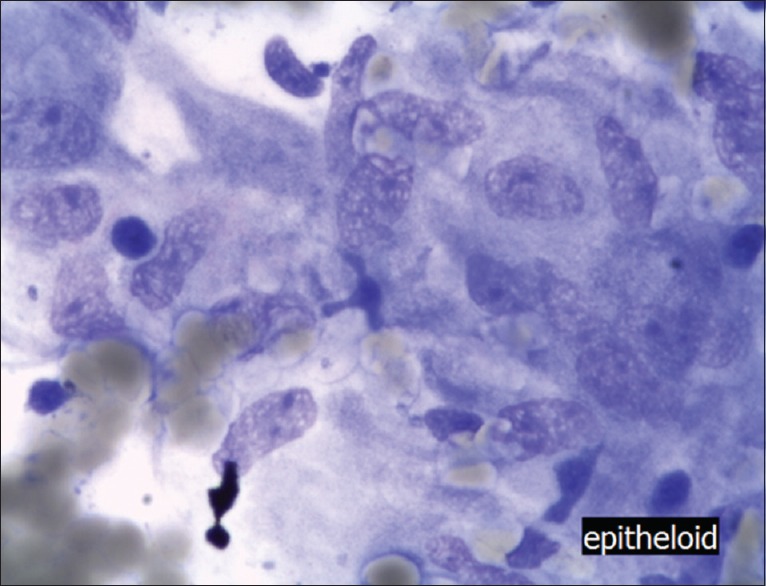
Figure 2.
Adenocarcinoma of the pancreas (May Grünwald Giemsa staining, ×400) Note that the nuclei are inhomogenous and reaching more than 2, 5 fold the size of erythrocytes (some are visible in the left upper end of the picture), mitotic figures are clearly to be seen in the cancerous cells
Figure 3.
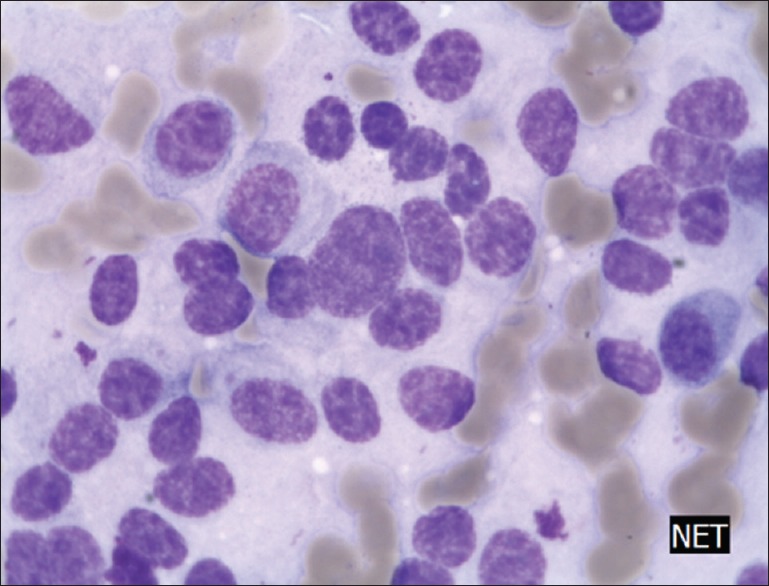
Neuroendocrine cells of the pancreas (May Grünwald Giemsa staining ×400) note that nuclei are roughly the size of erythrocytes (next to the tumor cells); however, cell connection is lost and the nuclei are slightly different from each other, nuclei are typically hyperchromatic
Figure 4.
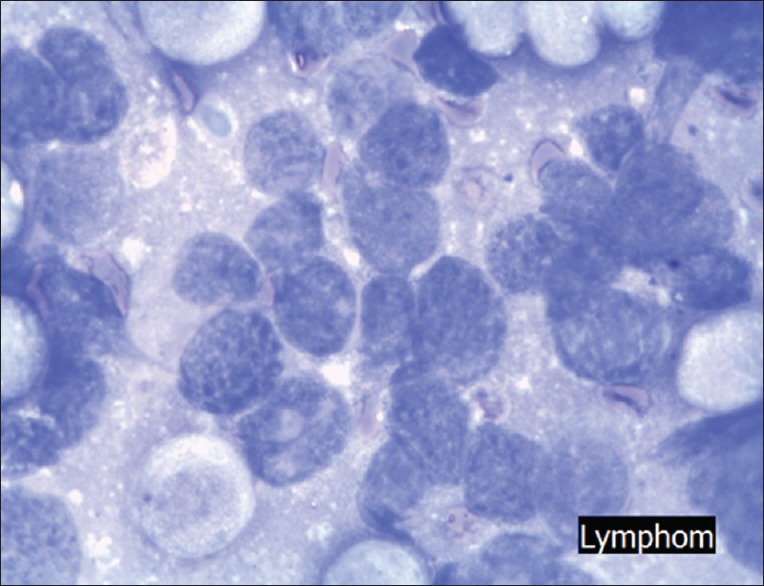
Lymphoma (May Grünwald Giemsa staining, ×400) cells are hypochromatic and not connected, nuclei are slightly different to each other however spread out closely
In lymph nodes, the cells of adenocarcinoma, squamous cell carcinoma, and small cell carcinoma can be detected as described before. Two more cell formations should be known to cover the majority of cases. The typical formation of epitheloid granulomas should be mentioned. In sarcoidosis, a cluster of cells with spindle-shaped or bean-like nuclei and a wide cytoplasma rim can be seen. The cores are homogenously configured and mostly located in the middle of the cells. Basically those cells can look like mature squamous cells but with a bean-like nuclei.
Lymphomas can be suspected if the slides are loaded with a massive amount of uniform single cells. Most often, the presence of lymphoma can already be recognized by gross visual examination of the slides after the staining method. Large number of cells are hypochromatic but not connected and nuclei are slightly different to each other. Under the microscope, the slide is full of single, fairly uniform appearing cells with only a small cytoplasmatic rim. The nuclei are mostly hypochromatic.
ADVANTAGES OF SELF-MADE CYTOLOGY IN CLINICAL PRACTICE
Performing basic clinical cytology oneself provides various advantages for a clinician.[9,27,28,29] First of all, the immediate result regarding smear quality can stimulate further FNA-passes if the material is nondiagnostic. This leads to a very high sensitivity and decreased need to repeat the EUS-FNA, reducing service demand. Frequently, on-site assessment allows an initial diagnosis, while in some cases, the changes can be so obvious that the result can be given to the patient and further diagnostic or treatment options immediately initialized. Performing cytology helps clinicians to interpret cytological reports in the right context. Sometimes, it is not easy even for an experienced cytopathologist to make the correct diagnosis and additional tests, for example, immunostaining, have to be performed. If so, the result must be seen within the clinical context and not considered the gold standard. The clinicians’ understanding of these technical and diagnostic challenges is markedly improved by performing cytology themselves. Clinician-performed cytology also improves the communication between clinicians and cytopathologists, particularly in controversial cases where the contact and level of information exchange needed is much higher. The clinician has the massive advantage of having all the relevant patient information, whereas the clinical information available to a pathologist is usually limited, even when the cytology request form is filled out properly.
Diagnostic potential of cytology
There is an ongoing debate among histologists and cytologists regarding which method is more reliable and suitable. Both methods have advantages and disadvantages and in the hands of an expert, each can achieve excellent results.
One recommendation has been to split material to use the advantages of both methods. However, dividing the specimen risks uneven distribution or worse “half of the material gives the half of the diagnosis.”
What relevance have cytology and histology in the context of EUS-guided FNA? One important aspect has yet to be mentioned. The design and the handling technique of the needle scrapes single cells or cell blocks of the tumor itself. Normally, the transition zone between tumor and healthy tissue is not included in the sample or is broken apart. This presents a major disadvantage for histological evaluation because one important criterion of malignancy, invasion into healthy tissue, will not be seen material obtained by EUS-FNA. Based on this, the decision of cancerous or noncancerous lesions has to be made on the cells themselves. This implies that the preparation and examination of the whole material for cytology can lead to the best results.
Histological specimen are usually prepared using formalin which causes protein denaturation and degeneration, but histological criteria of malignancy are much harder to detect using formalin-based histology methods then in air-dried cytological specimen. Using air-dried cell material and air-dried staining methods (e.g., MGG, Hämacolor or Diff-Quick-Stain), leads to a better, fine morphology and improves the diagnostic outcome. This is reflected by studies, which show a higher sensitivity for cytology in comparison to histology. In cytology, all diagnostic criteria can be easier to detect and a conclusion can virtually being made with a single tumor cell.[8] One advantage of histology is the greater reliability of immunohistochemistry staining methods. Although all immunohistochemical methods can also be used on air-dried cytological smears, the handling of the specimen seems to be more difficult than in imbedded material.[30] None the less, techniques for thin air-dried smears can be learned easily. A plentitude of studies is available demonstrating that immunocytochemistry can discriminate different tumor types as well as histological methods. Even modern receptor analysis and molecular diagnostics is possible on air-dried smears.[31,32,33,34]
Standardized cytological terminology
Although still widespread and comprehensible, the old Papanicolaou classification, originally used for gynecological smears, should be avoided in reporting cytology results of endoscopic FNA. In 2014, the Papanicolaou society proposed the following updated terminology:[35]
Nondiagnostic
Negative (for malignancy)
Atypical
Neoplastic: Benign or other
Suspicious (for malignancy)
Positive/malignant.
This new classification includes the term neoplastic, which is important especially for cystic pancreatic lesions, which do not have to be malignant although they are neoplastic. In our opinion, the recommended classification above, does not have any advantages to the original Papanicolaou classification [Table 2].
Table 2.
Comparison of original and new Papanicolaou classification
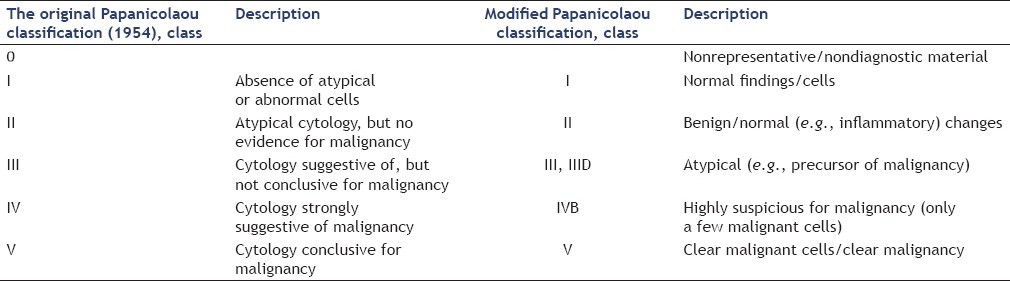
A slightly modified Papanicolaou classification (adding a Class 0 for nonrepresentative/nondiagnostic material and IVa for intraepithelial Neoplasia or Carcinoma in Situ), is used widely in Germany for gynecological as well as nongynecological cytology. The most important reason for the use of the modified Papanicolaou classification is that it is known to most clinicians and is explained in nearly every medical dictionary.
SUMMARY
The opportunity to perform on-site cytology themselves enables clinicians to optimize the diagnostic yield of EUS-guided FNA. It is an enhancement of the eye and deepening their visualization. Based on air-dried cell material, it is easy to learn and perform a cytological diagnosis. Preparation of the specimen is easily carried out though the initial cytological assessment of sample adequacy and interpretation has a steep learning curve, which is quite unusual for new methods in modern medicine. The clinician can easily become so involved that they no longer wish to miss out on assessing their own specimen. The most astonishing benefit, however, is improved communication with the pathology community where the acceptance of ROSE performed by endoscopists is higher than expected. Sometimes, we have to cross professional boundaries and learn new skills for the good of our patients. We would like to encourage all colleagues to learn this method themselves.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Nathan Atkinson for reviewing the manuscript.
REFERENCES
- 1.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 2.Jenssen C, Faiss S, Nürnberg D. Complications of endoscopic ultrasound and endoscopic ultrasound-guided interventions – Results of a survey among German centers. Z Gastroenterol. 2008;46:1177–84. doi: 10.1055/s-2008-1027334. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound-guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Dig Dis. 2008;26:356–63. doi: 10.1159/000177022. [DOI] [PubMed] [Google Scholar]
- 4.Pang NK, Chin SY, Nga ME, et al. Comparative validation of c-kit exon 11 mutation analysis on cytology samples and corresponding surgical resections of gastrointestinal stromal tumours. Cytopathology. 2009;20:297–303. doi: 10.1111/j.1365-2303.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 5.Spieler P, Ammann M, Schönegg R. Fine-needle aspiration cytology. Aspects of a minimally invasive diagnostic procedure. Pathologe. 2007;28:325–33. doi: 10.1007/s00292-007-0925-7. [DOI] [PubMed] [Google Scholar]
- 6.Jhala NC, Jhala DN, Chhieng DC, et al. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol. 2003;120:351–67. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 7.Mehmood S, Jahan A, Loya A, et al. Onsite cytopathology evaluation and ancillary studies beneficial in EUS-FNA of pancreatic, mediastinal, intra-abdominal, and submucosal lesions. Diagn Cytopathol. 2015;43:278–86. doi: 10.1002/dc.23207. [DOI] [PubMed] [Google Scholar]
- 8.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV – EUS-guided Interventions: General aspects and EUS-guided sampling (Long Version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]
- 9.Hocke M, Ignee A, Topalidis T, et al. Back to the roots – Should gastroenterologists perform their own cytology? Z Gastroenterol. 2013;51:191–5. doi: 10.1055/s-0032-1313148. [DOI] [PubMed] [Google Scholar]
- 10.Vilmann P, Hancke S, Henriksen FW, et al. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25:523–7. doi: 10.1055/s-2007-1010389. [DOI] [PubMed] [Google Scholar]
- 11.Wiersema MJ, Levy MJ, Harewood GC, et al. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc. 2002;56:275–8. doi: 10.1016/s0016-5107(02)70193-4. [DOI] [PubMed] [Google Scholar]
- 12.Jenssen C, Möller K, Wagner S, et al. Endoscopic ultrasound-guided biopsy: Diagnostic yield, pitfalls, quality management part 1: Optimizing specimen collection and diagnostic efficiency. Z Gastroenterol. 2008;46:590–600. doi: 10.1055/s-2008-1027413. [DOI] [PubMed] [Google Scholar]
- 13.Songür N, Songür Y, Bircan S, et al. Comparison of 19- and 22-gauge needles in EUS-guided fine needle aspiration in patients with mediastinal masses and lymph nodes. Turk J Gastroenterol. 2011;22:472–8. doi: 10.4318/tjg.2011.0322. [DOI] [PubMed] [Google Scholar]
- 14.Yamabe A, Irisawa A, Shibukawa G, et al. An experimental study to assess the best maneuver when using a reverse side-bevel histology needle for EUS-guided fine-needle biopsy. Endosc Int Open. 2016;4:E56–61. doi: 10.1055/s-0041-107801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhoun MF, Wani SB, Rastogi A, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 16.Villa NA, Berzosa M, Wallace MB, et al. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17–20. doi: 10.4103/2303-9027.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aadam AA, Oh YS, Shidham VB, et al. Eliminating the residual negative pressure in the endoscopic ultrasound aspirating needle enhances cytology yield of pancreas masses. Dig Dis Sci. 2016;61:890–9. doi: 10.1007/s10620-015-3860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 19.Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22:628–40. doi: 10.3748/wjg.v22.i2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uehara H, Sueyoshi H, Takada R, et al. Optimal number of needle passes in endoscopic ultrasound-guided fine needle aspiration for pancreatic lesions. Pancreatology. 2015;15:392–6. doi: 10.1016/j.pan.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Tadic M, Stoos-Veic T, Kusec R. Endoscopic ultrasound guided fine needle aspiration and useful ancillary methods. World J Gastroenterol. 2014;20:14292–300. doi: 10.3748/wjg.v20.i39.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Jaiwong K, Nimmanhaeminda K, Siriaree S, et al. Cytomorphologic comparison between rehydrated air-dried and conventional wet-fixed pap smears. J Med Assoc Thai. 2006;89:1811–6. [PubMed] [Google Scholar]
- 24.Denda T, Kamoshida S, Kawamura J, et al. Optimal antigen retrieval for ethanol-fixed cytologic smears. Cancer Cytopathol. 2012;120:167–76. doi: 10.1002/cncy.21192. [DOI] [PubMed] [Google Scholar]
- 25.Furuhata A, Shirahase H, Shirai T, et al. Utility of rapid on-site cytology in endoscopic ultrasonography-guided fine needle aspiration of pancreatic masses. Rinsho Byori. 2012;60:429–34. [PubMed] [Google Scholar]
- 26.Lee YN, Moon JH, Kim HK, et al. A triple approach for diagnostic assessment of endoscopic ultrasound-guided fine needle aspiration in pancreatic solid masses and lymph nodes. Dig Dis Sci. 2014;59:2286–93. doi: 10.1007/s10620-014-3119-1. [DOI] [PubMed] [Google Scholar]
- 27.Hikichi T, Irisawa A, Bhutani MS, et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322–8. doi: 10.1007/s00535-009-0001-6. [DOI] [PubMed] [Google Scholar]
- 28.Savoy AD, Raimondo M, Woodward TA, et al. Can endosonographers evaluate on-site cytologic adequacy? A comparison with cytotechnologists. Gastrointest Endosc. 2007;65:953–7. doi: 10.1016/j.gie.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Varadarajulu S, Holt BA, Bang JY, et al. Training endosonographers in cytopathology: Improving the results of EUS-guided FNA. Gastrointest Endosc. 2015;81:104–10. doi: 10.1016/j.gie.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010;45:868–75. doi: 10.1007/s00535-010-0217-5. [DOI] [PubMed] [Google Scholar]
- 31.Jain D, Mathur SR, Guleria R, et al. Utility and pattern of positivity of p40 in the diagnosis of squamous cell carcinoma of the lung by cytology: The first study on fine needle aspiration smears. Cytopathology. 2014;25:330–5. doi: 10.1111/cyt.12105. [DOI] [PubMed] [Google Scholar]
- 32.Zheng G, Ettinger DS, Maleki Z. Utility of the quantitative Ki-67 proliferation index and CD56 together in the cytologic diagnosis of small cell lung carcinoma and other lung neuroendocrine tumors. Acta Cytol. 2013;57:281–90. doi: 10.1159/000346394. [DOI] [PubMed] [Google Scholar]
- 33.Wu HH, Jones KJ, Cramer HM. Immunocytochemistry performed on the cell-transferred direct smears of the fine-needle aspirates: A comparison study with the corresponding formalin-fixed paraffin-embedded tissue. Am J Clin Pathol. 2013;139:754–8. doi: 10.1309/AJCP8O7VIGSIXIVS. [DOI] [PubMed] [Google Scholar]
- 34.Collins BT, Wang JF, Bernadt CT. Utilization of p40 (DeltaNp63) with p63 and cytokeratin 5/6 immunohistochemistry in non-small cell lung carcinoma fine-needle aspiration biopsy. Acta Cytol. 2013;57:619–24. doi: 10.1159/000354213. [DOI] [PubMed] [Google Scholar]
- 35.Layfield LJ, Ehya H, Filie AC, et al. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11(Suppl 1):4. doi: 10.4103/1742-6413.133352. [DOI] [PMC free article] [PubMed] [Google Scholar]


