Abstract
Background:
Several studies have shown that the application of amniotic membrane as a biological dressing in the management of burns is accompanied by rapid re-epithelialisation. In this follow-up study, we aimed to evaluate the possible role of amniotic membrane as an adjunct to split thickness skin grafting on reducing itching and severity of hypertrophic scar formation.
Materials and Methods:
From October 2013 to January 2015, in a prospective follow-up study, 54 patients (108 limbs) with second and third degree burns, covering 4%–15% of total body surface area (TBSA), were included in the study. All patients needed split-thickness skin grafts for burn-wound coverage. Selected patients had symmetric burns on two (upper or lower) extremities. Then, in every patient, the extremities were randomly divided into two groups: In one limb, the skin graft was traditionally fixed with skin staples (control group) and in the other limb, the skin graft was covered with an amniotic membrane (amnion group). Therefore, in every patient, the graft was covered with an amniotic membrane in one extremity and fixed with skin staples in the other extremity. Finally, after 6 months, the degree of itching and hypertrophic scar formation was compared between the two groups.
Results:
The study group was composed of 108 limbs in 54 patients (27 males and 27 females) with a mean age of 23.54 ± 4.9 years and burn 9.03 ± 2.69% TBSA. The patients were divided into two groups: 54 limbs in amnion group and 54 limbs in control group. In 59.25% of the cases, patient had less itching in the extremity covered with amniotic membrane. Furthermore, in 64.81% of the cases, patients had less hypertrophic scar formation in the extremity covered with amniotic membrane. These differences were statistically significant (P < 0.001).
Conclusions:
Amniotic membrane used as an adjunct in split thickness skin grafting is a novel modality which significantly reduces scar formation and itching that can be greatly distressing to burn patients. However, still more prospective well designed studies are needed to prove it.
Keywords: Amniotic membrane, burn, follow-up study, hypertrophic scar, itching
INTRODUCTION
Hypertrophic scarring induced by burns causes physical and psychological trauma to patients which may interfere with a healthy and active life. Hypertrophic scars can be itchy or painful, decrease range of motion of different joints and reduce self-esteem due to aesthetic issues.[1,2] They result from a deranged interrupted normal wound healing process and are defined as elevated erythematous lesions confined within the wound margins, which begin to appear mostly between 2 to 6 months after burns.[2,3] Two major risk factors are associated with hypertrophic scar development: Wound depth and healing time. The desired time for optimum healing has been mentioned to be below 21 days.[1,2,3,4] Annually, four million new cases are involved with burn induced scar formation in the developed world. The reported incidence rate for hypertrophic scarring after burn is between 30% and 91% in the literature.[3] There are different treatments regarding this situation including pressure therapy, silicone application, intralesional or topical corticosteroids, laser therapy, cryotherapy, radiation, immunotherapy, surgery, antimetabolites, etc.[5,6] However, an optimal therapeutic method is not yet achieved because of high failure and recurrence rate and side effects such as hypopigmentation and atrophy associated with current treatments.[2,7] The high possibility of hypertrophic scar formation after burns and the lack of an effective therapeutic approach indicate the necessity for adequate preventive management. Another annoying problem in burn patients in the healing phase or beyond after that is pruritus. This occurs very commonly in these patients which can even persist for several years. It does not respond well enough to routinely used treatments makes the management difficult.[8,9] The main surgical approach towards burn wounds is autologous split thickness skin graft.[3] Immobilisation of the skin graft is an important key to its faster revascularisation and prevention of graft loss. In our previous study, we have shown the positive effect of amniotic membrane in fixation of skin grafts resulting in a shorter duration of graft take.[10] This positive effect can potentially decrease the healing time and reduce the severity of hypertrophic scar formation when a skin graft is employed. In this study, we aimed to evaluate the possible role of amniotic membrane as an adjunct in split thickness skin graft on reducing itching and severity of hypertrophic scar formation in selected patients with symmetric burns in the extremities. In one extremity, the skin graft was fixed with skin staples and in the other extremity, the graft was wrapped by an amniotic membrane.
MATERIALS AND METHODS
From October 2013 to January 2015, in a prospective follow-up study of our previous clinical trial,[10] 54 patients (108 limbs) with second- and third-degree burns, covering 4%–15% of total body surface area (TBSA) were included. All patients needed split-thickness skin grafts for burn-wound coverage.
Our excluding criteria were wound infection, age more than 60 years and history of cardiac disease, renal failure, diabetes mellitus and any other severe, underlying metabolic disorder. We received the approval of Shiraz University of Medical Sciences Ethical Committee. All the patients (or their parents) signed an informed consent. We selected patients who had symmetric burns on two upper or lower extremities. Then, in every patient, the extremities were randomly divided into two groups: in one limb, the skin graft was traditionally fixed with skin staples (control group) and in the other limb, the skin graft was covered with an amniotic membrane (amnion group). All procedures were performed by an experienced burn surgeon using a (1.5/1) meshed graft.
In the amnion group, the graft was placed on the wound bed, the amniotic membrane was wrapped around the grafted extremity and the dressing applied. The membrane adheres to itself when wrapped around the extremity, and there is no need to use any stitch or staple. With meticulous dissection during a preparation procedure, amniotic membrane pieces as large as 10–20 cm × 30–50 cm are retrievable. In children and in the upper extremity of adults, it is very easy to wrap the extremity with an amniotic membrane. In the lower extremity, usually it is possible to do the same. However, even in situ ations where the size of a single membrane is not enough, it is possible to use two or three pieces of amniotic membrane to cover the skin graft.[10]
Previously, we have published the technique of amniotic membrane processing.[10] We have a tissue bank at our burn centre for amniotic membranes retrieved from the elective caesarean sections of mothers without any sexually transmitted disease, endometritis, premature rupture of the membranes or meconium-stained amniotic fluid. The amnion is carefully separated from the chorion and placenta and washed thoroughly with normal saline until a whitish, smooth, transparent layer remains, which is then stored in normal saline–gentamicin solution at 4°C. If Human Immunodeficiency Virus (HIV), hepatitis B surface (HBS), hepatitis C virus (HCV) and venereal disease research laboratory (VDRL) tests are negative for both mother and umbilical cord, the amniotic membrane will be used. Routine cultures are obtained from stored membranes to rule out bacterial contamination.[10]
After 6 months, patients were examined by an experienced burn surgeon, who was blinded to the treatment. The thickness of the scars was measured by calliper and hypertrophic scar measured according to modified Vancouver scar scale. Furthermore, itching ranking from 0 (no itching) to 4 (severe itching) was done by the same surgeon.
The mean thickness of scars and itching ranks during the time were calculated and each pair (control and amnion group) were compared to find out whether or not using amniontic membrane in skin graft could reduce the scar and itching in comparison with its control group.
The collected data were presented as mean and standard deviation (SD) (mean % SD). Normality of data was evaluated by the one-sample Kolmogorov–Smirnov test. As the distribution of our data was skewed, the Wilcoxon signed-rank test was used to compare the itching and hypertrophic scar per cent in two groups. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 16.0 software and P < 0.05 was considered statistically significant.
RESULTS
The study group was composed of 108 limbs in 54 patients (27 males and 27 females) with a mean age of 23.54 % 4.9 years and burn 9.03 % 2.69% TBSA. The mechanism of burn was flame (63%), scald (18.5%) and flash (18.5%). The patients divided into two groups: 54 limbs in amnion group and 54 limbs in control group. The details of severity of itching and hypertrophic scar formation are shown in Tables 1 and 2, respectively. The mean % SD of itching grade was 0.92 % 0.57 and 1.64 % 0.70 in amnion and control group, respectively [Table 3]. The mean % SD of hypertrophic scar severity was 1 % 0.47 and 1.72 % 0.68 in amnion and control group, respectively [Table 4].
Table 1.
Severity of itching
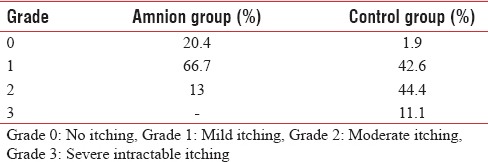
Table 2.
Severity of hypertrophic scar
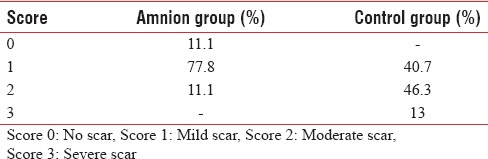
Table 3.
Itching grade in amnion and control group

Table 4.
Scar severity in amnion and control group

In 59.25% of the cases, patient had less itching in the extremity covered with amniotic membrane [Table 5]. Furthermore, in 64.81% of the cases, patient had less hypertrophic scar formation in the extremity covered with amniotic membrane [Table 6]. This differences were statistically significant (P < 0.001).
Table 5.
Itching comparison between amnion and control group

Table 6.
Scar severity comparison between amnion and control group

DISCUSSION
Burn scar hypertrophy is a distressing condition which reduces the quality of life in patients due to cosmetic and health impairments. Compared to scars caused by reasons other than burns, they do not follow the typical linear patterns and they need specific management.[11,12] The management of hypertrophic scars is still a matter of debate in spite of the progress that has been achieved in recent years. The treatment options are prolonged, painful and sometimes expensive. Different curative measures have been proposed, but an optimal approach is not yet achieved. An ideal time for healing is considered as 21 days.[7] In a study by Deitch et al., it was shown that when healing took place between 14 and 21 days, hypertrophic scar developed in about one-third of the patients and when healing time was over 21 days, 78% of patients developed hyperrophic scars.[13] Thus, a major goal in preventive management is to reduce the healing time. Different measures are proposed including silicone gel application, corticosteroid injection and pressure therapy.[3] In the study by Van den Kerckhove et al., it was shown that preventive 15 mm Hg pressure therapy by garment would significantly reduce the scar thickness compared to a 10 mm Hg pressure.[14] However, the limitations of pressure therapy include, patient discomfort and difficulty to use in specific anatomical areas.
Another annoying problem in burn patients is pruritus. Pruritus may be severe and disabling which can interfere with concentration or sleep. It is reported to be present in the rehabilitation phase as high as 87% in adults and 100% of children. The aetiology is not well understood and physicians are confronted with difficulties in the management.[8,15]
Since the first report by Davis in 1910, AM has been used as a biological dressing for burn wounds with good therapeutic and cosmetic outcomes.[16] There are different properties which make it popular for burn wound care, especially in developing countries. Amniotic membrane can potentially reduce inflammation. Adinolfi et al. discovered the deficiency of major histocompatibility complex antigens in amniotic membrane.[17] They are considered the key for foreign tissue rejection, so the consequence is a reduction in inflammation.
Furthermore, the study of Li et al. stated that amniotic membrane cells can suppress immunity by secreting factors which result in decreased chemotaxis of polymorphonuclear cells and reduced lymphocytic proliferation and induced apoptosis.[18]
On the other hand, amniotic membrane can decrease wound colonisation and consequent sepsis through the bactericidal effect of its lysozyme and antibacterial effect of the progesterone.[19] Amniotic membrane adheres closely to the burn wound bed due to its pliability that reduces fluid exudates and protects from environment germs which results in pain relief and prevention of bacterial colonisation.[20] Furthermore, it contains immunoglobulins and allantoin which are effective factors in prevention of infection.[21] Another positive effect of amniotic membrane is related to its haemostatic properties due to collagen which helps in prevention of haematoma formation and bacterial proliferation.[22]
Amniotic membrane has the potential to decrease scar hypertrophy by significantly reducing alpha smooth muscle action, which is a hallmark for myofibroblasts.[23] It accelerates re-epithelisation and leads to profuse granulation. The rapid healing may be due to a reduction in polymorphonuclear leucocyte infiltration caused by protease activity inhibition and angiogenic properties resulting in granulation. Also, the large amount of oestrogen in amniotic membrane may help in decreasing the healing time.[24,25,26] In Sawhney's study, hypertrophic scar was not observed in those burn patients with intermediate wounds who were treated by amnion. Also, oozing and healing time were reduced in patients who received amniotic membrane compared to those who were treated by silver sulphadiazine cream and the dressing change was more comfortable.[27]
In Singh's study, 7 out of fifty patients (14%) with partial-thickness burns who were treated with amnion showed hypertrophic scar and no change was seen in range of motion of joints and healing time was between 10 and 14 days.[28]
Amniotic membrane needs to be redressed every 3–4 days as compared to antibiotic dressing which needs to be changed at least once a day. This results in less traumatisation, less pain, less bleeding and less chance of scar formation. Another benefit is that healing advancement of the wound can be observed under the membrane.[29,30]
Major problems with amniotic membrane are as follows: first is the risk for viral disease transmission. We reduced this greatly by screening for HIV, HCV, HBS antigens besides VDRL. Also in the literature, there is no report of disease transmission through amniotic membrane. To omit the chance of being in the “window period,” long-time storage by developed preserving techniques will help.
Inconclusive tests must be repeated after 6 months. The second problem which annoys some of the patients is bad smell which is usually decreased after dressing changes. The third is the stiffness feeling that cause discomfort for some patients when topical heparin is used over the membrane, especially in facial wounds. It can be reduced by small incisions on amniotic membrane.[20,26,29]
Another issue which become more important in developing countries is the economical considerations. The preparation of amniotic membrane is easy and the costs of re-dressings are reduced for both patients and health-care system. Also, the financial benefits becomes more prominent as its usage has resulted in shorter hospitalisation, infection rates and nursing time.[16,22,26]
The main standard approach towards deep partial-thickness and full-thickness burns is excision and split-thickness skin graft as it significantly reduces leucocyte infiltration and bacterial counts.[20,31]
The graft must be fixed to the wound bed to improve the chance of revascularication. Different methods of graft fixation have been previously proposed including stitches, surgical drapes and skin staples. We have introduced a new technique for fixation which is the use of amniotic membrane to cover around the graft.[20] It was shown that AM significantly reduces the time for graft take, and hence it can potentially reduce the hypertrophic scar formation. In this study, we evaluated the effect of amniotic membrane as an adjunct in split thickness skin graft on itching and hypertrophic scar formation in patients with extremities burn. As this method is pioneered at our burn centre we did not find any related article in this regard in the literature.
Hence using amniotic membrane as an adjunct in split thickness skin graft is a novel modality in the prevention of hypertrophic scar formation and itching in burn patients. However, more prospective well designed studies are needed to prove it.
CONCLUSIONS
Amniotic membrane as an adjunct in split thickness skin grafting is a novel modality which significantly reduces scar formation and itching which can be greatly distressing to burn patients. However, still more prospective well designed studies are needed to prove it.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank Department of Surgery, Trauma Research Center, Shiraz Burn Research Center, and Ghotbeddin Burn Hospital personnel for their cooperation in this work. It should be mentioned that, this survey is based on the thesis of Dr. Shima Eskandari for MD degree, in Shiraz University of Medical Sciences.
REFERENCES
- 1.Cho YS, Jeon JH, Hong A, Yang HT, Yim H, Cho YS, et al. The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: A randomized controlled trial. Burns. 2014;40:1513–20. doi: 10.1016/j.burns.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Brewin MP, Lister TS. Prevention or treatment of hypertrophic burn scarring: A review of when and how to treat with the pulsed dye laser. Burns. 2014;40:797–804. doi: 10.1016/j.burns.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Bloemen MC, van der Veer WM, Ulrich MM, van Zuijlen PP, Niessen FB, Middelkoop E. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35:463–75. doi: 10.1016/j.burns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns. 2006;32:992–9. doi: 10.1016/j.burns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Arno AI, Gauglitz GG, Barret JP, Jeschke MG. New molecular medicine-based scar management strategies. Burns. 2014;40:539–51. doi: 10.1016/j.burns.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4:235–43. doi: 10.2165/00128071-200304040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Parrett BM, Donelan MB. Pulsed dye laser in burn scars: current concepts and future directions. Burns. 2010;36:443–9. doi: 10.1016/j.burns.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 8.LaSalle L, Rachelska G, Nedelec B. Naltrexone for the management of post-burn pruritus: A preliminary report. Burns. 2008;34:797–802. doi: 10.1016/j.burns.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Malenfant A, Forget R, Papillon J, Amsel R, Frigon JY, Choinière M. Prevalence and characteristics of chronic sensory problems in burn patients. Pain. 1996;67:493–500. doi: 10.1016/0304-3959(96)03154-5. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi AA, Johari HG, Eskandari S. Effect of amniotic membrane on graft take in extremity burns. Burns. 2013;39:1137–41. doi: 10.1016/j.burns.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433–8. doi: 10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 12.Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma. 1983;23:895–8. [PubMed] [Google Scholar]
- 14.Van den Kerckhove E, Stappaerts K, Fieuws S, Laperre J, Massage P, Flour M, et al. The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns. 2005;31:696–702. doi: 10.1016/j.burns.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Willebrand M, Low A, Dyster-Aas J, Kildal M, Andersson G, Ekselius L, et al. Pruritus, personality traits and coping in long-term follow-up of burn-injured patients. Acta Derm Venereol. 2004;84:375–80. doi: 10.1080/00015550410032941. [DOI] [PubMed] [Google Scholar]
- 16.Ravishanker R, Bath AS, Roy R. “Amnion Bank” – The use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns. 2003;29:369–74. doi: 10.1016/s0305-4179(02)00304-2. [DOI] [PubMed] [Google Scholar]
- 17.Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, et al. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–7. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:900–7. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 19.Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg Engl. 1979;61:444–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29:907–16. doi: 10.1097/BCR.0b013e31818b9e40. [DOI] [PubMed] [Google Scholar]
- 21.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–5. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 22.Adly OA, Moghazy AM, Abbas AH, Ellabban AM, Ali OS, Mohamed BA. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns. 2010;36:703–10. doi: 10.1016/j.burns.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Fraser JF, Cuttle L, Kempf M, Phillips GE, Hayes MT, Kimble RM. A randomised controlled trial of amniotic membrane in the treatment of a standardised burn injury in the merino lamb. Burns. 2009;35:998–1003. doi: 10.1016/j.burns.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Haberal M, Oner Z, Bayraktar U, Bilgin N. The use of silver nitrate-incorporated amniotic membrane as a temporary dressing. Burns. 1987;13:159–63. doi: 10.1016/0305-4179(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 25.Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48:477–8. doi: 10.1016/0007-1226(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan KM, Jayaraman V. Management of partial-thickness burn wounds by amniotic membrane: a cost-effective treatment in developing countries. Burns. 1997;23(Suppl 1):S33–6. doi: 10.1016/s0305-4179(97)90099-1. [DOI] [PubMed] [Google Scholar]
- 27.Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns. 1989;15:339–42. doi: 10.1016/0305-4179(89)90015-6. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Purohit S, Chacharkar MP, Bhandari PS, Bath AS. Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second-degree burns. Burns. 2007;33:505–10. doi: 10.1016/j.burns.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi A, Riazi H, Hasheminasab M, Sabet B, Mohammadi M, Abbasi S, et al. Amniotic membrane dressing vs. conventional topical antibiotic dressing in hospitalized burn patients. Iran Red Crescent Med J. 2009;11:66–70. [Google Scholar]
- 30.Branski LK, Herndon DN, Celis MM, Norbury WB, Masters OE, Jeschke MG. Amnion in the treatment of pediatric partial-thickness facial burns. Burns. 2008;34:393–9. doi: 10.1016/j.burns.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadi AA, Seyed Jafari SM, Kiasat M, Tavakkolian AR, Imani MT, Ayaz M, et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns. 2013;39:349–53. doi: 10.1016/j.burns.2012.07.010. [DOI] [PubMed] [Google Scholar]


