Abstract
Background:
Non-healing trophic ulcers in Hansen's disease patients is one of the major causes for disability. It has been shown that autologous platelet-rich fibrin matrix (PRFM) is effective in healing chronic non-healing leg ulcers.
Aim:
The objective of this study is to demonstrate the efficacy of autologous platelet-rich fibrin matrix (PRFM) in non-healing trophic ulcers in patients treated for Hansen's disease.
Design:
A prospective study.
Setting:
An institution-based clinic.
Participants:
Seven treated patients with Hansen's disease, with a mean age of 38.33 years, with nine non-healing trophic ulcer of more than 6 weeks duration.
Measurements:
Photographs were taken before treatment and at every subsequent sitting. Area and volume were calculated at baseline and every subsequent sitting till the closure was achieved.
Materials and Methods:
The healthy ulcers were treated with PRFM at weekly intervals, repeated once a week for a maximum of five sittings as per requirement.
Results:
The mean percentage improvement in the area was 93.52%, and volume was 97.74% at the end of the second sitting. All ulcers closed by a maximum of five sittings. No adverse events were noted.
Conclusion:
PRFM for the treatment of trophic ulcers in treated patients with Hansen's disease is a feasible, safe, simple and inexpensive method.
Keywords: Hansen's disease, platelet-rich fibrin matrix, trophic ulcer
INTRODUCTION
Non-healing trophic ulcer in Hansen's disease patients is one of the major causes for disability. Further, cost involved in the long-term management of such ulcers is substantially high. Minimising the duration of healing can be a major step in the rehabilitation of such patients.
Recent literature shows autologous platelet-rich fibrin matrix (PRFM) being rich in growth factors is effective in the treatment of chronic non-healing leg ulcers.[1,2] Hence, we studied a series of nine cases showing the usage of autologous PRFM in non-healing chronic trophic ulcers in patients with Hansen's disease.
MATERIALS AND METHODS
In the present study, we included seven patients with nine ulcers with the following inclusion and exclusion criteria from January 2015 to May 2015. Ethical clearance was taken from Institutional Ethical Committee.
Inclusion and exclusion criteria
Non-healing trophic ulcers of more than 6 weeks duration in Hansen's disease patients who had already been released from treatment were included in the study.
Patients with age group below 18 years, having a history of bleeding disorders, anaemia and other haematological disorders, platelet count <1.5 Lakhs/cumm, patients on anticoagulant medications (aspirin, warfarin, heparin), uncontrolled diabetes mellitus, with malignant ulcers, pregnant and lactating females were excluded from this study.
Procedure
A thorough examination of skin and nerves of patients was carried out to rule out active lesions after taking informed consent. Ulcer size in terms of length, breadth and depth was measured. Primary infection if any was taken care using antibiotics and surgical debridement wherever necessary before starting the treatment. Under strict aseptic precautions, ten ml of venous blood was drawn and added to a sterile centrifugation tube devoid of anticoagulant. Centrifugation was done at 3000 rpm (approximately 400 g) for ten minutes. Three layers were obtained following this: upper straw-coloured platelet poor plasma (PPP), red-coloured lower fraction containing red blood cells (RBCs) and the middle fraction containing the PRFM [Figure 1 – step 1]. The upper straw-coloured layer (PPP) was discarded. PRFM was separated from red corpuscles at the base using a sterile forceps and scissor, preserving a small RBC layer measuring around one mm in length, which was transferred onto a sterile gauze. The membrane does not tear when manipulated with forceps and scissor. However, excess force should not be applied. Middle membrane so obtained was compressed between two gauze pieces gently and applied on a healthy wound followed by application of a secondary non-absorbable dressing. Adequate rest was ensured during the treatment course. The secondary dressing of the patient and the dried PRFM was removed from the wound bed after a minimum of 5 days [Figure 1 – step 6].
Figure 1.
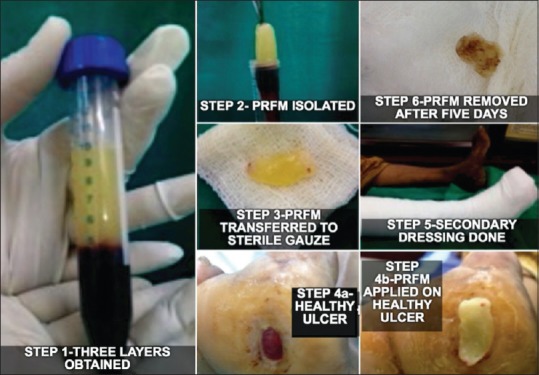
Procedure steps
The procedure was repeated every week up to a maximum of five sittings as per requirement. At the beginning and every week, healing of the ulcer was assessed, area and volume were calculated and photographs were taken. Wound area was calculated using the formula for an ellipse: Length × width × 0.7854 (an ellipse is closer to a wound shape than a square or rectangle). The use of an ellipse for calculating wound measurement has been used in randomised controlled trials in wound healing literature.[3,4] Volume was calculated using the formula (length × width × 0.7854) × depth.[4]
RESULTS
A total of seven patients with nine ulcers (two patients had two ulcers which were treated simultaneously) were treated with PRFM. The mean age of the patients was 38.33 years. Table 1 shows an overview of age-sex distribution with reference to wound duration. The sample comprised five male and two female patients. The duration of the ulcer ranged from 2 months to 1 year. Eight of nine ulcers required more than one application of PRFM, with a mean number of three applications. Table 2 shows details of measurement of ulcers with respect to each sitting. The mean percentage improvement in the area was 93.52%, and volume was 97.74% at the end of the second sitting. All ulcers closed by a maximum of five sittings [Figures 2–8]. No adjuvant treatment was required for treatment of the ulcers. No adverse events were reported as a result of PRFM treatment. In all cases, it was possible to complete the therapy within 60 min. The volume of ten ml of blood was adequate to cover an ulcer of maximum area 18.85 cm2 with 1 mm thickness of PRFM.
Table 1.
Age sex distribution with reference to wound duration
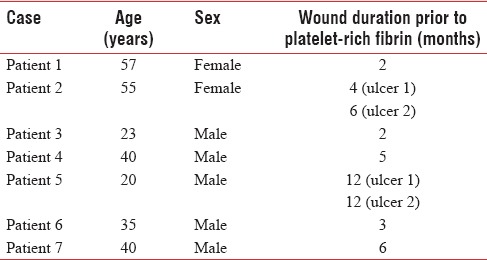
Table 2.
Measurement of ulcers with reference to each sitting
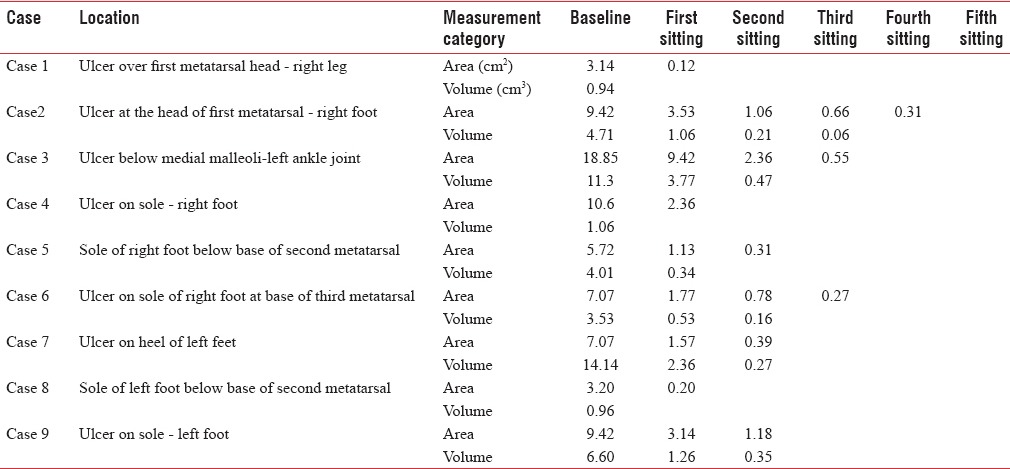
Figure 2.
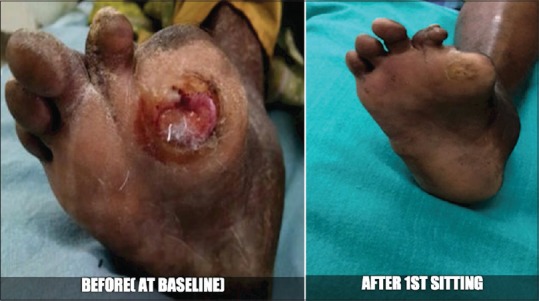
Case 1 - Before and after
Figure 8.
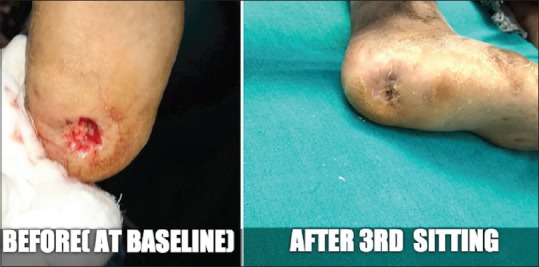
Case 7 - Before and after
Figure 3.
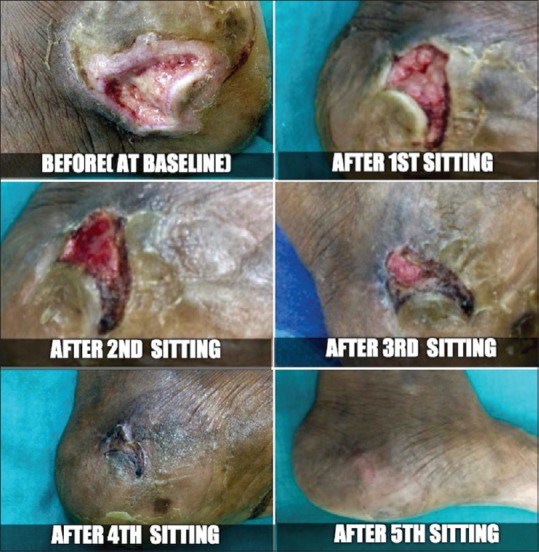
Case 2 - Before and after
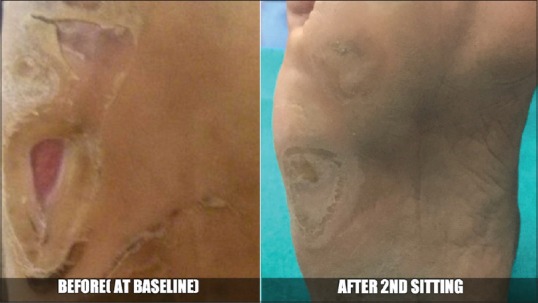
Figure 4.
Case 3 - Before and after
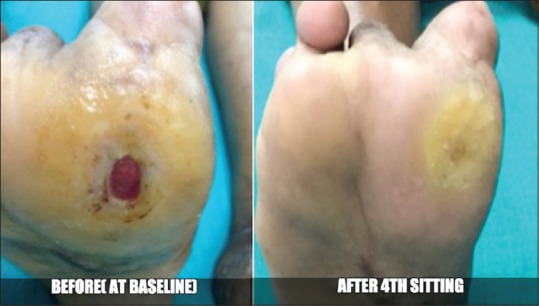
Figure 5.
Case 4 – Before and after
Figure 6.
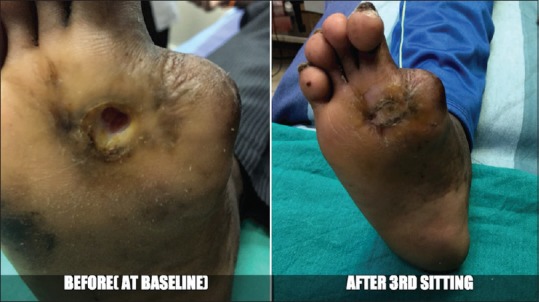
Case 5 - Before and after
Figure 7.
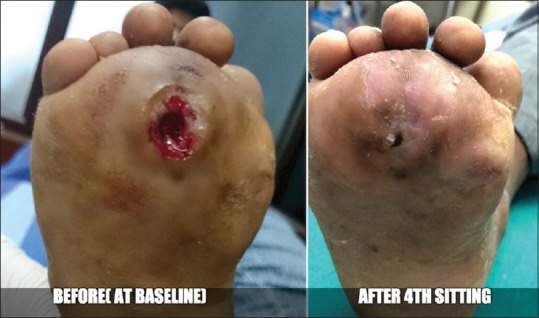
Case 6 - Before and after
DISCUSSION
Plantar ulceration is one of the most common disability in leprosy and occurs in about 10% of leprosy patients.[5] By shortening the wound healing phase, the quality of life of these patients can be improved, and they can be rehabilitated at the earliest. Various treatment modalities are available for trophic ulcers such as moist wound dressings, vacuum-assisted closure, hyperbaric oxygen therapy, reconstructive surgeries and topical application of growth factors,[6] in the form of platelet-rich plasma. Recently, the usefulness of PRFM has been published as a potential and inexpensive means for treatment of ulcers.[2]
The ulceration found in leprosy is a result of nerve damage and cutaneous anaesthesia and not as a consequence of the infection itself.[7] Platelets release growth factors and other secretory proteins which influence tissue healing.[8] It is, therefore, logical to assume, that the presence of more platelets in the wound bed will aid healing. Using purification techniques, the platelet concentration in plasma can be raised to >1.0 × 106/μl.[9]
Platelet-rich fibrin (PRF) was first developed by Choukroun et al.[10] in France for use in oral and maxillofacial surgery. PRF belongs to a new generation of platelet concentrates with simplified preparation and without any biochemical blood handling.[11] This technique neither requires anticoagulant nor bovine thrombin (nor any other gelling agent). It is just centrifuged blood without any addition. The absence of anticoagulant implies the activation in a few minutes of most platelets of the blood sample in contact with the tube walls and the release of the coagulation cascades. Fibrinogen is initially concentrated in the higher part of the tube, before the thrombin transforms it into fibrin clot, which is then concentrated in the middle of the tube, just between the red corpuscles at the bottom and acellular plasma at the top. Platelets are theoretically trapped massively in the fibrin meshes.[11] Hence, the success of this technique depends on speed of blood collection and transfer to centrifuge which was taken care of in our study.[12]
A study by Suchetha et al. showed that PRP has a higher platelet concentration when compared to PRF.[13] However, the superior effects of PRF when compared to PRP can be explained on the basis of study by Yazawa et al. which showed that, when incorporated into drug delivery systems such as fibrin, the mean concentration of growth factors in the platelet concentrates was three times or more than that observed with conventional platelet-rich plasma. Furthermore, the growth factors were released in a controlled manner over approximately 1 week.[14] Another study by Dohan et al. also proved a slower release of growth factors from PRFM than PRP and observed better healing properties with PRF.[15] Hence, in our study, we repeated PRFM once a week. In our case series, we achieved a complete closure of all ulcers in a maximum of 5 weeks. In a study by Sarvajnamurthy et al. mean duration of the healing of the chronic venous ulcers using PRP was in 5.1 weeks (standard deviation [SD] 3.1). The mean percentage improvement in the area and volume of the ulcer was 94.7% (SD 11.12) and 95.6% (SD 10.19) at the end of six sittings.[16] In our study, in spite of the chronic nature of the wounds, and associated neuropathy, the mean percentage improvement in the area and volume of ulcer at the end of second sitting was 93.52% and 97.74% which was quite significant. Almost similar results were seen in a retrospective study done by Steenvoorde et al. on the use of autologous PRF using Vivostat PRF on a range of hard-to-heal wounds. They achieved full closure in eight wounds and a reduction in diameter by up to 66% in three wounds, while the remaining two wounds only showed a reduction in depth with a mean number of 2.2 applications. The mean treatment period was 4.2 weeks with no adverse effects.[2] However, we could not come across other studies to compare our results of PRFM being used for the treatment of trophic ulcers in treated Hansen's disease patients. We could not compare the efficacy of PRP and PRF, but we achieved almost comparable results at the end of two sittings when compared to study a by Sarvajnamurthy et al. which took six sittings to achieve it.
CONCLUSION
PRFM for the treatment of trophic ulcers is a feasible, safe, simple and inexpensive method. A good patient compliance and satisfaction was seen as there were no problems with application and no complications were reported.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.O'Connell SM, Hessler K, Dardik H. Cascade ® autologous system platelet-rich fibrin matrix in the treatment of chronic leg ulcers. Adv Wound Care (New Rochelle) 2012;1:52–55. doi: 10.1089/wound.2011.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steenvoorde P, van Doorn LP, Naves C, Oskam J. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J Wound Care. 2008;17:60–3. doi: 10.12968/jowc.2008.17.2.28179. [DOI] [PubMed] [Google Scholar]
- 3.Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999;22:692–5. doi: 10.2337/diacare.22.5.692. [DOI] [PubMed] [Google Scholar]
- 4.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care. 2008;31:631–6. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan H. Leprosy: Disablement-its prevention and management. In: Valia RG, Valia AR, editors. IADVL Textbook of Dermatology. 3rd ed. Vol. 2. Mumbai: Bhalani Publishing House; 2010. pp. 2119–38. [Google Scholar]
- 6.Puri V, Venkateshwaran N, Khare N. Trophic ulcers-practical management guidelines. Indian J Plast Surg. 2012;45:340–51. doi: 10.4103/0970-0358.101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautman JR, Kirchheimer WF, Prabhakaran K, Hastings RC, Shannon EJ, Jacobson RR, et al. An overview of Carville research. Acta Leprol. 1981;84:1–29. [PubMed] [Google Scholar]
- 8.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 9.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Choukroun J, Adda F, Schoeffler C, Vervelle A. An opportunity in perio-implantology(in French): The PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 11.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16:284–93. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suchetha A, Lakshmi P, Bhat D, Mundinamane DB, Soorya KV, Bharwani GA. Platelet concentration in platelet concentrates and periodontal regeneration-unscrambling the ambiguity. Contemp Clin Dent. 2015;6:510–6. doi: 10.4103/0976-237X.169850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazawa M, Ogata H, Nakajima T, Mori T, Watanabe N, Handa M. Basic studies on the clinical applications of platelet-rich plasma. Cell Transplant. 2003;12:509–18. doi: 10.3727/000000003108747073. [DOI] [PubMed] [Google Scholar]
- 15.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Sarvajnamurthy S, Suryanarayan S, Budamakuntala L, Suresh DH. Autologous platelet rich plasma in chronic venous ulcers: Study of 17 cases. J Cutan Aesthet Surg. 2013;6:97–9. doi: 10.4103/0974-2077.112671. [DOI] [PMC free article] [PubMed] [Google Scholar]


