Abstract
Although biologic medications have demonstrated great efficacy for the treatment of psoriasis, a subset of patients fails to respond and others lose response later in the course. In treating a patient who has failed to respond to biologic therapy, clinicians must decide between dose escalation, switching biologics, and adding or switching to a non-biologic systemic drug or phototherapy. Although dose escalation is perhaps the simplest strategy and generally well-tolerated, it confers a tremendous cost burden because doubling the dosage is likely to double the wholesale price. We call for the development of rational strategies for the pricing of dose escalation in order to minimize this phenomenon. We also call for increased transparency surrounding negotiated pricing to ensure that all patients have access to the most effective, affordable treatment options available.
Key words: psoriasis, Biologic therapy, Dose escalation, cost effectiveness
Introduction
Over the past decade, biologic therapies against TNF-α, IL-17, and IL-12/IL-23 have revolutionized the treatment of chronic inflammatory diseases including psoriasis. Although these medications have demonstrated favorable efficacy and side effect profiles, a subset of patients fails to respond and others lose response over time. Even patients who have treatment success often desire to escalate their dose (Langley et al., 2015).
The prevalence of non-responders, defined as the failure to achieve a 75% reduction in Psoriasis Area and Severity Index (PASI) scores by week 12-16 of therapy, ranged from 51-66% for etanercept (Papp et al., 2005, Tyring et al., 2006), 20-32% for adalimumab (Menter et al., 2008, Saurat et al., 2008, Thaçi et al., 2010), 24-34% for ustekinumab (Leonardi et al., 2008, Papp et al., 2008), 18-33% for secukinumab (Langley et al., 2014), and 11-19% for ixekizumab (Gordon et al., 2016) in Phase III clinical trials. When treating a patient who has failed to respond to biologic therapy, which in the United States has usually been preceded by systemic medications per insurers’ appropriateness criteria, clinicians must decide between dose escalation, switching biologics, and adding or switching to a non-biologic systemic drug or phototherapy. In this era of renewed focus on cost and quality, this decision becomes increasingly important, especially because psoriasis patients may be taking these drugs for several decades.
Although dose escalation is perhaps the simplest strategy and has been shown to be well-tolerated in the majority of patients, even when outside labeling by the U.S. Food and Drug Administration (FDA; Brezinski and Armstrong, 2012), it confers a tremendous cost burden because doubling the dosage also doubles the wholesale price (RedBook Online). For example, the maintenance dose of etanercept is 50 mg weekly (Papp et al., 2005, Tyring et al., 2006), which costs approximately $53,909 per year in the United States. Increasing the dose to 50 mg twice weekly doubles the cost to $107,818 per year. Transitioning to another biologic, such as adalimumab or ustekinumab, is a far more cost-effective strategy with an annual cost that is approximately equivalent (RedBook Online; Table 1).
Table 1.
Costs of biologic drugs used to treat psoriasis, including the initial year of therapy, maintenance dosing, and escalated dosing
| Initial Dosing | 1st Year Cost of Therapy | Maintenance Dosing | Annual Cost | Escalated Dosing | Annual Cost | |
|---|---|---|---|---|---|---|
| Etanercept | 50 mg twice weekly for 3 months, then 50 mg weekly | $67,386 | 50 mg weekly | $53,909 | 50 mg twice weekly | $107,818 |
| Adalimumab | 80 mg once, then 40 mg every other week | $58,045 | 40 mg every other week | $53,899 | 40 mg weekly | $107,798 |
| Ustekinumab | ≤ 100 kg: 45 mg at weeks 0 & 4, then every 12 weeks | $58,966 | 45 mg every 12 weeks | $39,311 | 90 mg every 12 weeks | $78,622 |
| > 100 kg: 90 mg at Week 0 and Week 4, then every 12 weeks |
$117,933 | 90 mg every 12 weeks | $78,622 | 90 mg every 8 weeks | $117,933 | |
| Secukinumab | 300 mg weekly for 5 weeks, then 300 mg every 4 weeks | $70,195 | 300 mg every 4 weeks | $57,033 | ||
| Option to decrease to 150 mg every 4 weeks | ⁎$28,517 | |||||
| Ixekizumab | 160 mg once, then 80 mg every 2 weeks for 12 weeks, then 80 mg every 4 weeks | $83,714 | 80 mg every 4 weeks | $59,093 | 80 mg every 2 weeks | $118,185 |
Notes: Prices reflect average wholesale prices and may not include negotiated rebates or other discounts. Actual patient copays may be discordant with health system costs.
The 150 mg package is the same price as the 300 mg package, but the 300 mg package could theoretically be split into two 150 mg doses to decrease costs.
This issue is further complicated by the weight-based dosing of ustekinumab. For patients who weigh ≤ 100 kg, the maintenance dose is 45 mg every 12 weeks (a cost of $39,311 per year), which can later be escalated to 90 mg every 12 weeks if necessary (a cost of $78,622 per year). Patients who weigh more than 100 kg, however, initiate the higher dose from the beginning. Escalating to 90 mg every 8 weeks may improve efficacy in these patients, however, this dose is not FDA-approved and costs approximately $117,933 per year (Leonardi et al., 2008, Papp et al., 2008, RedBook Online [Internet], 2015).
Interestingly, treatment with secukinumab is designed to avoid dose escalation. All patients are initiated on 300 mg weekly for 5 weeks, followed by 300 mg every 4 weeks, which may later be decreased to 150 mg every 4 weeks in some patients. The annual cost of maintenance therapy for patients who remain on the 300 mg dose is approximately $57,033. Although this is one of the more expensive options, there is at least the reassurance that the cost will not double in the future due to dose escalation. In addition, the annual cost for patients who maintain the 150 mg dose is only $28,517, because even though the 150 mg and 300 mg dose packages are the same price, patients could theoretically get around this by purchasing the 300 mg package and administering the contents as two separate 150 mg doses (Langley et al., 2014, RedBook Online [Internet], 2015).
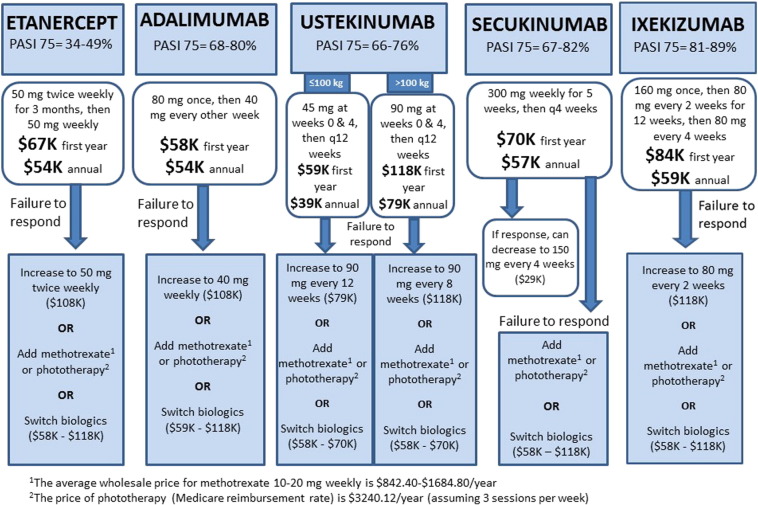
These considerations could lead to treatment algorithms designed to mimimize costs, which favor switching biologics over dose escalation (Figure 1). Unfortunately, although this is more affordable, there are potential clinical consequences such as the development of anti-drug antibodies (Hsu and Armstrong, 2013), which can limit a patient’s future treatment options. Switching to a biosimilar will also become an option in the future as these drugs emerge on the market. Unfortunately, they are only expected to decrease costs by 20-40% (Rumore and Vogenberg, 2016), and their long-term safety and efficacy have yet to be determined. Another option is to add or switch to a systemic medication such as methotrexate, which carries the risk of cumulative toxicity (Cather and Crowley, 2014). Starting methotrexate at the time of biologic initiation may also improve response and prevent the need for dose escalation later on, however clinicians should keep in mind the trade-off between any potential cost reduction and the safety profile of methotrexate.
Fig. 1.
Treatment algorithm for patients with psoriasis who fail to respond to initial biologic therapy. Treatment options for patients who fail initial biologic therapy or lose response over time include dose escalation, addition of a systemic drug or phototherapy, or transition to a different biologic.
In this era of increasingly hard choices, we have to fight to preserve access for our patients. Biologic drugs are expensive to develop, and the United States subsidizes these drugs for the rest of the world, making it an immense challenge to control prices. However, some patients may be best served by dose escalation, and we need to maintain affordability. There should also be more transparency around cost. In some cases, dose escalation may actually be cheaper because of negotiated pricing, but it is difficult for physicians and even health care systems to get access to this data.
In conclusion, biologics have demonstrated great success in the treatment of psoriasis, but carry with them a substantial cost burden. This is especially true for patients who fail to respond to starting doses, because dose escalation typically doubles the price of the medication. As a result, clinicians may be compelled to switch to a different biologic without first attempting to increase the dose. Moving forward, our field should support efforts to develop rational strategies for the pricing of dose escalation in order to minimize this phenomenon. These strategies would be substantially improved as well if there were increased transparency surrounding negotiated pricing, which would help ensure that all patients have access to the most effective, affordable treatment options available.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: AK has been a consultant and investigator for Amgen, Abbvie, Janssen, Novartis, Celgene, and Pfizer, has been a consultant for Eli Lilly, and receives fellowship funding from Janssen. KS received fellowship funding from Janssen.
References
- Brezinski E.A., Armstrong A.W. Off-label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS One. 2012;7(4):e33486. doi: 10.1371/journal.pone.0033486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cather J.C., Crowley J.J. Use of biologic agents in combination with other therapies for the treatment of psoriasis. Am J Clin Dermatol. 2014;15(6):467–478. doi: 10.1007/s40257-014-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K.B., Blauvelt A., Papp K.A., Langley R.G., Luger T., Ohtsuki M. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- Hsu L., Armstrong A.W. Anti-drug antibodies in psoriasis: a critical evaluation of clinical significance and impact on treatment response. Expert Rev Clin Immunol. 2013;9(10):949–958. doi: 10.1586/1744666X.2013.836060. [DOI] [PubMed] [Google Scholar]
- Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- Langley R.G., Lebwohl M., Krueger G.G., Szapary P.O., Wasfi Y., Chan D. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol. 2015;172(5):1371–1383. doi: 10.1111/bjd.13469. [DOI] [PubMed] [Google Scholar]
- Leonardi C.L., Kimball A.B., Papp K.A., Yeilding N., Guzzo C., Wang Y. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- Menter A., Tyring S.K., Gordon K., Kimball A.B., Leonardi C.L., Langley R.G. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Papp K.A., Langley R.G., Lebwohl M., Krueger G.G., Szapary P., Yeilding N. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- Papp K.A., Tyring S., Lahfa M., Prinz J., Griffiths C.E., Nakanishi A.M. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- RedBook Online [Internet] Truven Health Analytics: Micromedex Solutions. 2015. 2016 [cited 2015 September 11; 2016 July 13]. Available from http://micromedex.com/products/product-suites/clinical-knowledge/redbook.
- Rumore M.M., Vogenberg R.F. Biosimilars: still not quite ready for prime time. P T. 2016;41(6):366–375. [PMC free article] [PubMed] [Google Scholar]
- Saurat J.H., Stingl G., Dubertret L., Papp K., Langley R.G., Ortonne J.P. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158(3):558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- Thaçi D., Ortonne J.P., Chimenti S., Ghislain P.D., Argenberger P., Kragballe K. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163(2):402–411. doi: 10.1111/j.1365-2133.2010.09791.x. [DOI] [PubMed] [Google Scholar]
- Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]