Abstract
Female pattern hair loss is the most common cause of hair loss in women and one of the most common problems seen by dermatologists. This hair loss is a nonscarring alopecia in which loss occurs on the vertex scalp, generally sparing the frontal hairline. Hair loss can have significant psychosocial effects on patients, and treatment can be long and difficult. The influence of hormones on the pathogenesis of female pattern hair loss is not entirely known. The purpose of this paper is to review physiology and potential hormonal mechanisms for the pathogenesis of female pattern hair loss. We also discuss the current hormonal and hormone-modifying therapies that are available to providers as they partner with patients to treat this frustrating issue.
Introduction
Alopecia is a common issue that can cause significant morbidity because even though scalp hair is not biologically essential, it can have great psychological and social significance. The results of a 1993 Glamour magazine survey showed that more than half of women said, “If my hair looks good, I look attractive no matter what I’m wearing or how I look otherwise,” and “If my hair isn’t right, nothing else can make me feel that I look good” (Cash, 2001). Add to this the fact that more than 21 million women in the United States alone experience female pattern hair loss (FPHL), and it is not surprising that hair loss in women can be a serious cause of psychological stress and morbidity (Pickard-Holley, 1995, van Zuuren et al., 2016). In one study, 55% of affected women displayed symptoms of depression (Camacho and Garcia-Hernandez, 2002). In that same group, 89% of women experienced an improvement of those symptoms after treatment for hair loss (Camacho and Garcia-Hernandez, 2002).
However, the effects of alopecia reach far beyond symptoms of depression and include anxiety, obsessions, dissatisfaction with one’s appearance, and low self-esteem (Al-Mutairi and Eldin, 2011, Dlova et al., 2016, Hunt and McHale, 2005, Schmidt et al., 2001). There can be significant disturbance in a patient’s social life because they may change their hair style, clothing, or avoid social meetings (Al-Mutairi and Eldin, 2011). One study reported that 40% of surveyed women described marital problems and 63% had career-related issues that they ascribed to their hair loss (Hunt and McHale, 2005). These effects seem to occur regardless of patients’ age, race, or degree of hair loss (Dlova et al., 2016, Hunt and McHale, 2005, Schmidt et al., 2001). Another study of more than 200 women found that this psychologic morbidity occurs with equal frequency in women whose hair is typically covered by a headscarf (Erol et al., 2012).
Distress can also come from more than a change in body image. Dlova et al. (2016) found that in a group of black South African women, 52% reported serious worry that others would mistakenly assume that their hair loss was secondary to HIV infection or AIDS. It is critical that clinicians who care for such patients be compassionate and understanding but also have a solid understanding of hair loss so that reasonable expectations can be established and a therapeutic relationship can develop.
FPHL or androgenetic alopecia is the most common cause of hair loss in women and one of the most common chronic problems seen by dermatologists worldwide (Varothai and Bergfeld, 2014). FPHL is a nonscarring form of alopecia in which the frontal hairline is maintained, but there is progressive hair thinning at the vertex of the scalp. Thinning of the hair is secondary to alteration of the hair cycle with shortening of the anagen phase and simultaneous lengthening of telogen. This increase in the resting phase and decrease in the growth phase of the hair cycle results in the miniaturization of hair because long terminal hairs are gradually replaced by short vellus hairs (Messenger and Sinclair, 2006, Sinclair et al., 2011).
Pathophysiology
Despite the name androgenetic alopecia, the exact role of hormones is uncertain. It is well known that androgens affect the growth of the scalp and body hair and even Hippocrates observed 2,400 years ago that eunuchs did not experience baldness (Yip et al., 2011). However, hyperandrogenism cannot be the only pathophysiologic mechanism for FPHL because the majority of women with FPHL neither have abnormal androgen levels nor do they demonstrate signs or symptoms of androgen excess (Atanaskova Mesinkovska and Bergfeld, 2013, Schmidt and Shinkai, 2015, Yip et al., 2011). Furthermore, cases have been reported in which FPHL developed in patients with complete androgen insensitivity syndrome or hypopituitarism with no detectable androgen levels (Cousen and Messenger, 2010, Orme et al., 1999).
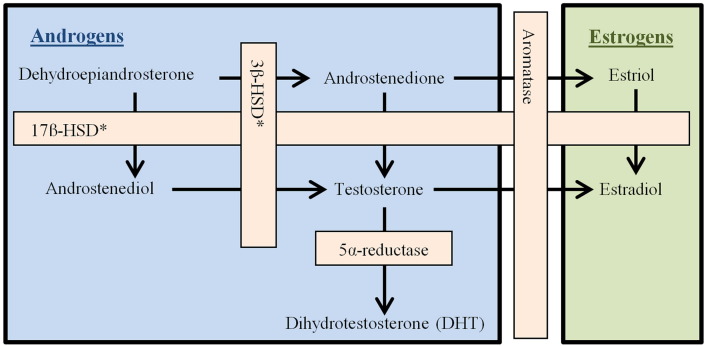
Male pattern hair loss has been established as androgen-dependent because it is associated with changes in the androgen receptor and responds to antiandrogen therapy (Ellis et al., 2002). With FPHL, genes that encode aromatase, which converts testosterone to estradiol, are also implicated (Yazdabadi et al., 2008, Yip et al., 2009). The process of androgen biosynthesis is depicted in Figure 1.
Figure 1.
Androgen biosynthesis.
Androstenedione, which is mostly produced in the ovary and adrenal glands, is converted to testosterone by 17β-hydroxysteroid dehydrogenase. Testosterone then circulates throughout the body to reach its target tissues. Androgen-metabolizing enzymes have been found in many parts of the hair follicle (Table 1; Bolognia et al., 2012). The presence of those enzymes makes the pilosebaceous unit a site of androgen metabolism and synthesis (Fazekas and Sandor, 1973). Circulating free testosterone either binds to intracellular androgen receptors in the hair bulb and dermal papilla, which facilitates miniaturization of the follicle, or is metabolized into dihydrotestosterone (DHT) by the enzyme 5-alpha-reductase. DHT then binds the same receptor but with much greater affinity (Kaufman, 2002, Levy and Emer, 2013). Of the androgens depicted in Figure 1, only DHT and testosterone bind to androgen receptors (Burger, 2002).
Table 1.
Androgen-metabolizing enzymes in the pilosebaceous unit
| Dermal Papilla | Aromatase, 17β-HSD, 5α-reductase (type II) |
| Outer Root Sheath | Aromatase, 17β-HSD, 5α-reductase (types I & II) |
| Inner Root Sheath | Aromatase, 5α-reductase (types I & II) |
| Sebaceous Gland | Aromatase, 5α-reductase (type I) |
| Sebaceous Duct | 5α-reductase (type II) |
17β-HSD, 17β-hydroxysteroid dehydrogenase.
Treatment
The process of androgen conversion and subsequent binding to its target receptor are targeted by many hormonal therapies that are currently available including 5-alpha-reductase inhibitors, androgen receptor blockers, and estrogen and oral contraceptive drugs.
5-alpha-reductase inhibitors
It is logical that some reduction in hair loss and miniaturization may occur if the conversion of testosterone to its stronger androgen, DHT, is prevented. The drug class of 5-alpha-reductase inhibitors stops that transition to DHT and leaves androgens that do not bind their receptors as tightly.
Finasteride is a 5-alpha-reductase type II inhibitor, and although it is approved by the U.S. Food and Drug Administration (FDA) for the treatment of male androgenetic alopecia, it is not approved for FPHL. Finasteride is significantly teratogenic and has been shown to cause feminization of male fetuses (Bowman et al., 2003) as well as sexual side effects, depression, headache, nausea, and hot flashes (Varothai and Bergfeld, 2014). The decreased conversion of testosterone to DHT causes a build-up of testosterone, which subsequently converts to estradiol and creates a relative estrogen excess, and this could theoretically increase the risk of breast cancer (Kelly et al., 2016). Studies that use low doses (1 mg daily) showed no significant benefit (Kim et al., 2012, Price et al., 2000). However, one study of 37 premenopausal women who were taking a 2.5-mg dose of finasteride daily with an oral contraceptive pill showed improvement of hair loss in 62% of patients (Iorizzo et al., 2006). Another study of 87 pre- and postmenopausal normoandrogenic patients who were taking a 5-mg dose of finasteride per day for 12 months showed a significant increase in both hair density and thickness (Yeon et al., 2011). The effectiveness of finasteride does not seem to differ between pre- and postmenopausal patients (Yeon et al., 2011). Finasteride is classified as pregnancy category X.
Dutasteride is a 5-alpha-reductase inhibitor that binds both types I and II enzymes. Compared with finasteride, its inhibition of type II enzymes is three times more potent; its inhibition of type I enzymes is 100 times more potent (Clark et al., 2004). Dutasteride is not approved for the treatment of FPHL by the FDA, and ongoing studies on the efficacy of the inhibitor are promising but largely focus on male patients (Gupta and Charrette, 2014, Olsen et al., 2006). A study of women after 3 years of therapy showed that dutasteride may be more effective than finasteride in women under 50 years of age as measured by hair thickness (not hair density) at the center and vertex scalp (Boersma et al., 2014). One case report of a 46-year-old female with FPHL showed some response after 6 months of treatment with a dose of 0.5-mg dutasteride daily despite a minimal response to treatment with finasteride and minoxidil (Olszewska and Rudnicka, 2005). Data with regard to the treatment side effects in women is extremely limited. Dutasteride is classified as pregnancy category X because of teratogenicity and should have the same theoretical risk of breast cancer as mentioned in relation to finasteride (Kelly et al., 2016).
Androgen receptor blockers
Spironolactone is a potassium-sparing diuretic that functions as a competitive aldosterone antagonist and inhibits the interaction of testosterone and DHT with intracellular androgen receptors in target tissues (van Zuuren et al., 2012, Yazdabadi and Sinclair, 2011). Spironolactone also weakly inhibits androgen synthesis (Price, 2003). The anti-androgen effect is more commonly used in hirsutism and acne but has been used successfully at 100- to 200-mg daily doses to treat FPHL (Sinclair et al., 2005). One retrospective study of survey data showed that nearly 75% of women reported stabilization or improvement of their hair loss after treatment with spironolactone (Famenini et al., 2015). Similar results were obtained in an open intervention study from 2005 (Sinclair et al., 2005). While the vast majority of published data discusses adult patients, one case report described the visible improvement of FPHL in a 9-year-old patient after 6 months of therapy (Yazdabadi et al., 2009).
Side effects of spironolactone include vomiting, diarrhea, dizziness with postural hypotension, breast tenderness, spotting, and electrolyte imbalance (Atanaskova Mesinkovska and Bergfeld, 2013). This androgen receoptor blocker is categorized as pregnancy category D.
Cyproterone acetate works in several ways. It not only competitively blocks DHT from binding to its receptors at target tissue (Gilman et al., 1990), but it is also a progestogen that lowers testosterone levels by decreasing the release of luteinizing and follicle-stimulating hormones through pituitary-mediated supression (Gilman et al., 1990, Varothai and Bergfeld, 2014). An open intervention study of 80 women who received treatment with spironolactone (200 mg daily) or cyproterone acetate (50 mg daily or 100 mg for 10 days per month if premenopausal) showed that three of four patients demonstrated an improvement or stabilization of their disease with no difference of effect between the therapies received (Sinclair et al., 2005).
When compared with no treatment, patients who received ethinyl estradiol 50 μg and cyproterone acetate 2 mg with cyproterone acetate 20 mg on days 5 to 20 of the menstrual cycle for 1 year had a significant increase in their percentage of anagen hairs with trends toward a larger shaft diameter of full anagen hairs and a decreased number of hairs that were less than 40 microns (Peereboom-Wynia et al., 1989). A 12-month randomized control trial of 66 women compared treatment with topical minoxidil 2% plus an oral contraceptive (ethinyl estradiol 30 μg + gestodene 75 μg) with treatment with cyproterone acetate 50 mg plus an oral contraceptive (ethinyl estradiol 35 μg + cyproterone acetate 2 mg) and demonstrated that treatment with cyproterone was more effective in hyderandrogenic patients but otherwise less effective (Vexiau et al., 2002). Side effects of cyproterone acetate include weight gain, breast tenderness, and a decreased libido (Kelly et al., 2016). Hepatotoxicity and development of multiple meningiomas may occur when doses exceed 25 mg daily (Medicines and Healthcare products Regulatory Agency, 2009). Cyproterone acetate is used widely in Europe and Canada, either in an isolated form or in combination with ethinyl estradiol, but it is only available in the United States as an orphan drug for the treatment of hirsutism (Carmina and Lobo, 2003, Jurzyk et al., 1992, Kelly et al., 2016). Cyproterone acetate is classified as pregnancy category X.
Flutamide is an oral anti-androgen that acts by competitively inhibiting the uptake of androgen and its nuclear binding in target tissues (Varothai and Bergfeld, 2014; Watson Pharma, 2011). It has been shown to be effective for the treatment of FPHL in hyperandrogenic women at a dose of 250 mg per day. One case report showed that treatment with flutamide was effective in a patient who had already failed to improve with spironolactone and minoxidil (Carmina and Lobo, 2003, Yazdabadi and Sinclair, 2011). After 2 years of therapy, 80% of patients were satisfied or highly satisfied with their treatment effect regardless whether they were taking concomitant oral contraceptives (Paradisi et al., 2011). Flutamide can cause hepatotoxicity and serial monitoring of liver function tests is recommended during treatment (Watson Pharma, 2011) even though data from one study on the safety and tolerability of flutamide showed that patient transaminase values returned to normal after treatment was discontinued and that levels did not rise while patients were treated with doses of 62.5 mg or 125 mg. Flutamide is classified as pregnancy category D.
Estrogen and oral contraceptive drugs
The role of estrogen and progestogen drugs in the treatment of hair loss and growth is also unclear. Estrogen is made when androstenedione or testosterone are modified by the enzyme aromatase. It is synthesized in the ovary and other peripheral tissues and then travels to its receptors, some of which are located in scalp hair follicles (Thornton et al., 2003a, Thornton et al., 2003b). At the scalp follicle, estradiol has been reported to induce aromatase activity (Hoffmann et al., 2002). Estrogen has been hypothesized to have a protective role against hair loss on the basis of the observation that patients with lower estrogen levels during menopause, postpartum, or treatment with aromatase inhibitors or selective estrogen receptor modulators are more likely to develop FPHL (Atanaskova Mesinkovska and Bergfeld, 2013, Park et al., 2014). Another supporting observation is that in the frontal hairline of women, which tends to be spared with FPHL, there is a higher level of aromatase enzyme when compared with the rest of the scalp (Levy and Emer, 2013). This variation in hair loss could be the result of locally increased levels of estradiol or decreased levels of testosterone and DHT that is secondary to greater amounts of conversion.
Estrogen and combined oral contraceptive (COC) drugs with estrogen or progestogen have been reported as effective, but data are limited (Adenuga et al., 2012, Raudrant and Rabe, 2003, Scheinfeld, 2008). They are thought to function through several mechanisms. Both components of COC drugs increase the levels of sex-hormone-binding globulin (Schindler, 2013). They also send negative feedback signals that suppress the hypothalamic secretion of gonadotropin and releases the hormone and pituitary secretion of the luteinizing and follicle-stimulating hormones, which results in a decreased androgen production (Gilman et al., 1990, Varothai and Bergfeld, 2014). These actions decrease androgen secretion from the ovary and the quantity of free, biologically active androgens, which reduces their effects on the hair follicles (Schindler, 2013). Our practice when prescribing COC drugs is a combination of ethinyl estradiol 20 mcg plus drospirenone 3 mg. Drospirenone is an analogue of spironolactone. This treatment combination is approved by the FDA for the treatment of acne but not alopecia.
Conclusions
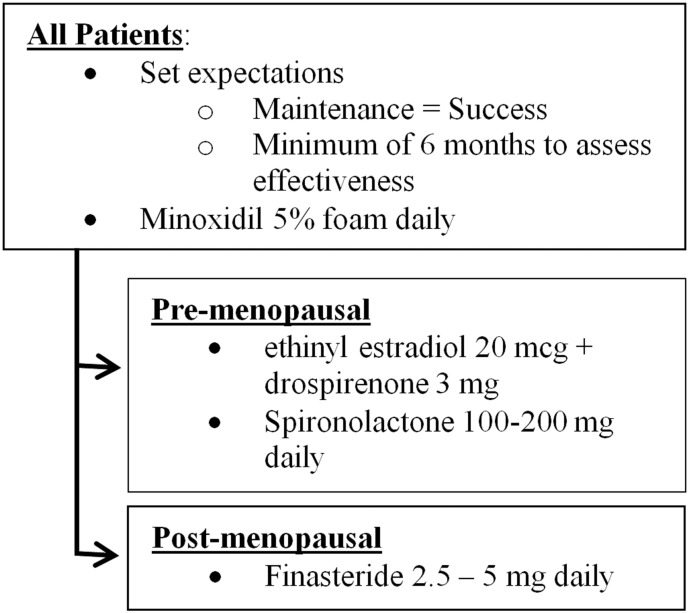
Our practice approach is depicted in Figure 2. All patients, unless contraindicated, initiate therapy with topical minoxidil 5% foam daily. This is the only therapy that is approved by the FDA for FPHL and has been shown to be safe and effective (van Zuuren et al., 2016, Varothai and Bergfeld, 2014).
Figure 2.
Approach to female pattern hair loss.
Additionally, two other considerations are important for a patient who receives treatment for FPHL. First, there is a set of reasonable expectations in patients. Maintaining the current hair density can be considered a successful treatment because women tend to have further thinning as they age (Harfmann and Bechtel, 2015). Second, it is important to ensure that patients understand that progress is slow, and months or years can be required to see a significant improvement (Boersma et al., 2014, Yeon et al., 2011). In our practice, we wait at least 6 months to assess treatment efficacy.
FPHL is common and can be distressing for patients. Further, treatment of FPHL can be long and difficult and what patients hope for or expect are not always the same as what clinicians consider successful therapy. However, patients are often appreciative as you partner with them to treat this frustrating disease.
References
- Adenuga P., Summers P., Bergfeld W. Hair regrowth in a male patient with extensive androgenetic alopecia on estrogen therapy. J Am Acad Dermatol. 2012;67:e121–e123. doi: 10.1016/j.jaad.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Al-Mutairi N., Eldin O.N. Clinical profile and impact on quality of life: Seven years experience with patients of alopecia areata. Indian J Dermatol Venereol Leprol. 2011;77:489–493. doi: 10.4103/0378-6323.82411. [DOI] [PubMed] [Google Scholar]
- Atanaskova Mesinkovska N., Bergfeld W.F. Hair: What is new in diagnosis and management? Female pattern hair loss update: diagnosis and treatment. Dermatol Clin. 2013;31:119–127. doi: 10.1016/j.det.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Boersma I.H., Oranje A.P., Grimalt R., Iorizzo M., Piraccini B.M., Verdonschot E.H. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2014;80:521–525. doi: 10.4103/0378-6323.144162. [DOI] [PubMed] [Google Scholar]
- Bolognia J.L., Jorizzo J.L., Schaffer J.V. 3rd ed. Elsevier; Amsterdam: 2012. Dermatology. [Google Scholar]
- Bowman C.J., Barlow N.J., Turner K.J., Wallace D.G., Foster P.M. Effects of in utero exposure to finasteride on androgen-dependent reproductive development in the male rat. Toxicol Sci. 2003;74:393–406. doi: 10.1093/toxsci/kfg128. [DOI] [PubMed] [Google Scholar]
- Burger H.G. Androgen production in women. Fertil Steril. 2002;77:S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Camacho F.M., Garcia-Hernandez M. Psychological features of androgenetic alopecia. J Eur Acad Dermatol Venereol. 2002;16:476–480. doi: 10.1046/j.1468-3083.2002.00475.x. [DOI] [PubMed] [Google Scholar]
- Carmina E., Lobo R.A. Treatment of hyperandrogenic alopecia in women. Fertil Steril. 2003;79:91–95. doi: 10.1016/s0015-0282(02)04551-x. [DOI] [PubMed] [Google Scholar]
- Cash T.F. The psychology of hair loss and its implications for patient care. Clin Dermatol. 2001;19:161–166. doi: 10.1016/s0738-081x(00)00127-9. [DOI] [PubMed] [Google Scholar]
- Clark R.V., Hermann D.J., Cunningham G.R., Wilson T.H., Morrill B.B., Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- Cousen P., Messenger A. Female pattern hair loss in complete androgen insensitivity syndrome. Br J Dermatol. 2010;162:1135–1137. doi: 10.1111/j.1365-2133.2010.09661.x. [DOI] [PubMed] [Google Scholar]
- Dlova N.C., Fabbrocini G., Lauro C., Spano M., Tosti A., Hift R.H. Quality of life in South African Black women with alopecia: a pilot study. Int J Dermatol. 2016;55:875–881. doi: 10.1111/ijd.13042. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Sinclair R., Harrap S.B. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11. doi: 10.1017/S1462399402005112. [DOI] [PubMed] [Google Scholar]
- Erol O., Can G., Aydiner A. Effects of alopecia on body image and quality of life of Turkish cancer women with or without headscarf. Support Care Cancer. 2012;20:2349–2356. doi: 10.1007/s00520-011-1338-y. [DOI] [PubMed] [Google Scholar]
- Famenini S., Slaught C., Duan L., Goh C. Demographics of women with female pattern hair loss and the effectiveness of spironolactone therapy. J Am Acad Dermatol. 2015;73:705–706. doi: 10.1016/j.jaad.2015.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas A.G., Sandor T. The metabolism of dehydroepiandrosterone by human scalp hair follicles. J Clin Endocrinol Metab. 1973;36:582–586. doi: 10.1210/jcem-36-3-582. [DOI] [PubMed] [Google Scholar]
- Gilman A.G., Rall T.W., Nies A.S., Taylor P., editors. Goodman and Gilman's the pharmacological basis of therapeutics. 8th ed. Permagon Press; New York: 1990. [Google Scholar]
- Gupta A.K., Charrette A. The efficacy and safety of 5alpha-reductase inhibitors in androgenetic alopecia: a network meta-analysis and benefit-risk assessment of finasteride and dutasteride. J Dermatolog Treat. 2014;25:156–161. doi: 10.3109/09546634.2013.813011. [DOI] [PubMed] [Google Scholar]
- Harfmann K.L., Bechtel M.A. Hair loss in women. Clin Obstet Gynecol. 2015;58:185–199. doi: 10.1097/GRF.0000000000000081. [DOI] [PubMed] [Google Scholar]
- Hoffmann R., Niiyama S., Huth A., Kissling S., Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp Dermatol. 2002;11:376–380. doi: 10.1034/j.1600-0625.2002.110413.x. [DOI] [PubMed] [Google Scholar]
- Hunt N., McHale S. The psychological impact of alopecia. BMJ. 2005;331:951–953. doi: 10.1136/bmj.331.7522.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorizzo M., Vincenzi C., Voudouris S., Piraccini B.M., Tosti A. Finasteride treatment of female pattern hair loss. Arch Dermatol. 2006;142:298–302. doi: 10.1001/archderm.142.3.298. [DOI] [PubMed] [Google Scholar]
- Jurzyk R.S., Spielvogel R.L., Rose L.I. Antiandrogens in the treatment of acne and hirsutism. Am Fam Physician. 1992;45:1803–1806. [PubMed] [Google Scholar]
- Kaufman K.D. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89–95. doi: 10.1016/s0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- Kelly Y., Blanco A., Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349–1364. doi: 10.1007/s40265-016-0629-5. [DOI] [PubMed] [Google Scholar]
- Kim W.J., Song M., Ko H.C., Kim B.S., Kim M.B. Efficacy of finasteride 1.25 mg on female pattern hair loss; pilot study. Ann Dermatol. 2012;24:370–372. doi: 10.5021/ad.2012.24.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L.L., Emer J.J. Female pattern alopecia: current perspectives. Int J Womens Health. 2013;5:541–556. doi: 10.2147/IJWH.S49337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicines and Healthcare products Regulatory Agency High-dose cyproterone acetate: potential risk of (multiple) meningiomas. Drug Safety Update. 2009;3:3–4. [Google Scholar]
- Messenger A.G., Sinclair R. Follicular miniaturization in female pattern hair loss: clinicopathological correlations. Br J Dermatol. 2006;155:926–930. doi: 10.1111/j.1365-2133.2006.07409.x. [DOI] [PubMed] [Google Scholar]
- Olsen E.A., Hordinsky M., Whiting D., Stough D., Hobbs S., Ellis M.L. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014–1023. doi: 10.1016/j.jaad.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Olszewska M., Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637–640. [PubMed] [Google Scholar]
- Orme S., Cullen D.R., Messenger A.G. Diffuse female hair loss: are androgens necessary? Br J Dermatol. 1999;141:521–523. doi: 10.1046/j.1365-2133.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Paradisi R., Porcu E., Fabbri R., Seracchioli R., Battaglia C., Venturoli S. Prospective cohort study on the effects and tolerability of flutamide in patients with female pattern hair loss. Ann Pharmacother. 2011;45:469–475. doi: 10.1345/aph.1P600. [DOI] [PubMed] [Google Scholar]
- Park J., Kim J.I., Yun S.K., Kim H.U., Ihm C.W. Pattern alopecia during hormonal anticancer therapy in patients with breast cancer. Ann Dermatol. 2014;26:743–746. doi: 10.5021/ad.2014.26.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peereboom-Wynia J.D., van der Willigen A.H., van Joost T., Stolz E. The effect of cyproterone acetate on hair roots and hair shaft diameter in androgenetic alopecia in females. Acta Derm Venereol. 1989;69:395–398. [PubMed] [Google Scholar]
- Watson Pharma . 2011. Product information: flutamide oral capsules, flutamide oral capsules (per DailyMed) [Google Scholar]
- Pickard-Holley S. The symptom experience of alopecia. Semin Oncol Nurs. 1995;11:235–238. doi: 10.1016/s0749-2081(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Price V.H. Androgenetic alopecia in women. J Investig Dermatol Symp Proc. 2003;8:24–27. doi: 10.1046/j.1523-1747.2003.12168.x. [DOI] [PubMed] [Google Scholar]
- Price V.H., Roberts J.L., Hordinsky M., Olsen E.A., Savin R., Bergfeld W. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43:768–776. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- Raudrant D., Rabe T. Progestogens with antiandrogenic properties. Drugs. 2003;63:463–492. doi: 10.2165/00003495-200363050-00003. [DOI] [PubMed] [Google Scholar]
- Scheinfeld N. A review of hormonal therapy for female pattern (androgenic) alopecia. Dermatol Online J. 2008;14:1. [PubMed] [Google Scholar]
- Schindler A.E. Non-contraceptive benefits of oral hormonal contraceptives. Int J Endocrinol Metab. 2013;11:41–47. doi: 10.5812/ijem.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T.H., Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672–690. doi: 10.1016/j.jaad.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Fischer T.W., Chren M.M., Strauss B.M., Elsner P. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038–1043. doi: 10.1046/j.1365-2133.2001.04195.x. [DOI] [PubMed] [Google Scholar]
- Sinclair R., Wewerinke M., Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466–473. doi: 10.1111/j.1365-2133.2005.06218.x. [DOI] [PubMed] [Google Scholar]
- Sinclair R., Patel M., Dawson T.L., Jr., Yazdabadi A., Yip L., Perez A. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165:12–18. doi: 10.1111/j.1365-2133.2011.10630.x. [DOI] [PubMed] [Google Scholar]
- Thornton M.J., Taylor A.H., Mulligan K., Al-Azzawi F., Lyon C.C., O’Driscoll J. Oestrogen receptor beta is the predominant oestrogen receptor in human scalp skin. Exp Dermatol. 2003;12:181–190. doi: 10.1034/j.1600-0625.2003.120209.x. [DOI] [PubMed] [Google Scholar]
- Thornton M.J., Taylor A.H., Mulligan K., Al-Azzawi F., Lyon C.C., O’Driscoll J. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J Investig Dermatol Symp Proc. 2003;8:100–103. doi: 10.1046/j.1523-1747.2003.12181.x. [DOI] [PubMed] [Google Scholar]
- van Zuuren E.J., Fedorowicz Z., Carter B. Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. Br J Dermatol. 2012;167:995–1010. doi: 10.1111/j.1365-2133.2012.11166.x. [DOI] [PubMed] [Google Scholar]
- van Zuuren E.J., Fedorowicz Z., Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2016;5:CD007628. doi: 10.1002/14651858.CD007628.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varothai S., Bergfeld W.F. Androgenetic alopecia: an evidence-based treatment update. Am J Clin Dermatol. 2014;15:217–230. doi: 10.1007/s40257-014-0077-5. [DOI] [PubMed] [Google Scholar]
- Vexiau P., Chaspoux C., Boudou P., Fiet J., Jouanique C., Hardy N. Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12-month randomized trial. Br J Dermatol. 2002;146:992–999. doi: 10.1046/j.1365-2133.2002.04798.x. [DOI] [PubMed] [Google Scholar]
- Yazdabadi A., Sinclair R. Treatment of female pattern hair loss with the androgen receptor antagonist flutamide. Australas J Dermatol. 2011;52:132–134. doi: 10.1111/j.1440-0960.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- Yazdabadi A., Magee J., Harrison S., Sinclair R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. Br J Dermatol. 2008;159:1300–1302. doi: 10.1111/j.1365-2133.2008.08820.x. [DOI] [PubMed] [Google Scholar]
- Yazdabadi A., Green J., Sinclair R. Successful treatment of female-pattern hair loss with spironolactone in a 9-year-old girl. Australas J Dermatol. 2009;50:113–114. doi: 10.1111/j.1440-0960.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- Yeon J.H., Jung J.Y., Choi J.W., Kim B.J., Youn S.W., Park K.C. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211–214. doi: 10.1111/j.1468-3083.2010.03758.x. [DOI] [PubMed] [Google Scholar]
- Yip L., Zaloumis S., Irwin D., Severi G., Hopper J., Giles G. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br J Dermatol. 2009;161:289–294. doi: 10.1111/j.1365-2133.2009.09186.x. [DOI] [PubMed] [Google Scholar]
- Yip L., Rufaut N., Sinclair R. Role of genetics and sex steroid hormones in male androgenetic alopecia and female pattern hair loss: an update of what we now know. Australas J Dermatol. 2011;52:81–88. doi: 10.1111/j.1440-0960.2011.00745.x. [DOI] [PubMed] [Google Scholar]