Abstract
Introduction
The transcription factor forkhead box protein A1 (FOXA1) plays a critical role in the proliferation of human breast cancer cells, particularly estrogen receptor alpha (ERα)-positive luminal breast cancer cells. However, genetic studies of the requirement for Foxa1 in mammary tumor formation in mice have been hampered by the lack of a conditional gene ablation.
Methods
We examined three mouse models of mammary-specific ablation of Foxa1 in ductal epithelial cells to identify the best system for complete and mammary-specific ablation of Foxa1.
Results
We found that MMTV-Cre and MMTV-rtTA;Tet-On-Cre led to partial deletion of Foxa1 and attenuated mammary duct formation, whereas Krt14-Cre led to complete ablation of Foxa1 and abolished mammary duct formation, in Foxa1loxP/loxP mice.
Conclusion
These results demonstrate that Foxa1 is essential for mammary duct formation, and reveal a series of mouse models in which mammary expression of Foxa1 can be attenuated or completely blocked. Our study also suggests a potentially powerful model for complete ablation of Foxa1 in mammary epithelial cells using Krt14-driven Cre expression in an inducible manner, such as Krt14-rtTA;Tet-On-Cre. This model system will facilitate further in vivo functional studies of Foxa1 or other factors in mammary gland development and tumor formation and progression.
Keywords: mammary gland, transgenic mice, MMTV, Krt14
INTRODUCTION
Breast cancer is the most common cancer and the second leading cause of cancer death in women all over the world (Torre et al., 2015). Breast cancer originates from the mammary gland epithelial cells and can be classified into several distinct transcriptional subtypes, of which the most common is the luminal subtype (Eroles et al., 2012). Luminal breast cancer cells are given rise from the amalgamation of epigenetic and genetic changes on luminal epithelial cells. It has been shown that the expression of estrogen receptor alpha (ERα) and forkhead box protein A1 (FOXA1) are strongly associated with luminal subtype of breast cancer, and that FOXA1-dependent ERα-mediated estrogen signaling promotes the growth of ERα-positive breast cancer cells (Badve et al., 2007; Carroll et al., 2005; Habashy et al., 2008; Hu et al., 2014; Laganiere et al., 2005). FOXA1, previously known as hepatocyte nuclear factor 3α (HNF3α), belongs to a large family of transcription factors, characterized by a common ‘winged helix’ DNA binding domain. In the past three decades, many studies have shown that Foxa factors were shown to control gene regulation during organogenesis, such as liver (Kaestner, 2010; Lee et al., 2005; Li et al., 2009), pancreas (Gao et al., 2008b; Monaghan et al., 1993), lung (Besnard et al., 2005; Kaestner et al., 1999b; Shih et al., 1999), prostate (Besnard et al., 2004; Mirosevich et al., 2005), kidney (Behr et al., 2004), renal pelvis, ureters, bladder, and testis (Peterson et al., 1997). High expression of FOXA1 has been found in human luminal subtype of breast cancer cell lines. Mammary gland ductal development depends upon growth and morphogenesis of luminal mammary epithelial cells (Asselin-Labat et al., 2007; Ewald et al., 2008), which occur in a hormone-dependent fashion, through the interaction of ERα and FOXA1 (Brisken and O’Malley, 2010). The regulatory network of ERα and FOXA1 in human breast cancer cells has been functionally evaluated using suppression of FOXA1 but with two different sets of results (Bernardo et al., 2013; Hurtado et al., 2011). Hurtado et al showed that suppression FOXA1 in human breast cancer cell lines, MCF7 and T47D, led to the inhibition of cell growth (Hurtado et al., 2011), whereas Bernardo et al found that suppression of FOXA1 in MCF7 and T47D cells not only inhibited their growth but also converted these cancer cells from the luminal to the basal subtypes (Bernardo et al., 2013). However, a defined role of Foxa1 in the early stage of mammary tumorigenesis has never been investigated, due to in part the fact that Foxa1−/− mice die within two weeks after birth (Kaestner et al., 1999a). Furthermore, an ex vivo culture of explanted Foxa1−/− mammary gland tissue has shown that Foxa1 is required for mammary duct morphogenesis (Bernardo et al., 2010). Thus, to better understand the role of Foxa1 in mammary tumorigenesis in vivo, a mouse model of mammary-specific ablation of Foxa1 is needed.
In the past several decades, genetically engineered mouse models have provided many powerful tools for improving our understanding in breast cancer biology, including initiation, progression, and metastasis. Conditional gene ablation in the mammary gland has frequently employed the mouse mammary tumor virus (MMTV) long terminal repeat (LTR) promoter-driven Cre recombinase (MMTV-Cre) (Wagner et al., 1997). Additionally, inducible MMTV-driven Cre expression, using the MMTV-rtTA;Tet-On-Cre transgene, has also been employed for mammary gland-specific manipulation of targeted genes to achieve tissue-specific gene expression or to avoid perinatal lethality (Gunther et al., 2002). Moreover, the whey acidic protein (WAP) gene promoter-driven Cre recombinase (WAP-Cre) was developed to modify mammary gland-specific gene expression, but the use of this model has been limited by the fact that WAP-Cre expression was only detected in alveolar epithelial cells of mammary tissue during lactation (Wagner et al., 1997). Cytokeratin proteins are markers of many epithelial cells, including mammary epithelial cells; Krt5-Cre and Krt8-Cre models have been used for tracing embryonic development of mammary glands (Van Keymeulen et al., 2011), and Krt14-Cre has shown to drive Cre expression in many epithelial cells, including mammary epithelia (Ahn et al., 2013; Bowman-Colin et al., 2013; Caffarel et al., 2012; Cang et al., 2007; Chakrabarti et al., 2012a; Chakrabarti et al., 2012b; Choi et al., 2009; Dassule et al., 2000; Gritli-Linde et al., 2007; Lindley et al., 2015; Mitchell and Serra, 2014).
Here, we compare gene ablation efficacy and the consequences on mammary duct formation in three mouse models with mammary gland-specific ablation of Foxa1, Foxa1loxP/loxP;MMTV-Cre, Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre, and Foxa1loxP/loxP; Krt14-Cre. We found that the novel Krt14-Cre system resulted in complete ablation of Foxa1 in mammary glands and concomitant abrogation of mammary duct formation, whereas the MMTV-driven Cre models only showed partial ablation of the loxP flanked Foxa1 gene with more limited effects on mammary gland development.
RESULTS
Incomplete suppression of Foxa1 in mammary glands leads to partial defects in mammary ductal formation
To achieve mammary-specific ablation of Foxa1, we mated the Foxa1loxP/loxP mice with MMTV-Cre mice. H&E staining showed that mammary glands in Foxa1loxP/loxP;MMTV-Cre mice had fewer mammary ducts compared to those in wild-type (WT) control Foxa1loxP/loxP mice (Fig. 1). Further staining for a ductal epithelial cell marker, cytokeratin 19 (CK19), confirmed that mammary glands in Foxa1loxP/loxP;MMTV-Cre mice indeed had significantly fewer ductal epithelial cells than the control mice (Fig. 1 and 2). When we examined Foxa1 expression in mammary glands by immunohistochemical staining, we found that Foxa1-positive staining was only found in ductal epithelial cells; however, MMTV-Cre only led to partial deletion of Foxa1 in Foxa1loxP/loxP;MMTV-Cre mice compared to controls (18.10 ± 3.69% vs 28.07 ± 4.18% positivity in Fig. 1 and 2). This was accompanied by partial reduction of ERα expression in Foxa1loxP/loxP;MMTV-Cre mice (11.32 ± 8.45% vs 22.06 ± 2.51% positivity in Fig. 1 and 2). Further whole-mount staining of mammary glands showed that partial ablation of Foxa1 in Foxa1loxP/loxP;MMTV-Cre mice also led to reduced branching of ductal trees and terminal end bud formation compared to the control WT mice (Fig. 3).
Fig. 1.
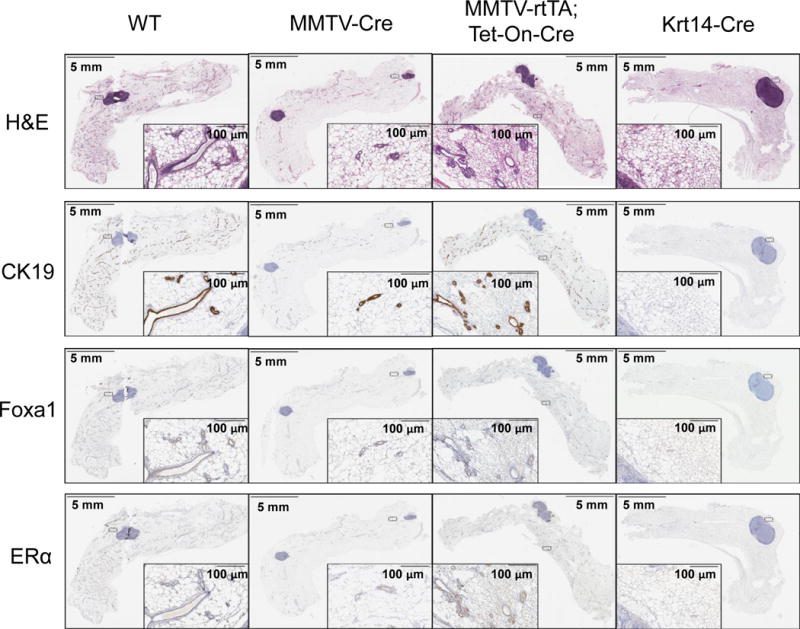
Foxa1 is required for the development of mammary ductal epithelial cells. Structures of mammary glands by H&E staining and expression of cytokeratin 19 (CK19), Foxa1, and ERα in the entire mammary glands from control wild-type (WT), Foxa1loxP/loxP;MMTV-Cre (MMTV-Cre), Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre (MMTV-rtTA;Tet-On-Cre), and Foxa1loxP/loxP;Krt14-Cre (Krt14-Cre) mice by immunohistochemical staining.
Fig. 2.
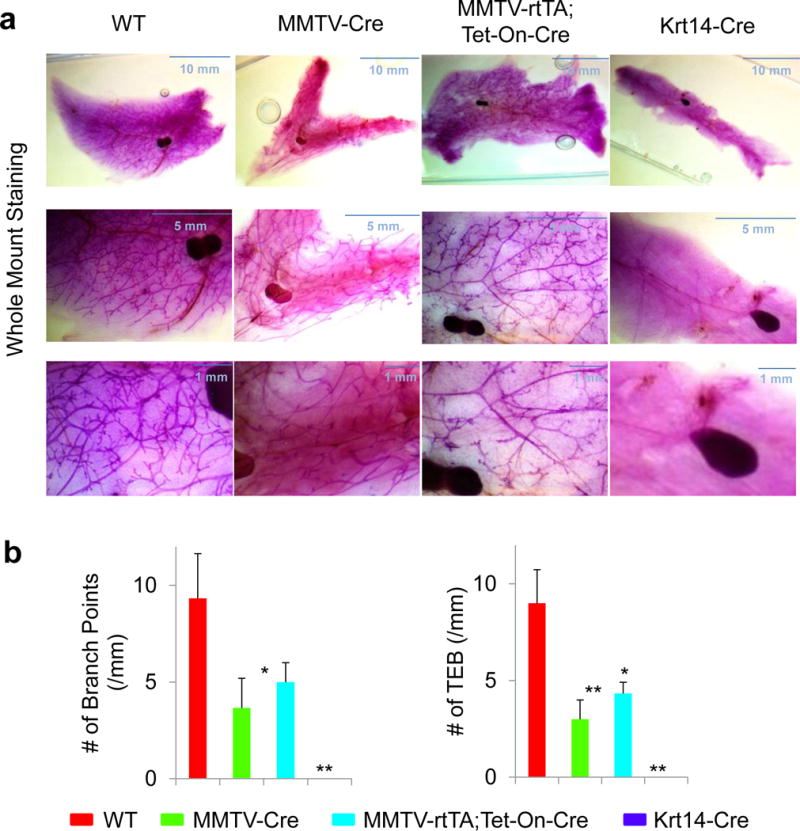
Foxa1 is required for mammary ductal branching and formation. (A) Whole-mount staining of mammary glands from control wild-type (WT), Foxa1loxP/loxP;MMTV-Cre (MMTV-Cre), Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre (MMTV-rtTA;Tet-On-Cre), and Foxa1loxP/loxP;Krt14-Cre (Krt14-Cre) mice. (B) Quantitative analysis of mammary ductal branching and terminal end bud (TEM) formation measured by the number of branch points and TEM per mm from control wild-type (WT, n=10), Foxa1loxP/loxP;MMTV-Cre (MMTV-Cre, n=3), Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre (MMTV-rtTA;Tet-On-Cre, n=5), and Foxa1loxP/loxP;Krt14-Cre (Krt14-Cre, n=3) mice. Values are expressed as means ± STD, *p<0.01, **p<0.001.
Fig. 3.
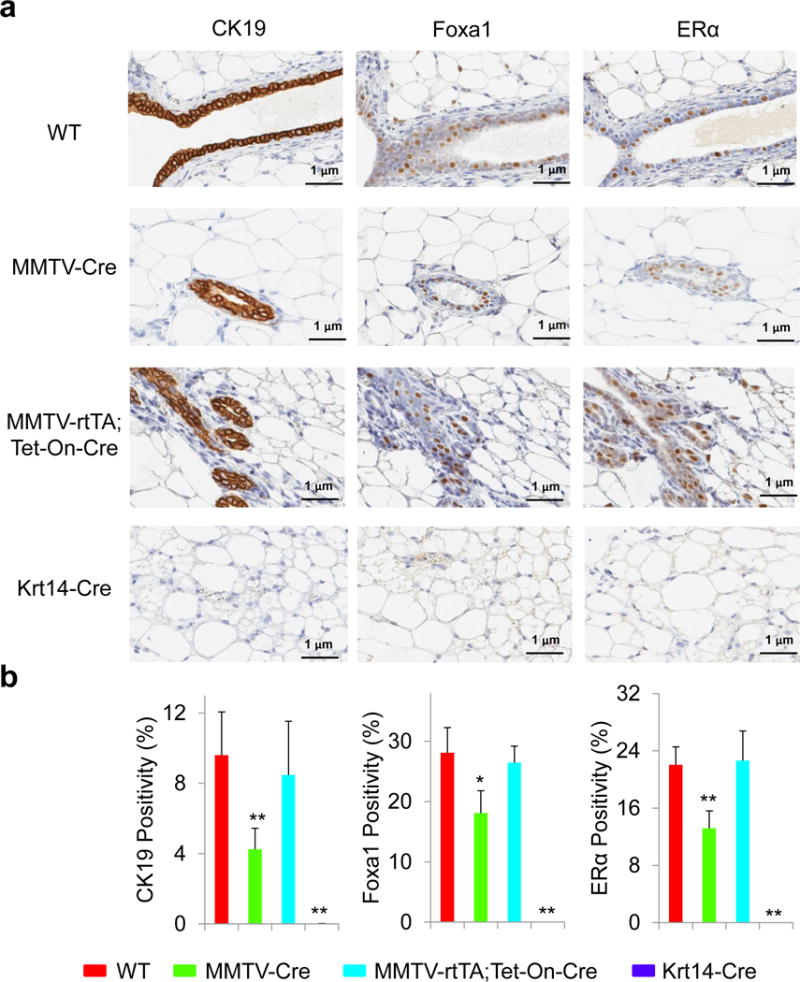
Quantitative analysis of CK19, Foxa1, and ERα expression in ductal epithelial cells. (A) Expression of CK19, Foxa1, and ERα in the ductal epithelial cells of mammary glands from control wild-type (WT), Foxa1loxP/loxP;MMTV-Cre (MMTV-Cre), Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre (MMTV-rtTA;Tet-On-Cre), and Foxa1loxP/loxP;Krt14-Cre (Krt14-Cre) mice by immunohistochemical staining. (B) The total positivity of CK19, Foxa1, and ERα expression in ductal epithelial cells in the entire mammary glands from control wild-type (WT, n=10), Foxa1loxP/loxP;MMTV-Cre (MMTV-Cre, n=3), Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre (MMTV-rtTA;Tet-On-Cre, n=5), and Foxa1loxP/loxP;Krt14-Cre (Krt14-Cre, n=3) mice. Values are expressed as means ± STD, *p<0.01, **p<0.001.
As the partial ablation of Foxa1 disturbed the development of mammary ducts, we also examined an inducible model of mammary-specific ablation of Foxa1 using Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre mice with treatment of 2 μg/ml doxycycline for 6 weeks. These mice only showed minimal ablation of Foxa1 and the frequency of CK19-positive and ERα-positive epithelial cells was not changed significantly (26.46 ± 2.75% Foxa1 and 20.32 ± 2.80% ERα positivity in Fig. 1 and 2). Interestingly, while the structures of ductal trees in these mice appeared normal histologically, the ductal branching and terminal end buds were slightly reduced in the mammary glands of the inducible transgenic model compared to the control WT mice (Fig. 3). Thus, these results suggest that MMTV-driven Cre recombinase may be a good model system for the genetic analysis of partial deletion of Foxa1 in mammary development and tumorigenesis.
Foxa1 is essential for ductal epithelium formation
To achieve complete ablation of Foxa1 in mammary epithelial cells, we mated the Foxa1loxP/loxP mice with Krt14-Cre mice. H&E staining showed that mammary fat pads in Foxa1loxP/loxP;Krt14-Cre mice were formed normally, but lacked mammary ducts, which was a striking difference when compared with the well developed mammary ducts and alveoli in wild-type (WT) control Foxa1loxP/loxP mice (Fig. 1). This observation was confirmed by CK19 staining (Fig. 1) that no mammary epithelial cells were present in Foxa1loxP/loxP;Krt14-Cre mice (Fig. 1 and 2). In addition, Foxa1 and ERα expression was absent from the mammary glands of Foxa1loxP/loxP;Krt14-Cre mice as expected if ductal epithelial cells are lacking completely (Fig. 1 and 2). Whole-mount staining of mammary glands confirmed complete ablation of Foxa1 in Foxa1loxP/loxP;Krt14-Cre mice, causing complete depletion of ductal tree formation (Fig. 3). Thus, Foxa1 is required and essential for mammary duct formation, and the Foxa1loxP/loxP;Krt14-Cre mouse model will be a useful tool to study Foxa1 functions in mammary tumorigenesis in vivo in the future.
Coordinative regulation of Foxa1 and ERα in mammary duct formation
Partial or complete ablation of Foxa1 in mammary glands in three mouse models produced corresponding reductions in ERα expression (Fig. 1 and 2), with high correlation (r = 0.91) (Fig. 4A). Additionally, the expression of these two factors, Foxa1 and ERα, was also highly correlated with CK19 expression of the ductal epithelial cell marker, r = 0.73 for Foxa1 and CK19, and r = 0.76 for ERα and CK19, respectively (Fig. 4B and 4C). Moreover, principal component analysis showed that Foxa1, ERα, and CK19 expression was clustered closely in mice with four groups of different genotypes, while the variance within each group was minimal (Fig. 4D), indicating that expression of these three genes is highly correlated. These results suggest that Foxa1 and ERα are required for coordinative regulation in mammary duct formation.
Fig. 4.
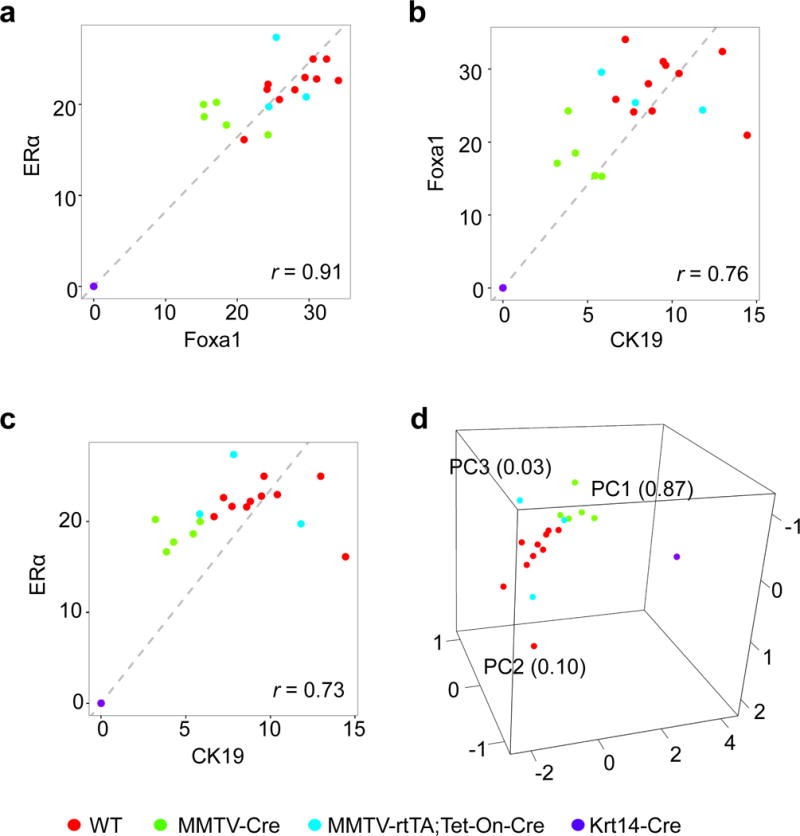
Correlation analysis of CK19, Foxa1, and ERα expression in mammary ductal formation. (A–C) correlation analysis of Foxa1 and ERα expression (A), Foxa1 and CK19 expression (B), and ERα and CK19 expression (C) from the positivity data (Fig. 2B) in the ductal epithelial cells of mammary glands in control wild-type (WT, n=10), Foxa1loxP/loxP;MMTV-Cre (MMTV-Cre, n=3), Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre (MMTV-rtTA;Tet-On-Cre, n=5), and Foxa1loxP/loxP;Krt14-Cre (Krt14-Cre, n=3) mice. D, The principal component analysis (PCA) of CK19, Foxa1, and ERα expression in mammary ductal formation of total 21 WT, MMTV-Cre, MMTV-rtTA;Tet-On-Cre, and Krt14-Cre mice.
DISCUSSION
Our study evaluated the roles of Foxa1 in mammary ductal branching and morphogenesis and provides the first fully in vivo demonstration that Foxa1 is essential for mammary ductal formation. A hallmark for luminal breast cancer is that FOXA1 is required for the growth of human ERα-positive breast cancer cells (Carroll et al., 2005; Laganiere et al., 2005). The fact that Foxa1-dependent and ERα-mediated estrogen signaling is required for mammary ductal development and progression of luminal breast cancer suggests that luminal breast cancer cells are still in a mammary lineage. Indeed, anti-estrogen therapy, such as tamoxifen, has been used to reverse the growth of luminal breast cancer cells. However, lack expression of both FOXA1 and ERα is a molecular signature for basal subtype of breast cancer. Thus, our study on the requirement of Foxa1 for the formation of mammary ductal epithelial cells indicates that suppression of FOXA1 and ERα in basal subtype of breast cancer might lead to cell differentiation away from the mammary lineage. This suggests that treatment of basal subtype of breast cancer should not target estrogen signaling.
Our observations of the highly correlated expression of Foxa1, ERα, and CK19 in mammary epithelial cells of the different models (Fig. 4) suggests the coordinative regulation of Foxa1 and ERα is essential for the development of mammary epithelial cells and ductal trees. Paralleled changes of ERα following partial and complete ablation of Foxa1 in mammary ductal epithelia suggest that Foxa1 is also required for the maintenance of ERα signature in mammary ducts (Figure 1, 2, and 4). Furthermore, we found that these gene expression changes were consistent to the changes in the ability of ductal tree formation in the mammary fat pad (Figure 1–4). However, genetic deficiency of FOXA1 has been barely seen in humans, thus, the investigation of Foxa1 functions in mouse mammary duct formation and tumorigenesis remains invaluable to provide key insights into the mechanisms of FOXA1 regulation in human breast cancer development.
Moreover, our study provides a model of duct-less mammary glands in Foxa1loxP/loxP;Krt14-Cre mice, suggesting that mammary fat pad formation is independent of Foxa1, as only the ductal epithelia were affected. Foxa1loxP/loxP;Krt14-Cre mice live normally without mammary ducts, suggesting that removing or terminating the formation of mammary ducts in females at high risks of breast cancer could be a potential therapy.
In conclusion, our study shows that Foxa1 is essential for mammary duct formation in vivo. Although Krt14-Cre abrogated the mammary duct formation in Foxa1loxP/loxP mice, it also completely deleted Foxa1 in mammary epithelial cells. Thus, ablation of Foxa1 in a matured and well developed mammary glands using Krt14-driven inducible Cre expression, Foxa1loxP/loxP;Krt14-rtTA;Tet-On-Cre, would be promising for the future investigation of Foxa1 in mammary tumorigenesis in vivo, which will also benefit other genetic studies of mammary development and tumorigenesis.
METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Mayo Clinic. The experiment was carried out under controlled conditions with a 12-h light/dark cycle. Cages with filters were used along with sterile bedding, ad libitum diet, and water. Animals were maintained on a normal chow. The derivation of Foxa1loxP/loxP mice (from Dr. Klaus H. Kaestner, University of Pennsylvania) has been reported previously (Gao et al., 2008a). Foxa1loxP/loxP mice were mated with MMTV-Cre (from Jackson Laboratory), MMTV-rtTA;Tet-On-Cre (from Dr. Lewis A. Chodosh, University of Pennsylvania) (Gunther et al., 2002), or Krt14-Cre (from Jackson Laboratory) mice to obtain Foxa1loxP/loxP;MMTV-Cre, Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre, or Foxa1loxP/loxP;Krt14-Cre mice. Foxa1loxP/loxP mice were used as control wild-type (WT) mice. Genotypes of Foxa1loxP/loxP and Cre were determined by PCR. 2 μg/ml doxycycline in drinking water was given to Foxa1loxP/loxP;MMTV-rtTA;Tet-On-Cre mice from age of 6 weeks old to 12 weeks old to induce Cre expression. Mammary glands of all female mice (3 to 10 mice per group) were collected at 12 weeks old for the following analysis.
Whole-mount staining
Dissected mammary glands were placed on slides and dried for at least 30 minutes, and then fixed in Carnoy’s solution (60% ethanol, 30% chloroform, and 10% acetic acid) for over 4 hours and stored in 70% ethanol. Samples were then gradually rehydrated to 100% water, stained with carmin alum, dehydrated and cleared with xylene. The images of all slides were captured using AmScope microscope (Irvine, CA).
Immunohistochemical Staining
Excised mammary gland tissues were fixed by immersion in 10% buffered formalin overnight and then transferred to 70% ethanol for long-term fixation. Representative sections of fixed tissue were trimmed and embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), cytokeratin 19 (CK19) antibody (clone, TROMA-3), Foxa1 antibody (Santa Cruz, Dallas, TX), or ERα antibody (Santa Cruz) for histological examination. The TROMA-3 antibody was purchased from The University of Iowa, Iowa City, IA. All of the stained sections were imaged using Aperio ScanScope XT (Vista, CA, USA) and analyzed using Aperio ImageScope (v11.1.2.752) to determine the total positivity.
Statistical analysis
Student’s t test was applied to compare two groups. Data are expressed as means ± STD. A probability (p) value of 0.05 or less was considered to be the criterion for a significant difference by asterisks: *, p ≤ 0.01; **, p ≤ 0.001. Principal component analysis (PCA) (Pearson, 1901) was used to analyze the correlations among Foxa1, ERα, and CK19.
Acknowledgments
We thank Dr. Lewis Chodosh (University of Pennsylvania) kindly provided the MMTV-rtTA mouse model. We thank Erin E. Miller for the instructions on the dissection of mouse mammary glands from the laboratory of Dr. Derek C. Radisky. We also thank Brandy Edenfield for immunohistochemical staining. We thank Kelly Viola from OSPA at Mayo Clinic Jacksonville for helpful proofreading. Authors declare that no potential conflicts of interest were disclosed. Z.L. designed the project and wrote the manuscript. Y.L., Y.Z., B.S., X.W., and C.C. did the experiments and wrote the part of manuscript. D.K. provided the mouse model and revised the manuscript. K.H.K provided the mouse model and revised the manuscript. This study is supported by the grant # R00CA168983 from NCI to Z.L.
Grant Support: This study is supported by the grant # R00CA168983 from NCI to Z.L.
References
- Ahn Y, Sims C, Logue JM, Weatherbee SD, Krumlauf R. Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling. Development. 2013;140:583–593. doi: 10.1242/dev.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature cell biology. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Badve S, Turbin D, Thorat MA, Morimiya A, Nielsen TO, Perou CM, Dunn S, Huntsman DG, Nakshatri H. FOXA1 expression in breast cancer–correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13:4415–4421. doi: 10.1158/1078-0432.CCR-07-0122. [DOI] [PubMed] [Google Scholar]
- Behr R, Brestelli J, Fulmer JT, Miyawaki N, Kleyman TR, Kaestner KH. Mild nephrogenic diabetes insipidus caused by Foxa1 deficiency. J Biol Chem. 2004;279:41936–41941. doi: 10.1074/jbc.M403354200. [DOI] [PubMed] [Google Scholar]
- Bernardo GM, Bebek G, Ginther CL, Sizemore ST, Lozada KL, Miedler JD, Anderson LA, Godwin AK, Abdul-Karim FW, Slamon DJ, et al. FOXA1 represses the molecular phenotype of basal breast cancer cells. Oncogene. 2013;32:554–563. doi: 10.1038/onc.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5:193–208. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Kaestner KH, Whitsett JA. Stage-specific regulation of respiratory epithelial cell differentiation by Foxa1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L750–759. doi: 10.1152/ajplung.00151.2005. [DOI] [PubMed] [Google Scholar]
- Bowman-Colin C, Xia B, Bunting S, Klijn C, Drost R, Bouwman P, Fineman L, Chen X, Culhane AC, Cai H, et al. Palb2 synergizes with Trp53 to suppress mammary tumor formation in a model of inherited breast cancer. Proc Natl Acad Sci U S A. 2013;110:8632–8637. doi: 10.1073/pnas.1305362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2:a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarel MM, Zaragoza R, Pensa S, Li J, Green AR, Watson CJ. Constitutive activation of JAK2 in mammary epithelium elevates Stat5 signalling, promotes alveologenesis and resistance to cell death, and contributes to tumourigenesis. Cell death and differentiation. 2012;19:511–522. doi: 10.1038/cdd.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang Y, Zhang J, Nicholas SA, Kim AL, Zhou P, Goff SP. DDB1 is essential for genomic stability in developing epidermis. Proc Natl Acad Sci U S A. 2007;104:2733–2737. doi: 10.1073/pnas.0611311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukacisin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T, et al. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nature cell biology. 2012a;14:1212–1222. doi: 10.1038/ncb2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Wei Y, Romano RA, DeCoste C, Kang Y, Sinha S. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem cells. 2012b;30:1496–1508. doi: 10.1002/stem.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Developmental biology. 2009;329:227–241. doi: 10.1016/j.ydbio.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer treatment reviews. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008a;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008b;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Hallberg K, Harfe BD, Reyahi A, Kannius-Janson M, Nilsson J, Cobourne MT, Sharpe PT, McMahon AP, Linde A. Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Developmental cell. 2007;12:99–112. doi: 10.1016/j.devcel.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, Chodosh LA. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. Faseb J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Habashy HO, Powe DG, Rakha EA, Ball G, Paish C, Gee J, Nicholson RI, Ellis IO. Forkhead-box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer. 2008;44:1541–1551. doi: 10.1016/j.ejca.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Hu Q, Luo Z, Xu T, Zhang JY, Zhu Y, Chen WX, Zhong SL, Zhao JH, Tang JH. FOXA1: a promising prognostic marker in breast cancer. Asian Pac J Cancer Prev. 2014;15:11–16. doi: 10.7314/apjcp.2014.15.1.11. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev. 2010;20:527–532. doi: 10.1016/j.gde.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Katz J, Liu Y, Drucker DJ, Schutz G. Inactivation of the winged helix transcription factor HNF3alpha affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 1999a;13:495–504. doi: 10.1101/gad.13.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Katz J, Liu Y, Drucker DJ, Schutz G. Inactivation of the winged helix transcription factor HNF3alpha affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 1999b;13:495–504. doi: 10.1101/gad.13.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V. From the Cover: Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci U S A. 2005;102:11651–11656. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest. 2009;119:1537–1545. doi: 10.1172/JCI38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley LE, Curtis KM, Sanchez-Mejias A, Rieger ME, Robbins DJ, Briegel KJ. The WNT-controlled transcriptional regulator LBH is required for mammary stem cell expansion and maintenance of the basal lineage. Development. 2015;142:893–904. doi: 10.1242/dev.110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62:339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- Mitchell EH, Serra R. Normal mammary development and function in mice with Ift88 deleted in MMTV- and K14-Cre expressing cells. Cilia. 2014;3:4. doi: 10.1186/2046-2530-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Pearson K. LIII. On lines and planes of closest fit to systems of points in space. Philosophical Magazine Series. 1901;6(2):559–572. [Google Scholar]
- Peterson RS, Clevidence DE, Ye H, Costa RH. Hepatocyte nuclear factor-3 alpha promoter regulation involves recognition by cell-specific factors, thyroid transcription factor-1, and autoactivation. Cell Growth Differ. 1997;8:69–82. [PubMed] [Google Scholar]
- Shih DQ, Navas MA, Kuwajima S, Duncan SA, Stoffel M. Impaired glucose homeostasis and neonatal mortality in hepatocyte nuclear factor 3alpha-deficient mice. Proc Natl Acad Sci U S A. 1999;96:10152–10157. doi: 10.1073/pnas.96.18.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]


