Abstract
Vulvar dermatoses are common, potentially debilitating conditions that can be seen by a variety of medical specialists. Lichenoid vulvar diseases, namely lichen sclerosus (LS), lichen planus (LP), and lichen simplex chronicus (LSC), can all negatively impact patients’ quality of life and LS and LP also have an association with squamous cell carcinoma. It is essential that dermatologists are familiar with the unique features of each of these conditions to ensure the appropriate management and follow up. Herein, we provide an update on the epidemiology, clinical presentation, histopathology, and treatment of patients with vulvar LS, LP, and LSC.
Keywords: lichenoid vulvar disease, vulvar dermatoses, lichen sclerosus, lichen planus, erosive lichen planus, lichen simplex chronicus
Introduction
Lichenoid vulvar dermatoses, which include lichen sclerosus (LS), lichen planus (LP), and lichen simplex chronicus (LSC), are chronic inflammatory conditions that can manifest with a variety of symptoms, most commonly pruritus or pain. Although there are overlapping clinical characteristics of LS, LP, and LSC, each condition also has its own unique features (Table 1). An accurate diagnosis is essential for appropriate disease management and patient education because the natural history of these conditions differs. In this article, we provide an update on the epidemiology, clinical characteristics, and treatment of patients with lichenoid vulvar dermatoses with an emphasis on clinical pearls for dermatologists who may not be familiar with the management of vulvar disease.
Table 1.
Distinguishing characteristics of vulvar lichen sclerosus, erosive lichen planus, and lichen simplex chronicus
| Lichen Sclerosus | Erosive Lichen Planus | Lichen Simplex Chronicus | |
|---|---|---|---|
| Age of Onset | All ages with common onset in both prepubertal and postmenopausal patients | Not in children | All ages |
| Extragenital Involvement | Rarely | Yes | Yes |
| Associated Symptoms | Pruritus | Pain | Pruritus |
| Clinical Features | Porcelain-white papules or plaques; can have associated purpura, hyperkeratosis, fissures, erosions, or ulcerations | Erosions with white reticulations | Lichenified plaques |
| Common Distribution in Genital Area | Interlabial sulci, labia minora, labia majora, clitoris, clitoral hood, perineum, and perianal area | Medial aspect of labia minora and vestibule | Labia majora |
| Vaginal Involvement | No | Yes | No |
| Histopathology | Hyperkeratosis, epidermal atrophy, and homogenization of collagen in the papillary dermis, overlying a lymphocytic infiltrate | Lymphocytic and lichenoid dermatitis, wedge shaped hypergranulosis, numerous cytoid bodies, and pointed rete ridges | Epidermal thickening, hyperkeratosis, spongiosis, and acanthosis |
| Risk of Progression to Squamous Cell Carcinoma | Yes | Yes | No |
Lichen sclerosus
Nomenclature
LS is a chronic inflammatory disease that was first identified in 1887 (Fistarol and Itin, 2013). It has since been referred to as kraurosis vulvae, vulvar dystrophy, white spot disease, lichen albus, guttate scleroderma, and lichen sclerosus et atrophicus (Fistarol and Itin, 2013, Schlosser and Mirowski, 2015).
Epidemiology
The exact prevalence of LS is unknown. It is estimated that the prevalence ranges from 0.1% to 1.7% with a higher prevalence reported in gynecology practices compared with dermatology practices (Goldstein et al., 2005, Wallace, 1971). A recent study determined the incidence of vulvar LS at 14.6 per 100,000 woman-years (Bleeker et al., 2016). Because LS can be asymptomatic and not always identified by physicians, its prevalence is likely greater than what has been reported. Although LS can occur at any age, it typically has a bimodal onset in prepubertal or postmenopausal women and is the most frequent among women who are postmenopausal (Wallace, 1971). Prepubertal LS was previously believed to resolve by puberty; however, several studies have refuted this notion (Powell and Wojnarowska, 2002, Smith and Fischer, 2009). In Smith and Fischer’s prospective study of 12 patients with prepubertal LS, 75% of patients still had active disease after menarche (Smith and Fischer, 2009).
Etiology
The exact etiology of LS has not been fully elucidated; however, it is thought to be an immune-mediated disorder. LS has been associated with autoimmune diseases in up to 28% of patients (Cooper et al., 2008). Autoimmune thyroiditis is the most common concomitant autoimmune disorder, followed by alopecia areata, vitiligo, and pernicious anemia (Birenbaum and Young, 2007, Cooper et al., 2008). A familial predisposition has been reported in 12% of patients and an association with human leukocyte antigen DQ7, DQ8, and DQ9 has also been reported (Powell and Wojnarowska, 2001, Sherman et al., 2010). Although autoantibodies that target extracellular matrix protein may be elevated in the sera of patients with anogenital LS, it is thought that these autoantibodies are secondary and not directly pathogenic in the disease process (Fistarol and Itin, 2013, Oyama et al., 2003). Additionally, Howard et al. (2004) found that one third of patients in their cohort of 96 patients with vulvar LS had autoantibodies that targeted the basement membrane zone (BMZ). However, Gambichler et al. (2011) later reported no significant difference in BMZ autoantibody levels between patients with LS and control subjects.
Clinical findings
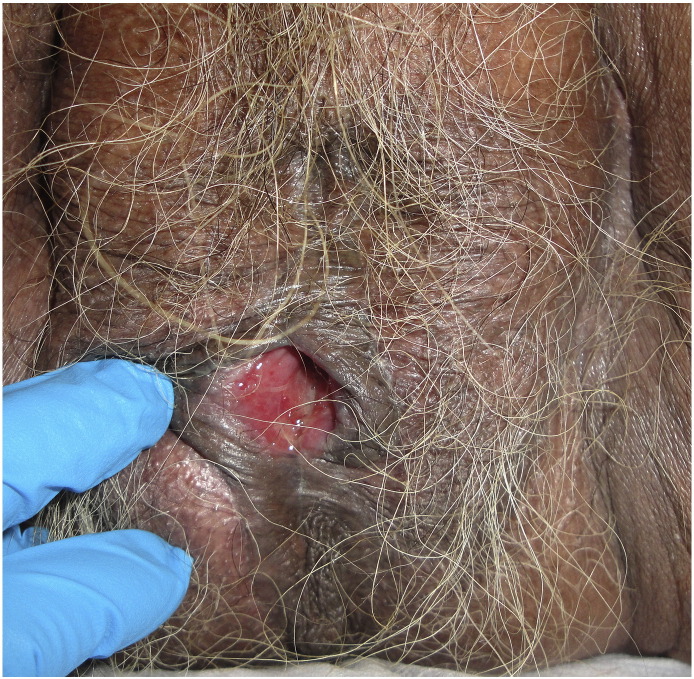
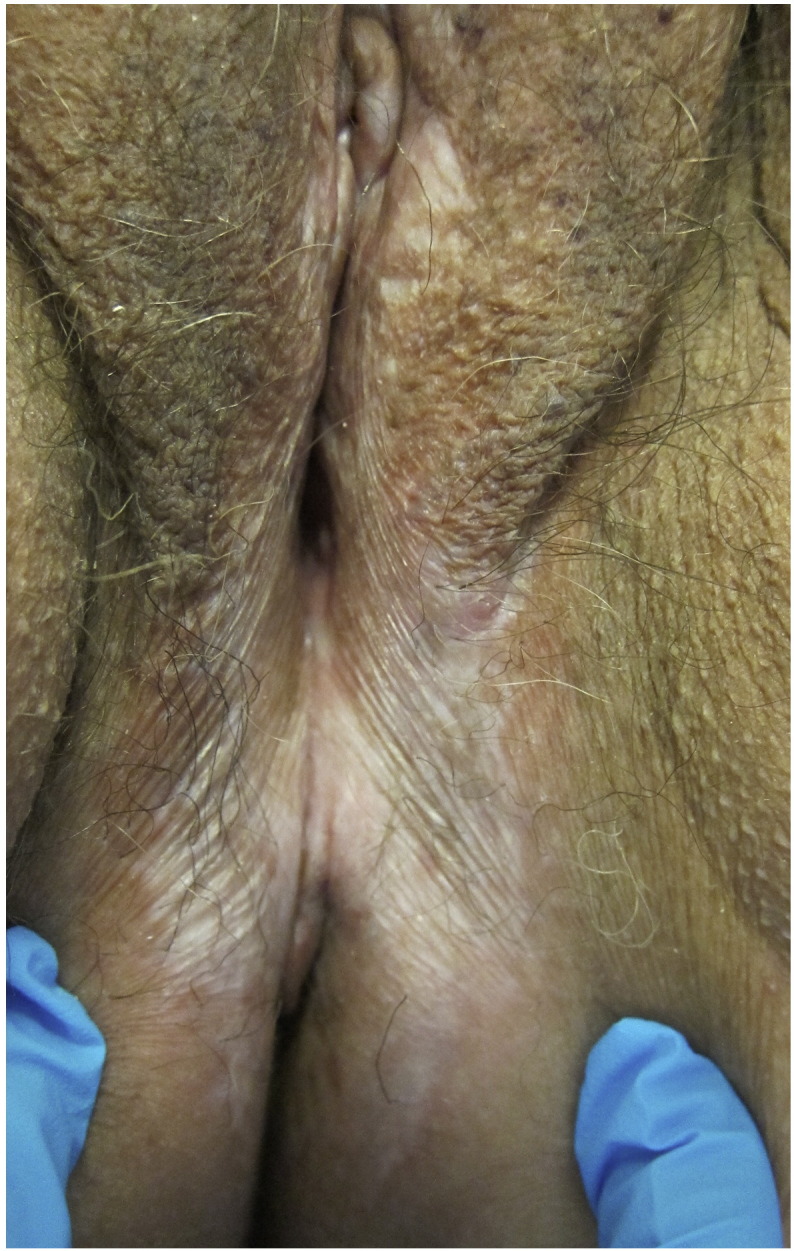
LS primarily affects the vulvar and perianal area and spares the vagina, although there have been rare reports of biopsy-proven LS that involve the vagina (Zendell and Edwards, 2013). LS can occur extragenitally in up to 13% of patients and presents exclusively extragenital in 6% of cases (Thomas et al., 1996, Wallace, 1971). Classically, vulvar LS appears as porcelain-white papules or plaques, which may have associated purpura, hyperkeratosis, fissures, erosions, or ulcerations (Fig. 1, Fig. 2; Fistarol and Itin, 2013). A wrinkled or cigarette paper-like appearance is characteristic (Schlosser and Mirowski, 2015). Clitoral hood edema may be present as well as lichenification if patients scratch affected areas (Schlosser and Mirowski, 2015). Vulvar LS usually involves the interlabial sulci, labia minora, labia majora, clitoris, clitoral hood, perineum, and perianal area, forming a figure-of-eight pattern (Fistarol and Itin, 2013). Additionally, LS can extend into the genitocrural folds, buttocks, and thighs (Fistarol and Itin, 2013).
Fig. 1.
Lichen sclerosus: White atrophic plaques that extend from the vulva to the perianal area. The cigarette paper-like appearance and figure-of-eight pattern are highlighted.
Fig. 2.
Lichen sclerosus: White thickened plaques with loss of architecture as demonstrated by the obliteration of the labia minora, narrowing of the introitus, and scarring of the clitoral hood.
In a retrospective cohort study of 81 women, 76% of patients had labial involvement including 70% who had concurrent clitoral involvement (Lorenz et al., 1998). Perineal and anal involvement were present in 68% and 32% of patients, respectively (Lorenz et al., 1998). LS can leave scars later in the disease course and result in the fusion of the labia minora with the labia majora, complete resorption of the labia minora, fusion of the clitoral hood, and/or scarring of the introitus, which may lead to sexual dysfunction and in extreme cases interference with urination (Fistarol and Itin, 2013, Schlosser and Mirowski, 2015).
In children, LS often presents with vulvar purpura or ecchymosis, which may mimic sexual abuse (Powell and Wojnarowska, 2001). Additionally, children with LS may present with gastrointestinal complaints due to perianal involvement. In a study of 18 girls with anogenital LS, 89% reported at least one gastrointestinal complaint of whom 67% experienced constipation (Edwards, 2011, Maronn and Esterly, 2005).
LS can lead to vulvar lentiginosis, which appears as dark brown or black macules with irregular borders (Fistarol and Itin, 2013). Vulvar lentiginosis can be readily distinguished from melanoma on the basis of biopsy results. However, it is important to note that genital nevi in the setting of LS can mimic melanoma both clinically and histologically. Thus, pathologists should be informed if a genital nevus has been biopsied in the setting of LS. Although vulvar melanoma can occur in setting of LS, it is extremely rare (Fistarol and Itin, 2013).
Patients with LS can experience debilitating symptoms with pruritus as a hallmark of this condition (Fistarol and Itin, 2013). LS can also cause pain, burning, and dyspareunia (Fistarol and Itin, 2013). LS is asymptomatic in up to 39% of adult patients (Goldstein et al., 2005). In contrast, LS in children is usually symptomatic (Powell and Wojnarowska, 2001).
Pathology
Pathognomonic histological changes in patients with LS include hyperkeratosis, epidermal atrophy, and homogenization of collagen in the papillary dermis (Schlosser and Mirowski, 2015). Histopathology in patients with early LS, however, may be nonspecific and difficult to differentiate from LP because both involve a lymphocytic, lichenoid interface dermatitis (Fung and LeBoit, 1998). Fung and Leboit (1998) identified light microscopic criteria to help differentiate early LS from early LP. A psoriasiform lichenoid pattern, epidermotropism, atrophy, basement membrane thickening, and a decrease in papillary dermal elastic fibers with vertically oriented fibers were more common in LS lesions compared with LP ones (Fung and LeBoit, 1998).
Diagnosis
A diagnosis of LS can be made clinically; however, a biopsy can aid in the diagnosis or rule out other conditions in the differential diagnosis. As described previously, histology may be nonspecific, particularly in patients with early LS. In children with LS, a biopsy should ideally be restricted to treatment-refractory cases.
Disease course
LS is a chronic, relapsing condition that can result in loss of vulvar architecture and development of vulvar squamous cell carcinoma (VSCC). A recent study of 3038 women with histologically confirmed vulvar LS determined the 20-year incidence of VSCC at 6.7% (Bleeker et al., 2016). Age of ≥ 70 years and presence of vulvar intraepithelial neoplasia at the time of diagnosis were independent risk factors for the development of VSCC (Bleeker et al., 2016).
Treatment
Ultra-potent topical steroid medications are the mainstay of treatment for patients with LS and there is strong evidence supporting their efficacy. Ointment is the preferred vehicle because it is less irritating compared with other forms of treatment (Guerrero and Venkatesan, 2015). In a study of 327 women with vulvar LS who received ultra-potent topical steroid medications, 96% experienced an improvement in symptoms, 23% had a complete return to normal skin, and 68% had a partial return (Cooper et al., 2004a).
Ultra-potent topical steroid medications should be used as a first-line treatment in children as well and there are data to support their safety and efficacy in the prepubertal population (Ellis and Fischer, 2015, Fischer and Rogers, 1997). Intralesional corticosteroid medications can be effective, especially for thick, hyperkeratotic plaques (Mazdisnian et al., 1999). Emollients can also be helpful to improve symptoms and maintain remission (Cattaneo et al., 1996).
Topical calcineurin inhibitors have documented efficacy in the treatment of patients with vulvar LS and can be considered to treat patients who are refractory to or intolerant of topical corticosteroid medications. Clobetasol has been found to be a superior treatment (Funaro et al., 2014, Goldstein et al., 2011).
Topical testosterone, a treatment that was used in the past, has demonstrated no statistically significant difference when compared with petroleum jelly (Sideri et al., 1994). Topical and systemic retinoid treatments, phototherapy, and photodynamic therapy have also been described in the treatment of patients with vulvar LS (Bousema et al., 1994, Rasi et al., 2010, Shi et al., 2016, Terras et al., 2014, Virgili et al., 1995). Lastly, surgery may be beneficial in cases of scarring and disfigurement as a result of LS (Goldstein and Burrows, 2007).
Maintenance treatment and follow-up
Until recently, data on appropriate follow-up and maintenance practices for patients with vulvar LS were limited. Lee et al. (2015) addressed these knowledge gaps in a prospective cohort study of 507 women with biopsy-proven LS treated with corticosteroid medications over a mean time period of 4.7 years. In the study, compliant patients had 0 cases of VSCC but partially compliant cases had a statistically significant increased risk of VSCC at 4.7%. Compliant patients also had significantly lower rates of scarring and symptoms compared with partially compliant patients. Importantly, maintenance treatment with topical corticosteroid medications was well-tolerated. This study is the largest to date to report on the outcomes of topical corticosteroid treatment for patients with LS and demonstrates that long-term maintenance therapy is warranted (Lee et al., 2015). Lee et al. recommended that once initial treatment with topical corticosteroid medications resulted in normal skin color and texture, the topical corticosteroid strength should be reduced. Patients should be evaluated every 3 months until their topical corticosteroid regimen is stable and their skin color and texture are normal. Additionally, patients should be followed indefinitely and seen at least annually (Lee et al., 2015).
Ellis and Fischer (2015) also demonstrated that after initial induction treatment with ultra-potent topical steroid medications, maintenance therapy in children with individualized regimens (using low or mid-potency topical steroid medications) results in improved patient outcomes. In their cohort of 46 children, those who were adherent to maintenance therapy were more likely to achieve complete disease suppression and less likely to have scarring compared with those who were nonadherent (Ellis and Fischer, 2015).
Lichen planus
Nomenclature
LP is a chronic autoimmune disorder that can affect the skin and mucous membranes including the vulva and vagina (Schlosser and Mirowski, 2015). In 1982, Pelisse et al. designated the term vulvovaginal gingival syndrome for vulvovaginal LP with concomitant oral involvement (Eisen, 1994, Pelisse et al., 1982). Desquamative inflammatory vaginitis (DIV) had been used to describe cases of erosive vulvovaginal LP; however, DIV now refers to a distinct entity that can have overlapping signs and symptoms with vulvovaginal LP (Lotery and Galask, 2003).
Epidemiology
Large population-based studies that describe the epidemiology of vulvar LP (VLP) are lacking. In a study of 3350 women at a vulvar specialty clinic over a 13-year time period, 3.7% of patients had biopsy-proven VLP of whom 17.6% had erosive disease (Micheletti et al., 2000). The incidence of VLP has not been studied.
VLP most commonly affects women during their 5th or 6th decade of life (Ball and Wojnarowska, 1998, Cooper and Wojnarowska, 2006, Eisen, 1994). The erosive variant of VLP does not occur in children, which can help distinguish it from other vulvar diseases such as LS (Kirtschig et al., 2005).
Erosive VLP can occur simultaneously with oral LP (OLP). Up to 57% of patients with OLP have concomitant VLP (Belfiore et al., 2006, Eisen, 2002). Additionally, up to 68% of women with erosive VLP have concomitant OLP (Kennedy and Galask, 2007, Kirtschig et al., 2005, Santegoets et al., 2010). Concomitant cutaneous LP can also be present. In a retrospective study of 44 patients with erosive VLP, 20% had concomitant cutaneous LP (Kirtschig et al., 2005).
Erosive VLP is associated with other autoimmune diseases. In a case-control study of 126 women with erosive VLP, 29% had one or more associated autoimmune disorders (Cooper et al., 2008). Thyroid disease is the most common concomitant autoimmune disorder, followed by alopecia areata and celiac disease (Cooper et al., 2008).
Concomitant vulvar diseases often occur in patients with VLP. In a study by Kennedy and Galask (2007) of 113 patients with erosive VLP, 34% had simultaneous contact dermatitis, 25% atrophic vaginitis (more recently termed genitourinary syndrome of menopause), and 18% candidiasis.
Although there is a well-described association between LP and HCV in certain countries such as Japan, there are no large epidemiological studies that evaluate the association between VLP and HCV specifically (Nagao et al., 1995). Kirtschig et al. (2005) and Cooper et al. (2004b) did not find any association between HCV antibodies and VLP in their studies of patients in the United Kingdom.
Etiology
LP is thought to be a T-cell mediated autoimmune disease that is characterized by the destruction of keratinocytes in the basal layer of the epidermis (Cooper et al., 2005). However, the exact etiology remains unclear. Although a study by Cooper et al. (2005) of a cohort of 56 women with biopsy-proven erosive LP showed that 61% of patients had antibodies to BP180, it is believed that these antibodies are likely a secondary phenomenon and not causative.
Clinical features
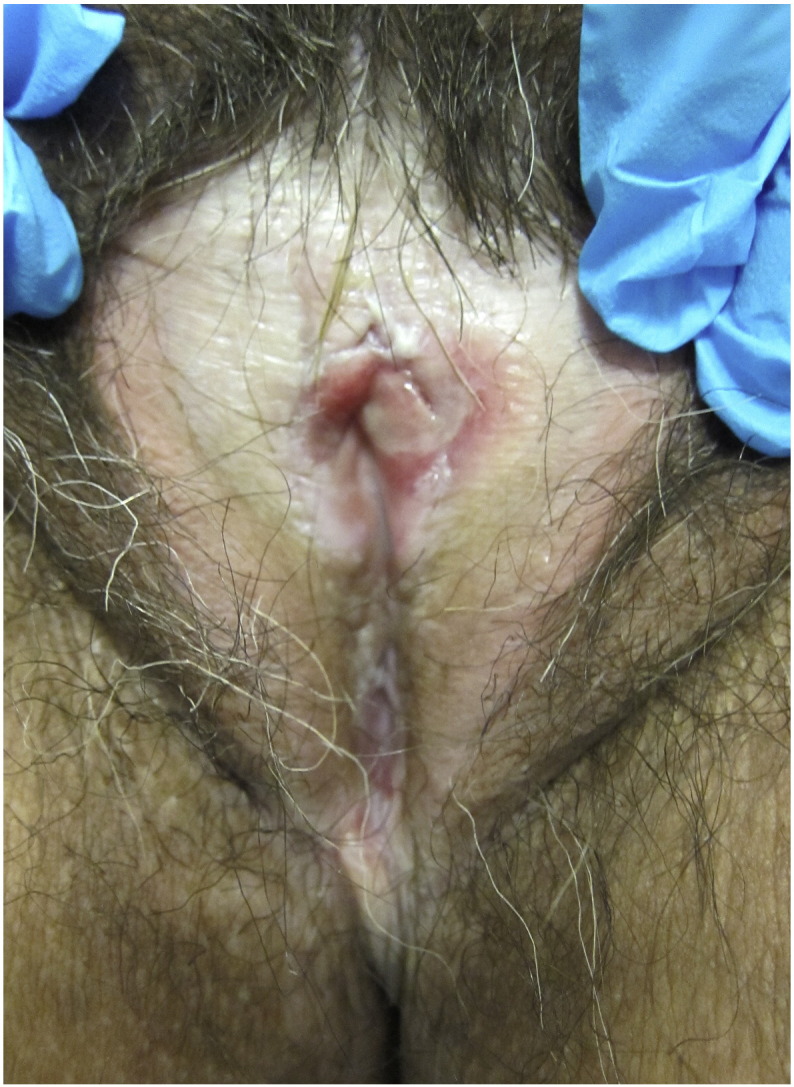
There are three variants of LP that can affect the vulva: classic type or papulosquamous LP, hypertrophic LP, and erosive LP. Papulosquamous LP appears as violaceus papules or plaques with overlying white reticulations that are known as Wickham striae (Guerrero and Venkatesan, 2015). Hypertrophic LP presents as hyperkeratotic lesions, which can mimic squamous cell carcinoma (SCC; Lewis and Bogliatto, 2013). Finally, erosive LP is the most common variant that affects the vulva. Erosions with a white lacy edge are observed and often affect the medial aspect of the labia minora and vaginal orifice (Fig. 3; Kirtschig et al., 2005, Lewis, 1998, Lewis and Bogliatto, 2013). The vagina is frequently involved (Lewis and Bogliatto, 2013). In a study of 20 patients with erosive VLP, 70% had vaginal involvement. Thus, it is important that women with erosive VLP are evaluated for vaginal involvement with speculum examinations. Erosive VLP can result in an alteration of the normal architecture including the development of vaginal adhesions, stenosis, and in severe cases complete closure of the vaginal canal (Lewis, 1998). Vulvo-vaginal gingival syndrome is a form of erosive LP that involves the vulva, vagina and mouth. This subtype of erosive LP often causes scarring and is difficult to treat (Setterfield et al., 2006).
Fig. 3.
Erosive lichen planus: Erosion that involves the vestibule. Additionally, the normal vulvar architecture has been destroyed.
Women with erosive VLP can present with pain, dyspareunia, and dysuria while women with nonerosive forms of VLP typically experience pruritus (Guerrero and Venkatesan, 2015). Those with vaginal involvement may experience vaginal discharge. Both vulvar and vaginal LP can be asymptomatic (Lewis and Bogliatto, 2013).
Pathology
Like LS, a biopsy of an LP lesion can reveal a lymphocytic, lichenoid dermatitis (Fung and LeBoit, 1998). Histological features that can differentiate LP from LS include a wedge-shaped hypergranulosis, numerous cytoid bodies, and pointed rete ridges (Fung and LeBoit, 1998). A biopsy may be nondiagnostic particularly in cases of erosive LP where there may be a loss of the epidermis.
Diagnosis
Although a diagnosis of VLP can be made clinically, particularly in patients with the classic type, a biopsy may be necessary in certain situations. Patients with the hypertrophic form of VLP should have a biopsy to rule out SCC, which this variant can mimic. Patients with the erosive variant should also undergo a biopsy to rule out other diseases in the differential diagnosis of erosive vulvar disease including bullous pemphigoid, pemphigus vulgaris, and mucus membrane pemphigoid. With erosive VLP, a biopsy should be performed at the edge of the lesion. As discussed previously, Cooper et al. (2005) showed that immunoglobins A and G indirect immunofluorescence was positive in 61% of cases of erosive VLP but direct immunofluorescence was negative in all cases.
Disease course
The natural history of VLP differs depending on the specific subtype. The classic and hypertrophic variants often respond to therapy and do not cause scarring. The erosive variant of VLP is challenging to treat and can cause scarring (Guerrero and Venkatesan, 2015). Although there is a well-described association between oral LP and SCC, this association is not as well-studied in patients with erosive VLP; however, studies have revealed an incidence of SCC of up to 3% in patients with erosive VLP (Cooper and Wojnarowska, 2006, Simpson and Murphy, 2012).
Treatment
As the treatment approach for classic and hypertrophic forms of VLP is similar to the approach for these variants in other locations of the body, the focus of treatment discussions will be on erosive VLP, which often presents a therapeutic challenge.
First-line treatment for patients with erosive VLP is typically ultra-potent corticosteroid ointment with variable outcomes reported in the literature that range from the majority being persistently symptomatic with treatment to the majority being asymptomatic (Cooper and Wojnarowska, 2006, Kirtschig et al., 2005). Whether maintenance treatment reduces the risk of scarring, SCC, and symptoms long term has not been investigated.
Topical calcineurin inhibitors have demonstrated improvement in symptoms in cases that are refractory to topical corticosteroid medications; however, a recurrence rate of 84% has been reported (Byrd et al., 2004, Lonsdale-Eccles and Velangi, 2005).
When the vagina is involved, topical steroid medications can administered with a rectal suppository in the vagina but use of high potency steroid medications must be limited given the risk of systemic absorption (Guerrero and Venkatesan, 2015). Physicians should be aware of the risk for superinfection with candida or herpes in patients who apply topical corticosteroid medications to the vagina.
For patients with severe disease, oral corticosteroid medications may be given to bridge to other immunosuppressive medications such as mycophenolate mofetil, methotrexate, or azathioprine (Guerrero and Venkatesan, 2015). Metronidazole and rituximab have also been used to treat patients with mucosal LP but data on these treatments are limited. In a study of patients with mucosal and/or cutaneous LP, metronidazole resulted in improvements in 66.6% of patients with mucosal involvement. The study did not specify whether mucosal involvement included the vagina (Rasi et al., 2010). Heelan et al. (2015) reported two cases of refractory erosive VLP who were successfully treated with rituximab.
Photodynamic therapy (PDT) is a recently described treatment for patients with VLP and was found to have an equivalent efficacy to clobetasol ointment in a randomized controlled trial of 40 patients (Helgesen et al., 2015). Finally, patients with labial adhesions may benefit from surgery (Guerrero and Venkatesan, 2015). Also, the ongoing hELP trial on systemic therapy for patients with vulvar erosive lichen planus aims to evaluate whether systemic therapy in addition to topical therapy is an effective second-line treatment for patients with this condition (Simpson et al., 2016).
Lichen simplex chronicus
Epidemiology
LSC is a chronic inflammatory disorder that can involve the vulva (Thorstensen and Birenbaum, 2012, Torgerson et al., 2015). Vulvar LSC (VLSC) is common and comprises up to 35% of patient visits to vulvar specialty clinics (O'Keefe et al., 1995, Thorstensen and Birenbaum, 2012). The incidence and prevalence of VLSC have not been studied to date. VLSC most commonly affects adults but can present in children as well (Lynch, 2004).
Etiology
VLSC can occur as either a primary condition or secondary to other dermatologic diseases (Moyal-Barracco and Wendling, 2014). Primary VLSC is considered a variant of atopic dermatitis and patients who are affected often have an atopic diathesis (Stewart, 2010). Both primary and secondary VLSC are consequences of the itch-scratch cycle (Corazza et al., 2015, Stewart, 2010). In patients with primary VLSC, the itch-scratch cycle is frequently triggered by skin irritation, which leads to pruritus and scratching. The scratching results in skin thickening and additional irritation, which subsequently worsens the pruritus. In patients with secondary VLSC, an underlying vulvar disorder contributes to this cycle (Stewart, 2010).
Clinical findings
Patients with VLSC report intense pruritus that may disturb sleep (ACOG, 2008). Heat, sweat, and friction may worsen the pruritus and symptoms can remain despite the removal of triggers (Stewart, 2010). During a physical examination, VLSC can be identified as lichenified plaques that commonly present on the labia majora but can also involve the labia minora, and perineum (Fig. 4). The vagina is spared (Lynch, 2004). Excoriations are frequently present. There can be erythema, hyper- or hypopigmentation, or scale (Stewart, 2010). Because VLSC can disrupt the normal skin barrier, there may be secondary infections present (Stewart, 2010).
Fig. 4.
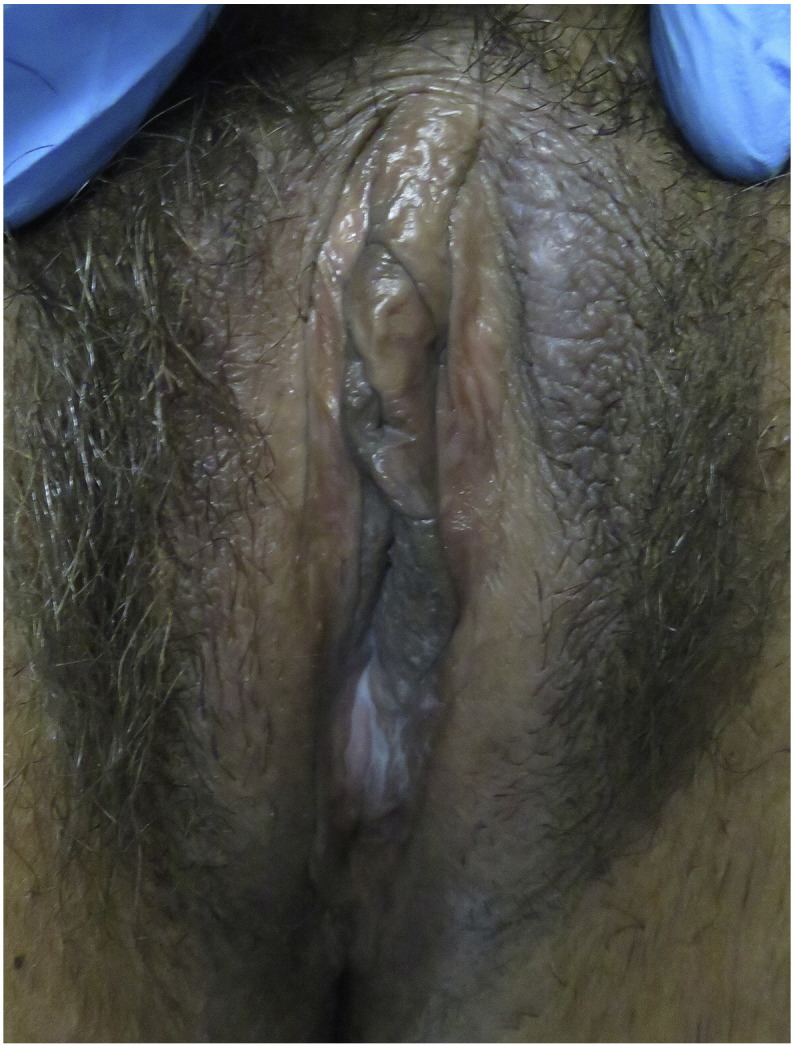
Lichen simplex chronicus: Lichenification and hyperpigmentation along the anterior medial aspect of the left labia majora.
Diagnosis
The diagnosis of VLSC is often based on patient history and physical examination; however, results from a skin biopsy can also aid in the diagnosis for equivocal cases (Stewart, 2010, Thorstensen and Birenbaum, 2012). It is important to evaluate for the presence of another vulvar disorder such as vulvar candidiasis, lichen sclerosus, psoriasis, or contact dermatitis that may be underlying secondary VLSC (Lynch, 2004).
Pathology
Histopathologically, VLSC lesions have epidermal thickening, hyperkeratosis, spongiosis, and acanthosis (Burrows et al., 2008). Notably, VLSC often lacks the vertical fibrosis that is seen in extragenital LSC and has prominent fibroblasts (Chan and Zimarowski, 2015).
Disease course
VLSC is a chronic disease and recurrence rates are high even with treatment. Patients may need therapy for years (Lynch, 2004).
Treatment
Patient education is an essential component in the management of VLSC. It is important to discuss triggers with patients such as urine or fecal incontinence, sweat, certain fabrics and pantyliners (Corazza et al., 2015, Lynch, 2004). Patients should also avoid excessive use of cleansers or irritating topical ointments, which can contribute to itching and pain (Lynch, 2004).
The management of VLSC also involves treatment of any underlying vulvar disorders, repair of barrier function, reduction of inflammation, and elimination of the itch-scratch cycle (Lynch, 2004). It is important to treat any underlying vulvar disorder; however, this may not necessarily treat the VLSC and additional treatment may need to be pursued (Lynch, 2004). Repair of the barrier can be accomplished with emollients. High potency topical steroid medications may be used to reduce inflammation, which is often present even if the skin is not erythematous (Thorstensen and Birenbaum, 2012).
Calcineurin inhibitors have also demonstrated efficacy in the treatment of patients with VLSC (Kelekci et al., 2008). Topical lidocaine can be used for immediate relief (Stewart, 2010) and antihistamines can break the itch scratch cycle (Lynch, 2004). Sedative antihistamines may be particularly beneficial to avoid night time scratching. Amitriptyline, gabapentin, and selective serotonin reuptake inhibitors can be effective to treat pruritus as well (Gencoglan et al., 2010, Lynch, 2004, O'Connell et al., 2008). Lastly, there has been one case report of a patient with recalcitrant VLSC who was successfully treated with hand-held phototherapy, which may represent an option for patients who are refractory to other treatments (Virgili et al., 2014).
Lastly, because anxiety and depression are prevalent in patients with VLSC, it is important to evaluate the psychological health of this population (Lynch, 2004). Lynch describes that patients with VLSC will report that their disease began during a time of stress and worsens with increasing stress levels. Thus, it is essential that the psychological component of this disease is addressed (Lynch, 2004).
Conclusion
It is important for dermatologists to be familiar with the diagnosis and management of lichenoid vulvar dermatoses, which are often symptomatic and in the case of LS or LP can cause scarring. Furthermore, LS and LP are associated with VSCC and there is evidence that maintenance treatment for LS can prevent VSCC as well as improve patient outcomes with regard to symptoms and scarring. Further research is warranted on the epidemiology and management of VSCC that is associated with LP.
Our review also highlights the therapeutic challenge that erosive VLP can present. Emerging research including the hELP trial will hopefully shed light on effective treatment options for patients with VLP who are refractory to treatment. Lastly, our review touches on the multifaceted nature of VLSC and the various components, both psychological and medical, that go into its management.
Footnotes
Funding sources: Dr. Pomeranz serves on the scientific advisory board for Procter and Gamble and receives royalties from UptoDate.
References
- Ball S.B., Wojnarowska F. Vulvar dermatoses: Lichen sclerosus, lichen planus, and vulval dermatitis/lichen simplex chronicus. Semin Cutan Med Surg. 1998;17:182–188. doi: 10.1016/s1085-5629(98)80012-6. [DOI] [PubMed] [Google Scholar]
- Belfiore P., Di Fede O., Cabibi D., Campisi G., Amaru G.S., De Cantis S. Prevalence of vulval lichen planus in a cohort of women with oral lichen planus: An interdisciplinary study. Br J Dermatol. 2006;155:994–998. doi: 10.1111/j.1365-2133.2006.07480.x. [DOI] [PubMed] [Google Scholar]
- Birenbaum D.L., Young R.C. High prevalence of thyroid disease in patients with lichen sclerosus. J Reprod Med. 2007;52:28–30. [PubMed] [Google Scholar]
- Bleeker M.C., Visser P.J., Overbeek L.I., van Beurden M., Berkhof J. Lichen sclerosus: Incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomark Prev. 2016;25:1224–1230. doi: 10.1158/1055-9965.EPI-16-0019. [DOI] [PubMed] [Google Scholar]
- Bousema M.T., Romppanen U., Geiger J.M., Baudin M., Vaha-Eskeli K., Vartiainen J. Acitretin in the treatment of severe lichen sclerosus et atrophicus of the vulva: A double-blind, placebo-controlled study. J Am Acad Dermatol. 1994;30:225–231. doi: 10.1016/s0190-9622(94)70021-4. [DOI] [PubMed] [Google Scholar]
- Burrows L.J., Shaw H.A., Goldstein A.T. The vulvar dermatoses. J Sex Med. 2008;5:276–283. doi: 10.1111/j.1743-6109.2007.00703.x. [DOI] [PubMed] [Google Scholar]
- Byrd J.A., Davis M.D., Rogers R.S., III Recalcitrant symptomatic vulvar lichen planus: Response to topical tacrolimus. Arch Dermatol. 2004;140:715–720. doi: 10.1001/archderm.140.6.715. [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Carli P., De Marco A., Sonni L., Bracco G., De Magnis A. Testosterone maintenance therapy. Effects on vulvar lichen sclerosus treated with clobetasol propionate. J Reprod Med. 1996;41:99–102. [PubMed] [Google Scholar]
- Chan M.P., Zimarowski M.J. Vulvar dermatoses: A histopathologic review and classification of 183 cases. J Cutan Pathol. 2015;42:510–518. doi: 10.1111/cup.12541. [DOI] [PubMed] [Google Scholar]
- Cooper S.M., Wojnarowska F. Influence of treatment of erosive lichen planus of the vulva on its prognosis. Arch Dermatol. 2006;142:289–294. doi: 10.1001/archderm.142.3.289. [DOI] [PubMed] [Google Scholar]
- Cooper S.M., Gao X.H., Powell J.J., Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702–706. doi: 10.1001/archderm.140.6.702. [DOI] [PubMed] [Google Scholar]
- Cooper S.M., Kirtschig G., Jeffery K.J., Wojnarowska F. No association between hepatitis B or C viruses and vulval lichen planus in a UK population. BJOG. 2004;111:271–273. doi: 10.1111/j.1471-0528.2004.00050.x. [DOI] [PubMed] [Google Scholar]
- Cooper S.M., Dean D., Allen J., Kirtschig G., Wojnarowska F. Erosive lichen planus of the vulva: Weak circulating basement membrane zone antibodies are present. Clin Exp Dermatol. 2005;30:551–556. doi: 10.1111/j.1365-2230.2005.01866.x. [DOI] [PubMed] [Google Scholar]
- Cooper S.M., Ali I., Baldo M., Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: A case-control study. Arch Dermatol. 2008;144:1432–1435. doi: 10.1001/archderm.144.11.1432. [DOI] [PubMed] [Google Scholar]
- Corazza M., Borghi A., Minghetti S., Toni G., Virgili A. Effectiveness of silk fabric underwear as an adjuvant tool in the management of vulvar lichen simplex chronicus: Results of a double-blind randomized controlled trial. Menopause. 2015;22:850–856. doi: 10.1097/GME.0000000000000410. [DOI] [PubMed] [Google Scholar]
- Edwards L. Pediatric genital disease. In: Edwards L.P., editor. Genital dermatology atlas. 2nd ed. Lippincott, Williams & Wilkins; Philadelphia: 2011. [Google Scholar]
- Eisen D. The vulvovaginal-gingival syndrome of lichen planus. The clinical characteristics of 22 patients. Arch Dermatol. 1994;130:1379–1382. [PubMed] [Google Scholar]
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol. 2002;46:207–214. doi: 10.1067/mjd.2002.120452. [DOI] [PubMed] [Google Scholar]
- Ellis E., Fischer G. Prepubertal-onset vulvar lichen sclerosus: The importance of maintenance therapy in long-term outcomes. Pediatr Dermatol. 2015;32:461–467. doi: 10.1111/pde.12597. [DOI] [PubMed] [Google Scholar]
- Fischer G., Rogers M. Treatment of childhood vulvar lichen sclerosus with potent topical corticosteroid. Pediatr Dermatol. 1997;14:235–238. doi: 10.1111/j.1525-1470.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Fistarol S.K., Itin P.H. Diagnosis and treatment of lichen sclerosus: An update. Am J Clin Dermatol. 2013;14:27–47. doi: 10.1007/s40257-012-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaro D., Lovett A., Leroux N., Powell J. A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J Am Acad Dermatol. 2014;71:84–91. doi: 10.1016/j.jaad.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Fung M.A., LeBoit P.E. Light microscopic criteria for the diagnosis of early vulvar lichen sclerosus: A comparison with lichen planus. Am J Surg Pathol. 1998;22:473–478. doi: 10.1097/00000478-199804000-00013. [DOI] [PubMed] [Google Scholar]
- Gambichler T., Hoxtermann S., Skrygan M., Eberz B., Regauer S., Scola N. Occurrence of circulating anti-bullous pemphigoid antibodies in patients with lichen sclerosus. J Eur Acad Dermatol Venereol. 2011;25:369–370. doi: 10.1111/j.1468-3083.2010.03739.x. [DOI] [PubMed] [Google Scholar]
- Gencoglan G., Inanir I., Gunduz K. Therapeutic hotline: Treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194–198. doi: 10.1111/j.1529-8019.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- Goldstein A.T., Burrows L.J. Surgical treatment of clitoral phimosis caused by lichen sclerosus. Am J Obstet Gynecol. 2007;196:e121–e124. doi: 10.1016/j.ajog.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Goldstein A.T., Marinoff S.C., Christopher K., Srodon M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J Reprod Med. 2005;50:477–480. [PubMed] [Google Scholar]
- Goldstein A.T., Creasey A., Pfau R., Phillips D., Burrows L.J. A double-blind, randomized controlled trial of clobetasol versus pimecrolimus in patients with vulvar lichen sclerosus. J Am Acad Dermatol. 2011;64:e99–104. doi: 10.1016/j.jaad.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Venkatesan A. Inflammatory vulvar dermatoses. Clin Obstet Gynecol. 2015;58:464–475. doi: 10.1097/GRF.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Heelan K., McAleer M.A., Roche L., McCreary C., Murphy M. Intractable erosive lichen planus treated successfully with rituximab. Br J Dermatol. 2015;172:538–540. doi: 10.1111/bjd.13537. [DOI] [PubMed] [Google Scholar]
- Helgesen A.L., Warloe T., Pripp A.H., Kirschner R., Peng Q., Tanbo T. Vulvovaginal photodynamic therapy vs. topical corticosteroids in genital erosive lichen planus: A randomized controlled trial. Br J Dermatol. 2015;173:1156–1162. doi: 10.1111/bjd.14033. [DOI] [PubMed] [Google Scholar]
- Howard A., Dean D., Cooper S., Kirtshig G., Wojnarowska F. Circulating basement membrane zone antibodies are found in lichen sclerosus of the vulva. Australas J Dermatol. 2004;45:12–15. doi: 10.1111/j.1440-0960.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- Kelekci H.K., Uncu H.G., Yilmaz B., Ozdemir O., Sut N., Kelekci S. Pimecrolimus 1% cream for pruritus in postmenopausal diabetic women with vulvar lichen simplex chronicus: A prospective non-controlled case series. J Dermatolog Treat. 2008;19:274–278. doi: 10.1080/09546630801955341. [DOI] [PubMed] [Google Scholar]
- Kennedy C.M., Galask R.P. Erosive vulvar lichen planus: Retrospective review of characteristics and outcomes in 113 patients seen in a vulvar specialty clinic. J Reprod Med. 2007;52:43–47. [PubMed] [Google Scholar]
- Kirtschig G., Wakelin S.H., Wojnarowska F. Mucosal vulval lichen planus: Outcome, clinical and laboratory features. J Eur Acad Dermatol Venereol. 2005;19:301–307. doi: 10.1111/j.1468-3083.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Lee A., Bradford J., Fischer G. Long-term management of adult vulvar lichen sclerosus: A prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061–1067. doi: 10.1001/jamadermatol.2015.0643. [DOI] [PubMed] [Google Scholar]
- Lewis F.M. Vulval lichen planus. Br J Dermatol. 1998;138:569–575. doi: 10.1046/j.1365-2133.1998.02164.x. [DOI] [PubMed] [Google Scholar]
- Lewis F.M., Bogliatto F. Erosive vulval lichen planus--a diagnosis not to be missed: A clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214–219. doi: 10.1016/j.ejogrb.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Lonsdale-Eccles A.A., Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: A prospective case series. Br J Dermatol. 2005;153:390–394. doi: 10.1111/j.1365-2133.2005.06544.x. [DOI] [PubMed] [Google Scholar]
- Lorenz B., Kaufman R.H., Kutzner S.K. Lichen sclerosus. Therapy with clobetasol propionate. J Reprod Med. 1998;43:790–794. [PubMed] [Google Scholar]
- Lotery H.E., Galask R.P. Erosive lichen planus of the vulva and vagina. Obstet Gynecol. 2003;101:1121–1125. doi: 10.1016/s0029-7844(02)02383-9. [DOI] [PubMed] [Google Scholar]
- Lynch P.J. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatol Ther. 2004;17:8–19. doi: 10.1111/j.1396-0296.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- Maronn M.L., Esterly N.B. Constipation as a feature of anogenital lichen sclerosus in children. Pediatrics. 2005;115:e230–e232. doi: 10.1542/peds.2004-1544. [DOI] [PubMed] [Google Scholar]
- Mazdisnian F., Degregorio F., Mazdisnian F., Palmieri A. Intralesional injection of triamcinolone in the treatment of lichen sclerosus. J Reprod Med. 1999;44:332–334. [PubMed] [Google Scholar]
- Micheletti L., Preti M., Bogliatto F., Zanotto-Valentino M.C., Ghiringhello B., Massobrio M. Vulval lichen planus in the practice of a vulval clinic. Br J Dermatol. 2000;143:1349–1350. doi: 10.1046/j.1365-2133.2000.03935.x. [DOI] [PubMed] [Google Scholar]
- Moyal-Barracco M., Wendling J. Vulvar dermatosis. Best Pract Res Clin Obstet Gynaecol. 2014;28:946–958. doi: 10.1016/j.bpobgyn.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Nagao Y., Sata M., Tanikawa K., Itoh K., Kameyama T. Lichen planus and hepatitis C virus in the northern Kyushu region of Japan. Eur J Clin Investig. 1995;25:91–94. doi: 10.1111/j.1365-2362.1995.tb01966.x. [DOI] [PubMed] [Google Scholar]
- O'Connell T.X., Nathan L.S., Satmary W.A., Goldstein A.T. Non-neoplastic epithelial disorders of the vulva. Am Fam Physician. 2008;77:321–326. [PubMed] [Google Scholar]
- O'Keefe R.J., Scurry J.P., Dennerstein G., Sfameni S., Brenan J. Audit of 114 non-neoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780–786. doi: 10.1111/j.1471-0528.1995.tb10842.x. [DOI] [PubMed] [Google Scholar]
- Oyama N., Chan I., Neill S.M., Hamada T., South A.P., Wessagowit V. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118–123. doi: 10.1016/S0140-6736(03)13863-9. [DOI] [PubMed] [Google Scholar]
- Pelisse M., Leibowitch M., Sedel D., Hewitt J. A new vulvovaginogingival syndrome. Plurimucous erosive lichen planus. Ann Dermatol Venereol. 1982;109:797–798. [PubMed] [Google Scholar]
- Powell J., Wojnarowska F. Childhood vulvar lichen sclerosus: An increasingly common problem. J Am Acad Dermatol. 2001;44:803–806. doi: 10.1067/mjd.2001.113474. [DOI] [PubMed] [Google Scholar]
- Powell J., Wojnarowska F. Childhood vulvar lichen sclerosus. The course after puberty. J Reprod Med. 2002;47:706–709. [PubMed] [Google Scholar]
- ACOG Practice Bulletin No. 93: Diagnosis and management of vulvar skin disordersObstet Gynecol. 2008;111:1243–1253. doi: 10.1097/AOG.0b013e31817578ba. [DOI] [PubMed] [Google Scholar]
- Rasi A., Behzadi A.H., Davoudi S., Rafizadeh P., Honarbakhsh Y., Mehran M. Efficacy of oral metronidazole in treatment of cutaneous and mucosal lichen planus. J Drugs Dermatol. 2010;9:1186–1190. [PubMed] [Google Scholar]
- Santegoets L.A., Helmerhorst T.J., van der Meijden W.I. A retrospective study of 95 women with a clinical diagnosis of genital lichen planus. J Low Genit Tract Dis. 2010;14:323–328. doi: 10.1097/LGT.0b013e3181d73622. [DOI] [PubMed] [Google Scholar]
- Schlosser B.J., Mirowski G.W. Lichen sclerosus and lichen planus in women and girls. Clin Obstet Gynecol. 2015;58:125–142. doi: 10.1097/GRF.0000000000000090. [DOI] [PubMed] [Google Scholar]
- Setterfield J.F., Neill S., Shirlaw P.J., Theron J., Vaughan R., Escudier M. The vulvovaginal gingival syndrome: A severe subgroup of lichen planus with characteristic clinical features and a novel association with the class II HLA DQB1*0201 allele. J Am Acad Dermatol. 2006;55:98–113. doi: 10.1016/j.jaad.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Sherman V., McPherson T., Baldo M., Salim A., Gao X.H., Wojnarowska F. The high rate of familial lichen sclerosus suggests a genetic contribution: An observational cohort study. J Eur Acad Dermatol Venereol. 2010;24:1031–1034. doi: 10.1111/j.1468-3083.2010.03572.x. [DOI] [PubMed] [Google Scholar]
- Shi L., Miao F., Zhang L.L., Zhang G.L., Wang P.R., Ji J. Comparison of 5-aminolevulinic acid photodynamic therapy and clobetasol propionate in treatment of vulvar lichen sclerosus. Acta Derm Venereol. 2016;96:684–688. doi: 10.2340/00015555-2341. [DOI] [PubMed] [Google Scholar]
- Sideri M., Origoni M., Spinaci L., Ferrari A. Topical testosterone in the treatment of vulvar lichen sclerosus. Int J Gynaecol Obstet. 1994;46:53–56. doi: 10.1016/0020-7292(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Simpson R.C., Murphy R. Is vulval erosive lichen planus A premalignant condition? Arch Dermatol. 2012;148:1314–1316. doi: 10.1001/2013.jamadermatol.84. [DOI] [PubMed] [Google Scholar]
- Simpson R.C., Murphy R., Bratton D.J., Sydes M.R., Wilkes S., Nankervis H. Systemic therapy for vulval Erosive Lichen Planus (the 'hELP' trial): Study protocol for a randomised controlled trial. Trials. 2016;17:2. doi: 10.1186/s13063-015-1133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.D., Fischer G. Childhood onset vulvar lichen sclerosus does not resolve at puberty: A prospective case series. Pediatr Dermatol. 2009;26:725–729. doi: 10.1111/j.1525-1470.2009.01022.x. [DOI] [PubMed] [Google Scholar]
- Stewart K.M. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669–680. doi: 10.1016/j.det.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Terras S., Gambichler T., Moritz R.K., Stucker M., Kreuter A. UV-A1 phototherapy vs clobetasol propionate, 0.05%, in the treatment of vulvar lichen sclerosus: A randomized clinical trial. JAMA Dermatol. 2014;150:621–627. doi: 10.1001/jamadermatol.2013.7733. [DOI] [PubMed] [Google Scholar]
- Thomas R.H., Ridley C.M., McGibbon D.H., Black M.M. Anogenital lichen sclerosus in women. J R Soc Med. 1996;89:694–698. doi: 10.1177/014107689608901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen K.A., Birenbaum D.L. Recognition and management of vulvar dermatologic conditions: Lichen sclerosus, lichen planus, and lichen simplex chronicus. J Midwifery Womens Health. 2012;57:260–275. doi: 10.1111/j.1542-2011.2012.00175.x. [DOI] [PubMed] [Google Scholar]
- Torgerson R., Litin S., Bundrick J.B. Clinical pearls in dermatology. Dis Mon. 2015;61:297–307. doi: 10.1016/j.disamonth.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Virgili A., Corazza M., Bianchi A., Mollica G., Califano A. Open study of topical 0.025% tretinoin in the treatment of vulvar lichen sclerosus. One year of therapy. J Reprod Med. 1995;40:614–618. [PubMed] [Google Scholar]
- Virgili A., Minghetti S., Borghi A., Corazza M. Phototherapy for vulvar lichen simplex chronicus: An 'off-label use' of a comb light device. Photodermatol Photoimmunol Photomed. 2014;30:332–334. doi: 10.1111/phpp.12120. [DOI] [PubMed] [Google Scholar]
- Wallace H.J. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9–30. [PubMed] [Google Scholar]
- Zendell K., Edwards L. Lichen sclerosus with vaginal involvement: Report of 2 cases and review of the literature. JAMA Dermatol. 2013;149:1199–1202. doi: 10.1001/jamadermatol.2013.4885. [DOI] [PubMed] [Google Scholar]