Summary
Necroptosis is a form of cell death that can be observed downstream of death receptor or pattern recognition receptor signaling under certain cellular contexts, or in response to some viral and bacterial infections. The receptor interacting protein kinases-1 (RIPK1) and RIPK3 are at the core of necroptotic signaling, among other proteins. Because this pathway is normally halted by the pro-apoptotic protease caspase-8 and the IAP ubiquitin ligases, how and when necroptosis is triggered in physiological settings are ongoing questions. Interestingly, accumulating evidence suggests that RIPK3 has functions beyond the induction of necroptotic cell death, especially in the areas of tissue injury and sterile inflammation. Here, we will discuss the role of RIPK3 in a variety of physiological conditions, including necroptotic and non-necroptotic cell death, in the context of viral and bacterial infections, tissue damage, and inflammation.
Keywords: cell death, necroptosis, RIPK3, inflammation, tissue damage, infection
Introduction
Billions of cells die in multicellular organisms each day as part of tissue homeostasis and clearance of old or damaged cells. The majority of these cell death events occur via apoptosis, a well-described form of programmed cell death dependent on the caspases, a family of cysteine-aspartic proteases (1). Apoptosis is generally considered immunologically silent or even tolerogenic; the immune system typically does not perceive this form of cell death as a threat, and cells that have undergone apoptosis are quickly and neatly cleared by local phagocytes. Historically, apoptosis has been contrasted with the unprogrammed process of necrosis, in which cells are killed by overwhelming chemical or environmental insult. Necrosis involves cell lysis and the release of reactive molecules normally contained by the plasma membrane, including a nebulous class of molecules called danger (or sometimes “damage”)-associated molecular patters (DAMPs). DAMPs are perceived as signatures of damage by the innate immune system, and can trigger inflammatory responses; for this reason, necrosis is considered to be a driver of inflammation (2).
In recent years, the dichotomy between programmed, non-inflammatory apoptosis and unprogrammed, inflammatory necrosis has been challenged by the description of additional forms of cell death that are both programmed and inflammatory. Among these is the process of “programmed necrosis,” also called necroptosis. Necroptosis shares morphological similarities with passive necrosis: cells undergoing necroptosis swell and burst, leading to the release of DAMPs (3, 4). This morphological similarity has led to the idea that necroptosis, like necrosis, is an inflammatory form of cell death. Necroptosis is triggered by activation of the RIP kinases, RIPK1 (5, 6) and RIPK3 (7–9). Importantly, recent work has indicated that activation of these kinases can trigger transcriptional responses in addition to—and in some cases accompanying—necroptotic cell death. Furthermore, evidence from knockout mice implies that the lytic nature of necroptosis may not be the key driver of inflammation and immune responses to cell death, and that inflammatory transcriptional signaling carried out by the RIP kinases in dying cells may determine how necroptotic cells are perceived by the immune system. In this review, we will discuss the inflammatory effects of necroptotic cell death, and evidence for the relative contributions of DAMPs and transcriptional responses to necroptotic stimuli. We will highlight the signaling underlying this cell death program, as well as non-death outcomes of RIPK activation.
RIPK3-dependent necroptosis: the “canonical” function of RIPK3
The pathway by which cells are induced to die by necroptosis has been worked out in detail in recent years, and has been extensively described elsewhere (10–12). Here we present an abbreviated description of this pathway, in an effort to introduce the key enzymes and regulatory mechanisms involved. The core of the necroptotic pathway involves the activities of the RIP kinases, RIPK1 and RIPK3, and the phosphorylation-driven activation by the latter of the pseudokinase mixed lineage kinase domain-like (MLKL).
RIPK1 and RIPK3 interact with each other via the RIP homotypic interaction motif (RHIM) that both possess. RHIM-RHIM interactions between RIPK1 and RIPK3 lead to formation of the “necrosome,” an oligomeric cytosolic complex in which reciprocal phosphorylation between RIPK1 and RIPK3 (13) can lead to MLKL recruitment and activation. Importantly, in addition to a RHIM domain, RIPK1 also contains a death domain (DD) through which it can recruit the adapter FADD, the protease caspase-8, and the caspase paralog cFLIP to the necrosome. Recruitment of these proteins by RIPK1, along with the activity of the IAP ubiquitin ligases, inhibits RIPK1/RIPK3 oligomerization and signaling and prevents necroptosis (14–17). For this reason, necroptosis is generally observed in conditions in which caspase-8 and/or the IAPs are absent or inhibited. Consistent with this, genetic ablation of caspase-8 leads to embryonic lethality in mice due to hyperactivation of necroptosis during development; this lethality is rescued by co-ablation of RIPK3 (16, 18).
Upon necrosome-driven activation, RIPK3 phosphorylates the pseudokinase MLKL, which serves as the executioner of necroptosis (19–23). Phosphorylation of MLKL leads to a conformational switch that exposes its N-terminal four-helix bundle domain and leads to the oligomerization of MLKL (23–27). It has been proposed that this oligomerization leads to a net positive charge of the multi-MLKL complex, and therefore active MLKL is recruited to negatively charged membranes, leading to membrane disruption (28). While the exact mechanism by which MLKL leads to cell membrane disruption is still unclear, it is well appreciated that MLKL is essential for necroptotic death to occur (27). Notably, knockout of MLKL also rescues the embryonic lethality observed in caspase-8 knockout animals, consistent with the idea that caspase-8 deficiency causes embryonic lethality via RIPK3- and MLKL-mediated necroptosis (29).
If the necrosome represents the core of the necroptotic pathway, what signals control its formation? Necrosome formation involves RHIM-dependent interactions, and the activation of RHIM-containing proteins is therefore required to initiate necroptotic signaling. Four RHIM-containing proteins have been described in the mammalian proteome: in addition to RIPK1 and RIPK3, the adaptor protein TRIF contains a RHIM, and the innate immune sensor DNA-dependent activator of IFN-regulatory factors (DAI) contains three putative RHIMs (30). RIPK3 acts as a signaling adaptor for the activation of MLKL (among other functions, discussed below), and signals leading to RIPK3 activation involve activation of RIPK1, TRIF, and/or DAI. We briefly consider each of these signaling pathways here, and discuss specific physiological settings in which they can be activated in this review.
RIPK1 participates in a wide array of receptor signaling pathways, notably those initiated by the “death receptors” TNFR1, Fas, and the TRAIL receptors (31, 32). In these contexts, RIPK1 has a kinase-independent role in the initiation of inflammatory transcriptional programs mediated by NF-κB (33, 34) and the MAP kinases (35, 36). However, these receptor signals can also lead to translocation of RIPK1 into the cytosol, where it can interact with necrosome components. As discussed above, whether cytosolic RIPK1 is able to form a stable complex with RIPK3 to trigger necroptosis depends on the status of caspase-8 and cFLIP, which inhibit this process. Importantly, upon receptor activation, cFLIP is upregulated by the inflammatory transcription programs in which RIPK1 participates (37, 38); this represents one mechanism by which the pro-inflammatory activity of RIPK1 exerts feedback inhibition on its pro-death activity. This also highlights a theme to which we return repeatedly: that the signals that initiate necroptosis can also drive inflammatory transcription.
Necroptosis can also be triggered by TRIF (39, 40), a signaling adaptor that contains both a TIR domain and a RHIM. TRIF is activated downstream of TLR3 and TLR4, which sense double-stranded RNA (dsRNA) or lipopolysaccharide (LPS), respectively. Analogously to TNFR1, signaling through either of these TLRs normally leads to pro-inflammatory and pro-survival responses in the cell due to NF-κB and IRF-3 activation (41), but when cells are sensitized to necroptosis by inhibition of capase-8/cFLIP, TRIF can activate RIPK1 and/or RIPK3. Notably, because TRIF contains a RHIM domain, it can directly activate RIPK3 in the absence of RIPK1 (39, 40); however, RIPK1 can also be recruited to TRIF (42), and this recruitment may alter the outcome of TRIF-RIPK3 signaling by promoting transcriptional responses and engaging the inhibitory effects of caspase-8 and cFLIP (recall that the latter depend on the DD of RIPK1 for recruitment). Again, necroptotic signaling is initiated by proteins— TRIF and RIPK1— that can also drive inflammatory transcription.
The final RHIM containing protein encoded by mammals is DAI (also known as ZBP1 or DLM-1). DAI was initially described as a sensor of DNA in the unusual Z-form conformation (Z-DNA) (43), and has since been shown to elicit cell death and transcriptional responses to DNA viruses via its Z-DNA binding domain (44, 45). However, more recent data have shown that DAI can trigger necroptosis downstream of influenza A virus (IAV)(46, 47), a single-stranded negative-sense segmented RNA virus (discussed later in this review), as well as in sterile settings in which the ability of RIPK1’s scaffolding function to suppress RIPK3 is disrupted (48, 49). As neither of these functions is consistent with the sensing of Z-DNA, it remains unclear how DAI is activated in these settings.
RIPK3-dependent apoptosis
In addition to the induction of necroptosis, the necrosome can also trigger apoptosis in some settings; this effect depends on the recruitment of caspase-8, and appears to predominate when RIPK3 is inactive or MLKL is absent, precluding necroptotic signaling. In this context, RIPK3 serves as a pro-apoptotic adaptor and recruits RIPK1 and FADD, forming a platform that leads to the activation of caspase-8 and subsequently, apoptosis (50, 51). In fact, while RIPK3-deficient animals develop normally (52), mice engineered to express a version of catalytically inactive RIPK3 (D161N) die at day E11.5 from aberrant apoptosis. Casp8−/−Ripk3D161N/D161N mice are viable, demonstrating the role of caspase-8 in promoting apoptosis during embryogenesis of these animals (50). Similar effects are observed when a small-molecule inhibitor of RIPK3 is used (51). Of interest, mice with a different knock-in version of catalytically inactive RIPK3 (K51A) not only survive to birth, but are viable, fertile, and immunocompetent, and phenocopy Ripk3−/− mice in rescuing the embryonic lethality of Casp8−/− animals (51). Importantly, while RIPK3K51A does not induce RIPK3-dependent apoptosis in the manner observed with RIPK3D161N, treatment of cells expressing RIPK3K51A with RIPK3 inhibitors unleashes a pro-apoptotic activity even in the K51A mutant (51). Together, these findings lead to a model in which chemical inhibition of RIPK3, or presence of the D161N mutation, alters the conformation of RIPK3 stabilized the necrosome but prevents signaling to MLKL. This platform is thereby able to mediate sustained interactions with RIPK1 and caspase-8, promoting apoptosis. Interestingly, a similar form of RIPK3-dependent “reverse signaling” was recently described during influenza infection, as described below.
The role of RIPK3 in viral infections
Having defined the core pathways leading to necrosome formation, the question arises: when are these pathways engaged physiologically? As noted, necrosome formation and apoptosis are antagonized by the caspase-8/FLIP complex and the IAPs in many settings, so when does necroptosis occur under natural conditions? Mice lacking RIPK3 are particularly susceptible to certain viruses, providing genetic evidence that RIPK3 is important in controlling viral infections (7, 44, 53). However, there have been hints that RIPK3 has functions beyond cell death, and that its activation can lead to transcriptional responses (discussed below), which may also contribute to the clearance of viruses. Thus, in some situations it is unclear whether RIPK3 is important in viral clearance because of cell death, transcriptional functions, or a combination of both. Ongoing efforts to compare Ripk3−/− animals to mice lacking MLKL and/or caspase-8 will help to clarify this point.
One of the first studies to demonstrate that RIPK3 is important in the control of viral infection was also one of the first to pinpoint RIPK3 as a key protein in the necroptotic pathway. In 2009, Cho and colleagues used an RNA interference screen to identify RIPK3 as a pro-necrotic kinase activated during vaccinia virus (VacV) infection (7). This seminal study found that wild-type (WT) T cells infected with VacV were sensitive to TNF-induced necroptosis, while RIPK3-deficient T cells were protected. Furthermore, they demonstrated that RIPK3-deficient mice had higher viral tissue titers upon VacV infection compared to their WT counterparts, illustrating the role of RIPK3 in controlling VacV replication. Interestingly, a much earlier study found that VacV infection of murine fibroblasts sensitized these cells to a “necrotic-like” death upon TNF treatment, and that this sensitization required expression of the viral caspase inhibitor, B13R (54). This finding predated the clear description and naming of necroptosis, but it seems clear that the authors were observing necroptotic cell death. Together, these findings provide compelling evidence that RIPK3-dependent necroptosis can occur when caspases, and therefore apoptosis, are blocked by viral inhibitors, and that this cell death pathway contributes to the control of VacV infection.
The idea that necroptosis provides protection against infection by DNA viruses is reinforced by the finding that murine cytomegalovirus (MCMV) encodes a RHIM-containing inhibitor of necroptosis, as well as an inhibitor of caspase activation. The MCMV M36 gene encodes a caspase-8 inhibitor (viral inhibitor of caspase-8-induced apoptosis, or vICA)(55); while blockade of caspase-8 would normally prime a cell for necroptosis, the MCMV M45 gene encodes an inhibitor of RIP activation (vIRA)(56). vIRA contains a RHIM domain, and as such, can inhibit RIPK3-dependent necrosis (57). In fact, only MCMV strains that lack vIRA, or that express a RHIM-mutant version of vIRA (M45mutRHIM) can induce necroptosis, as vIRA is no longer able to block RIPK3 activation. These viruses are attenuated in mice (58, 59); however, this attenuation is reversed in RIPK3-deficient mice (58), demonstrating that necroptosis can control viral infection, and that vIRA is essential for MCMV to block RIPK3-dependent necrosis. Importantly, in 2012 it was demonstrated that DAI, a PRR thought to sense the DNA of viral genomes, sensitizes cells to necroptosis upon MCMV infection (44). DAI contains three RHIM domains, and RHIM-A is responsible for mediating interactions between DAI and RIPK3. Upton et al. demonstrated that DAI and RIPK3 form a complex during MCMV infection and this leads to necroptosis; in fact, like in RIPK3-deficient mice, MCMV M45mutRHIM virus attenuation is also reversed in mice lacking DAI (44).
Like its murine counterpart, human CMV (HCMV) encodes inhibitors of both apoptosis and necroptosis. HCMV UL36 encodes vICA, which is important for the suppression of apoptosis in infected human cells (60). Curiously, however, an HCMV immediate early 1 (IE1) gene product is responsible for suppressing necroptosis downstream of RIPK3 phosphorylation and activation of MLKL, although the specific identity of the inhibitor is unknown (61). This differs from MCMV’s strategy for blocking necroptosis, which utilizes vIRA to inhibit RIPK3 activation through a RHIM-dependent mechanism. This finding is interesting for at least two reasons: first, it highlights the diversity of strategies employed by viruses to inhibit necroptosis. Second, the finding that the inhibition mediated by HCMV targets MLKL activation rather than necrosome formation identifies RIPK3- and MLKL-dependent cell death, rather than other potential aspects of necrosome signaling, as the target of viral inhibition in this setting.
Herpes simplex virus (HSV)-1 and HSV-2 are other examples of human pathogens that have evolved to inhibit cell death pathways during infection (62, 63). The UL39 gene of HSV-1 encodes the protein ICP6, which is also known as HSV-1 ribonucleotide reductase (RNR) subunit 1 (R1). Likewise, HSV-2 encodes a similar protein, ICP10. ICP6 and ICP10 are interesting because they can not only block apoptosis through their C-terminal caspase-8-binding domain, but can also block necroptosis by inhibiting RIPK1 and RIPK3 interactions via their own N-terminal RHIM domain (64, 65). Although this mechanism of necroptosis suppression is reminiscent of vIRA encoded by MCMV, it is interesting to note that in the case of HSV, a single gene product can impede both apoptosis and necroptosis in human cells. Notably, both ICP6 and ICP10 have been shown to activate, rather than inhibit, necroptosis in murine cells through a RHIM-dependent mechanism, demonstrating a species specificity of RHIM signaling outcome (65).
Although the examples noted thus far have pointed to a role for DAI- and RIPK3-mediated necroptosis in eliminating DNA viruses, RIPK3-dependent cell death has recently been shown to be important for the control of the segmented RNA virus influenza type A (IAV). In addition to showing that cells lacking RIPK3 are resistant to IAV-induced death, Nogusa et al. demonstrated that IAV infection leads to both apoptotic and necroptotic signaling from the necrosome, in a manner analogous to the “reverse signaling” observed upon RIPK3 inhibition (discussed above)(53). Upon IAV infection, mouse embryonic fibroblasts (MEF) were found to undergo necroptosis; however, in the absence of MLKL, IAV infection instead triggered apoptosis, which depended on RIPK3, RIPK1, FADD and caspase-8. Interestingly, the authors found that necroptosis contributed to IAV control, but that RIPK3-dependent apoptosis could compensate for loss of necroptosis, as illustrated by the fact that both RIPK3-deficient mice and Mlkl−/−Fadd−/− double knockout (DKO) mice (which are deficient in both apoptotic and necroptotic signaling), are more susceptible to IAV than either WT or Mlkl−/− mice alone (53). It is interesting that in the case of IAV, MLKL-deficiency does not phenocopy RIPK3-deficiency in mice, and that RIPK3-dependent apoptosis contributes to protection against this virus when MLKL is absent. Given that CMV and HSV encode inhibitors of apoptotic and necroptotic signaling, future studies using viruses lacking both inhibitors may clarify the relative contribution of apoptotic and necroptotic necrosome signaling to viral control in vivo.
Subsequently, multiple groups demonstrated that IAV activates RIPK3 and triggers necroptosis through DAI—a molecule that that was previously thought to directly sense DNA (46, 47). How DAI senses IAV infection is somewhat disputed, however; one initial report indicated that DAI senses the viral proteins NP and PB1 (46), while another indicated that DAI recognizes and binds nascent IAV RNA genomes (47). The contribution of DAI— and indeed of necroptosis itself—in controlling IAV infection in vivo is also somewhat unclear. DAI-deficient mice have been shown to be either susceptible or resistant to IAV infection by different groups (46, 47), and while Nogusa et al. found that RIPK3-deficient mice exhibited increased susceptibility to IAV (53), an earlier report also showed that excess RIPK3 activation could cause detrimental tissue damage during IAV infection in mice lacking cIAP2 in the lung (66).
As noted previously, RIPK3 may have functions beyond cell death in the context of viral infections. Interestingly, Harris and colleagues demonstrated a temporal role of RIPK3 in intestinal epithelial cells (IECs) infected with the RNA virus coxsackievirus B3 (CVB)(67). Early in infection, RIPK3 facilitates CVB replication by regulating flux through the autophagic pathway, necessary for the life cycle of the virus. At later points of infection, however, the CVB-encoded cysteine protease 3Cpro cleaves RIPK3, rendering it incapable of transducing the necroptotic signal. Of interest, the C-terminal RHIM-containing cleavage product of RIPK3 was shown to induce non-necrotic cell death, while the N-terminal kinase domain-containing product was unable to trigger any form of cell death (67). Although it is unclear what type of non-necrotic cell death occurs once 3Cpro cleaves RIPK3, it is tempting to speculate that perhaps the fragment of RIPK3 containing the RHIM domain is sufficient to serve as a pro-apoptotic adaptor and recruit RIPK1, FADD, and caspase-8 – thus leading to apoptosis.
Taken together, these examples clearly illustrate that RIPK3 forms an important component of host defense to an array of viral infections, as illustrated by the increased susceptibility of RIPK3-deficient mice. Many of these studies have taken as a starting point the canonical role of RIPK3 in triggering necroptosis, and have led to the idea that this form of cell death can eliminate the replicative niche and promote antiviral immunity. However, emerging data—including the demonstration of RIPK3-dependent apoptosis as a contributor to host defense against IAV—indicate that necrosome-mediated signaling is not limited to necroptosis. Thus, while RIPK3 mediates defense against viral infection, the nature of the signaling required for this defense remains the subject of ongoing investigation.
RIPK3 and necroptosis in the context of bacterial infections
Cell death signaling also contributes to the immune response to bacterial infections, although the role of RIPK3 and necroptosis in these contexts is less clear. Many bacteria encode inhibitors of apoptotic signaling, such as NleB1 from enteropathogenic E. coli (EPEC), that block caspase activation and apoptosis in infected cells (68). This inhibition may prime infected cells for RIPK3-dependent necroptosis and elimination of the cellular space necessary for intracellular bacteria survival. However, the literature on RIPK3-depedent necroptosis and its contribution to antibacterial host defense is less conclusive, and probably highly context-dependent, as discussed below.
EPEC encodes a type III secretion system effector known as EspL, a cysteine protease that was recently shown to cleave the RHIM domains of RIPK1 and RIPK3, as well as TRIF and DAI, during infection—thus, inhibiting necroptosis and inflammation (69). Infection of WT mice with the EPEC-like pathogen Citrobacter rodentium results in intestinal colonization, which is attenuated in bacteria lacking EspL (69). This finding demonstrates the direct targeting of RHIM-dependent signaling by a bacterial effector protein, providing strong evidence that RHIM signaling contributes to host defense against bacterial pathogens. However, it remains unclear which aspects of RHIM-mediated signaling— which may include apoptotic, necroptotic, and inflammatory transcriptional signals— are at play in this context.
Staphylococcus aureus has been shown to activate RIPK3- and MLKL-mediated necroptosis. One study has reported that necroptosis contributes to bacterial clearance from infected skin, in part by eliminating cells that contribute to pathological inflammation (70). Interestingly, while MLKL-deficient mice have high bacterial loads, RIPK3-deficient mice show increased bacterial clearance and decreased inflammation, which hints at a necroptosis-independent function for RIPK3 in promoting bacterial infection. Likewise, in a pulmonary model of S. aureus infection, Ripk3−/− mice have reduced bacterial loads (71).
Another example where RIPK3 deficiency may be beneficial is in the context of Salmonella enterica serovar Typhimurium infection. S. Typhimurium is a gram-negative bacterium that can induce multiple forms of cell death in macrophages, including caspase-1-dependent pyroptosis and RIPK3-dependent necroptosis, most likely as a means to promote its own spread (72). Consistent with this idea, RIPK3-deficient mice were moderately protected from S. Typhimurium infection compared to their WT counterparts (73). Likewise, in a zebrafish model of infection by Mycobacterium tuberculosis, RIPK3-dependent necroptosis in macrophages was shown to contribute to the release and dissemination of bacteria (74). These findings stand in contrast to the bacterial inhibition of RHIM-dependent necroptosis and inflammation exerted by EPEC, and highlight the fact that roles for these pathways in bacterial infection vary among pathogens and host tissues.
Contributions of RIPK3 to tissue damage
As discussed, there is clear evidence that RIPK3 signaling— necroptotic or otherwise— can contribute to host defense against viral infection. However, the inflammatory and cell death responses triggered by RIPK3 represent a double-edged sword, and their aberrant activation may contribute to tissue pathology. Consistent with this idea, there is accumulating evidence that acute or chronic organ injury can lead to RIPK3-dependent contributions to the pathophysiology of a variety of diseases (reviewed in (75)). Interestingly, evidence from knockout mice has highlighted the differing contributions of MLKL and RIPK3 to these pathologies, supporting the idea that non-necroptotic functions of RIPK3 may underlie some of the observed effects.
RIPK3 signaling has been shown to be a critical contributor to damage in ischemia/reperfusion injury (IRI) in a variety of settings, including neonatal-hypoxia ischemic brain injury (76, 77), ischemic stroke (78), renal ischemia-reperfusion injury (IRI) (79, 80), and in cardiac ischemia and infarction (81–83). Implication of RIPK3 in these models led to the idea that necroptotic cell death contributes to tissue damage; however, more recent studies on MLKL knockout mice, in which necroptosis is deficient but other aspects of RIPK3 signaling persist, has led to a reevaluation of this idea. In 2012, Linkermann et al. used a high mortality model of renal ischemia to demonstrate that administration of the RIPK1 inhibitor Nec-1 to mice prior to renal IRI prolonged survival compared phosphate-buffered saline (PBS)-treated controls (80). Later, it was further demonstrated that catalytically inactive RIPK1 knock-in (D138N) mice, or RIPK3-deficient mice, were protected in a model of kidney IRI (84). Interestingly, however, MLKL deficiency did not afford mice the same level of protection in the kidney IRI model, which implies that there are other roles of RIPK3 beyond necroptotic cell death at work in this model (84).
Similarly, using a mouse model of myocardial infarction (MI), Luedde et al. demonstrated that RIPK3 is upregulated and can lead to necroptosis after MI. In addition, they showed that RIPK3-deficient mice have diminished inflammatory responses and reactive oxygen species (ROS) production in infarcted hearts compared to their WT counterparts (83). These data were further verified by Newton et al. in 2016 (84). However, these studies did not include a comparison with MLKL-deficient mice, so it is difficult to conclude whether the observed damage was mediated by MLKL or by RIPK3 transcriptional and inflammatory responses.
Yet another example of detrimental RIPK3-dependent signaling is the neurodegenerative disease amyotrophic lateral sclerosis (ALS). Loss of the protein optineurin (Optn), which has been implicated in ALS, sensitizes glial cells of the CNS to necroptosis, leading to demyelination and axonal degeneration (85). This is clearly demonstrated in mouse embryonic fibroblasts (MEFs) derived from Optn−/− mice, as well as in the spinal cords of Optn−/− mice, which have increased levels of complex-associated RIPK1, RIPK3, and phospho-MLKL, which can be used as a marker of necroptosis. Interestingly, the axonal pathology and hindlimb weakness of Optn-deficient mice was rescued in Optn−/−Ripk1D138N/D138N mice, as well as Optn−/−Ripk3−/− double mutant mice, and treatment of Optn−/− mice with Nec-1s (a highly specific inhibitor of RIPK1 catalytic activity) led to a reduction of neuropathology (85). Together, these data suggest that uncontrolled RIPK3-dependent necroptosis can be harmful in the context of ALS, and have led to efforts to target this pathway pharmacologically in the clinic.
RIPK3-dependent death has also been reported to contribute to hepatocyte cell death from ethanol-induced injury (86). Liver biopsies from patients with alcoholic liver disease (ALD) showed higher RIPK3 expression compared with controls. RIPK3 was also shown to be upregulated in the livers of ethanol-fed mice, and RIPK3-deficient mice were significantly protected against ethanol-induced liver injury and inflammation (cytokine levels of MCP-1, IL-6, and TNFα) compared to WT mice. Interestingly, however, administration of Nec-1 did not reduce ethanol-induced hepatocyte injury, as measured by plasma ALT/AST levels (86). This may indicate that RIPK3-dependent necroptosis proceeds independent of RIPK1 kinase activity in this setting, though other functions of RIPK3 signaling could also contribute to this pathology.
These examples illustrate that in situations of tissue injury, RIPK3 activation can lead to detrimental effects in the host. However, in several of the settings highlighted above, it has emerged that the observed effects are RIPK3-dependent but MLKL-independent, implicating non-death functions of the necrosome in the observed pathology. Additional studies, including work with newly-available mouse models in which specific functional domains of RIPK1 and/or RIPK3 are mutated, will provide insight into the nature of the signals driving tissue damage in these settings.
The role of RIPK3 in inflammation
While RIPK3 is best known for its role in cell death, the findings discussed above— and in particular the distinct phenotypes observed between Ripk3−/− and Mlkl−/− animals in both viral infection and sterile tissue pathology—has made it clear that RIPK3 participates in additional signaling pathways. The question of how RIPK3 signaling and necroptosis participate in host immune responses and immunopathology hinges on how necrosome signaling contributes to inflammation and the initiation of innate immune responses. In turn, this question can be roughly divided into two sub-topics. First, how does necroptosis contribute to inflammation? And second, what non-necroptotic signals— such as activation of inflammatory transcription pathways— also emanate from the necrosome? These two questions clearly bear on each other: cell death would be predicted to terminate inflammatory transcription, and targets of inflammatory transcription may inhibit cell death. Having highlighted physiological settings in which RIPK3 signaling is important, we will now consider evidence for the pathways mediated by RIPK3, including both necroptotic and transcriptional effects.
Because necroptosis is a lytic form of cell death, it is thought that the release of danger associated molecular patterns (DAMPs) from necroptotic cells contributes to inflammation. Examples of DAMPs include intracellular components such as the chromatin-associated protein high-mobility group box 1 (HMGB-1), heat shock proteins (HSPs), F-actin, as well as DNA, RNA, and ATP; as these molecules are normally contained within cells, their release may be sensed as a signature of tissue damage, leading to activation of local innate immune cells and thus to inflammation (2). It is thought that bystander cells sense these molecules and events through the same PRRs that sense pathogen-associated molecular patterns (PAMPs) or through specialized DAMP receptors, and initiate an inflammatory response. Thus, necroptotic cell death has been considered pro-inflammatory due to DAMP release.
An example that hints at the importance of DAMPs from necroptotic cells comes from an in vivo model of TNF-induced toxicity, systemic inflammatory response syndrome (SIRS). When Ripk3−/− mice are treated with high doses of TNF, they fare better than their WT counterparts in terms of both morbidity and mortality (87). Likewise, pre-treatment of WT mice with Nec-1 or use of mice lacking the kinase activity of RIPK1 provides similar protection to high doses of TNF (50, 84, 87). This suggests that necroptosis mediates cellular damage that eventually leads to mortality; in fact, levels of certain DAMPs and cytokines are reduced in RIPK3-deficient mice in this model (87). This finding is in keeping with the idea that lytic cell death and resultant inflammation and tissue damage are a key outcome of RIPK3 signaling. However, another study found that loss of MLKL was not as effective as RIPK3-deficiency in the high-dose TNF model (84), suggesting that RIPK3 and RIPK1 may be regulating more than simply MLKL-dependent death.
In keeping with the notion that RIPK3 controls more than just necroptotic death, RIPK3 has also been implicated in the activation of inflammatory transcription. Using transient overexpression systems, early studies of RIPK1 and RIPK3 found that while RIPK1 potently activated NF-κB porter genes, RIPK3 was comparatively less effective, and could even inhibit TNF-induced NF-κB activation (88–90). Nonetheless, RIPK3 was also identified in a screen for proteins that could activate an NF-κB reporter gene (91). In 2004, Newton et al. showed that RIPK3-deficient cells did not differ in their ability to engage NF-κB signaling in response to TNF, LPS, peptidoglycan, or downstream of B- or T- cell antigen receptor crosslinking (52). As we now appreciate RIPK3’s role in cell death, early studies demonstrating that RIPK3 has an inhibitory role in RIPK1- or TNF-induced NF-κB activation can now be revisited with the lens of necroptosis— in fact, it makes sense that RIPK3 overexpression or activation would dampen NF-κB responses, as cell death would cause termination of biological processes. In keeping with this view, it was recently shown that necroptosis leads to the suppression of TNF- or LPS-induced inflammation by ceasing cytokine and chemokine production (92).
However, somewhat contrary to the idea that necroptotic death terminates inflammation, there is evidence that RIPK3 activation can lead to de novo synthesis of pro-inflammatory cytokines and chemokines. Using a system of direct RIPK3 activation, independent of upstream receptor signaling, Yatim et al. demonstrated that chemically-enforced RIPK3 oligomerization leads to the recruitment of RIPK1 and subsequent NF-κB activation (93). The authors went on to demonstrate that this NF-κB signaling in necroptotic cells determines the ability of dendritic cells (DCs) to cross-prime CD8+ T cells with antigens derived from the dying cells. Further, a more recent study showed that the kinase activity of RIPK1, as well as RIPK3 itself, contributed to TRIF-dependent inflammatory transcription upon TLR4 activation in bone marrow-derived macrophages (94). RIPK1 has well-described roles in NF-κB activation, but these canonical functions are independent of the kinase activity of RIPK1, or of RIPK3 engagement (reviewed in (95)). How RIPK3 activity and necrosome formation contribute to NF-κB or other transcriptional pathways is still poorly understood.
Moreover, there is evidence that RIPK3 leads to cytokine expression downstream of injury and promotes tissue repair. In a 2014 publication, Moriwaki et al. demonstrated that surprisingly, the presence of RIPK3 protects against dextran sodium sulfate (DSS)-induced colitis, and that RIPK3 is required for tissue repair by inducing an axis of IL-23, IL-1β and IL-22 downstream of DSS-induced injury (96). However, contrary to these results, another study found that loss of RIPK3 had no effect on DSS-induced colitis (84). Different DSS-administration and recovery protocols, as well as duration of health monitoring, and differences in colony microbiota between institutions, might contribute to the differences observed. Nonetheless, it is interesting to speculate that RIPK3 may play a role in promoting tissue injury (or repair) and inflammation, independent of its role in cell death.
Genetic models have highlighted RIPK3-dependent inflammation that both does and does not depend on MLKL-dependent cell death. Most clearly, loss of caspase-8 causes embryonic lethality in mice, which is rescued by co-ablation of either RIPK3 or MLKL, supporting the notion that it is unchecked MLKL-dependent cell death, and not some other function of RIPK3, that leads to lethality in Casp8−/− animals. However, Casp8−/−Mlkl−/− double-knockout mice display a phenotype somewhat distinct from that observed in Casp8−/−Ripk3−/− double knockout animals, including an accelerated accumulation of aberrant lymphocyte populations and increased inflammatory cytokines in the serum (29). These differences may reflect death-independent RIPK3 signaling, which would still be intact in Casp8−/−Mlkl−/− animals. Other models also highlight the dichotomy of death-dependent and independent functions for RIPK3. Skin inflammation due to RIPK1 ablation can be ameliorated in mice by deleting either RIPK3 or MLKL, supporting a role for MLKL-dependent cell death in this setting (97). However, in other genetic models of inflammation this does not hold true. A20 (also known as TNFAIP3) is a pro-survival protein, and A20-deficient mice suffer systemic inflammation and die shortly after birth (98). Interestingly, while RIPK3 deficiency or catalytically inactive RIPK1 (Ripk1KD/KD) delay the mortality of A20−/− mice, MLKL deficiency does not (84). This indicates that MLKL-dependent necroptosis does not contribute significantly to the morbidity and mortality associated with A20 deficiency; instead, other as-yet-unclear roles of necrosome signaling appear to mediate these effects.
While it is now appreciated that RIPK3 can act in concert with RIPK1 to engage in pro-inflammatory, non-cell death signaling, proteins that regulate the necrosome, such as caspase-8 and cFLIP, may also be involved in inflammatory processes. Recent work has demonstrated an unexpected, cell-intrinsic role for caspase-8 in the initiation of inflammatory transcription, in addition to its functions in initiation apoptosis and suppressing necroptosis. This was shown to be important during the host response to Yersinia and Salmonella infection, as well as downstream of direct activation of TLR4 by LPS (99). The mechanism by which caspase-8 activation leads to upregulation of pro-inflammatory cytokines is not clear, although it is known that caspase-8 enzymatic activity is necessary, and a recent publication implicated the caspase-8/cFLIP heterodimer as the species involved, by showing that a version of caspase-8 that cannot undergo interdomain cleavage was able to support the transcriptional, but not apoptotic, functions of this enzyme. It is intriguing that this caspase-8/cFLIP heterodimer is also the complex that is recruited to the necrosome to suppress necroptosis (99). Future work will undoubtedly focus on putative roles for necrosome formation in caspase-8-dependent transcriptional pathways.
The RIP kinases and caspase-8 can contribute to inflammation via non-transcriptional mechanisms, as well. Interestingly, one such example is in the case of inflammasome activation and the processing of pro-IL-1β. While pro-IL-1β is an inflammatory cytokine that is canonically cleaved— and thereby activated— by caspase-1, under certain conditions it can be cleaved by caspase-8 (reviewed in (100)). Moreover, RIPK3 can promote IL-1β processing and secretion in response to LPS stimulation by assembling a complex including caspase-8, FADD, RIPK1, and requiring TRIF, in macrophages and DCs (101). The kinase activity of RIPK1 and RIPK3 is not required for this atypical non-apoptotic caspase-8 activation and in fact, treatment of cells with a RIPK3 kinase inhibitor enhances LPS-induced caspase-8 activation and thus IL-1β processing (101). Furthermore, recent studies have demonstrated an additional link between necroptosis in inflammasome activation, by showing that MLKL activation, which leads to membrane disruption, can activate the NLRP3 inflammasome in a cell-intrinsic manner by altering ion homeostasis (102, 103). This finding implies that induction of necroptosis is linked inextricably to inflammasome assembly, and that caspase-1 activation and processing of IL-1β and IL-18 likely accompany necroptotic cell death when all are present.
So what can we conclude from these complex and sometimes contradictory results? It seems clear that necroptosis can occur physiologically, and that in some settings this can contribute to host defense. It also seems apparent that non-necroptotic functions of RIPK1 and RIPK3 are important contributors to both beneficial immune responses and pathophysiology, though the precise nature of these signals remain to be elucidated.
Concluding Remarks
RIPK3 has traditionally been considered a pro-necrotic protein, although we now appreciate that it has non-necroptotic roles in a variety of physiological settings. From an evolutionary perspective, it may be that RIPK3 evolved as a pro-inflammatory protein alongside its homolog RIPK1, and this pathway was re-purposed to induce inflammatory death upon caspase-8 inhibition or absence (reviewed in (104)). Because programmed necrosis can occur in response to pathogen infection, it follows that perhaps this form of cell death evolved as a “backup” to apoptosis when pathogens themselves evolved mechanisms to inhibit caspase-8. Whether this is the case, however, remains an open question.
Furthermore, separating RIPK3 signaling from caspase-8 and RIPK1 remains challenging. RIPK1 has been shown to play a crucial role in promoting NF-κB activation and inflammation downstream of TNFR1 signaling, and recently was shown to be important for the inflammation and cross-priming potential of necroptotic cells after direct RIPK3 activation. Caspase-8 also has distinct transcriptional and post-translational roles in inflammation. As both RIPK1 and caspase-8 can promote inflammation, it will be important to elucidate RIPK3’s role in inflammation both independent of and in concert with these other proteins. Further, persistent questions remain about the relative contributions of MLKL-dependent necroptosis and non-death functions of RIPK3 during host defense and tissue damage. Studies comparing RIPK3-deficient and MLKL-deficient mice in a variety of disease models will be necessary to elucidate the contribution of necroptosis versus RIPK3 non-death signaling.
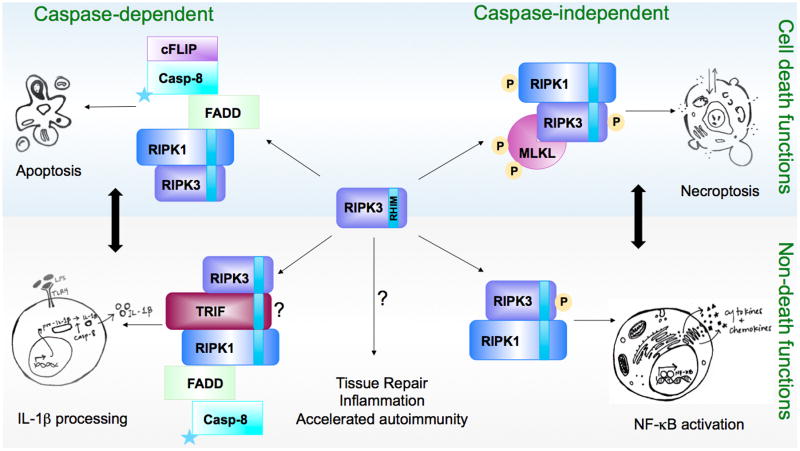
Figure 1.
The cell death and non-death functions of RIPK3. RIPK3 can participate in different complexes, both known and unknown, depending on the cellular setting. These multi-protein complexes can lead to cell death (top blue panel) and/or inflammatory outcomes (bottom gray panel). RIPK3-dependent processes can be further divided into caspase-dependent and caspase-independent contexts. It is important to note that the cell death and non-death functions of RIPK3 are not necessarily mutually exclusive, and in fact, cell death and inflammation may be a coordinated response (dark black arrows). For example, NF-kB activation has been shown to accompany necroptotic cell death in a model of direct RIPK3 activation, and the resulting transcriptional program is necessary for the immunogenicity of antigen derived from necroptotic cells (93). Stars represent catalytic activity of caspase-8.
Acknowledgments
We thank Michelle Brault and Brian Daniels for editorial comments. This work is supported by NIH grants 3R01AI108685-01S1 (SO), 1R01AI108685-01 and 1R21CA185681 (AO).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature reviews Immunology. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanden Berghe T, Vanlangenakker N, Parthoens E, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell death and differentiation. 2010;17:922–930. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 4.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature reviews Molecular cell biology. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Y, Choksi S, Shen HM, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. The Journal of biological chemistry. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 7.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 10.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton K, Manning G. Necroptosis and Inflammation. Annu Rev Biochem. 2016;85:743–763. doi: 10.1146/annurev-biochem-060815-014830. [DOI] [PubMed] [Google Scholar]
- 13.Li J, McQuade T, Siemer AB, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feoktistova M, Geserick P, Kellert B, et al. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Molecular cell. 2011 doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geserick P, Hupe M, Moulin M, et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187:1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberst A, Dillon CP, Weinlich R, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orozco S, Yatim N, Werner MR, et al. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell death and differentiation. 2014;21:1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser WJ, Upton JW, Long AB, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L, Wang H, Wang Z, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Jitkaew S, Cai Z, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Zhou Z, Li L, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. The Journal of biological chemistry. 2013;288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Huang Z, Ren J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy JM, Czabotar PE, Hildebrand JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrand JM, Tanzer MC, Lucet IS, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Z, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Li W, Ren J, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Dondelinger Y, Declercq W, Montessuit S, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Diaz S, Dillon CP, Lalaoui N, et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser WJ, Upton JW, Mocarski ES. Viral modulation of programmed necrosis. Curr Opin Virol. 2013;3:296–306. doi: 10.1016/j.coviro.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 33.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 35.Kim JW, Joe CO, Choi EJ. Role of receptor-interacting protein in tumor necrosis factor-alpha -dependent MEKK1 activation. The Journal of biological chemistry. 2001;276:27064–27070. doi: 10.1074/jbc.M009364200. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 37.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiser WJ, Sridharan H, Huang C, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. The Journal of biological chemistry. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ullah MO, Sweet MJ, Mansell A, Kellie S, Kobe B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J Leukoc Biol. 2016;100:27–45. doi: 10.1189/jlb.2RI1115-531R. [DOI] [PubMed] [Google Scholar]
- 42.Meylan E, Burns K, Hofmann K, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 43.Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 44.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell host & microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rebsamen M, Heinz LX, Meylan E, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO reports. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuriakose T, Man SM, Malireddi RK, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016:1. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thapa RJ, Ingram JP, Ragan KB, et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell host & microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton K, Wickliffe KE, Maltzman A, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–133. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 49.Lin J, Kumari S, Kim C, et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newton K, Dugger DL, Wickliffe KE, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 51.Mandal P, Berger SB, Pillay S, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nogusa S, Thapa RJ, Dillon CP, et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell host & microbe. 2016;20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menard C, Wagner M, Ruzsics Z, et al. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J Virol. 2003;77:5557–5570. doi: 10.1128/JVI.77.10.5557-5570.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brune W, Menard C, Heesemann J, Koszinowski UH. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science. 2001;291:303–305. doi: 10.1126/science.291.5502.303. [DOI] [PubMed] [Google Scholar]
- 57.Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. The Journal of biological chemistry. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell host & microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lembo D, Donalisio M, Hofer A, et al. The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J Virol. 2004;78:4278–4288. doi: 10.1128/JVI.78.8.4278-4288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. The Journal of biological chemistry. 2015;290:11635–11648. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo H, Omoto S, Harris PA, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell host & microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Z, Wu SQ, Liang Y, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell host & microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Rodrigue-Gervais IG, Labbe K, Dagenais M, et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell host & microbe. 2014;15:23–35. doi: 10.1016/j.chom.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Harris KG, Morosky SA, Drummond CG, et al. RIP3 Regulates Autophagy and Promotes Coxsackievirus B3 Infection of Intestinal Epithelial Cells. Cell host & microbe. 2015;18:221–232. doi: 10.1016/j.chom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Pearson JS, Giogha C, Muhlen S, et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat Microbiol. 2017;2:16258. doi: 10.1038/nmicrobiol.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitur K, Wachtel S, Brown A, et al. Necroptosis Promotes Staphylococcus aureus Clearance by Inhibiting Excessive Inflammatory Signaling. Cell Rep. 2016;16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kitur K, Parker D, Nieto P, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu ZQ, Zhao WH. Type 1 interferon-associated necroptosis: a novel mechanism for Salmonella enterica Typhimurium to induce macrophage death. Cell Mol Immunol. 2013;10:10–12. doi: 10.1038/cmi.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao H, Jaffer T, Eguchi S, Wang Z, Linkermann A, Ma D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015;6:e1975. doi: 10.1038/cddis.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavez-Valdez R, Martin LJ, Northington FJ. Programmed Necrosis: A Prominent Mechanism of Cell Death following Neonatal Brain Injury. Neurol Res Int. 2012;2012:257563. doi: 10.1155/2012/257563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 79.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25:2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linkermann A, Brasen JH, Himmerkus N, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 81.Koshinuma S, Miyamae M, Kaneda K, Kotani J, Figueredo VM. Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia-reperfusion injury. J Anesth. 2014;28:235–241. doi: 10.1007/s00540-013-1716-3. [DOI] [PubMed] [Google Scholar]
- 82.Oerlemans MI, Liu J, Arslan F, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 83.Luedde M, Lutz M, Carter N, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 84.Newton K, Dugger DL, Maltzman A, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell death and differentiation. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ito Y, Ofengeim D, Najafov A, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–608. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duprez L, Takahashi N, Van Hauwermeiren F, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 89.Yu PW, Huang BC, Shen M, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9:539–542. doi: 10.1016/s0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- 90.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. The Journal of biological chemistry. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 91.Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-kappaB by the T cell receptor complex. EMBO J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kearney CJ, Cullen SP, Tynan GA, et al. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell death and differentiation. 2015;22:1313–1327. doi: 10.1038/cdd.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yatim N, Jusforgues-Saklani H, Orozco S, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Najjar M, Saleh D, Zelic M, et al. RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4. Immunity. 2016;45:46–59. doi: 10.1016/j.immuni.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell death and differentiation. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 96.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dannappel M, Vlantis K, Kumari S, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Philip NH, DeLaney A, Peterson LW, et al. Activity of Uncleaved Caspase-8 Controls Anti-bacterial Immune Defense and TLR-Induced Cytokine Production Independent of Cell Death. PLoS Pathog. 2016;12:e1005910. doi: 10.1371/journal.ppat.1005910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gurung P, Kanneganti TD. Novel roles for caspase-8 in IL-1beta and inflammasome regulation. Am J Pathol. 2015;185:17–25. doi: 10.1016/j.ajpath.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J Immunol. 2015;194:1938–1944. doi: 10.4049/jimmunol.1402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gutierrez KD, Davis MA, Daniels BP, et al. MLKL Activation Triggers NLRP3-Mediated Processing and Release of IL-1beta Independently of Gasdermin-D. J Immunol. 2017 doi: 10.4049/jimmunol.1601757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conos SA, Chen KW, De Nardo D, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brault M, Oberst A. Controlled detonation: evolution of necroptosis in pathogen defense. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]