Abstract
Hirsutism is defined as excessive terminal hair growth in a male pattern in females. It typically affects 5 to 10% of reproductive-age women. Excessive hair growth can often cause significant psychological and emotional distress. As a result, hirsutism is a common presenting complaint to healthcare professionals, including dermatologists, as women search for cosmetic and medical solutions to their problem. Hirsutism results from excess production of androgens, often from ovarian or adrenal sources. It is typically associated with a metabolic syndrome like polycystic ovarian syndrome (PCOS), but can be idiopathic or medication-induced. This article provides an endocrine perspective for the evaluation and management of hirsutism.
Introduction
True hirsutism is medically defined as excessive terminal hair appearing in a male pattern in females (Ferriman and Gallwey, 1961, Hartz et al., 1979, McKnight, 1964). Hirsutism affects between 5 and 10% of reproductive-age women in the general population and can often be a sign of conditions of androgen excess. Excessive hair growth can cause a tremendous amount of emotional distress for patients (Barth et al., 1993, Sonino et al., 1993). Feelings of self-consciousness often stem from what is considered the “ideal hair pattern” within our culture and society. For example, in the United States, “ideal” is considered as no terminal hair except for the scalp, eyebrows, eyelashes, and pubis (Loriaux, 2012). Using this definition, a large number of normal women would be considered to have hirsutism. The terms hirsutism and hypertrichiosis are sometimes used interchangeably. However, hypertrichiosis is defined as excessive hair growth, terminal or vellus, in non-androgen-dependent areas of the body. Hypertrichiosis can be congenital or acquired.
This text will outline an appropriate evaluation of a patient with hirsutism.
Pathogenesis
Hair growth is regulated by a number of local and systemic factors, growth factors, cytokines, and sex steroids (Azziz et al., 2000, Danilenko et al., 1996, Harmon and Nevins, 1993, Moore et al., 1991, Peus and Pittelkow, 1996, Philpott et al., 1994). Thyroid and growth hormones have also been shown to alter hair-growth patterns. Sex steroids, particularly androgens, play an important role in the type of hair that forms and how hair is distributed over the human body. As androgen levels increase during puberty, vellus follicles in specific areas develop into terminal hair (Akiyama et al., 1996). Additionally, androgens act to increase sebum secretion, resulting in increased oiliness of the skin and hair. Finally, androgens prolong the anagen phase of body hair and shorten the anagen phase of scalp hair (Ebling, 1986, Randall, 1994, Rosenfield, 2005).
Hirsutism results from an interaction between androgen levels and the sensitivity of the hair follicle to androgens (Rosenfield, 2005). The level and duration of exposure to androgens, the local 5-alpha-reductase activity, and the intrinsic sensitivity of the hair follicle to androgen action determine whether vellus hair is converted to terminal hair (Hawryluk and English, 2009). Excess androgen levels lead to increased terminal hair growth in most androgen-sensitive sites (e.g., upper lip, chin, chest, back, and upper abdominal area). Although androgen excess underlies most cases of hirsutism, there is only a modest correlation between the quantity of hair growth and androgen levels (Azziz et al., 2000).
Causes of hirsutism
Hirsutism is often the initial, and possibly only, sign of an underlying androgen disorder. Therefore, understanding the various causes of hirsutism will help guide the evaluation of these patients (Table 1).
Table 1.
Causes of Hirsutism.
| Polycystic ovarian syndrome |
|---|
| Congenital adrenal hyperplasia |
| - classical |
| - nonclassical |
| Cushing’s disease |
| Ovarian tumors |
| Adrenal tumors |
| - Adenoma |
| - Carcinoma |
| Growth hormone excess |
| Insulin resistance |
| Stromal hyperthecosis |
| Medications |
| - Cyclosporine |
| - Testosterone injections, creams, patches |
| - Progestins |
| - Diazoxide |
| - Minoxidil |
| - Phenytoin |
| - Danazol |
| - Glucocorticoids |
| - Anabolic steroids |
Information from Loriaux L. (2012). An Approach to the Patient with Hirsutism. J Clin Endocrinol Metab 97(9):2957-2968
Polycystic ovarian syndrome
Polycystic ovarian syndrome (PCOS) is the most common cause of hirsutism in women. Hirsutism typically develops at puberty. Characteristics of PCOS include menstrual irregularities or infertility, insulin resistance (in the form of diabetes or metabolic syndrome), clinical signs of androgen excess (including acne and hirsutism), and biochemical confirmation of hyperandrogenism. Documentation of polycystic ovaries is not necessary for the diagnosis of polycystic ovarian syndrome.
The exact cause of PCOS is uncertain, but it is thought to be multifactorial. PCOS develops when the ovaries are stimulated to produce excessive amounts of androgens. This can occur through excessive luteinizing hormone (LH) release or hyperinsulinemia. Elevated insulin levels increase gonadotrophin releasing hormone (GnRH) pulse frequency, LH over follicle-stimulating hormone dominance, and androgen production, and decrease sex hormone-binding globulin (SHBG) (Mayo Clinic Staff, 2011).
Idiopathic hirsutism
Approximately half of all women with mild hirsutism have idiopathic hirsutism (Reingold and Rosenfield, 1987). It is usually a diagnosis of exclusion. Women diagnosed with idiopathic hirsutism often have normal serum androgen concentrations, no menstrual irregularities, and no identifiable cause of hirsutism (Azziz et al., 2000, Glickman and Rosenfield, 1984, Moore et al., 1983).
Congenital adrenal hyperplasia
Congenital adrenal hyperplasias are a group of autosomal recessive disorders affecting enzymes responsible for cortisol production. Classic congenital adrenal hyperplasia can present in three forms: 21-hydroxylase deficiency, 11-hydroxylase deficiency, and 3-beta hydroxysteroid dehydrogenase deficiency. Of these, 21-hydroxylase deficiency is the most common (Loriaux, 2012). These disorders are usually recognized at birth or in early infancy, as they tend to present with androgen excess, hirsutism, genital ambiguity, and cortisol deficiency.
Nonclassical or late-onset form of this disorder is milder and develops in late childhood or early adulthood. Women affected with this condition typically present with hirsutism and occasionally primary amenorrhea or infertility, but they lack evidence of cortisol deficiency. Symptoms can often resemble PCOS. The nonclassic form is almost always due to 21-hydroxylase deficiency leading to increase production of both 17-hydroxyprogesterone and androstenedione.
Ovarian tumors
Androgen-secreting tumors make up only 5% of all ovarian tumors. They cause hirsutism, typically later in life, and tend to progress more rapidly. Serum testosterone levels are greater than 150 to 200 ng/dL (Friedman et al., 1985, Meldrum and Abraham, 1979, Moltz et al., 1984).
Adrenal tumors
These are rare causes of hyperandrogenism. Most of these tumors are adrenal carcinomas and typically cause hypercortisolism and excess androgen secretion (dihydroepiandrostenedione [DHEA] and DHEA sulfate [DHEA-S]). Therefore, patients typically present with rapid onset of Cushing's syndrome and hyperandrogenism.
Cushing's disease
Cushing’s disease is typically a result of an adrenocorticotrophic hormone-secreting pituitary adenoma resulting in excessive secretion of not only cortisol, but also adrenal androgens, by the adrenal gland. Hirsutism is a common symptom of this disorder along with weight gain, hypertension, rounding of the face, abdominal striae, and irregular menstruation.
Hyperthecosis
Hyperthecosis occurs when an area of luteinization develops along with stromal hyperplasia in the ovary. The luteinized thecal cells overproduce androgens, more specifically testosterone, resulting in hirsutism and virilization. The cause is unknown.
Insulin resistance
This is a condition in which cells fail to respond normally to insulin. Severe hyperinsulinemia causes increased GnRH pulse frequency, increased androgen production, and decreased SHBG, resulting in elevated levels of free or active testosterone (Mayo Clinic Staff, 2011). Women who have severe insulin resistance or hyperinsulinemia often develop hirsutism.
Medications
There are many drugs that may be associated with the development of hirsutism (Table 1).
Evaluation
Most women with hirsutism typically present to their physician’s office with cosmetic concerns regarding excessive hair growth. Many of these women may have underlying conditions of androgen excess. Therefore, it is important to perform a thorough history, physical examination, and biochemical evaluation to identify any underlying endocrine disorders.
History
Initial questions should focus on the duration of symptoms. Recent onset, worsening hirsutism, or onset later in life rather than near puberty should raise suspicion for androgen-secreting tumors. Evaluation should also focus on signs of virilization, including deepening of the voice, acne, increased muscle mass, male pattern hair loss, and clitoromegaly.
As almost every woman with excessive androgens will have irregular menstrual cycles, obtaining an appropriate menstrual history is vital. Women with polycystic ovarian syndrome often present with irregular menses. Women with regular menses may have idiopathic hirsutism. Ethnicity should also be considered while obtaining a history. Women of Mediterranean descent on average have greater quantities of body hair compared with most Asian women, who have relatively little body hair (Carmina et al., 1992).
Finally, family history of hirsutism, infertility, and obesity, along with medication use, should be reviewed.
Physical exam
When evaluating a woman with hirsutism, the Ferriman–Gallwey (FG) score is a simple and commonly used method to quantify hair growth (Ferriman and Gallwey, 1961). This method evaluates nine androgen sensitive sites and grades them from 0 to 4 (Ferriman and Gallwey, 1961). Scores above eight are considered abnormal in Caucasian and African American females. Scores between 8 and 15 are usually considered to be mild hirsutism, whereas scores greater than 25 indicate severe hirsutism (Ferriman and Gallwey, 1961). Some limitations of this scoring system include: (a) the variation in hair growth between different ethnic groups; (b) failure to account for regional hirsutism; and (c) the fact that many women may have treated their excessive hair growth with cosmetic measures, such as chemical depilatories, electrolysis, laser therapy, etc.
Evaluation of physical signs of insulin resistance such as acanthosis nigricans and Cushing's syndrome (i.e., thinning of the skin, rounding of the face, dorsal fat pad, abdominal striae, or easy bruising) is required. Male pattern baldness, increase in muscle mass, acne, and deepening of the voice are signs of virilization. Abdominal and pelvic exams should be performed to look for masses and to evaluate clitoral size.
Biochemical testing
The goal behind biochemical evaluation of women with hirsutism is to identify women with markedly elevated androgen levels suggestive of androgen-secreting tumors. Serum total testosterone levels and DHEA-S levels are usually obtained to rule out ovarian and adrenal tumors, respectively.
The Endocrine Society guidelines suggest against the routine evaluation of androgen levels in women with mild hirsutism. The guidelines suggest biochemical testing only in women with moderate to severe hirsutism, sudden onset hirsutism, or rapidly progressive hirsutism. The guidelines also recommend further biochemical testing in evaluation of hirsutism when associated with infertility, irregular menses, central obesity, or acanthosis nigricans (Martin et al., 2008).
Total testosterone provides the best initial evaluation of androgen production in hirsute women. Direct free testosterone assays are not accurate. Further evaluation of free testosterone by equilibrium dialyses could be indicated if total testosterone is significantly elevated. A total testosterone level greater than 150 ng/dl warrants further investigation, because it is suggestive of an ovarian or adrenal testosterone-secreting tumor or ovarian hyperthecosis.
DHEA-S levels greater than 700 mcg/dl raise suspicion for hormone-secreting adrenocortical carcinoma. 17-hydroxyprogesterone levels should be checked in women with a high likelihood of congenital adrenal hyperplasia, such as women with a positive family history or who belong to high risk ethnic groups (Hispanic, Slavic, Ashkenazi Jew).
In women with menstrual abnormalities, a prolactin level should be obtained. A 24-hour urine free cortisol exam is recommended in women with signs of cortisol excess. A pelvic ultrasound is indicated in women with a suspected ovarian androgen-secreting tumor. Computed tomography of the adrenals is recommended for women with a suspected adrenal tumor with significant elevation in testosterone levels.
Management of hirsutism
Mild or moderate hirsutism does not always require treatment. The 2008 Endocrine Society guidelines have coined the term “patient-important hirsutism” to emphasize the patient’s perception of the severity of the condition and the importance of taking patient preference into consideration when decision is made to initiate treatment (Martin et al., 2008). In any case, the underlying cause of excess hair growth should be identified when possible.
Women should be educated about cosmetic as well as pharmacological therapies, the possible need for long-term treatment, and the potential side effects of the different drugs or procedures. Realistic expectations should be set for the patient at the initiation of therapy. Patients should be advised that pharmacologic therapy will likely result in slower hair growth and less-frequent need for hair removal as opposed to complete elimination of hair follicles. Monitoring response to treatment should be done by obtaining a FG score at baseline and, if possible, at every visit. The most important outcome is likely the patient’s perception of improvement. There are no recommendations to follow androgen levels during treatment. Treatment can be continued during the reproductive years as long as the patient desires and should be stopped if pregnancy is contemplated.
Oral contraceptives
Estrogen-progestin combinations are usually considered safe and cost-effective. Their mechanism of action stems from progestin’s ability to suppress LH production and, subsequently, ovarian androgen production. Estrogen increases the levels of SHBG, consequently decreasing the levels of free testosterone and other SHBG-bound androgens. Oral contraceptives (OCPs) also inhibit adrenal androgens by interfering with their synthesis. OCPs are usually started with a combination of ethinyl estradiol (0.03 to 0.035 mg) in combination with a progestin that has low androgenic or antiandrogenic properties.
Two progestins with antiandrogenic properties, cyproterone acetate (CPA) and drosperinone, have been compared for treatment of PCOS.
In a recent study, 52 women with PCOS were randomized to 0.035 mg of ethinyl estradiol with 2 mg of CPA or 0.03 mg of ethinyl estradiol with 3 mg of drosperinone for 12 months. The study demonstrated a median decline in the total modified FG score of 35% in the CPA group, as opposed to an 18% median decline in the drosperinone group at 12 months (p = .035). The study concluded that OCPs with CPA are more effective for the treatment of hirsutism in PCOS (Kahraman et al., 2014).
Antiandrogen Therapy
Spironolactone is an aldosterone antagonist related structurally to progestins. It competes with dihydrotestosterone (DHT) for binding androgen receptors. Spironolactone also has an inhibitory effect on 5-alpha reductase and, at doses higher than 200 mg per day, inhibits action of various enzymes involved in androgen biosynthesis. Starting dose is usually 50 mg twice daily, which is then increased to 100 mg twice daily if needed. The main side effects include hyperkalemia, irregular menses, and teratogenicity.
Finasteride is a type-2, 5-alpha reductase inhibitor. Only a partial inhibitory effect is anticipated with finasteride as it does not affect type-1, 5-alpha reductase inhibitors. Usual dose for hirsutism is 5 mg per day, although some data suggest that a higher dose of 7.5 mg per day might be more effective. The main side effect is the feminization of a male fetus because DHT is involved in the development of male external genitalia. When used in women of reproductive age, it should be used with effective contraception.
Flutamide is a nonsteroidal antiandrogen. It blocks androgen receptors, preventing the binding of DHT. Usual dose is 250 to 750 mg per day, which is equal in efficacy to spironolactone 100 mg/day or finasteride 5 mg/day. Flutamide is associated with hepatotoxicity, which may be dose dependent. As such, it is not recommended as first- line therapy and the lowest effective dose should be used with careful monitoring of liver function.
Cyproterone is a 17-hydroxyprogesterone acetate derivative with strong progestogenic effects. It competes with DHT for the androgen receptor and decreases serum LH and ovarian androgen production. It is used at a low dose of 2 mg as the progestin part of combination oral contraceptives, or at a higher dose of 12.5 to 100 mg per day as monotherapy. It is not available in the United States.
In a systematic review conducted in 2008 by Swiglo et al., five trials were identified comparing antiandrogen therapy to placebo. Meta-analysis of these trials showed that compared to placebo, antiandrogens (spironolactone, finasteride, and flutamide) significantly reduced hirsutism, lowering FG scores by 3.9 (95% CI [2.3, 5.4]), with no apparent difference among the three medications, although head-to-head comparisons of the three antiandrogens were not performed (Sahin et al., 1998, Swiglo et al., 2008). In a randomized control trial comparing OCPs (containing low-dose cyproterone) to antiandrogens (finasteride), there was no significant difference in the FG score (Sahin et al., 1998).
The 2008 Endocrine Society guidelines suggest OCPs as first-line therapy to treat “patient-important” hirsutism. The guidelines recommend against antiandrogen monotherapy unless effective contraception is used, due to teratogenic effects. In women who are not contemplating fertility or who cannot conceive, the use of OCPs or antiandrogens will depend on patient preference. The guidelines also suggest against the use of flutamide, given its hepatotoxicity (Martin et al., 2008).
Insulin-sensitizing agents
Both metformin and thiazolidinediones have been used to attenuate hyperinsulinemia and decrease androgen levels. The role of these medications in the absence of metabolic disturbances remains controversial.
The best available evidence on metformin is based on a systematic review of nine trials comparing metformin or troglitazone to placebo. The study showed a modest effect on hirsutism, with a decrease in the FG scores by 1.5.
A separate subgroup analysis of eight trials comparing metformin to placebo found no significant difference (Cosma et al., 2008). The 2008 Endocrine Society guidelines suggest against the use of insulin-lowering agents as therapy for hirsutism (Martin et al., 2008).
Other therapies
GnRH agonists inhibit gonadotropins and decrease ovarian androgen production, leading to decreased hirsutism but also lower estrogen levels. They are usually used in conjunction with low-dose estrogen–progestin pills to eliminate the effects of estrogen deficiency. Weak evidence suggests that GnRH therapy is more effective than placebo for the treatment of hirsutism, but there seems to be no evidence to support its use over OCPs or antiandrogens. The medication itself is expensive and requires injections. The Endocrine Society guidelines suggest against its use except in women with severe hyperandrogenemia who do not respond to or cannot tolerate OCPs and antiandrogens (Martin et al., 2008).
Steroids are usually used long term in women with classic 21-hydroxylase deficiency. They suppress adrenal androgen production and control hirsutism, while maintaining ovulatory cycles. Trials comparing glucocorticoids to antiandrogens and OCPs in women with the nonclassic form of 21-hydroxylase deficiency (NCCAH) found glucocorticoids to be more effective in suppressing adrenal androgens, but less effective in controlling hirsutism (Spritzer et al., 1990).
The Endocrine Society guidelines suggest against the use of glucocorticoids for routine management of hirsutism in women without classic or nonclassic form of congenital adrenal hyperplasia related to 21-hydroxylase deficiency.
The use of glucocorticoids in women with NCCAH is suggested for those who do not respond to or cannot tolerate OCPs or antiandrogens, or those who seek ovulation induction (Spritzer et al., 1990).
Combination therapy
A systematic review identified five trials comparing antiandrogens combined with OCPs versus OCPs alone. A meta-analysis of these trials showed no significant difference in hirsutism scores between the two treatment groups. However, pooling of spironolactone and finasteride comparisons revealed a significant effect compared with OCP alone (–1.7, 95 % CI [0.1, 3.3]) (Sahin et al., 1998). A meta-analysis of two trials comparing flutamide and metformin showed significantly lower hirsutism scores in patients using both medications compared to patients on metformin alone (4.6, 95% CI [1.3, 7.9]; Swiglo et al., 2008). The 2008 Endocrine Society guidelines suggest adding an antiandrogen if hirsutism persists after 6 months of monotherapy with OCPs alone (Martin et al., 2008).
Topical treatment
Eflornithine hydrochloride 13.9% (Vaniqa) is a topical preparation that inhibits hair growth by irreversibly inhibiting ornithine decarboxylase. It does not remove hair, but rather slows hair growth. It can be used alone or in conjunction with other therapies.
Randomized control trials have reported a more significant hair growth reduction when comparing a combination of laser therapy with eflornithine versus laser therapy and placebo cream, especially early in the treatment course (Hamzavi et al., 2007). The Endocrine Society guidelines suggest the addition of eflornithine to photoepilation therapy in women who desire a more rapid initial response (Martin et al., 2008).
Nonpharmacologic methods
Temporary methods
Temporary methods such as shaving, plucking, or waxing are effective, safe, and inexpensive. They can be used alone or in combination with pharmacologic therapy.
Permanent methods
These include photoepilation (laser or intense pulsed light) or electrolysis.
Women with hyperandrogenemia will experience hair regrowth, and the 2008 Endocrine Society guidelines suggest concomitant use of pharmacologic treatment to prevent recurrence (Martin et al., 2008).
Lifestyle modifications
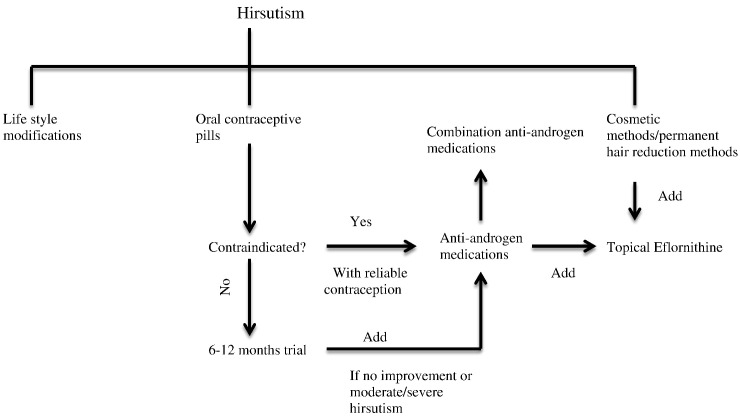
Lifestyle modifications including weight loss have been suggested in the treatment of hirsutism. A meta-analysis by Moran et al. (2011) identified four studies addressing the effect of lifestyle modifications on hirsutism in women with PCOS. Lifestyle modifications (e.g., diet, exercise, behavioral changes, or combined treatments) showed significant improvement of excess hair growth by the FG score compared with minimal or no treatment (mean difference –1.19 (95% CI [–2.35, –0.03], p = .04). The treatment group also showed a significant difference in total testosterone levels, weight, and waist circumference (Fig. 1).
Fig. 1.
Management of hirsutism.
The authors concluded that lifestyle modifications improved hyperandrogenism in women with PCOS and its clinical manifestations, including hirsutism. Other reviews on the treatment of hirsutism have also suggested counseling patients on smoking cessation. Smoking could potentially interfere with the treatment of hirsutism, especially when OCPs are being considered (Somani and Turvy, 2014).
Conclusion
Successful management of hirsutism often requires an interdisciplinary approach. This can often be achieved through involvement of physicians (endocrinologists, gynecologists, dermatologists), psychologists, and cosmeticians. The goal with this multifocal approach is not only to tackle cosmetic worries through medical therapy and mechanical hair removal, but also to address the woman's concerns regarding self-image and psychological stresses that are associated with excessive body hair.
In summary, hirsutism is a common dermatologic complaint that is often seen in young women of reproductive age. It can cause significant psychological distress. It is important for healthcare providers to understand the pathophysiology as well as the appropriate evaluation of this disorder to rule out underlying disease or tumors. When medical therapy is used for hirsutism, the provider must educate the patient regarding the usual or expected time frame of decreased hair growth. Close follow-up for assessment and tolerance of medical therapy and efficacy is critical, as is emotional support of the patient.
Footnotes
This article is a reprint of a previously published article. For citation purposes, please use the original publication details; International Journal of Women's Dermatology 1 (2015) 90-94. DOI of original item: 10.1016/j.ijwd.2015.04.003.
References
- Akiyama M., Smith L.T., Holbrook K.A. Growth factor and growth factor receptor localization in the hair follicle bulge and associated tissue in human fetus. J Invest Dermatol. 1996;106:391–396. doi: 10.1111/1523-1747.ep12343381. [DOI] [PubMed] [Google Scholar]
- Azziz R., Carmina E., Sawaya M.E. Idiopathic hirsutism. Endocr Rev. 2000;21:347–362. doi: 10.1210/edrv.21.4.0401. [DOI] [PubMed] [Google Scholar]
- Barth J.H., Catalan J., Cherry C.A., Day A. Psychological morbidity in women referred for treatment of hirsutism. J Psychosom Res. 1993;37:615–619. doi: 10.1016/0022-3999(93)90056-l. [DOI] [PubMed] [Google Scholar]
- Carmina E., Koyama T., Chang L., Stanczyk F.Z., Lobo R.A. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992;167:1807–1812. doi: 10.1016/0002-9378(92)91779-a. [DOI] [PubMed] [Google Scholar]
- Cosma M., Swiglo B.A., Flynn D.N., Kurtz D.M., Labella M.L., Mullan R.J. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135–1142. doi: 10.1210/jc.2007-2429. [DOI] [PubMed] [Google Scholar]
- Danilenko D.M., Ring B.D., Pierce G.F. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol Med Today. 1996;2:460–467. doi: 10.1016/1357-4310(96)10045-9. [DOI] [PubMed] [Google Scholar]
- Ebling F.J.G. Hair follicles and associated glands as androgen targets. Clin Endocrinol Metab. 1986;15:319–339. doi: 10.1016/s0300-595x(86)80028-7. [DOI] [PubMed] [Google Scholar]
- Ferriman D., Gallwey J.D. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- Friedman C.I., Schmidt G.E., Kim M.H., Powell J. Serum testosterone concentrations in the evaluation of androgen-producing tumors. Am J Obstet Gynecol. 1985;153:44–49. doi: 10.1016/0002-9378(85)90587-3. [DOI] [PubMed] [Google Scholar]
- Glickman S.P., Rosenfield R.L. Androgen metabolism by isolated hairs from women with idiopathic hirsutism is usually normal. J Invest Dermatol. 1984;82:62–66. doi: 10.1111/1523-1747.ep12259135. [DOI] [PubMed] [Google Scholar]
- Hamzavi I., Tan E., Shapiro J., Lui H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54–59. doi: 10.1016/j.jaad.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Harmon C.S., Nevins T.D. IL-1 alpha inhibits human hair follicle growth and hair fiber production in whole-organ cultures. Lymphokine Cytokine Res. 1993;12:197–203. [PubMed] [Google Scholar]
- Hartz A.J., Barboriak P.N., Wong A., Katayama K.P., Rimm A.A. The association of obesity with infertility and related menstrual abnormalities in women. Int J Obes. 1979;3:57–73. [PubMed] [Google Scholar]
- Hawryluk E.B., English J.C., III Female adolescent hair disorders. J Pediatr Adolesc Gynecol. 2009;22(4):271–281. doi: 10.1016/j.jpag.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Kahraman K., Sukur Y.E., Atabekoglu C.S., Ates C., Taskin S. Comparison of two oral contraceptive forms containing cyproterone acetate and drospirenone in the treatment of patients with polycystic ovary syndrome: a randomized clinical trial. Arch Gynecol Obstet. 2014;290:321–328. doi: 10.1007/s00404-014-3217-5. [DOI] [PubMed] [Google Scholar]
- Loriaux L. An approach to the patient with hirsutism. J Clin Endocrinol Metab. 2012;97(9):2957–2968. doi: 10.1210/jc.2011-2744. [DOI] [PubMed] [Google Scholar]
- Martin K.A., Chang R.J., Ehrmann D.A., Ibanez L., Lobo R.A., Rosenfield R.L. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105–1120. doi: 10.1210/jc.2007-2437. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic Staff Polycystic ovary syndrome (PCOS) [Internet] 2011. http://www.mayoclinic.org/diseases-conditions/pcos/basics/definition/con-20028841?DSECTION=all Cited 2011 November 15, Available from:
- McKnight E. The prevalence of “hirsutism” in young women. Lancet. 1964;1:410–413. doi: 10.1016/s0140-6736(64)92789-8. [DOI] [PubMed] [Google Scholar]
- Meldrum D.R., Abraham G.E. Peripheral and ovarian venous concentrations of various steroid hormones in virilizing ovarian tumors. Obstet Gynecol. 1979;53:36–43. [PubMed] [Google Scholar]
- Moltz L., Pickartz H., Sörensen R., Schwartz U., Hammerstein J. Ovarian and adrenal vein steroids in seven patients with androgen-secreting ovarian neoplasms: selective catheterization findings. Fertil Steril. 1984;42:585–593. doi: 10.1016/s0015-0282(16)48143-4. [DOI] [PubMed] [Google Scholar]
- Moore A., Magee F., Cunningham S., Culliton M., McKenna T.J. Adrenal abnormalities in idiopathic hirsutism. Clin Endocrinol (Oxf) 1983;18:391–399. doi: 10.1111/j.1365-2265.1983.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Moore G.P., Du Cros D.L., Isaacs K., Pisansarakit P., Wynn P.C. Hair growth induction: roles of growth factors. Ann N Y Acad Sci. 1991;642:308–325. doi: 10.1111/j.1749-6632.1991.tb24397.x. [DOI] [PubMed] [Google Scholar]
- Moran L.J., Hutchison S.K., Norman R.J., Teede H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;7:CD007506. doi: 10.1002/14651858.CD007506.pub3. [DOI] [PubMed] [Google Scholar]
- Peus D., Pittelkow M.R. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14:559–572. doi: 10.1016/s0733-8635(05)70384-3. [DOI] [PubMed] [Google Scholar]
- Philpott M.P., Sanders D.A., Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102:857–861. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- Randall V.A. Androgens and human hair growth. Clin Endocrinol (Oxf) 1994;40:439–457. doi: 10.1111/j.1365-2265.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Reingold S.B., Rosenfield R.L. The relationship of mild hirsutism or acne in women to androgens. Arch Dermatol. 1987;123:209–212. [PubMed] [Google Scholar]
- Rosenfield R.L. Hirsutism. N Engl J Med. 2005;353:24. doi: 10.1056/NEJMcp033496. [DOI] [PubMed] [Google Scholar]
- Sahin Y., Bayram F., Kelestimur F., Muderrs I. Comparison of cyproterone acetate plus ethinyl estradiol and finasteride in the treatment of hirsutism. J Endocrinol Invest. 1998;21:348–352. doi: 10.1007/BF03350769. [DOI] [PubMed] [Google Scholar]
- Somani N., Turvy D. Hirsutism: an evidence-based treatment update. Am J Clin Dermatol. 2014;15:247–266. doi: 10.1007/s40257-014-0078-4. [DOI] [PubMed] [Google Scholar]
- Sonino N., Fava G.A., Mani E., Belluardo P., Boscaro M. Quality of life of hirsute women. Postgrad Med. 1993;69:186–189. doi: 10.1136/pgmj.69.809.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer P., Billaud L., Thalabard J.C., Birman P., Mowszowicz I., Raux-Demay M.C. Cyproterone acetate versus hydrocortisone treatment in late-onset adrenal hyperplasia. J Clin Endocrinol Metab. 1990;70:642–646. doi: 10.1210/jcem-70-3-642. [DOI] [PubMed] [Google Scholar]
- Swiglo B.A., Cosma M., Flynn D.N., Kurtz D.M., Labella M.L., Mullan R.J. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1153–1160. doi: 10.1210/jc.2007-2430. [DOI] [PubMed] [Google Scholar]