Abstract
Skin of color comprises a diverse and expanding population of individuals. In particular, women of color represent an increasing subset of patients who frequently seek dermatologic care. Acne, melasma, and alopecia are among the most common skin disorders seen in this patient population. Understanding the differences in the basic science of skin and hair is imperative in addressing their unique needs. Despite the paucity of conclusive data on racial and ethnic differences in skin of color, certain biologic differences do exist, which affect the disease presentations of several cutaneous disorders in pigmented skin. While the overall pathogenesis and treatments for acne in women of color are similar to Caucasian men and women, individuals with darker skin types present more frequently with dyschromias from acne, which can be difficult to manage. Melasma is an acquired pigmentary disorder seen commonly in women with darker skin types and is strongly associated with ultraviolet (UV) radiation, genetic factors, and hormonal influences. Lastly, certain hair care practices and hairstyles are unique among women of African descent, which may contribute to specific types of hair loss seen in this population, such as traction alopecia, trichorrhexis nodosa and central centrifugal cicatricial alopecia (CCCA).
Introduction
Skin of color, which refers to individuals with Fitzpatrick skin types (FST) IV through VI, represents a rapidly expanding population in the United States as well as in many nations across the world. It includes Asians, Latinos (Hispanics), Africans, African-Americans, Afro-Caribbeans, Middle Easterners, Native Americans, Alaskan natives, Pacific Islanders, Native Hawaiians and Mediterraneans. In the United States alone, just over one third of the United States population reported their race and ethnicity as something other than non-Hispanic white in the 2010 census (US Census Bureau, 2010). As this patient population increases, it is critical to recognize the vast clinical presentations of skin and hair disorders in patients with pigmented skin. The epidemiology of skin diseases in individuals of color has not been extensively studied (Taylor, 2003). Table 1 provides recent nationally representative data from dermatology practice surveys among skin of color patients (Davis et al., 2012). While acne and unspecified dermatitis or eczema are in the top five cutaneous diagnoses for all major U.S. racial and ethnic groups, dyschromia also remains a leading condition among African Americans and Hispanics (Davis et al., 2012). African Americans are unique in that alopecia remains a top diagnosis within this ethnic group (Davis et al., 2012). In particular, there are unique challenges specific to treating skin and hair conditions in women of color. Delays in treatment or misdiagnoses may lead to possible sequelae, such as postinflammatory hyperpigmentation (PIH), hypertrophic scarring, keloid formation, and permanent alopecia. The optimal treatments for women of color seeking dermatologic care continue to evolve over time. It is the authors’ aim that a better understanding of the common skin and hair disorders afflicted by women of color will ultimately lead to more satisfactory outcomes in this patient population.
Table 1.
Top Ten Dermatologic Conditions Among Caucasian Versus Skin of Color Patients in the United States.⁎
| Caucasians | African Americans | Asian or Pacific Islanders | Hispanics or Latinos |
|---|---|---|---|
| Actinic keratosis | Acne vulgaris | Acne vulgaris | Acne vulgaris |
| Acne vulgaris | Unspecified dermatitis or eczema | Unspecified dermatitis or eczema | Unspecified dermatitis or eczema |
| Benign neoplasm of the skin | Seborrheic dermatitis | Benign neoplasm of the skin | Psoriasis |
| Unspecified dermatitis or eczema | Atopic dermatitis | Psoriasis | Benign neoplasm of the skin |
| Non-melanoma skin cancer | Dyschromia | Seborrheic keratosis | Viral warts |
| Seborrheic keratosis | Psoriasis | Atopic dermatitis | Actinic keratosis |
| Viral warts | Alopecia | Viral warts | Seborrheic keratosis |
| Psoriasis | Keloid scar | Urticaria | Sebaceous cyst |
| Rosacea | Viral warts | Sebaceous cyst | Rosacea |
| Sebaceous cyst | Sebaceous cyst | Seborrheic dermatitis | Dyschromia |
Listed in order of decreasing frequency. Table adapted from Davis SA, Narahari S, Feldman SR, Huang W, Pichardo-Geisinger RO, McMichael AJ. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-73.
Structure & function of skin of color
The heterogeneity seen among persons of color can be best explained via the differences in the structure, function, and physiology of the skin and hair. Literature on ethnic and racial differences in skin and hair structure, physiology, and function is lacking. Although data in this area is limited, the literature does offer some insight on the physiologic and structural variations that exist. It is important to understand these differences since disease presentations are influenced by these variations and several cutaneous disorders are much more commonly seen in skin of color (Davis et al., 2012).
Melanocyte biology
It is well known that ethnic and racial differences in skin color are not due to the number of melanocytes, but rather are due to variations in the size, number, and aggregation of melanosomes within keratinocytes and melanocytes (Taylor, 2002). Melanocytes synthesize melanin in melanosomes which are specialized organelles. Pigmented melanosomes are transferred from one melanocyte to between 30–35 adjacent keratinocytes in the basal layer. Two types of melanin exist: eumelanin, a dark brown–black pigment and pheomelanin, a yellow–reddish pigment. The constitutive levels of pheomelanin and eumelanin are generally believed to be due to genetics (Taylor, 2002). Eumelanin is more important in determining the degree of pigmentation than pheomelanin (Wakamatsu et al., 2006). Lighter melanocytes have higher pheomelanin content than dark melanocytes (Wakamatsu et al., 2006). In one study by Wakamatsu et al, Caucasians had the least amount of eumelanin, Asian Indians had more, and African-Americans had the highest amount (Wakamatsu et al., 2006). Paracrine factors secreted from fibroblasts and keratinocytes have also been shown to play important roles in the regulation of skin pigmentation. In particular, neuregulin-1 (NRG1) which is highly expressed and secreted by fibroblasts derived from Fitzpatrick type VI skin has been found by Choi et al to play an important role in regulating the constitutive pigmentation of human skin by increasing the proliferation and pigmentation of melanocytes (Choi et al., 2012). These authors subsequently characterized the bioactive motif of NRG1 that is involved in modulating melanin production in melanocytes by increasing melanin production (Choi et al., 2012).
Melanosomes also differ among races. In black skin, they are larger and more active in producing melanin and are packaged, distributed, and broken down differently than in Caucasian skin (Taylor, 2002). The number of melanosomes transferred to keratinocytes has also been shown to be significantly higher in skin of African descent versus white skin as made evident by higher expression of the RAB27A melanosome transport molecule in darkly pigmented melanocytes (Yoshida-Amano et al., 2012). Protease-activated receptor 2 (PAR-2) is another contributor to skin pigmentation. This receptor is involved in various biologic processes including cutaneous pigmentation since its activation stimulates production of pigment (Babiarz-Magee et al., 2004). In addition, its expression has been found to be different in Caucasian skin when compared to darker skin. One study demonstrated that PAR-2 and its activator trypsin are expressed in higher levels in darker skin and was also found to have higher cleavage ability in highly pigmented skin (Babiarz-Magee et al., 2004).
Melanin provides protection from ultraviolet (UV) light. Fitzpatrick skin phototypes IV–VI are less susceptible to photoaging, which is most likely due to the photoprotective properties of melanin. Previous studies have illustrated that the mean UVA transmission through the epidermis is 17.5% in pigmented skin and is 55.5% for Caucasian skin (Kligman, 1994). The UVB transmission through the epidermis is 5.7% for pigmented skin compared to 29.4% for Caucasian skin (Kligman, 1994).
Biology of the epidermis and dermis
The structure and function of the epidermis and dermis is similar among various races and ethnicities, but important differences do exist.
The stratum corneum (SC) serves as the skin’s mechanical barrier and prevents water loss. Over the years, there has been conflicting data in the literature regarding racial differences in the SC. Early studies have shown that the stratum corneum of black skin is more compact than that of white skin (Taylor, 2002). In one study conducted by Chu and Kollias, corneocyte detachment was more prevalent with age and was found to be more severe on the dorsal forearm in Caucasian subjects (Chu and Kollias, 2011). In addition, corneocyte detachment values were lower for African-Americans than for Caucasians (Chu and Kollias, 2011). The authors also concluded that the SC appears different between body sites of different exposures, ages, and persons of different pigmentation groups, but there are minimal differences in water-handling properties (Chu and Kollias, 2011). In a study investigating differences in SC lipids among different ethnicities, the ceramide/cholesterol ratio was highest in Asians, whites had intermediate values, and Africans had the lowest values (Jungersted et al., 2010). Another study showed that East Asian and to some extent Caucasian skin had low maturation and relatively weak skin barrier (Muizzuddin et al., 2010). African American skin had low ceramide levels and high protein cohesion in the uppermost layers of the SC (Muizzuddin et al., 2010). Transepidermal water loss (TEWL) is defined as the amount of water vapor loss from the skin, excluding sweat. The difference in TEWL between different races has yet to be established due to conflicting studies. TEWL has been found to be greater in black skin compared to white skin in the majority of studies, but the opposite has also been reported (Muizzuddin et al., 2010).
The dermis of blacks is thicker and more compact than that of white skin (Girardeau-Hubert et al., 2012). The papillary and reticular layers of the dermis are more distinct, contain larger collagen fiber bundles, and the fiber fragments are sparse in whites. In contrast, the dermis of black skin contains closely stacked, smaller collagen fiber bundles with a surrounding ground substance (Girardeau-Hubert et al., 2012). Girardeau-Hubert et al showed on histology that African skin had greater convoluted appearance of the dermal epidermal junction (DEJ) than Caucasian skin and on immunostaining laminin 332, type IV and VII collagens, and nidogen proteins at the DEJ were lower in African skin compared with Caucasian skin (Girardeau-Hubert et al., 2012).
Hair biology
Three major categories of hair exist according to ethnic origin: Asian, Caucasian, and African. Genetics are thought to be responsible for the majority of variations seen across races and ethnic groups in hair morphology. It has been previously noted that the four hair types are helical, spiral, straight, and wavy (Taylor, 2002). The majority of blacks have the spiral hair type (Taylor, 2002). This impart explains why African hair is more difficult to comb than Caucasian hair. Regarding hair shape, Asian and Caucasian hair is more cylindrical in contrast to African hair, which resembles an oval twisted rod that has frequent twists that have random reversals in direction and pronounced flattening. Asian hair has the greatest diameter with circular geometry while black hair shows an elliptic section along the shaft and a high degree of irregularity in the hair diameter. Caucasian hair is intermediate in diameter and section shape. Black hair breaks more easily than Caucasian hair due to the lesser tensile strength and also has lower moisture content (Ji et al., 2013). A study by Ji et al evaluated the change of integral hair lipids after UVR in Africans, Asians, and Europeans. The study found that Asian hair had more integral hair lipids than European and African hair, but lipid composition overall was similar across the groups (Ji et al., 2013). African hair in general showed the most hair surface damage (Ji et al., 2013). The study also revealed that after UV irradiation, European and African hair exhibited more damage because they had less integral hair lipids when compared to Asian hair, which had less damage (Ji et al., 2013). As measured by high-performance thin-layer chromatography, integral hair lipids in whole hair follicles are composed of fatty acid, phytosphingosine, ceramide, cholesterol, cholesterol sulfate, and cholesterol oleate in decreasing order (Lee et al., 2005). These findings reiterate the importance of hair care in blacks as this hair type is naturally more brittle and more susceptible to breakage. Although keratins and their respective amino acids have been reported to be similar across the three groups, one study showed high variation in keratin protein expression among Kenyans, Koreans, Caucasians, African Americans allowing identification of individual profiles (Laatsch et al., 2014). On the contrary, differences between these groups were less marked and relied largely on levels of keratin-associated proteins (Laatsch et al., 2014). Table 2 summarizes the most established differences in the structure and function of the skin and hair between Caucasians and African Americans (Wakamatsu et al., 2006, Yoshida-Amano et al., 2012, Chu and Kollias, 2011, Jungersted et al., 2010, Girardeau-Hubert et al., 2012, Ji et al., 2013, Badreshia-Bansal and Taylor, 2009, Weigand et al., 1974, La Ruche and Cesarini, 1992, Lindelof et al., 1998).
Table 2.
Comparison of the Structure and Function of Skin and Hair Between Caucasians and African Americans.⁎
| Caucasians | African Americans | Reference | |
|---|---|---|---|
| Stratum corneum thickness | Equal | Equal | Weigand et al. (1974) |
| Stratum corneum layers | Less | More | Weigand et al. (1974) |
| Stratum corneum lipids | Low | High | La Ruche and Cesarini (1992) |
| Corneocyte detachment | High | Low | Chu and Kollias (2011) |
| Ceramide concentration | High | Low | Jungersted et al. (2010) |
| Melanin content | Low | High | Wakamatsu et al. (2006) |
| Melanosomes | Small, aggregated | Large, dispersed; higher number transferred to keratinocytes | Yoshida-Amano et al. (2012) |
| Dermis | Thin, less compact | Thick, more compact | Girardeau-Hubert et al. (2012) |
| Papillary and reticular layers | More distinct | Less distinct | Girardeau-Hubert et al. (2012) |
| Collagen fiber bundles | Larger | Smaller, closely stacked | Girardeau-Hubert et al. (2012) |
| Fiber fragments | Sparse | Prominent, numerous | Girardeau-Hubert et al. (2012) |
| Hair shaft structure | Straight or slightly curved | Tightly coiled, spiral | Lindelof et al. (1998) |
| Cross-section of hair | Slightly less round than Asian hair | Oval or elliptical shape | Lindelof et al. (1998) |
| Tensile strength of hair | Higher | Lower | Ji et al. (2013) |
Table modified from Badreshia-Bansal S, Taylor S. The Structure and Function of Skin of Color. In: Kelly AP, Taylor SC, eds. Dermatology for skin of color. New York, NY: McGraw-Hill companies. 2009:71-77.
Adult-Female acne
Acne vulgaris is the most commonly reported dermatological complaint among non-Caucasian patients (Halder et al., 1983a, Davis and Callender, 2010, Callender et al., 2014a). Adult acne is traditionally defined as the presence of acne beyond the age of 25 years and is divided into persistent and late-onset acne (Callender et al., 2014a). Although once previously regarded as a disease of adolescence, acne vulgaris may persist from adolescence beyond the age of 25 years (“persistent acne”) or manifest itself for the first time beyond the age of 25 years (“late-onset adult acne”) (Khunger and Kumar, 2012). Among adult cases of acne, women tend to be affected more frequently than men; 12-22% of US women have adult acne, compared to 3% of US men (Goulden et al., 1999). One photographic study found clinical acne to be highly prevalent in African American (37%), Hispanic/Latinas (32%), Asian (30%), and Continental Indian (23%) women (Perkins et al., 2011). Another study reported that adult subjects with acne (mostly women) comprised the majority of dermatologic office visits (61.9%) while adolescents (aged 15-17 years old) comprised only 36.5% of visits (Yentzer et al., 2010). In addition, females account for two-thirds of dermatologic visits for acne and one-third of all office visits for acne are by women over the age of 25 years old (Yentzer et al., 2010).
A recent cross-sectional, web-based survey of 208 women with adult facial acne was performed to evaluate the racial differences in clinical characteristics, perceptions, behaviors and psychosocial impact of acne. In this study, non-Caucasian women experienced substantially more PIH than Caucasian women (Callender et al., 2014a). In addition, lesion clearance was the most important aspect of acne clearing for Caucasian women (57.9% vs non-Caucasian 31.7%), whereas clearance of PIH was most important for many non-Caucasian females (41.6% vs Caucasian 8.4%) (Callender et al., 2014a). These findings further emphasize the importance of developing an individualized, tailored treatment approach when caring for women of color with acne.
Several previous studies have suggested that adult female acne has a significant negative psychosocial impact in women. A recent cross-sectional, web-based survey reported the facial acne in adult women negatively affected their overall health-related quality of life, was associated with mild to moderate symptoms of depression and/or anxiety, and impacted their ability to concentrate on work or school (Tanghetti et al., 2014). Taken together, these findings demonstrate the crucial role dermatologists serve in addressing the symptom burden of acne in adult women.
Pathogenesis
The multifactorial pathogenesis of acne vulgaris in women of color is similar to that of Caucasian women and men. The four key components central to acne pathophysiology include follicular hyperkeratinization, sebum production, Propionibacterium acnes overgrowth, and inflammation (Fisk et al., 2014). In addition, many previous studies have evaluated differences in skin biology characteristics between racial ethnic groups. For example, one small study of 60 women found an overall higher density of P. acnes in African American subjects (Warrier et al., 1996). Another study demonstrated that pore size was positively correlated with acne in African American, Asian and Continental Indian women (Perkins et al., 2011). However, to date, results from studies evaluating potential differences in sebaceous gland activity and size and sebum production between racial groups remain contradictory. Additional factors such as genetics, diet, cosmetics, tobacco, stress, medications, and hormonal influences from estrogens and androgens also play a role in adult women with acne (Davis and Callender, 2010, Fisk et al., 2014).
Clinical features
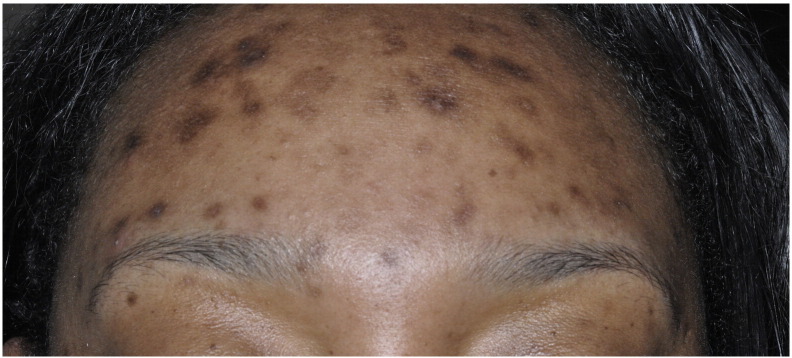
Acne vulgaris in the adult female patient typically presents with primary lesions on the lower one-third of the face, especially near the jaw line and chin (Kamangar and Shinkai, 2012) (Fig. 1). Clinical features that are more frequently encountered in women of color include dyspigmentation and scarring (Yin and McMichael, 2014). In one study, these aforementioned events were more common in African Americans and Hispanic women than all other ethnicities (Perkins et al., 2011).
Fig. 1.
Acne in an African American female (Courtesy of Susan C. Taylor, MD, et al; from Treatments for Skin of Color, copyright Elsevier 2011).
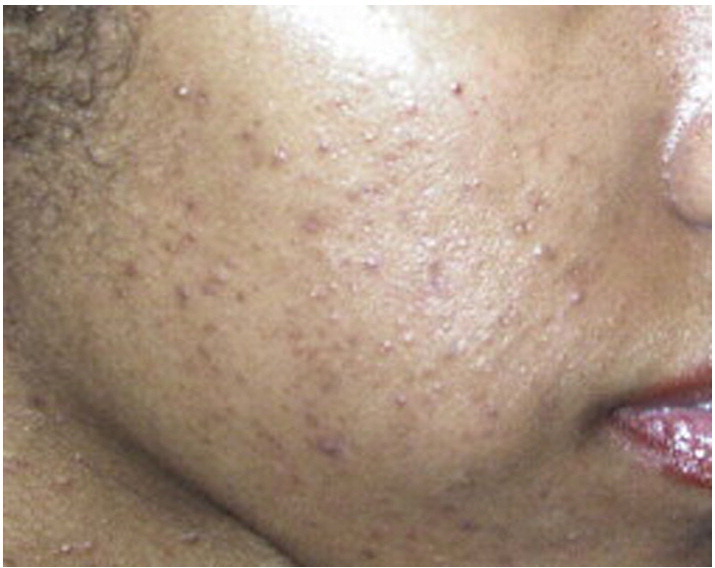
PIH is often the presenting complaint among women of color with darker skin types with acne (Davis and Callender, 2010, Yin and McMichael, 2014) (Fig. 2). While epidermal lesions may persist for up to one year, dermal pigmentation may persist for years and may be a cause of significant distress (Yin and McMichael, 2014). In 2013, a new instrument to measure PIH from acne vulgaris, known as the postacne hyperpigmentation index (PAHPI), was developed and found to have good reliability and validity in skin of color subjects (Savory et al., 2014).
Fig. 2.
Acne and Postinflammatory Hyperpigmentation (Courtesy of Valerie Callender, MD; Callender Dermatology & Cosmetic Center, Glenn Dale, MD).
A study performed by Halder et al. (1996) demonstrated histopathological evidence of marked inflammation in all types of acne lesions on biopsy specimens, including comedones that lacked evidence of inflammation clinically. This intensified level of inflammation on histopathology in skin of color subjects may account for their increased propensity to develop PIH. Other complications of acne seen more commonly in women of color include keloidal scarring and persistent erythema.
Women of color with acne may also develop distinctive subtypes of acne secondary to unique cultural practices integral to their ethnic background. Pomade acne, commonly seen in African Americans, presents as closed comedones and papules along the frontal hairline, forehead, and temples (Davis and Callender, 2010, Yin and McMichael, 2014) (Fig. 3). Pomade acne occurs secondary to long-term daily use of oils and thick emollients used for moisturizing ethnic hair. Acne cosmetica is also frequently encountered among women of color and presents as comedones due to the use of certain cosmetic products that are used to conceal acne lesions and PIH (Davis and Callender, 2010, Yin and McMichael, 2014). In addition, steroid acne may be seen in patients using skin-lightening agents that contain potent topical corticosteroids in efforts to improve PIH (Callender, 2004).
Fig. 3.
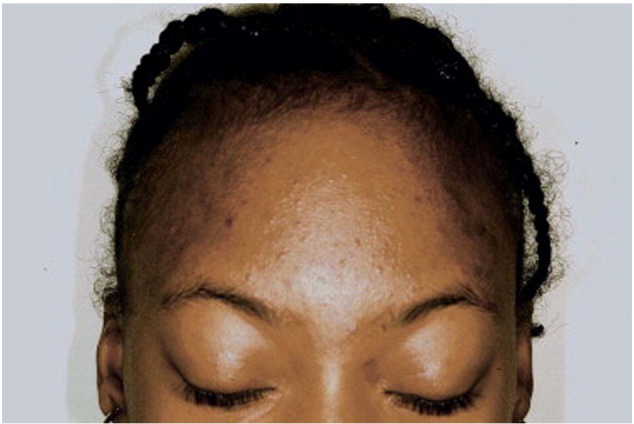
Pomade acne. Note the closed comedones on the forehead and temples. (Courtesy of Valerie Callender, MD; from Treatments for Skin of Color by Susan C. Taylor, MD, et al, copyright Elsevier 2011).
Treatment
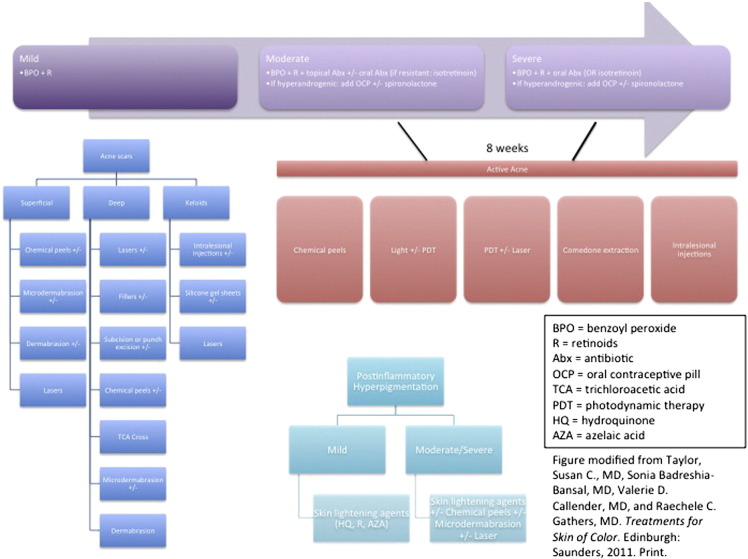
The treatment of acne in women of color is generally similar to that of Caucasian patients. However, an appropriate understanding of the sequelae of acne is the key distinguishing characteristic in women of color. Potential sequelae, such as PIH and keloid scarring, occur more frequently in individuals with skin of color (Shah and Alexis, 2010). More severe forms of acne (especially truncal) can be associated with keloids and may be long lasting or permanent (Shah and Alexis, 2010). Therefore, early and efficacious anti-inflammatory agents to minimize pigmentary abnormalities and keloid scarring must be balanced with their potential for adverse effects (e.g. avoidance of potentially irritating topical agents that might further aggravate pre-existing PIH.) As with all acne patients, one must first determine the predominant type of acne lesion(s) and obtain a history of the patient’s skin type (normal, oily, dry or combination). An acne/PIH algorithm is provided in Fig. 4 (Taylor et al., 2011).
Fig. 4.
Acne and PIH algorithm (Modified from Treatments for Skin of Color by Susan C. Taylor, MD, et al, copyright Elsevier 2011).
Topical retinoids
Topical retinoids, which include adapalene, tretinoin, and tazarotene, are the mainstay of treatment in mild to moderate acne vulgaris in women of color. Retinoids are beneficial for women of color as they treat not only the active acne lesions, but also help ameliorate PIH (Davis and Callender, 2010). Their mechanism of action includes inhibition of toll-like receptor (TLR)-2, reduction of the formation of hyperproliferative keratins, and inhibition of AP-1 pathway, thereby reducing inflammation (Taylor and Summers, 2009). An additional property of topical retinoids that makes these agents appealing for use in skin of color patients is their ability to improve PIH by inhibition of melanosome transfer and facilitating melanin dispersion and removal (Davis and Callender, 2010, Yin and McMichael, 2014).
A major concern with the use of topical retinoids in skin of color includes the potential for inducing a retinoid dermatitis, which is characterized by xerosis, peeling, and erythema of the skin (Taylor and Summers, 2009). There are several practical tips that may mitigate this adverse effect and prevent further worsening of PIH. These include initiating patients on lower concentrations of topical retinoids, choosing cream or lotion (over gel), instructing an every-other-day application regimen with increased frequency as tolerated by the patient, and the addition of a topical gentle moisturizer (Davis and Callender, 2010, Yin and McMichael, 2014, Taylor and Summers, 2009).
A study of 171 Mexican subjects with FST III and IV were evaluated in a single-center, randomized, double-blinded, placebo-controlled clinical trial evaluating the efficacy and safety of adapalene and tretinoin (Tirado-Sanchez et al., 2013). The authors found that adapalene 0.3% gel and tretinoin 0.05% gel were comparable in efficacy in reducing both inflammatory and noninflammatory lesions, while tolerance was better with adapalene 0.1% gel than with adapalene 0.3% and tretinoin 0.05% gel in Mexican subjects.
Grimes and Callender (2006) performed a randomized, double-blinded, vehicle-controlled study consisting of 74 subjects of darker racial ethnic groups with acne to assess the efficacy of tazarotene in the treatment of PIH. In this study, once-daily application of tazarotene 0.1% cream achieved significant reduction in overall PIH disease severity compared with vehicle and in the intensity and area of hyperpigmentation within 18 weeks (Grimes and Callender, 2006).
Topical antimicrobials
Benzoyl peroxide is effective in treating acne due to its suppression of bacterial proliferation, hyperkeratinization and inflammation (Fisk et al., 2014). It may be used as monotherapy or in combination with topical retinoids and antimicrobials. A recent, multicenter, randomized, double blind, placebo controlled study (Alexis et al., 2014) was performed in black subjects with moderate acne to evaluate the efficacy and safety of adapalene 0.1%/benzoyl peroxide (BPO) 2.5% combination gel. The authors reported that significantly more black subjects had decreased median total, inflammatory and noninflammatory lesion counts with adapalene-BPO combination than with vehicle and significant reductions were detected as early as week 1. In addition, adapalene-BPO was safely tolerated in the black subjects and no cases of treatment-induced PIH were observed in this study (Alexis et al., 2014).
Topical antimicrobials such as clindamycin and erythromycin are used primarily for inflammatory acne in women of color. Due to the potential development of resistant strains of P. acnes to these agents, they are often recommended for use in combination with other topical agents such as BPO or retinoids (Davis and Callender, 2010). A recent, multi-center, 12-week, double-blinded study consisting of 797 subjects with moderate to severe acne was performed to assess the efficacy and cutaneous tolerability of clindamycin phosphate 1.2%/benzoyl peroxide 2.5% gel among FST I through III as compared with FST IV through VI (Callender, 2012). Treatment success and median reductions in inflammatory, noninflammatory and total lesions at week 12 were comparable between both groups. Subjects with FST IV through VI were not found to be more susceptible to cutaneous irritation from this combination treatment as compared to FST I through III (Callender, 2012).
A recent, randomized, double-blinded, placebo-controlled clinical trial consisting of 33 subjects with FST IV through VI was performed to evaluate the efficacy and safety of clindamycin phosphate 1.2% and tretinoin 0.025% gel for the treatment of acne and acne-induced PIH (Callender et al., 2012a). The authors concluded that this combination gel was safe and effective for skin of color patients with acne.
Azelaic acid
Topical azelaic acid, a dicarboxyclic acid, minimizes both inflammatory and noninflammatory lesions in acne vulgaris. It is an excellent treatment option for women of color with acne-induced PIH given its ability to inhibit tyrosinase (Davis and Callender, 2010, Fisk et al., 2014). In a pilot study by Kircik, azelaic acid 15% gel applied twice daily for 16 weeks was found to improve both acne and PIH in subjects with FST IV through VI (Kircik, 2011).
Dapsone
Topical dapsone is an effective treatment for acne vulgaris due to its anti-inflammatory and antimicrobial properties (Draelos et al., 2007). A double-blinded, randomized, vehicle-controlled crossover study of 64 subjects, consisting mostly of African Americans, was undertaken to evaluate the risk of hemolysis in acne patients with glucose-6-phosphate dehydrogenase deficiency (G6PD) who were treated with dapsone 5% gel vs vehicle gel (Piette et al., 2008). The authors reported only a 0.32 g/dL decrease in hemoglobin levels from baseline to 2 weeks; however, this was not accompanied by alterations in other laboratory parameters indicative of hemolysis (Piette et al., 2008). The authors concluded that the risk of hemolytic anemia with topical dapsone 5% gel is minimal in patients with G6PD deficiency.
Oral agents
Similar to the general population, oral antibiotics are indicated in women of color for moderate to severe inflammatory acne given their anti-inflammatory and anti-microbial properties (Yin and McMichael, 2014). The most common classes of antibiotics used include macrolides and tetracyclines. Fleischer et al (Fleischer et al., 2006) evaluated an extended release formulation of minocycline hydrochloride for its safety and efficacy in a phase 2 dose finding study and two phase 3 safety and efficacy trials. The designs were prospective, multicenter, randomized, double-blinded, and placebo-controlled and included 1038 participants with moderate to severe acne. Study participants received minocycline 1 mg/kg daily or placebo over 12 weeks. The number of inflammatory lesions was reduced and significant improvement was noted in the Evaluator’s Global Severity Assessment scores (Fleischer et al., 2006). In both the placebo and treatment groups, acute vestibular adverse events (AVAEs) were comparable. The authors concluded that extended release minocycline reduces dose dependent AVAEs, inflammatory lesions, and improves overall appearance of acne patients.
Recently, a randomized controlled trial (RCT) comparing the efficacy of oral doxycycline and oral azithromycin in acne treatment showed that doxycycline is the better treatment option for acne (Ullah et al., 2014). Subjects with moderate acne received either azithromycin 500 mg daily before meals for 4 consecutive days monthly for 3 months or doxycycline 100 mg daily after meals for 3 months and followed up after 3 months. An excellent response was noted in 3.1% and a good response was observed in 22.8% in the azithromycin group subjects. In the doxycycline group, 11.4% had excellent and 55.4% had a good response (Ullah et al., 2014).
Another recent non-inferiority RCT by Tan et al. (2014) compared the efficacy and safety of oral isotretinoin vs. doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (D+A/BPO) in severe nodular acne over 20 weeks. The authors found that D+A/BPO showed a favorable composite efficacy/safety profile compared with isotretinoin and concluded that this combination can be used as an alternative to isotretinoin in severe nodular acne (Tan et al., 2014).
Both doxycycline and minocycline have been associated with gastrointestinal side effects, photosensitivity and photo-onycholysis (Kircik, 2010). Three distinct types of cutaneous minocycline-induced pigmentation have been described, which include blue-black pigmentation confined to sites of scarring or inflammation on the face (Type I), blue-gray circumscribed pigmentation of normal skin on the lower legs and forearms (Type II), and diffuse muddy brown pigmentation of normal skin accentuated in sun-exposed areas (Type III) (Mouton et al., 2004). Minocycline-induced cutaneous hyperpigmentation ranges in frequency from 2.4% to 14.8% in several longitudinal studies (Soung et al., 2007). In addition, minocycline has been associated with the development of autoantibodies, including anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA), and anti-phospholipid antibodies with or without associated clinical symptoms (Kircik, 2010). Of note, minocycline has been recently associated with drug-induced lupus, with the majority of cases (84%) occurring in young women taking minocycline for acne (Kircik, 2010). Therefore, in women of color, one must carefully weigh the benefits and risks of these agents, paying particular attention to the risk of photosensitivity, gastrointestinal concerns, skin hyperpigmentation, and autoimmune and hypersensitivity syndromes (Davis and Callender, 2010).
Any adult female patient with difficult-to-treat acne should be evaluated for a possible underlying endocrine disorder, especially hyperandrogenism. Polycystic ovarian syndrome (PCOS) is the most common cause of hyperandrogenism and cutaneous signs of PCOS include acne, hirsutism, androgenetic alopecia, seborrhea and acanthosis nigricans (Kamangar and Shinkai, 2012). One recent study supports the concept that women often report worsening of acne prior to the onset of their menses, with 65% of all participants reporting perimenstrual acne flare (Geller et al., 2014). The subset of women with acne exacerbations related to their menstrual cycle may be the best candidates to benefit from hormonal therapy such as oral contraceptive pills.
Isotretinoin, an oral retinoid approved by the US Food and Drug Administration, is the treatment of choice for severe nodulocystic acne in skin of color (Davis and Callender, 2010). Special attention and education is mandatory for women of childbearing age given its serious systemic toxicities, which include teratogenicity (pregnancy category X), dyslipidemia, pancreatitis, hepatotoxicity, and pseudotumor cerebri (Davis and Callender, 2010, Yin and McMichael, 2014). An interesting study done by Kelly and Sampson (1987) was performed to evaluate the response of African American patients with recalcitrant nodulocystic acne to isotretinoin treatment. Of the 10 subjects studied, the author reported an early onset flare in areas of the face that were initially devoid of lesions prior to treatment. In addition, the author also reported an improvement in PIH at the completion of the study (Kelly and Sampson, 1987). The subjects in this study were also noted to develop a temporary ashen or grayish facial hue between the second and eighth week of therapy, which was likely attributed to the drying and desquamative effects of isotretinoin. In order to avoid non-compliance and treatment failures, it is critical to educate patients with darker skin prior to initiation of isotretinoin that dyschromia may occur.
Cosmetic therapies
Atrophic acne scarring is another potential sequela of acne in skin of color patients that presents a therapeutic challenge (Shah and Alexis, 2010). A study consisting of 30 subjects with atrophic ice pick scars was performed to assess the efficacy and safety of the chemical reconstruction of skin scars (CROSS) technique in FST IV and V using high strength trichloroacetic acid (TCA) focally into the depth of the scar to induce collagen production (Khunger et al., 2011). The subjects were initially primed for two weeks before the procedure with hydroquinone 4% cream in the morning and tretinoin 0.025% cream in the night. Focal application of 100% TCA with a wooden toothpick in each individual scar at two weekly intervals for four sessions resulted in excellent improvement in 73.3% of subjects. Transient hypopigmentation was observed in one subject and hyperpigmentation in two subjects. At three-month follow-up, no significant adverse effects such as prolonged pigmentary changes or scarring were noted. However, given the increased risk of dyschromias and keloid scarring in skin of color patients, the current authors emphasize that concentrations of TCA greater than 35% should be used with extreme caution in such patients as higher concentrations are thought to be melanotoxic.
Laser technology can also be an effective adjunct therapy in the management of acne in women of color. Gold performed an evaluation on five subjects with using a novel superficial and deep carbon dioxide (CO2) fractional laser in FST IV and V with acne scarring (Gold, 2012). Although this study included a small sample size, all subjects had clinically significant improvement in their skin and no reports of PIH were noted. A recent single-center prospective study assessed the clinical efficacy and safety of the 1450-nm diode laser for the treatment of acne scarring in patients with FST IV to VI (Semchyshyn et al., 2013). While the authors found the nonablative 1450-nm diode laser to be effective in improving the appearance of atrophic acne scars in these subjects, PIH was noted in 56% of the subjects (Semchyshyn et al., 2013). Therefore, while lasers may be beneficial in treating acne-related scarring, similar to most other surgical procedures, caution must be utilized with use of lasers in women of color given the risks of postinflammatory hyper- and hypopigmentation and scarring.
Recently, the efficacy and safety of polymethylmethacrylate (PMMA) suspended in bovine collagen was evaluated for the correction of atrophic facial acne scars in a study by Karnik et al. (2014). The study was a multicenter, double blind, RCT that included 147 participants with more than 20% reporting Fitzpatrick skin types V and VI. Those with at least 4 moderate to severe rolling, atrophic scars were included. Subjects were randomized to PMMA-collagen or saline injections and underwent 2 injection sessions. Follow up was for 6 months. Scar severity was assessed using the Acne Scar Rating Scale (ASRS). The study included 1288 scars with 863 in the PMMA-collagen group and 425 in the control group. Successful results were achieved in 64% of the PMMA-collagen group compared with 33% of the control group. The study found no significant differences in efficacy or safety for darker skin types. Upon assessment of adverse events (particularly those concerning for darker skin types which include hypertrophic scarring, hypopigmentation, or hyperpigmentation), none were seen. Overall, the authors concluded that PMMA-collagen is an effective treatment for facial atrophic acne scars and maintains an excellent safety profile (Karnik et al., 2014). With regards to darker skin types, the authors highlight that the complete lack of dyspigmentation along with excellent treatment outcomes makes PMMA-collagen a great treatment modality for darker skin types (Karnik et al., 2014).
A summary of clinical studies on acne in skin of color is provided in Table 3.
Table 3.
Review of Acne in Skin of Color.
| Authors | Study Design | Total # of Subjects (N) | Summary of Results | Level of Evidence | Strength of Recommendation |
|---|---|---|---|---|---|
| Tirado-Sanchez et al. (2013) | Single-center, randomized, double-blinded placebo-controlled study | 171 subjects (F=94; M = 77) | At 90 days of treatment, the efficacy rates of tretinoin 0.05% gel, adapalene 0.3% gel and adapalene 0.1% gel were 80%, 70%, and 59% respectively. Tolerance was better with adapalene 0.1% gel than with adapalene 0.3% and tretinoin 0.05% gel (P = 0.001). |
I | A |
| Grimes and Callender (2006) | Multicenter, randomized, double-blinded, vehicle-controlled study | 74 subjects (F=65; M=9) | Once-daily tazarotene 0.1% cream was significantly more effective than vehicle in lessening PIH overall (P = 0.010), and in reducing the intensity (P = 0.044) and area of hyperpigmented lesions (P = 0.026) within 18 weeks. | I | A |
| Alexis et al. (2014) | Multicenter, randomized, double-blinded, placebo-controlled study | 238 subjects (F=166; M=72) | After 12 weeks, significant reductions in total, inflammatory and noninflammatory lesion counts were observed with adapalene 0.1%/BPO 2.5% gel than vehicle. After 12 weeks, most subjects reported no dryness (> 89.9%), no erythema (> 90.9%), no scaling (> 96.0%), or no stinging/burning (> 94.9) with adapalene-BPO treatment. |
I | A |
| Callender (2012) | Multicenter, randomized, double-blinded, vehicle-controlled study | 797 subjects (F=408; M=389) | Treatment success with clindamycin phosphate 1.2%/BPO 2.5% gel was comparable between FST I-III and FST IV-VI at week 12. Patients with FST IV-VI were not found to be more susceptible to cutaneous irritation than patients with FST I-III. |
I | A |
| Callender et al. (2012a) | Multicenter, randomized, double-blinded, placebo-controlled study | 33 subjects (F=26; M=7) | Clindamycin phosphate 1.2%/tretinoin 0.025% gel-treated patients had a greater decrease in inflammatory lesion counts from baseline than vehicle group at week 12 (P = 0.05). | I | B |
| Kircik (2011) | Single-center, open-label pilot study | 20 subjects (F=15; M=5) | At week 16, azelaic acid 15% gel applied twice daily resulted in 92% of subjects with at least a one-point improvement in IGA for acne and 100% of subjects had at least a 2-point improvement in IGA for PIH. | II-iii | B |
| Piette et al. (2008) | Multicenter, randomized, double-blinded, vehicle-controlled crossover study | 64 subjects (F=35; M=29) | Subjects with glucose-6-phosphate dehydrogenase deficiency (G6PD) treated with dapsone 5% gel had only a 0.32 g/dL decrease in hemoglobin levels from baseline to 2 weeks; however, no changes were noted in reticulocytes, haptoglobin, bilirubin or lactate dehydrogenase levels. | I | A |
| Fleischer et al. (2006) | Multicenter, randomized, double-blinded, placebo-controlled study | 1038 subjects (F=449; M=589) | An extended release formulation of minocycline hydrochloride was evaluated in subjects with moderate to severe acne in a phase 2 dose finding study and two phase 3 safety and efficacy trials. Study participants received minocycline 1 mg/kg daily or placebo over 12 weeks. The number of inflammatory lesions was reduced and significant improvement was noted in the Evaluator’s Global Severity Assessment scores. In both the placebo and treatment groups, acute vestibular adverse events (AVAEs) were comparable. The authors concluded that extended release minocycline reduces dose dependent AVAEs, inflammatory lesions, and improves overall appearance of acne patients. |
I | A |
| Ullah et al. (2014) | Single-center, randomized controlled study | 386 subjects (F=215; M=171) | Subjects with moderate acne received either azithromycin 500 mg daily before meals for 4 consecutive days monthly for 3 months or doxycycline 100 mg daily after meals for 3 months and followed up after 3 months. An excellent response was noted in 3.1% and a good response was observed in 22.8% in the azithromycin group. In the doxycycline group, 11.4% had excellent and 55.4% had a good response. The authors concluded that doxycycline is the better treatment option for acne. | I | A |
| Tan et al. (2014) | Multicenter, randomized, controlled, noninferiority, investigator-blinded study | 266 subjects (F=39; M=227) | The efficacy and safety of oral isotretinoin versus doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (D+A/BPO) was compared in subjects with severe nodular acne over 20 weeks. The authors found that D+A/BPO showed a favorable composite efficacy/safety profile compared with isotretinoin and concluded that this combination can be used as an alternative to isotretinoin in severe nodular acne. | I | A |
| Kelly and Sampson (1987) | Case series | 10 subjects | 10 African American subjects with recalcitrant nodulocystic acne treated with isotretinoin developed an early onset flare of nodulocystic lesions at sites initially devoid of acne lesions at weeks 2-4. At completion of the study, an improvement in PIH was noted. Between weeks 2-8, most subjects developed a reversible ashen or grayish facial hue (due to the drying and desquamative effects of isotretinoin). |
II-iii | B |
| Khunger et al. (2011) | Case series | 30 subjects (F=20; M=10) | Subjects with FST IV and V with atrophic ice pick acne scars were primed for 2 weeks (with hydroquinone 4% cream in the morning and tretinoin 0.025% cream at night) prior to receiving focal application of 100% trichloroacetic acid (TCA) to each scar at 2-week intervals for 4 sessions. Excellent improvement was seen in 73.3% of subjects, while 20% showed good improvement and 6.7% had fair results. Transient hypopigmentation was observed in 1 patient and hyperpigmentation was observed in 2 patients. No significant prolonged pigmentary changes or scarring were noted at 3 month follow-up. |
II-iii | C |
| Gold (2012) | Case series | 5 subjects (F=3; M=2) | Subjects with FST IV and V treated with a novel superficial and deep AcuPulse MultiMode carbon dioxide (CO2) fractional laser had clinically significant improvement in acne and no reports of PIH were noted. | II-iii | C |
| Semchyshyn et al. (2013) | Case series | 20 subjects | Subjects with FST IV through VI treated with a nonablative 450-nm diode laser found it to be effective in improving the appearance of atrophic acne scars, however, PIH was common in 56% of subjects. | II-iii | C |
| Karnik et al. (2014) | Multicenter, randomized, double-blinded, controlled study | 147 subjects (F = 90; M = 57) | Subjects with at least 4 moderate to severe rolling, atrophic scars randomly received polymethylmethacrylate (PMMA) suspended in bovine collagen (PMMA-collagen) or saline injections. Subjects underwent up to 2 injection sessions and were followed up for 6 months. Success was achieved by 64% of those treated with PMMA-collagen compared with 33% of control subjects (P = .0005). The treatment showed excellent safety with generally mild, reversible adverse events. No significant differences in efficacy or safety were noted between genders, for darker skin types, or in older age groups. | I | B |
* In accordance with the US Preventive Services Task Force levels of evidence for grading clinical trials (Sheth and Pandya, 2011b), (see Appendix A).
Abbreviations: F, female; M, male; BPO, benzoyl peroxide; PIH, postinflammatory hyperpigmentation; FST, Fitzpatrick skin type; IGA, investigator global assessment.
Melasma
Melasma is an acquired hypermelanosis of the skin characterized by hyperpigmented macules and patches distributed symmetrically on sun-exposed areas of the body, particularly on the face. Most cases occur in women of reproductive age with darker skin tones (Fitzpatrick skin type IV–VI) living in areas of intense ultraviolet (UV) radiation, but people of all ages, races, and skin colors may be affected. Its pathogenesis is not completely understood. The majority of cases have been associated with identifiable risk factors such as genetic predisposition, ultraviolet (UV) radiation and hormonal influences including oral contraceptives and pregnancy, anti-seizure and phototoxic medications, and thyroid disease (Grimes, 1995, Sheth and Pandya, 2011a). In fair skinned individuals, the pigmentation may reduce or resolve completely after parturition, whereas, it may persist in women with darker skin.
Based on the distribution of the facial lesions, melasma is classified into malar, centrofacial, mandibular and combination patterns of these (Fig. 5, Fig. 6). In the Indian subcontinent, a majority of patients show a malar distribution compared with disease pattern in Western population where centrofacial pattern predominates (Sheth and Pandya, 2011a, Sardesai et al., 2013). Based on Wood's lamp examination, it has been classified as epidermal, dermal, mixed, and indeterminate variants in Type IV-VI skin (Grimes, 1995, Sheth and Pandya, 2011a). While a Wood’s lamp examination was previously thought to precisely predict epidermal versus dermal pigment deposition, recent studies have shown that dermal melanin deposition is common and may be underrecognized given that many patients often exhibit a mixed pattern (Grimes, 1995, Sheth and Pandya, 2011a, Sardesai et al., 2013).
Fig. 5.
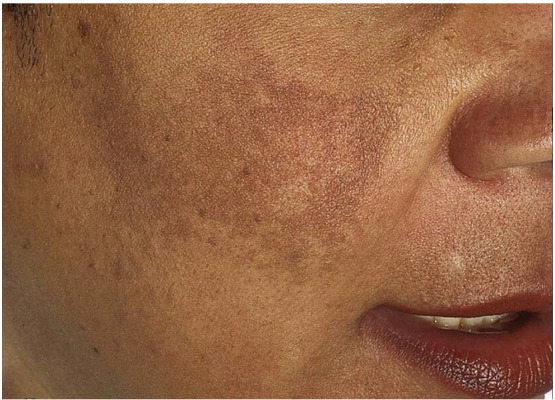
Melasma, malar variant (Courtesy of Jean Bolognia, MD; from Dermatology, copyright Elsevier, 3rd ed., 2012).
Fig. 6.
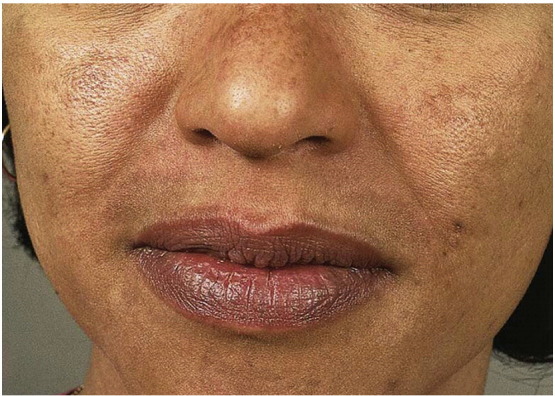
Melasma, centrofacial variant with sparing of the philtrum (Courtesy of Jean Bolognia, MD; from Dermatology, copyright Elsevier, 3rd ed., 2012).
Medical management
A broad-spectrum UVA- and UVB-protective sunscreen with a sun protection factor (SPF) of at least 30 along with a physical blocker, such as titanium dioxide or zinc oxide, should be applied daily in patients with melasma. In addition to protection from ultraviolet radiation (UVR), visible light protection is also warranted as it also impacts pigmentation. Recently, it has been shown that short wavelengths of visible light (415 nm) can induce prolonged hyperpigmentation in comparison to long wavelengths (630 nm), which do not impact pigmentation in healthy volunteers (Boukari et al., 2015). Boukari et al. (2015) recently conducted a RCT in real-life settings during the spring and summer evaluating the protective properties against melasma relapses of a sunscreen protecting against UVA/UVB and the shorter wavelengths of visible light compared to a sunscreen with UVA/UVB protection but without visible light protection. The study included 40 melasma patients representing FST III –V. Subjects were randomized to receive either Formula A (tinted and contained iron oxides) or Formula B, which were initially analyzed with photospectrometric analysis to assess the absorption potential of visible light. Patients applied 1 dose of the respective product twice daily and an additional dose every 2 hours, and 30 minutes before exposure to daylight. Patients were instructed to apply 1 teaspoon of sunscreen to the face. The main clinical assessment was the Melasma Area and Severity Index (MASI) score. The median increase of the MASI score from baseline to month 6 was more important with Formula B than with Formula A. Also, the tinted sunscreen only provided a partial protection in the targeted visible wavelengths according to the photospectrometric analysis. Given their results, the authors emphasize the impact that visible light has on melasma pathogenesis. Thus, they note the importance of developing more effective filters that protect against these wavelengths (Boukari et al., 2015). Additional sun protective measures, such as limited time outdoors, use of protective clothing and hats, and sun avoidance when possible are also essential to the treatment of melasma.
Currently, there is no single universally effective therapeutic agent for the treatment of melasma as the condition is recurrent and challenging. Conventional treatment of melasma includes elimination of any possible causative factors coupled with the use of a broad-spectrum sunscreen and skin lightening agents such as topical retinoids, hydroquinone, kojic acid, azelaic acid, deoxyarbutin, or ascorbic acid as monotherapy or in combination formulations using the modified Kligman’s formula.
Triple combination (TC) therapy, comprised of hydroquinone, a retinoid, and a fluorinated corticosteroid, is considered to be a highly effective and safe treatment for melasma in skin of color (Rivas and Pandya, 2013). One of the most successful TC therapy regimens consists of a fixed combination of 4% hydroquinone, 0.05% tretinoin and 0.01% fluocinolone acetonide (Sheth and Pandya, 2011b). Taylor et al. (2003) conducted one of the initial studies demonstrating the high efficacy of this TC by comparing nightly use of TC cream with nightly use of three dual-combination creams (4% hydroquinone plus 0.05% tretinoin, 0.05% plus 0.01% fluocinolone acetonide, and 4% hydroquinone plus 0.01% fluocinolone acetonide). After 8 weeks, complete clearance was achieved in 26.1% of subjects treated with TC cream in comparison with 9.5% of subjects using hydroquinone plus tretinoin, 1.9% of subjects using tretinoin plus fluocinolone, and 2.5% of subjects using hydroquinone plus fluocinolone (Taylor et al., 2003). In addition, complete or near complete clearance was achieved in 77% of patients on the TC therapy versus a maximum of 46.8% in subjects using the dual combination preparations. Adverse effects such as erythema, peeling, irritation and dryness were highest in the combination regimens containing tretinoin, however, these side effects were transient and mild (Taylor et al., 2003). Several additional publications have subsequently demonstrated the safety and efficacy of this TC cream, particularly in darker-skinned racial ethnic groups (Torok, 2006, Torok et al., 2005a, Chan et al., 2008).
Given the relapsing nature of melasma, maintenance regimens are often necessary to sustain efficacy after initial stabilization. A study conducted by Arellano et al. (2012) was performed to determine the maintenance therapy for patients in whom TC therapy had shown to be effective. The authors found that the twice-weekly TC therapy group had a lower relapse rate compared with the tapering regimen group (3 times weekly for the first month, twice weekly for the second month and once weekly for the fourth month) (Arellano et al., 2012). Torok et al. (2005b) conducted a 12-month extension of an 8-week multicenter RCT consisting of 327 subjects evaluating the safety and efficacy of once daily TC cream in facial melasma. The study concluded that TC cream had a favorable safety profile and when applied intermittently over a long time period is tolerable, safe, and effective for moderate to severe facial melasma (Torok et al., 2005b).
Other combination creams have also been studied and show promising results. In a randomized study conducted by Lim, 40 Chinese female subjects were treated with 2% kojic acid in a gel containing 10% glycolic acid and 2% hydroquinone (Lim, 1999). The gel was applied to one-half of the face twice daily and compared with a gel containing only 10% glycolic acid and 2% hydroquinone applied to the other half of the face twice daily for 12 weeks. The authors found that in 60% of the subjects in the kojic acid group, more than 50% of melasma lesions had cleared versus 47.5% of subjects in the 10% glycolic acid and 2% hydroquinone group (Lim, 1999). More recently, a hydroquinone-free, multimodal complex skin lightening agent has been developed to address dyschromias such as melasma. This agent acts via targeting multiple pathways in melanin synthesis including reduction in melanocyte activation, melanin synthesis, melanin transfer to keratinocytes, and epidermal melanin (Makino et al., 2013). Grimes (2014) presented a case report of six female subjects with Fitzpatrick skin types IV - V with moderate epidermal facial melasma treated with a hydroquinone-free, multimodality skin brightener. Patients applied the agent twice daily and were evaluated at 4, 8, and 12 weeks. The Investigator Overall Hyperpigmentation scores improved by an average of 22% and the MASI scores improved by an average 38%. In addition, 100% of the participants showed at week 12 at least a 25% increase in Global Improvement. The skin brightener was well tolerated without pruritus, erythema, edema, scaling, or burning/stinging reported (Grimes, 2014).
In summary, it is recommended that patients with mild melasma use non-phenolic therapy such as azelaic acid (AZ), arbutin, kojic acid, tretinoin, adapalene and glycolic acid or any combination of these or 4% hydroquinone as monotherapy. For moderate to severe melasma, TC therapy is the gold standard. These agents are often used in concurrence with other physical therapies.
Chemical peels
A wide variety of agents are available for chemical peels, however, the choice is rather limited while treating a patient with Fitzpatrick skin type IV - VI. Use of medium-depth peels is advised with caution and deep phenol peels are avoided owing to risk of scarring and prolonged hyperpigmentation (Sarkar et al., 2012, Salam et al., 2013). Among the superficial peels, 20–30% salicylic acid in ethanol is widely considered to be the safest peel in the ethnic skin population (Salam et al., 2013). Based on current available evidence, glycolic acid peels (20-70%) every 4-6 weeks are a useful adjunctive therapy in refractory cases of epidermal melasma (Sheth and Pandya, 2011b, Sarkar et al., 2012, Salam et al., 2013). It is the most widely utilized peeling agent in skin of color and has proven safety as well as efficacy. Superficial trichloroacetic acid (TCA) peels (10-25%) should be used with caution in darker skin types due to the risk of pigment dyschromias and scarring. The most common sequela with the use of chemical peels for melasma in ethnic skin is PIH, which can either occur between the treatment sessions or after stopping treatment. Pre-peel priming with hydroquinone and tretinoin for 2-4 weeks enhances the effect of the peeling agent apart from decreasing the PIH, with hydroquinone considered superior to tretinoin as a priming agent (Nanda et al., 2004). Recommendations of chemical peels for melasma in skin of color are provided in Table 4.
Table 4.
Levels of evidence and strength of recommendations for various peeling agents in ethnic skin (Sarkar et al., 2012).
| Peeling agent | Level of evidence | Strength of recommendation |
|---|---|---|
| Glycolic acid peel | II-i | A |
| Lactic acid peel | II-iii | B |
| Salicylic acid peel | II-iii | B |
| Trichloroacetic acid peel | II-iii | B |
| Jessner’s solution | II-iii | B |
| Phytic acid peel | III | C |
| Pyruvic acid peel | III | C |
* In accordance with the US Preventive Services Task Force levels of evidence for grading clinical trials (Sheth and Pandya, 2011b), (see Appendix A).
Light and laser therapy (Arora et al., 2012, Sardana and Garg, 2014)
Published data or recommendations on the safety and effectiveness of lasers in Fitzpatrick skin types V – VI are limited by complications of post procedure hyper- or hypopigmentation. Several of the lasers utilized as options for the treatment of melasma, including the pigment-specific lasers (Q-switched, long-pulsed lasers and intense pulsed light or IPL), ablative lasers (Er:YAG) and fractional lasers have had conflicting results and are anecdotal. Most of the studies reported in Asians have been performed on Fitzpatrick skin types III – IV and demonstrate that lasers may cause worsening of the disease, minimally improve it, or produce unwanted PIH (Jang et al., 2011).
A recent 2013 evidence-based review of treatments for melasma demonstrated overall that laser and light therapy yields varied results and that relapse is a frequent occurrence after treatment (Rivas and Pandya, 2013). While IPL therapy has been shown to be effective in improving melasma, it is characterized by a high relapse rate (Wang et al., 2004). QS-Nd:YAG lasers are associated with an increased risk of rebound hyperpigmentation and a very high rate of relapse following treatment (Wattanakrai et al., 2012). In general, laser and light therapies are considered third-line agents for the treatment of melasma and further research is needed. Most physicians recommend priming the patient’s skin with triple combination prior to laser therapy and continue to use this regimen after treatment sessions. Low fluence and low treatment density non-ablative fractional lasers and Q-switched 1064 nm can be considered as third-line treatment for patients who have not improved sufficiently after 3–6 months of topical treatment (Table 5).
Table 5.
Levels of evidence and strength of recommendations for lasers and light based devices for treating melasma in skin of color.
| Type of laser | Level of evidence | Strength of recommendation | Side effects and complications | |
|---|---|---|---|---|
| Q switched lasers | Q switched Ruby (Jang et al., 2011, Hilton et al., 2013) | II-iii | C | PIH and recurrence of melasma |
| Post operative discomfort | ||||
| Q switched Alexandrite (Fabi et al., 2014, Rusciani et al., 2005) | II-iii | C | PIH | |
| Q switched Nd:Yag-“laser toning/laser facial” (Wattanakrai et al., 2010, Bansal et al., 2012, Sim et al., 2014) | I | B | Erythema, burning, swelling, whitening of hair Spotty depigmentation | |
| Rebound hyperpigmentation | ||||
| Recurrence of melasma | ||||
| Light devices | IPL (Zaleski et al., 2012) | II-iii | C | Erythema |
| Pain | ||||
| PIH | ||||
| Fractional Ablative lasers | Erbium:YAG (Attwa et al., 2014, Manaloto and Alster, 1999) | II-iii | C | PIH |
| CO2 (Jalaly et al., 2014, Neeley et al., 2010) | II-iii | C | PIH | |
| Rebound melasma | ||||
| Vascular lasers | PDL (Passeron et al., 2011) | II-iii | C | PIH |
| Fractional lasers Non- ablative (Wind et al., 2010) |
Non-ablative 1,550 nm fractional laser therapy | I | C | Erythema, burning sensation, edema, pain and PIH |
| 1,927-nm thulium fiber laser (Polder and Bruce, 2012) | II-iii | C | Moderate erythema and mild edema |
* In accordance with the US Preventive Services Task Force levels of evidence for grading clinical trials (Sheth and Pandya, 2011b), (see Appendix A).
Abbreviations: IPL, intense pulsed light; PDL, pulsed dye laser; PIH, postinflammatory hyperpigmentation.
Alopecia
Hair care practices
Hair and scalp disorders in women of African descent are particularly challenging, partially due to a paucity of research into the pathophysiology of these disorders, and also because of poor understanding of the basic hair care practices in this patient population. Below we discuss some of the more common grooming practices that could significantly impact the clinical presentation of hair and scalp disorders, and compliance with treatment regimens.
Several hairstyles common in people of African descent result in tension at the root of the hair follicles. These include cornrows (formed by braiding the hair tightly into individual sections) and dreadlocks (a type of styling allows the hair to knot into individual twistlike structures) (Ogunleye et al., 2014). In either of these styles, human or synthetic hair may be added for increased length and volume (Callender et al., 2004). Hair weaving is another common hairstyle, where the hair is cornrowed and additional hair is sewn or glued to the cornrows. These methods of styling have all been associated with traction alopecia or marginal scalp hair loss (Grimes, 2000).
Straightening of the hair has traditionally been the style of choice in black women, as it is thought to increase styling options and manageability of the hair (Ogunleye et al., 2014, Callender, 2002). This can be achieved via thermal straightening or chemical straightening. Thermal straightening, also known as hot combing or pressing, involves temporarily rearranging hydrogen bonds within the hair shafts (Callender et al., 2004). The hair is coated with a lubricating oil and then combed with a hot metal comb heated to 300 to 500°F (LoPresti et al., 1968). An alternative to the hot comb is the flat iron. Here, the hair is placed between 2 hot smooth, ceramic plates with temperatures ranging from 180 to 450°F (Fig. 7).
Fig. 7.

(Left to right) Electric curling iron, hot comb and ceramic flat iron (Courtesy of Valerie Callender, MD; from Treatments for Skin of Color by Susan C. Taylor, MD, et al, copyright Elsevier 2011).
Chemical relaxers produce permanent hair straightening by rearranging disulfide bonds within the shaft; using sodium hydroxide in lye relaxers, and guanidine hydroxide in no-lye relaxers (Fig. 8). These often result in irritant contact dermatitis, trichorrhexis nodosa, and fragile, easily damaged hair (Swee et al., 2000). A newer method of hair straightening, known as the Brazilian keratin treatment, is a process in which a liquid keratin solution is sealed into the hair using a flat iron. Formaldehyde, the preservative used in this process, can be a significant health concern to both stylists and clients.
Fig. 8.
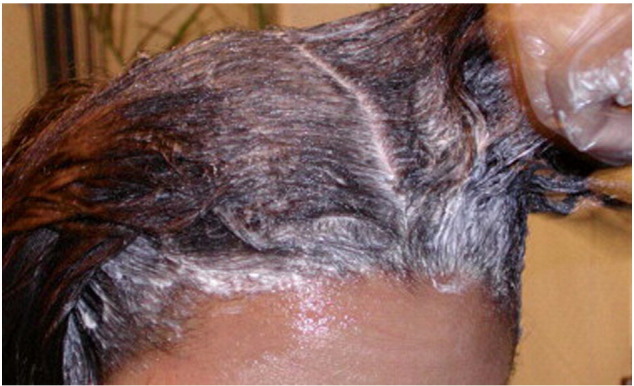
Chemical relaxer application to hair (Courtesy of Susan C. Taylor, MD, et al; from Treatments for Skin of Color, copyright Elsevier 2011).
In terms of hair cleansing, African American women tend to shampoo less frequently than other ethnicities, approximately once every 1 to 2 weeks (Hall et al., 2013). This is partially due to lower levels of sebum on the hair shaft, with resulting dryness and breakage if shampooed too frequently. Also, the hairstyles described above are time consuming and costly, and so the hair is washed infrequently in an attempt to maintain these hairstyles for longer periods of time (Callender, 2002, McMichael, 2003).
Traction Alopecia
Traction alopecia (TA) is a form of alopecia loss caused by repetitive or prolonged tension to the hair. Although early disease is non-scarring, longstanding TA can lead to permanent hair loss. TA is most frequently reported in individuals of African descent (Khumalo et al., 2007a, Khumalo et al., 2007b) and is closely linked to the unique hair grooming practices and increased susceptibility of the hair follicles in this patient population (Mulinari-Brenner and Bregfeld, 2001).
The earliest clinical sign of traction on the scalp is perifollicular erythema, which may progress to follicular papules and pustules. Eventually, yellow-white cylinders, known as peri-pilar casts, encircle the hair shaft at sites of traction (Samrao et al., 2011, Rollins, 1961, Fox et al., 2007, Tosti et al., 2010). Subsequently, clinically apparent alopecia develops, with decreased follicular markings in late stage disease (Samrao et al., 2011).
TA often affects the frontal and temporal scalp, as well as anterior and superior to the ears (Fig. 9). Patients usually report a history of tight braids, weaves, cornrows, ponytails, chignons or religious head coverings. There is a significantly higher risk of developing TA when traction is applied to chemically treated hair, such as relaxers and dyes; but does not appear to be affected by the frequency of relaxer application (Khumalo et al., 2007a, Khumalo et al., 2008). The presence of retained hairs along the frontal and/or temporal hairline, termed the “fringe sign,” is a common finding in patients with TA of the marginal hairline, and can help in making a clinical diagnosis of TA (Samrao et al., 2011).
Fig. 9.
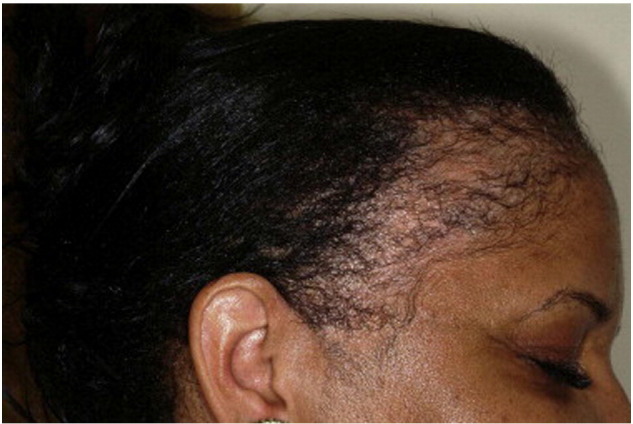
Traction alopecia (Courtesy of Valerie Callender, MD; from Treatments for Skin of Color by Susan C. Taylor, MD, et al, copyright Elsevier 2011).
As the early stage of TA is reversible, it is very important that the public be educated on the increased risk of TA in patients with symptomatic traction an combined hairstyles, such as the tight braiding of relaxed hair. It has been suggested that to influence the practice of at risk individuals, scientific data should be translated into simple messages like "tolerate pain from a hairstyle and risk hair loss" and "no braids or weaves on relaxed hair" (Mirmirani and Khumalo, 2014).
An unusual variant of TA resulting from the use of wefted hair extensions, termed the “horseshoe” pattern, has been reported (Ahdout and Mirmirani, 2012). Wefts consist of multiple strands of hair held together by a band of fine threads, and are attached directly to the hairline by being sewn, bonded, glued, or clipped. TA has also been reported in Hispanic women with long hair who wear tight ponytails, and in Japanese women and ballerinas who frequently wear their hair in a tight bun. In these cases, the hair loss may be confined to the occipital or temporal scalp (Trueb, 1995, Samrao et al., 2010a).
In patients who give a remote or unclear history of tight hairstyles, the clinical differential diagnosis includes androgenetic alopecia, telogen effluvium, trichotillomania and central centrifugal cicatricial alopecia. When TA is localized to the marginal hairline, frontal fibrosing alopecia and the ophiasis pattern of alopecia areata should be considered (Heath and Taylor, 2012).
Treatment options for TA depend on duration of the disease process. Firstly, patients should be advised that TA may lead to permanent progressive alopecia (James et al., 2007). In early disease, the hairstyle should be loosened, and chemicals, heat and combined hairstyles should be avoided. Intralesional corticosteroids directed at the periphery of hair loss, suppress peri-follicular inflammation (Callender et al., 2004). Oral or topical antibiotics may also be used in early disease for their anti-inflammatory effect (Callender et al., 2004). Topical minoxidil may also be considered in conjunction with intralesional corticosteroids (Khumalo and Ngwanya, 2007). In longstanding disease, dermal scarring leads to permanent alopecia. In these advanced cases, surgical options such as hair transplantation may be considered (Callender et al., 2004, Ozcelik, 2005).
Acquired Trichorrhexis Nodosa
Trichorrhexis nodosa (TN), a hair shaft disorder, is characterized by fragility and nodes along the shaft. The hair appears lusterless and dry and patients may complain of “whitish spots” along the hair (Martin and Sugathan, 2011) (Fig. 10). The underlying pathogenic mechanism seems to be the loss of cuticle cells along the shaft. As a result, the cortical fibers lose their protection, and split longitudinally into numerous small fibers. On microscopic examination, there is cuticular cell disruption and fraying and breaking of the cortical fibers, with the resulting appearance likened to two paint brushes crushed against each other, hence the description “paint brush fracture” (Martínez de Lagrán et al., 2009, Rudnicka et al., 2013, Miyamoto et al., 2009).
Fig. 10.
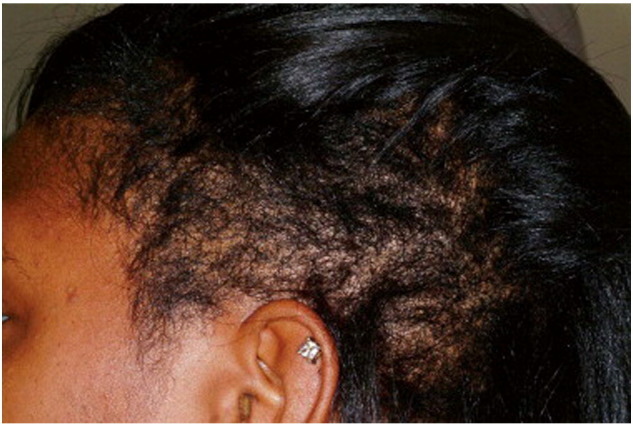
Trichorrhexis nodosa in the temporal scalp region (Courtesy of Valerie Callender, MD; from Treatments for Skin of Color by Susan C. Taylor, MD, et al, copyright Elsevier 2011).
TN may be congenital or acquired. Acquired TN results from repeated trauma to the hair (Khumalo et al., 2007a, Khumalo et al., 2007b, Khumalo et al., 2008, Mulinari-Brenner and Bregfeld, 2001, Samrao et al., 2010a, Samrao et al., 2011, Rollins, 1961, Fox et al., 2007, Tosti et al., 2010, Mirmirani and Khumalo, 2014, Ahdout and Mirmirani, 2012, Trueb, 1995, Heath and Taylor, 2012, James et al., 2007, Callender et al., 2004, Khumalo and Ngwanya, 2007, Ozcelik, 2005, Martin and Sugathan, 2011), such as from harsh hair combing practices, chemical trauma, excessive brushing, and application of heat. While acquired TN usually affects the distal shaft, there has been a recently reported case of recurrent generalized proximal trichorrhexis nodosa in a Nigerian female with a history of severe atopic dermatitis, affecting the scalp (Ogunbiyi et al., 2014). Occasionally, acquired TN may also result from iron deficiency or hypothyroidism (Lurie et al., 1996).
Chemical trauma to the hair includes excessive exposure to bleaching, perming, shampooing and dyeing. In African type hair, chemical “relaxers” are used to straighten the tightly curled hair. In addition to disrupting the hair cuticle, these relaxers also reduce cysteine levels within the hair shaft (Khumalo et al., 2010). Cysteine is a component of the disulfide bonds that are responsible for the inherent strength of hair (Khumalo et al., 2010).
Heating devices such as hot combs and flat irons are used, not only in African Americans, but in all ethnic groups, to obtain sleek, straight hair (Khumalo et al., 2010). Here, hydrogen bonds in the cortex of the hair shaft are temporarily broken and then reform (Bolduc and Shapiro, 2001, Quinn et al., 2003). Unfortunately, damage to the protective cuticle is a common consequence; with hair breakage reported in up to 18% of African American women using a hot comb (Grimes, 2000).
Treatment of acquired trichorrhexis nodosa focuses on avoiding the offending agents. Patients should be advised to use combs with straight elongated bristles, along with hair oils and conditioners, to minimize tension and friction along the hair shaft. In chemical induced TN, hair relaxers and dyes should be applied only by a licensed professional. Patients should be advised of the significantly increased risk of hair breakage when dyeing chemically straightened hair. Also, hot combs and flat irons should be used no more frequently than once per week, on dry hair only, at temperatures below 175°C (Mirmirani, 2010, Detwiler et al., 1994, Ruetsch and Kamath, 2004).
Central Centrifugal Cicatricial Alopecia
Central Centrifugal Cicatricial Alopecia (CCCA), a chronic, progressive and inflammatory form of hair loss, seen more commonly in women of African descent and rarely in men (Sperling and Sau, 1992). It has been previously known as “follicular degeneration syndrome,” “hot comb alopecia,” and chemically induced scarring alopecia (Sperling and Sau, 1992, LoPresti et al., 1968, Nicholson et al., 1993). The currently recognized term, CCCA, was later coined by the North American Hair Research Society (NAHRS) (Olsen et al., 2003).
The true prevalence of this condition is still unknown but may vary from 2.7% to 5.7% and increases with age (Ogunleye et al., 2014). However, it has been reported that CCCA patients are among the top five reasons why African- Americans seek dermatologic evaluation (Halder et al., 1983b, Alexis et al., 2007). The true etiology of CCCA remains to be elucidated and is most likely multifactorial. Possible contributing factors include intense heat and traction to the scalp, the application of chemical relaxers, the intrinsically curly nature of African hair follicles and the decreased number of dermal elastic fibers in African hair compared with that of white individuals (Nicholson et al., 1993, Gathers et al., 2009, Nnoruka, 2005, Ackerman et al., 2000, Montagna and Carlisle, 1991). Genetics also appears to play a significant role, and an autosomal dominant inheritance pattern with partial penetrance has been proposed (Dlova and Forder, 2012, Dlova et al., 2014). Lastly, the intrinsically curly nature of African hair follicles likely plays a role in CCCA development. African hair is elliptical in nature, with frequent twists and decreased tensile strength (Franbourg et al., 2003). This intrinsic risk for breakage is compounded with harsh hair grooming practices which force the hair to flow in an unnatural direction for prolonged periods of time (Callender and Onwudiwe, 2011).
In CCCA, the scarring alopecia occurs mainly on the vertex of the scalp with symmetric spread in a centrifugal pattern (Fig. 11, Fig. 12, Fig. 13). The clinical presentation and distribution of hair loss may mimic that of androgenetic alopecia, but the lack of follicular openings indicates a scarring process (Fig. 14). Possible signs and symptoms include papules, pustules, tenderness or pruritus of the scalp and may be totally asymptomatic. A sign of early or occult CCCA may present as hair breakage (Callender et al., 2012b). While patients may not have frank alopecia at presentation, a history of short, broken hair should prompt further investigation and a confirmatory biopsy. Dermatoscopic evaluation may aid in identifying the optimal site from which to obtain a biopsy specimen. On dermoscopy, the presence of a peripilar white halo is a feature that suggests CCCA in an African American patient with mild central thinning. The peripilar white halo corresponds on pathology to the lamellar fibrosis surrounding the outer root sheath (Miteva and Tosti, 2013, Miteva and Tosti, 2014).
Fig. 11.
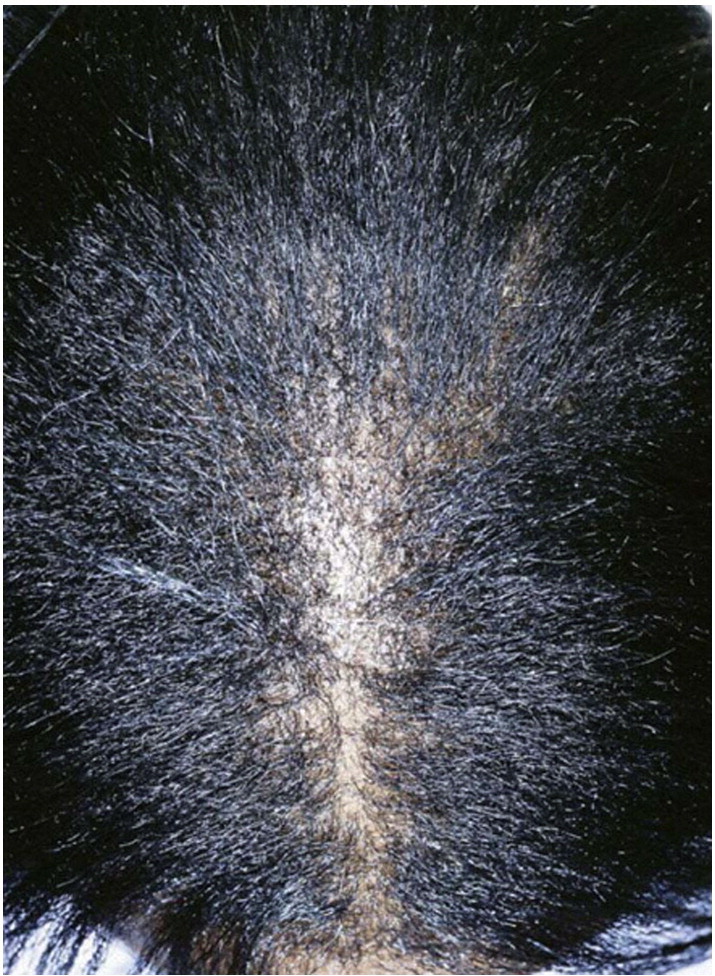
Early stage CCCA (Courtesy of Susan C. Taylor, MD, et al; from Treatments for Skin of Color, copyright Elsevier 2011).
Fig. 12.
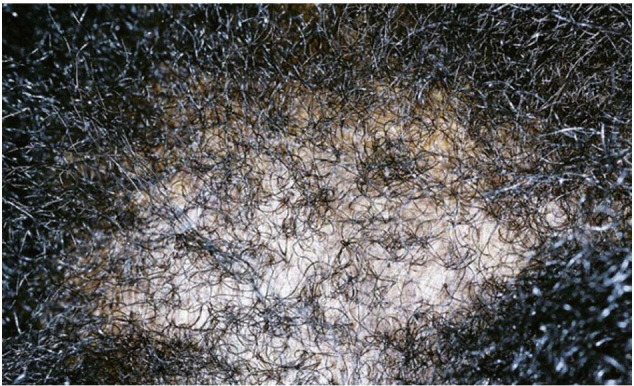
CCCA (Courtesy of Susan C. Taylor, MD, et al; from Treatments for Skin of Color, copyright Elsevier 2011).
Fig. 13.
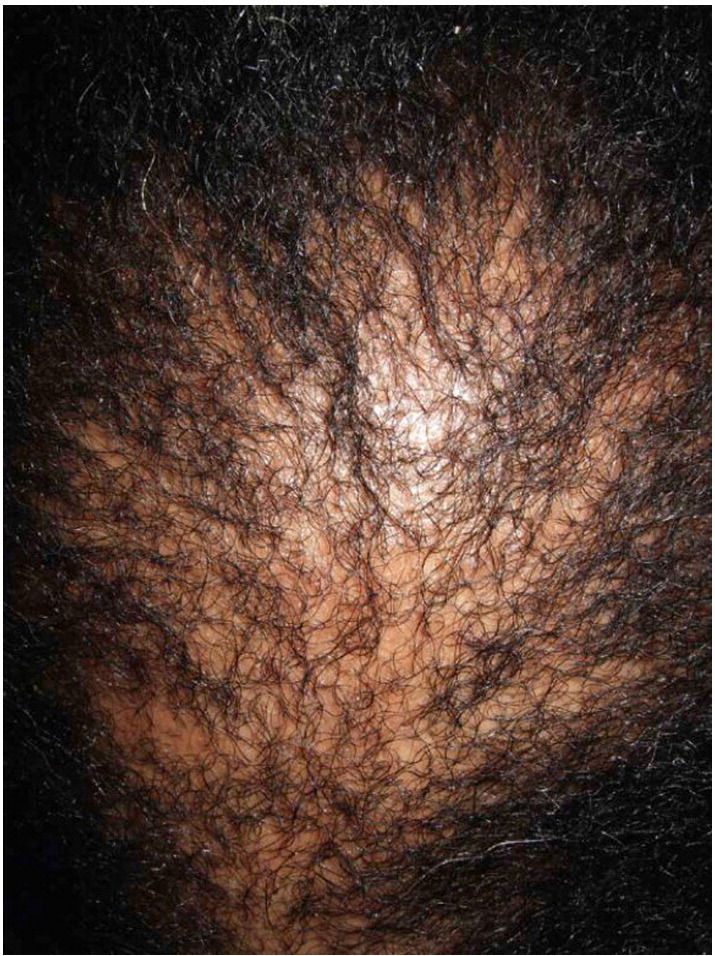
CCCA in an African American woman (Courtesy of Jean Bolognia, MD; from Dermatology, copyright Elsevier, 3rd ed., 2012).
Fig. 14.
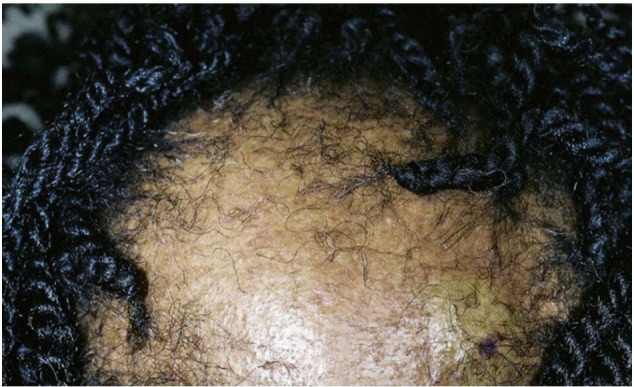
Late stage CCCA (Courtesy of Susan C. Taylor, MD, et al; from Treatments for Skin of Color, copyright Elsevier 2011).
In terms of associated disorders and hair grooming practices, results of a cross-sectional survey by Kyei et al found a statistically significant correlation with type 2 diabetes mellitus, bacterial skin infections and hairstyles like braids and weaves. Interestingly, no significant difference was noted in the use of chemical relaxers and hot combs. There was also a strong positive correlation between the age of the subjects and degree of hair loss (Kyei et al., 2011). A study by Olsen et al found no significant correlation between chemical relaxer and hot comb use, seborrheic dermatitis, or bacterial infections (Olsen et al., 2011).
Treatment of CCCA is focused on attempting to terminate the progression of disease with hopes of possible regrowth if permanent scarring has not occurred. Firstly, discontinuation of potentially damaging hair-grooming practices should be encouraged. High potency topical corticosteroids or intralesional corticosteroid injections may help with symptom alleviation. For active spreading disease, anti-inflammatory antibiotics such as doxycycline and antimalarials such as hydroxychloroquine may slow disease activity (Summers et al., 2011).
In patients with stable disease controlled with medical therapy for at least one year, and who also display a lack of inflammation on scalp biopsy, hair transplantation can be a safe well-tolerated procedure for CCCA in women of color (Callender et al., 2014b). However, it is important to note that the presence of scarring can decrease the transplanted graft survival rate, and regrowth of the transplanted hair is slow (Callender, 2006, Callender and Young, 2008). Counseling includes styles that seek to camouflage the injury including wigs, color sticks/crayons or micropigmentation (Callender et al., 2004).
Frontal Fibrosing Alopecia
Frontal fibrosing alopecia (FFA), first described in 1994 (Kossard, 1994), is a primary scarring alopecia that is considered a clinical variant of lichen planopilaris (Kossard et al., 1997). It occurs mainly in postmenopausal women (Kossard, 1994), although premenopausal women (Moreno-Ramirez and Camacho Martinez, 2005, Faulkner et al., 2002, Banka et al., 2014, Vañó-Galván et al., 2014) and men (Kossard and Shiell, 2005, Nusbaum and Nusbaum, 2010, Stockmeier et al., 2002, Dlova and Goh, 2013, Ramaswamy et al., 2012) may also be affected.
Most data on FFA comes from studies and case reports including a majority of white and Asian patients. It is important to note that, although uncommon, FFA does occur in individuals of African descent (Miteva et al., 2012, Dlova et al., 2013). This lack of data on FFA in black patients may be due to misdiagnosis as traction alopecia (Miteva et al., 2012), which has overlapping clinical features with FFA and is a very common entity in this patient population.
On presentation, there is follicular hyperkeratosis, perifollicular erythema and scarring of the frontotemporal hairline (Fig. 15). Up to 75% of patients may also complaint of eyebrow loss, which may be the initial complaint at presentation (Ladizinski et al., 2013, Tan and Messenger, 2009, Samrao et al., 2010b). Other affected areas include the eyelashes, axillary and pubic regions, the occipital scalp, and vellus hairs, which may present as facial noninflammatory papules, or follicular red dots in the glabellar region (Ladizinski et al., 2013, Tan and Messenger, 2009, Samrao et al., 2010b, Chew et al., 2010, Molina et al., 2011, Pirmez et al., 2014). Furthermore, it has been proposed that the triad of eyelash loss, body hair involvement, and facial papules is associated with severe FFA, and may be used to predict prognosis and to highlight patients who would require systemic therapy (Vañó-Galván et al., 2014).
Fig. 15.
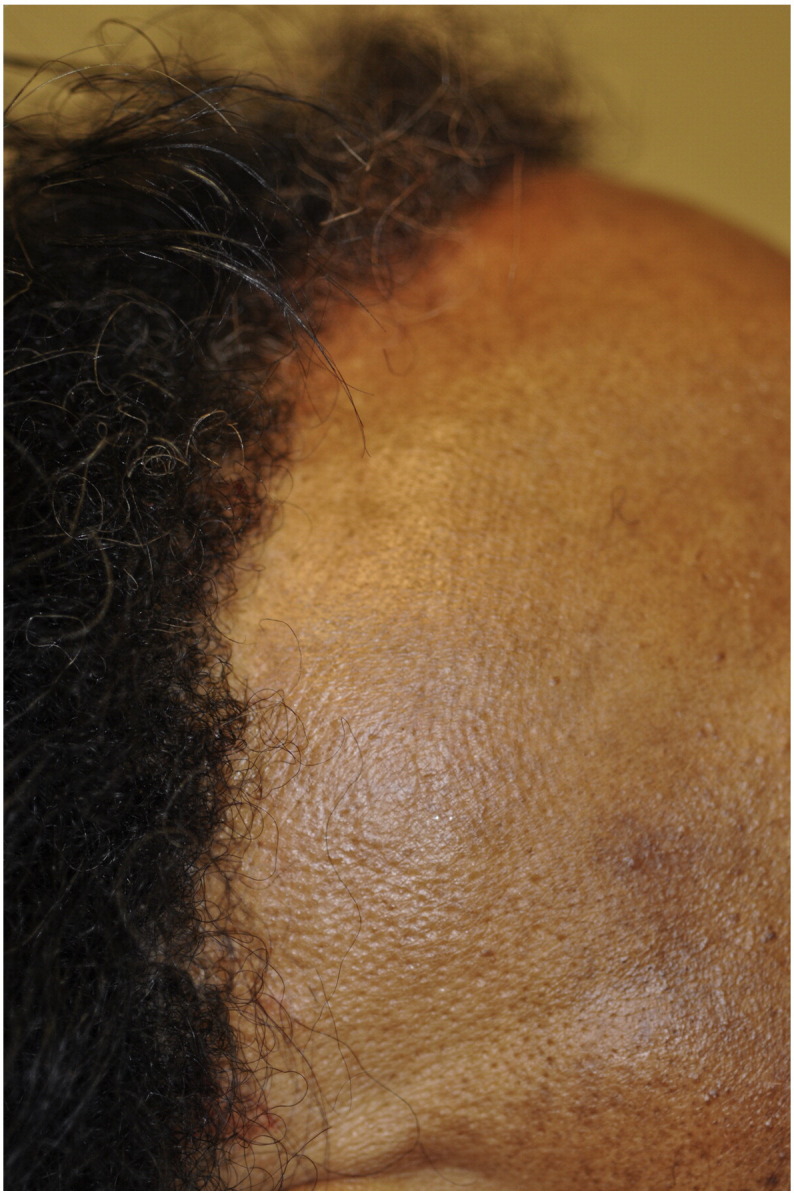
FFA in an African American female. Note the symmetrical band-like loss of hair on the frontotemporal hairline and scalp. (Courtesy of Valerie Callender, MD; Callender Dermatology & Cosmetic Center, Glenn Dale, MD).
An interesting clinical finding of FFA is termed the “lonely hair sign” (Tosti et al., 2011). There has been debate as to whether this sign is specific to FFA, as it is common to see hairs that are spared from the inflammatory processes in all types of cicatricial alopecias (Camacho, 2012). However, it is important to note that this term describes the presence of one or few isolated terminal hairs, particularly in the middle of the forehead; as FFA is the only cicatricial alopecia characterized by progressive recession of the frontotemporal hairline (Tosti, 2012). Another useful tool in the diagnosis of FFA is dermoscopy (Inui et al., 2008); which shows loss of follicular orifices and perifollicular erythema, a direct marker of FFA activity (Toledo-Pastrana et al., 2013).
Several disorders reported to possibly be associated with FFA include thyroid disease, vitiligo, lichen planus pigmentosus, lupus erythematosus (MacDonald et al., 2012, Miteva et al., 2011, Berliner et al., 2014, Del Rei et al., 2014, Khan et al., 2013, Dlova, 2013, Gaffney et al., 2013). Lichen planus pigmentosus has been identified as a herald sign for imminent FFA in black South African patients (Dlova, 2013). Because of the significant prevalence of hypothyroidism in FFA subjects in the their study, Vañó-Galván et al strongly suggest including thyroid hormone laboratory studies in the initial workup of patients with FFA (Vañó-Galván et al., 2014).
While there are presently no clinical trials for the management of FFA, several treatments have been reported. These include topical and intralesional corticosteroids, topical calcineurin inhibitors, hydroxychloroqine, mycophenolate mofetil and oral 5-alpha reductase inhibitors (5aRi) (Moreno-Ramirez and Camacho Martinez, 2005, Samrao et al., 2010b, Tan et al., 2004, Katoulis et al., 2009, Chiang et al., 2010, Cho et al., 2010). The efficacy of these treatment modalities are inconsistent. In patients who do not undergo any treatment, recession of the anterior hairline ranges from 0.95-1.08 cm per year (Toledo-Pastrana et al., 2013, Miteva and Tosti, 2012). Before initiating treatment, it is important that the patient understands that the main goal is to halt progression of hair loss. In reported cases of improvement, hair regrowth was minimal, and always located at the hairline.
In recently published reviews, oral finasteride or dutasteride showed good response in 44-47% (Vañó-Galván et al., 2014, Racz et al., 2013). This was followed by intralesional steroids and oral antimalarials. Therefore, it has been suggested that 5aRi may be a useful maintenance treatment to stabilize FFA (Ladizinski et al., 2013), and can be used together with intralesional corticosteroids when signs of activity such as perifollicular erythema or follicular hyperkeratosis are present (Vañó-Galván et al., 2014).
Conclusion
People of color constitute a large group that will continue to expand. This article has reviewed the most updated, evidence-based literature regarding the pathophysiology and treatment of disorders prevalent in women of color, particularly acne, melasma and alopecia. Important biological differences in skin and hair structure do exist in individuals of color and this may account for the increased incidence of keloids and pigmentary disorders. Prompt diagnosis and initiation of targeted therapy is imperative as these steps may prevent the potential sequelae common in women of color such as dyschromias and scarring.
In general, the pathogenesis and treatments for acne in women of color are similar to the general population; however, there are a few special considerations. Given the increased risk of PIH and keloid scarring in women with darker skin types, early aggressive reduction of inflammation is essential in treatment. Therefore, dermatologists should consider topical or oral agents with prominent anti-inflammatory effects to reduce acne-associated inflammation. In addition, in order to avoid exacerbation of PIH, physicians must be cautious with the selection of topical acne agents that are known to cause irritation. Sun protective measures, as well as effective skin lightening agents such as hydroquinone and azelaic acid, are important adjunctive therapies in the treatment of PIH.
The tendency for skin of color to develop PIH also limits the therapeutic modalities for melasma. Topical and aesthetic procedures are typically used in combination to achieve the best cosmetic outcome. While topical therapies remain the first choice in therapy, chemical peels and lasers are the second and third lines of management, respectively.
In women of color, particularly African Americans, a basic understanding of the diverse hair care practices is essential as many alopecias in this patient population may be associated with unique culturally-related hair styling methods. CCCA is the most common primary scarring alopecia in African American women. While topical and intralesional corticosteroids, oral anti-inflammatory agents and hair transplantation are potential treatments, patient education is paramount to halt progression of the disease process. As our understanding of these common dermatological disorders in women of color continue to unfold, advances in treatment are likely to develop.
Footnotes
This article has no funding source.
We declare the following conflicts of interest: Dr. Valerie Callender is a Consultant and Speaker for Allergan, Inc. The remaining authors do not have any conflicts of interest to disclose.
This article is a reprint of a previously published article. For citation purposes, please use the original publication details; International Journal of Women's Dermatology 1 (2015) 59-75. DOI of original item: 10.1016/j.ijwd.2015.04.002.
Appendix A. US Preventive Services Task Force levels of evidence for grading clinical trials (Sheth and Pandya, 2011b)
| Level of evidence | Quality of evidence |
|---|---|
| I | Evidence obtained from at least one properly designed, randomized controlled trial |
| II-i | Evidence obtained from well designed controlled trials without randomization |
| II-ii | Evidence obtained from well designed cohort or case control analytical studies, preferably from more than one center or research group |
| II-iii | Evidence obtained from multiple time series with or without the intervention; dramatic results in uncontrolled experiments could also be regarded as this type of evidence |
| III | Opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees |
| IV | Evidence inadequate because of problems of methodology (eg, sample size or length of comprehensiveness of follow-up or conflicts in evidence) |
| Strength of recommendations | |
|---|---|
| A | There is good evidence to support the use of the procedure |
| B | There is fair evidence to support the use of the procedure |
| C | There is poor evidence to support the use of the procedure |
| D | There is fair evidence to support the rejection of the use of the procedure |
| E | There is good evidence to support the rejection of the use of the procedure |
References
- Ackerman A.B., Walton N.W., Jones R.E., Charissi C. Hot comb alopecia: “follicular degeneration syndrome” in African-American women is traction alopecia! Dermatopathol Pract Concept. 2000;6:6–21. [Google Scholar]
- Ahdout J., Mirmirani P. Weft hair extensions causing a distinctive horseshoe pattern of traction alopecia. J Am Acad Dermatol. 2012;67:e294–e295. doi: 10.1016/j.jaad.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Alexis A.F., Sergay A.B., Taylor S.C. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387–394. [PubMed] [Google Scholar]
- Alexis A.F., Johnson L.A., Kerrouche N., Callender V.D. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170–174. [PubMed] [Google Scholar]
- Arellano I., Cestari T., Ocampo-Candiani J. Preventing melasma recurrence: prescribing a maintenance regimen with an effective triple combination cream based on long-lasting clinical severity. J Eur Acad Dermatol Venereol. 2012;26:611–618. doi: 10.1111/j.1468-3083.2011.04135.x. [DOI] [PubMed] [Google Scholar]
- Arora P., Sarkar R., Garg V.K., Arya L. Lasers for treatment of melasma and post-inflammatory hyperpigmentation. J Cutan Aesthet Surg. 2012;5:93–103. doi: 10.4103/0974-2077.99436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwa E., Khater M., Assaf M., Haleem M.A. Melasma treatment using an erbium:YAG laser: a clinical, immunohistochemical, and ultrastructural study. Int J Dermatol. 2014;54:235–244. doi: 10.1111/ijd.12477. [DOI] [PubMed] [Google Scholar]
- Babiarz-Magee L., Chen N., Seiberg M., Lin C.B. The expression and activation of protease-activated receptor-2 correlate with skin color. Pigment Cell Res. 2004;17:241–251. doi: 10.1111/j.1600-0749.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- Badreshia-Bansal S., Taylor S. The Structure and Function of Skin of Color. In: Kelly A.P., Taylor S.C., editors. Dermatology for skin of color. McGraw-Hill companies; New York, NY: 2009. pp. 71–77. [Google Scholar]
- Banka N., Mubki T., Bunagan M.J., McElwee K., Shapiro J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53:1324–1330. doi: 10.1111/ijd.12479. [DOI] [PubMed] [Google Scholar]
- Bansal C., Naik H., Kar H.K., Chauhan A. A Comparison of Low-Fluence 1064-nm Q-Switched Nd: YAG Laser with Topical 20% Azelaic Acid Cream and their Combination in Melasma in Indian Patients. J Cutan Aesthet Surg. 2012;5:266–272. doi: 10.4103/0974-2077.104915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner J.G., McCalmont T.H., Price V.H., Berger T.G. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:e26–e27. doi: 10.1016/j.jaad.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Bolduc C., Shapiro J. Hair care products: waving, straightening, conditioning, and coloring. Clin Dermatol. 2001;19:431–436. doi: 10.1016/s0738-081x(01)00201-2. [DOI] [PubMed] [Google Scholar]
- Boukari F., Jourdan E., Fontas E., Montaudié H., Castela E., Lacour J.P., Passeron T. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189–190. doi: 10.1016/j.jaad.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Callender V.D. African-American scalp disorders and treatment considerations. Skin Aging. 2002;10:12–14. [Google Scholar]
- Callender V.D. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184–195. doi: 10.1111/j.1396-0296.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- Callender V.D. Hair transplantation for pigmented skins. In: Halder R.M., editor. Dermatology and Dermatological Therapy of Pigmented Skins. Taylor & Francis; Boca Raton, FL: 2006. pp. 245–257. [Google Scholar]
- Callender V.D. Fitzpatrick Skin Types and Clindamycin Phosphate 1.2%/Benzoyl Peroxide Gel: Efficacy & Tolerability of Treatment in Moderate to Severe Acne. J Drugs Dermatol. 2012;11:643–648. [PubMed] [Google Scholar]
- Callender V.D., Onwudiwe O. Prevalence and etiology of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:972–974. doi: 10.1001/archdermatol.2011.205. [DOI] [PubMed] [Google Scholar]
- Callender V.D., Young C.M. Alopecias and hair restoration in women. In: Grimes P.E., Soriano T., Hexsel D.M., Kim J., editors. Aesthetics and Cosmetic Surgery for Darker Skin Types. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 287–295. [Google Scholar]
- Callender V.D., McMichael A.J., Cohen G.F. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164–176. doi: 10.1111/j.1396-0296.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- Callender V.D., Young C.M., Kindred C., Taylor S.C. Efficacy and safety of clindamycin phosphate 1.2% and tretinoin 0.025% gel for the treatment of acne and acne-induced post-inflammatory hyperpigmentation in patients with skin of color. J Clin Aesthet Dermatol. 2012;5:25–32. [PMC free article] [PubMed] [Google Scholar]
- Callender V.D., Wright D.R., Davis E.C., Sperling L.C. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047–1052. doi: 10.1001/archdermatol.2011.3428. [DOI] [PubMed] [Google Scholar]
- Callender V.D., Alexis A.F., Daniels S.R., Kawata A.K., Burk C.B., Wilcox T.K., Taylor S.C. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19–31. [PMC free article] [PubMed] [Google Scholar]
- Callender V.D., Lawson C.N., Onwudiwe O.C. Hair transplantation in the surgical treatment of central centrifugal cicatricial alopecia. Dermatol Surg. 2014;40:1125–1131. doi: 10.1097/DSS.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Camacho F.M. Lonely hair sign: not specific for frontal fibrosing alopecia. Arch Dermatol. 2012;148:1208–1209. doi: 10.1001/archdermatol.2012.1736. [DOI] [PubMed] [Google Scholar]
- Chan R., Park K.C., Lee M.H. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide, 0.01%, hydroquinone, 4%, tretinoin, 0.05%) compared with hydroquinone, 4% cream in Asian patients with moderate to severe melasma. Br J Dermatol. 2008;159:697–703. doi: 10.1111/j.1365-2133.2008.08717.x. [DOI] [PubMed] [Google Scholar]
- Chew A.L., Bashir S.J., Wain E.M. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63(4):653–660. doi: 10.1016/j.jaad.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Chiang C., Sah D., Cho B.K., Ochoa B.E., Price V.H. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62:387–392. doi: 10.1016/j.jaad.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Cho B.K., Sah D., Chwalek J., Roseborough I., Ochoa B., Chiang C., Price V.H. Efficacy and safety of mycophenolate mofetil for lichen planopilaris. J Am Acad Dermatol. 2010;62:393–397. doi: 10.1016/j.jaad.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Choi W., Kolbe L., Hearing V.J. Characterization of the bioactive motif of neuregulin-1, a fibroblast-derived paracrine factor that regulates the constitutive color and the function of melanocytes in human skin. Pigment Cell Melanoma Res. 2012;25:477–481. doi: 10.1111/j.1755-148X.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M., Kollias N. Documentation of normal stratum corneum scaling in an average population: Features of differences among age, ethnicity and body site. Br J Dermatol. 2011;164:497–507. doi: 10.1111/j.1365-2133.2010.10120.x. [DOI] [PubMed] [Google Scholar]
- Davis E.C., Callender V.D. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24–38. [PMC free article] [PubMed] [Google Scholar]
- Davis S.A., Narahari S., Feldman S.R., Huang W., Pichardo-Geisinger R.O., McMichael A.J. Top dermatologic conditions in patients of color: An analysis of nationally representative data. J Drugs Dermatol. 2012;11:466–473. [PubMed] [Google Scholar]
- Del Rei M., Pirmez R., Sodré C.T., Tosti A. Coexistence of frontal fibrosing alopecia and discoid lupus erythematosus of the scalp in 7 patients: just a coincidence? J Eur Acad Dermatol Venereol. 2014 doi: 10.1111/jdv.12642. [Published Jul 30, 2014, Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Detwiler S.P., Carson J.L., Woosley J.T., Gambling T.M., Briggaman R.A. Bubble hair: case caused by an overheating hair dryer and reproducibility in normal hair with heat. J Am Acad Dermatol. 1994;30:54–60. doi: 10.1016/s0190-9622(94)70008-7. [DOI] [PubMed] [Google Scholar]
- Dlova N.C. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439–442. doi: 10.1111/j.1365-2133.2012.11146.x. [DOI] [PubMed] [Google Scholar]
- Dlova N.C., Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(S1):17–20. doi: 10.1111/j.1365-4632.2012.05557.x. [20-3] [DOI] [PubMed] [Google Scholar]
- Dlova N.C., Goh C.L. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2013;54:81–83. doi: 10.1111/j.1365-4632.2012.05821.x. [DOI] [PubMed] [Google Scholar]
- Dlova N.C., Jordaan H.F., Skenjane A., Khoza N., Tosti A. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939–941. doi: 10.1111/bjd.12424. [DOI] [PubMed] [Google Scholar]
- Dlova N.C., Jordaan F.H., Sarig O., Sprecher E. Autosomal dominant inheritance of central centrifugal cicatricial alopecia in black South Africans. J Am Acad Dermatol. 2014;70:679–682. doi: 10.1016/j.jaad.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Draelos Z.D., Carter E., Maloney J.M., Elewski B., Poulin Y., Lynde C., Garrett S., United States/Canada Dapsone Gel Study Group Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1–439.e10. doi: 10.1016/j.jaad.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Fabi S.G., Friedmann D.P., Niwa Massaki A.B., Goldman M.P. A randomized, split-face clinical trial of low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1,064 nm) laser versus low-fluence Q-switched alexandrite laser (755 nm) for the treatment of facial melasma. Lasers Surg Med. 2014;46:531–537. doi: 10.1002/lsm.22263. [DOI] [PubMed] [Google Scholar]
- Faulkner C.F., Wilson N.J., Jones S.K. Frontal fibrosing alopecia associated with cutaneous lichen planus in a premenopausal woman. Australas J Dermatol. 2002;43:65–67. doi: 10.1046/j.1440-0960.2002.00558.x. [DOI] [PubMed] [Google Scholar]
- Fisk W.A., Lev-Tov H.A., Sivamani R.K. Epidemiology and Management of Acne in Adult Women. Curr Dermatol Rep. 2014;3:29–39. [Google Scholar]
- Fleischer A.B., Jr., Dinehart S., Stough D., Plott R.T., Solodyn Phase 2 Study Group. Solodyn Phase 3 Study Group Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78:21–31. [PubMed] [Google Scholar]
- Fox G.N., Stausmire J.M., Mehregan D.R. Traction folliculitis: an underreported entity. Cutis. 2007;79:26–30. [PubMed] [Google Scholar]
- Franbourg A., Hallegot P., Baltenneck F., Toutain C., Leroy F. Current research on ethnic hair. J Am Acad Dermatol. 2003;48:S115–S119. doi: 10.1067/mjd.2003.277. [DOI] [PubMed] [Google Scholar]
- Gaffney D.C., Sinclair R.D., Yong-Gee S. Discoid lupus alopecia complicated by frontal fibrosing alopecia on a background of androgenetic alopecia. Br J Dermatol. 2013;169:217–218. doi: 10.1111/bjd.12287. [DOI] [PubMed] [Google Scholar]
- Gathers R.C., Jankowski M., Eide M., Lim H.W. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574–578. doi: 10.1016/j.jaad.2008.10.064. [DOI] [PubMed] [Google Scholar]
- Geller L., Rosen J., Frankel A., Goldenberg G. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30–34. [PMC free article] [PubMed] [Google Scholar]
- Girardeau-Hubert S., Pageon H., Asselineau D. In vivo and in vitro approaches in understanding the differences between caucasian and african skin types: specific involvement of the papillary dermis. Int J Dermatol. 2012;51(S1):1–4. doi: 10.1111/j.1365-4632.2012.05553.x. [DOI] [PubMed] [Google Scholar]
- Gold M.H. Clinical evaluation of the safety and efficacy of a novel superficial and deep carbon dioxide fractional system in the treatment of patients with skin of color. J Drugs Dermatol. 2012;11:1331–1335. [PubMed] [Google Scholar]
- Goulden V., Stables G.I., Cunliffee W.J. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577–580. [PubMed] [Google Scholar]
- Grimes P.E. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453–1457. doi: 10.1001/archderm.131.12.1453. [DOI] [PubMed] [Google Scholar]
- Grimes P.E. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659–665. doi: 10.1016/s0733-8635(05)70217-5. [DOI] [PubMed] [Google Scholar]
- Grimes P.E. Novel skin brightener used as monotherapy for moderate melasma in skin of color. J Drugs Dermatol. 2014;13:364–366. [PubMed] [Google Scholar]
- Grimes P.E., Callender V.D. Tazarotene Cream for Postinflammatory Hyperpigmentation and Acne Vulgaris in Darker Skin: A Double-Blind, Randomized, Vehicle-Controlled Study. Cutis. 2006;77:45–50. [PubMed] [Google Scholar]
- Halder R.M., Grimes P.E., McLaurin C.L., Kress M.A., Kenney J.A. Incidence of common dermatoses in a predominantly black dermatology practice. Cutis. 1983;32:388–390. [PubMed] [Google Scholar]
- Halder R.M., Grimes P.E., McLaurin C.I., Kress M.A., Kenney J.A. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388–390. [PubMed] [Google Scholar]
- Halder R.M., Holmes Y.C., Bridgeman-Shah S., Kligman A.M. A clinicohistopathologic study of acne vulgaris in black females. J Invest Dermatol. 1996;106:888. [Google Scholar]
- Hall R.R., Francis S., Whitt-Glover M., Loftin-Bell K., Swett K., McMichael A.J. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310–314. doi: 10.1001/jamadermatol.2013.1946. [DOI] [PubMed] [Google Scholar]
- Heath C.R., Taylor S.C. Alopecia in an ophiasis pattern: traction alopecia versus alopecia areata. Cutis. 2012;89:213–216. [PubMed] [Google Scholar]
- Hilton S., Heise H., Buhren B.A., Schrumpf H., Bölke E., Gerber P.A. Treatment of melasma in Caucasian patients using a novel 694-nm Q-switched ruby fractional laser. Eur J Med Res. 2013;18:43. doi: 10.1186/2047-783X-18-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S., Nakajima T., Shono F., Itami S. Dermoscopic findings in frontal fibrosing alopecia: report of four cases. Int J Dermatol. 2008;47:796–799. doi: 10.1111/j.1365-4632.2008.03681.x. [DOI] [PubMed] [Google Scholar]
- Jalaly N.Y., Valizadeh N., Barikbin B., Yousefi M. Low-power fractional CO2 laser versus low-fluence Q-switch 1,064 nm Nd:YAG laser for treatment of melasma: a randomized, controlled, split-face study. Am J Clin Dermatol. 2014;15:357–363. doi: 10.1007/s40257-014-0080-x. [DOI] [PubMed] [Google Scholar]
- James J., Saladi R.N., Fox J.L. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497–498. doi: 10.3122/jabfm.2007.05.070076. [DOI] [PubMed] [Google Scholar]
- Jang W.S., Lee C.K., Kim B.J., Kim M.N. Efficacy of 694-nm Q-switched ruby fractional laser treatment of melasma in female Korean patients. Dermatol Surg. 2011;37:1133–1140. doi: 10.1111/j.1524-4725.2011.02018.x. [DOI] [PubMed] [Google Scholar]
- Ji J.H., Park T.S., Lee H.J., Kim Y.D., Pi L.Q., Jin X.H., Lee W.S. The ethnic differences of the damage of hair and integral hair lipid after ultra violet radiation. Ann Dermatol. 2013;25:54–60. doi: 10.5021/ad.2013.25.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungersted J.M., Hogh J.K., Hellgren L.I., Jemec G.B., Agner T. Ethnicity and stratum corneum ceramides. Br J Dermatol. 2010;163:1169–1173. doi: 10.1111/j.1365-2133.2010.10080.x. [DOI] [PubMed] [Google Scholar]
- Kamangar F., Shinkai K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012;51:1162–1174. doi: 10.1111/j.1365-4632.2012.05519.x. [DOI] [PubMed] [Google Scholar]
- Karnik J., Baumann L., Bruce S., Callender V., Cohen S., Grimes P., Joseph J., Shamban A., Spencer J., Teldaldi R., Werschler W., Smith S. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77–83. doi: 10.1016/j.jaad.2014.02.034. [DOI] [PubMed] [Google Scholar]
- Katoulis A., Georgala S., Bozi E., Papadavid E., Kalogeromitros D., Stavrianeas N. Frontal fibrosing alopecia: treatment with oral dutasteride and topical pimecrolimus. J Eur Acad Dermatol Venereol. 2009;23:580–582. doi: 10.1111/j.1468-3083.2008.02963.x. [DOI] [PubMed] [Google Scholar]
- Kelly A.P., Sampson D.D. Recalcitrant nodulocystic acne in black Americans: treatment with isotretinoin. J Natl Med Assoc. 1987;79:1266–1270. [PMC free article] [PubMed] [Google Scholar]
- Khan S., Fenton D.A., Stefanato C.M. Frontal fibrosing alopecia and lupus overlap in a man: guilt by association? Int J Trichol. 2013;5:217–219. doi: 10.4103/0974-7753.130420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khumalo N.P., Ngwanya R.M. Traction alopecia: 2% topical minoxidil shows promise. Report of two cases. J Eur Acad Dermatol Venereol. 2007;21:433–434. doi: 10.1111/j.1468-3083.2006.01933.x. [DOI] [PubMed] [Google Scholar]
- Khumalo N.P., Jessop S., Gumedze F., Ehrlich R. Hairdressing and the prevalence of scalp disease in African adults. Br J Dermatol. 2007;157:981–988. doi: 10.1111/j.1365-2133.2007.08146.x. [DOI] [PubMed] [Google Scholar]
- Khumalo N.P., Jessop S., Gumedze F., Ehrlich R. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106–110. doi: 10.1111/j.1365-2133.2007.07987.x. [DOI] [PubMed] [Google Scholar]
- Khumalo N.P., Jessop S., Gumedze F., Ehrlich R. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432–438. doi: 10.1016/j.jaad.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Khumalo N.P., Stone J., Gumedze F., McGrath E., Ngwanya M.R., de Berker D. 'Relaxers' damage hair: evidence from amino acid analysis. J Am Acad Dermatol. 2010;62:402–408. doi: 10.1016/j.jaad.2009.04.061. [DOI] [PubMed] [Google Scholar]
- Khunger N., Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78:335–341. doi: 10.4103/0378-6323.95450. [DOI] [PubMed] [Google Scholar]
- Khunger N., Bhardwaj D., Khunger M. Evaluation of CROSS technique with 100% TCA in the management of ice pick acne scars in darker skin types. J Cosmet Dermatol. 2011;10:51–57. doi: 10.1111/j.1473-2165.2010.00526.x. [DOI] [PubMed] [Google Scholar]
- Kircik L.H. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;9:1407–1411. [PubMed] [Google Scholar]
- Kircik L.H. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586–590. [PubMed] [Google Scholar]
- Kligman A.M. Solar elastosis in relation to pigmentation. In: Fitzpatrick T.B., Pathak M.A., Harber L.C., Seiji M., Kukita A., editors. Sunlight and Man: Normal and Abnormal Photobiologic Response. University of Tokyo Press; Tokyo: 1994. pp. 157–163. [Google Scholar]
- Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770–774. [Erratum in: Arch Dermatol. 1994;130:1407] [PubMed] [Google Scholar]
- Kossard S., Shiell R.C. Frontal fibrosing alopecia developing after hair transplantation for androgenetic alopecia. Int J Dermatol. 2005;44:321–325. doi: 10.1111/j.1365-4632.2004.02251.x. [DOI] [PubMed] [Google Scholar]
- Kossard S., Lee M.S., Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59–66. doi: 10.1016/s0190-9622(97)70326-8. [DOI] [PubMed] [Google Scholar]
- Kyei A., Bergfeld W.F., Piliang M., Summers P. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909–914. doi: 10.1001/archdermatol.2011.66. [DOI] [PubMed] [Google Scholar]
- Laatsch C.N., Durbin-Johnson B.P., Rocke D.M., Mukwana S., Newland A.B., Flagler M.J., Davis M.G., Eigenheer R.A., Phinney B.S., Rice R.H. Human hair shaft proteomic profiling: Individual differences, site specificity and cuticle analysis. PeerJ. 2014;2:e506. doi: 10.7717/peerj.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladizinski B., Bazakas A., Selim M.A., Olsen E.A. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749–755. doi: 10.1016/j.jaad.2012.09.043. [DOI] [PubMed] [Google Scholar]
- La Ruche G., Cesarini J. Histology and physiology of black skin. Ann Dermatol Venerol. 1992;119:567–574. [PubMed] [Google Scholar]
- Lee W.S., Oh T.H., Chun S.H., Jeon S.Y., Lee E.Y., Lee S., Park W.S., Hwang S. Integral lipid in human hair follicle. J Investig Dermatol Symp Proc. 2005;10:234–237. doi: 10.1111/j.0022-202X.2005.10113.x. [DOI] [PubMed] [Google Scholar]
- Lim J.T. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol Surg. 1999;25:282–284. doi: 10.1046/j.1524-4725.1999.08236.x. [DOI] [PubMed] [Google Scholar]
- Lindelof B., Forslind B., Hedblad M. Human hair form morphology revealed by light and scanning electron microscopy and computer aided three-dimensional reconstruction. Arch Dermatol. 1998;124:1359–1362. [PubMed] [Google Scholar]
- LoPresti P., Papa C.M., Kligman A.M. Hot comb alopecia. Arch Dermatol. 1968;98:234–238. [PubMed] [Google Scholar]
- Lurie R., Hodak E., Ginzburg A., David M. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358–359. [PubMed] [Google Scholar]
- MacDonald A., Clark C., Holmes S. Frontal fibrosing alopecia: A review of 60 cases. J Am Acad Dermatol. 2012;67:955–961. doi: 10.1016/j.jaad.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Makino E.T., Mehta R.C., Garruto J., Gotz V., Sigler M.L., Herndon J.H. Clinical efficacy and safety of a multimodality skin brightener composition compared with 4% hydroquinone. J Drugs Dermatol. 2013;12:s21–s26. [PubMed] [Google Scholar]
- Manaloto R.M., Alster T. Erbium:YAG laser resurfacing for refractory melasma. Dermatol Surg. 1999;25:121–123. doi: 10.1046/j.1524-4725.1999.08103.x. [DOI] [PubMed] [Google Scholar]
- Martin A.M., Sugathan P. Localised acquired trichorrhexis nodosa of the scalp hair induced by a specific comb and combing habit - a report of three cases. Int J Trichol. 2011;3:34–37. doi: 10.4103/0974-7753.82138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Lagrán Z., González-Hermosa M.R., Díaz-Pérez J.L. Localized trichorrhexis nodosa. Actas Dermosifiliogr. 2009;100:522–524. [PubMed] [Google Scholar]
- McMichael A.J. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48:S127–S133. doi: 10.1067/mjd.2003.278. [DOI] [PubMed] [Google Scholar]
- Mirmirani P. Ceramic flat irons: improper use leading to acquired trichorrhexis nodosa. J Am Acad Dermatol. 2010;62:145–147. doi: 10.1016/j.jaad.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Mirmirani P., Khumalo N.P. Traction alopecia: how to translate study data for public education–closing the KAP gap? Dermatol Clin. 2014;32:153–161. doi: 10.1016/j.det.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Miteva M., Tosti A. The follicular triad: a pathological clue to the diagnosis of early frontal fibrosing alopecia. Br J Dermatol. 2012;166:440–442. doi: 10.1111/j.1365-2133.2011.10533.x. [DOI] [PubMed] [Google Scholar]
- Miteva M., Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013;27:1299–1303. doi: 10.1111/j.1468-3083.2012.04530.x. [DOI] [PubMed] [Google Scholar]
- Miteva M., Tosti A. Dermatoscopic features of central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2014;71:443–449. doi: 10.1016/j.jaad.2014.04.069. [DOI] [PubMed] [Google Scholar]
- Miteva M., Aber C., Torres F., Tosti A. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445–447. doi: 10.1111/j.1365-2133.2011.10382.x. [DOI] [PubMed] [Google Scholar]
- Miteva M., Whiting D., Harries M., Bernardes A., Tosti A. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208–210. doi: 10.1111/j.1365-2133.2012.10809.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Tsuboi R., Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109–110. doi: 10.1111/j.1346-8138.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- Molina L., Doche I. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147(12):1424–1427. doi: 10.1001/archdermatol.2011.321. [DOI] [PubMed] [Google Scholar]
- Montagna W., Carlisle K. The architecture of black and white facial skin. J Am Acad Dermatol. 1991;24:929–937. doi: 10.1016/0190-9622(91)70148-u. [DOI] [PubMed] [Google Scholar]
- Moreno-Ramirez D., Camacho Martinez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700–705. doi: 10.1111/j.1468-3083.2005.01291.x. [DOI] [PubMed] [Google Scholar]
- Mouton R.W., Jordaan H.F., Schneider J.W. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8–14. doi: 10.1111/j.1365-2230.2004.01421.x. [DOI] [PubMed] [Google Scholar]
- Muizzuddin N., Hellemans L., Van Overloop L., Corstjens H., Declercq L., Maes D. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. J Dermatol Sci. 2010;59:123–128. doi: 10.1016/j.jdermsci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Mulinari-Brenner F., Bregfeld F. Hair loss: an overview. Dermatol Nurs. 2001;13:269–278. [PubMed] [Google Scholar]
- Nanda S., Grover C., Reddy B.S. Efficacy of hydroquinone (2%) versus tretinoin (0.025%) as adjunct topical agents for chemical peeling in patients of melasma. Dermatol Surg. 2004;30:385–388. doi: 10.1111/j.1524-4725.2004.30106.x. [DOI] [PubMed] [Google Scholar]
- Neeley M.R., Pearce F.B., Collawn S.S. Successful treatment of malar dermal melasma with a fractional ablative CO2 laser in a patient with type V skin. J Cosmet Laser Ther. 2010;12:258–260. doi: 10.3109/14764172.2010.538412. [DOI] [PubMed] [Google Scholar]
- Nicholson A.G., Harland C.C., Bull R.H., Mortimer P.S., Cook M.G. Chemically induced cosmetic alopecia. Br J Dermatol. 1993;128:537–541. doi: 10.1111/j.1365-2133.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Nnoruka E.N. Hair loss: is there a relationship with hair care practices in Nigeria? Int J Dermatol. 2005;44(S1):13–17. doi: 10.1111/j.1365-4632.2005.02801.x. [DOI] [PubMed] [Google Scholar]
- Nusbaum B.P., Nusbaum A.G. Frontal fibrosing alopecia in a man: results of follicular unit test grafting. Dermatol Surg. 2010;36:959–962. doi: 10.1111/j.1524-4725.2010.01580.x. [DOI] [PubMed] [Google Scholar]
- Ogunbiyi A., Ogun O., Enechukwu N. Recurrent hair loss resulting from generalized proximal trichorrhexis nodosa in a nigerian female. Int J Trichol. 2014;6:83–84. doi: 10.4103/0974-7753.138599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunleye T.A., McMichael A., Olsen E.A. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173–181. doi: 10.1016/j.det.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Olsen E.A., Bergfeld W.F., Cotsarelis G., Price V.H., Shapiro J., Sinclair R., Solomon A., Sperling L., Stenn K., Whiting D.A., Bernado O., Bettencourt M., Bolduc C., Callender V., Elston D., Hickman J., Ioffreda M., King L., Linzon C., McMichael A., Miller J., Mulinari F., Trancik R., Members of the Workshop on Cicatricial Alopecia Summary of North American Hair Research Society (NAHRS)-sponsored Work- Shop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48(1):103–110. doi: 10.1067/mjd.2003.68. [DOI] [PubMed] [Google Scholar]
- Olsen E.A., Callender V.D., McMichael A., Sperling L., Anstrom K.J., Shapiro J., Roberts J., Durden F., Whiting D., Bergfeld W. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245–252. doi: 10.1016/j.jaad.2009.11.693. [DOI] [PubMed] [Google Scholar]
- Ozcelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthet Plast Surg. 2005;29:325–327. doi: 10.1007/s00266-005-0004-5. [DOI] [PubMed] [Google Scholar]
- Passeron T., Fontas E., Kang H.Y., Bahadoran P., Lacour J.P., Ortonne J.P. Melasma treatment with pulsed-dye laser and triple combination cream: A prospective, randomized, single-blind, split-face study. Arch Dermatol. 2011;147:1106–1108. doi: 10.1001/archdermatol.2011.255. [DOI] [PubMed] [Google Scholar]
- Perkins A.C., Cheng C.E., Hillebrand G.G., Miyamoto K., Kimball A.B. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054–1060. doi: 10.1111/j.1468-3083.2010.03919.x. [DOI] [PubMed] [Google Scholar]
- Piette W.W., Taylor S., Pariser D., Jarratt M., Sheth P., Wilson D. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564–1570. doi: 10.1001/archdermatol.2008.518. [DOI] [PubMed] [Google Scholar]
- Pirmez R., Donati A., Valente N.S., Sodré C.T., Tosti A. Glabellar red dots in frontal fibrosing alopecia: a further clinical sign of vellus follicle involvement. Br J Dermatol. 2014;170:745–746. doi: 10.1111/bjd.12683. [DOI] [PubMed] [Google Scholar]
- Polder K.D., Bruce S. Treatment of melasma using a novel 1,927-nm fractional thulium fiber laser: a pilot study. Dermatol Surg. 2012;38:199–206. doi: 10.1111/j.1524-4725.2011.02178.x. [DOI] [PubMed] [Google Scholar]
- Quinn C.R., Quinn T.M., Kelly A.P. Hair care practices in African American women. Cutis. 2003;72:280–282. [PubMed] [Google Scholar]
- Racz E., Gho C., Moorman P.W., Noordhoek Hegt V., Neumann H.A. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461–1470. doi: 10.1111/jdv.12139. [DOI] [PubMed] [Google Scholar]
- Ramaswamy P., Mendese G., Goldberg L.J. Scarring alopecia of the sideburns: a unique presentation of frontal fibrosing alopecia in men. Arch Dermatol. 2012;148:1095–1096. doi: 10.1001/archdermatol.2012.848. [DOI] [PubMed] [Google Scholar]
- Rivas S., Pandya A. Treatment of melasma with topical agents, peels and lasers: An evidence-based review. Am J Clin Dermatol. 2013;14:359–376. doi: 10.1007/s40257-013-0038-4. [DOI] [PubMed] [Google Scholar]
- Rollins T.G. Traction folliculitis with hair casts and alopecia. Am J Dis Child. 1961;101:639–640. doi: 10.1001/archpedi.1961.04020060097013. [DOI] [PubMed] [Google Scholar]
- Rudnicka L., Rakowska A., Kerzeja M., Olszewska M. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013;31:695–708. doi: 10.1016/j.det.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Ruetsch S.B., Kamath Y.K. Effects of thermal treatments with a curling iron on hair fiber. J Cosmet Sci. 2004;55:13–27. [PubMed] [Google Scholar]
- Rusciani A., Motta A., Rusciani L., Alfano C. Q-switched alexandrite laser-assisted treatment of melasma: 2-year follow-up monitoring. J Drugs Dermatol. 2005;4:770–774. [PubMed] [Google Scholar]
- Salam A., Dadzie O.E., Galadari H. Chemical peeling in ethnic skin: an update. Br J Dermatol. 2013;169(S3):82–90. doi: 10.1111/bjd.12535. [DOI] [PubMed] [Google Scholar]
- Samrao A., Chen C., Zedek D., Price V.H. Traction alopecia in a ballerina: clincopathologic features. Arch Dermatol. 2010;146:930–931. doi: 10.1001/archdermatol.2010.183. [DOI] [PubMed] [Google Scholar]
- Samrao A., Chew A.L., Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296–1300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- Samrao A., Price V.H., Zedek D., Mirmirani P. The "Fringe Sign" - A useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1. [PubMed] [Google Scholar]
- Sardana K., Garg V.K. Lasers are not effective for melasma in darkly pigmented skin. J Cutan Aesthet Surg. 2014;7:57–60. doi: 10.4103/0974-2077.129985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai V.R., Kolte J.N., Srinivas B.N. A clinical study of melasma and a comparison of the therapeutic effect of certain currently available topical modalities for its treatment. Indian J Dermatol. 2013;58:239. doi: 10.4103/0019-5154.110842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R., Bansal S., Garg V.K. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247–253. doi: 10.4103/0974-2077.104912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savory S.A., Agim N.G., Mao R., Peter S., Wang C., Maldonado G., Dietert J.B., Lieu T.J., Wang C., Pretzlaff K., Das S., Vandergriff T., Lopez I.E., Litzner B.R., Hynan L.S., Arellano-Mendoza M.I., Bergstresser P.R., Pandya A.G. Reliability assessment and validation of the postacne hyperpigmentation index (PAHPI), a new instrument to measure postinflammatory hyperpigmentation from acne vulgaris. J Am Acad Dermatol. 2014;70:108–114. doi: 10.1016/j.jaad.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Semchyshyn N., Prodanovic E., Varade R. Treating acne scars in patients with Fitzpatrick skin types IV to VI using the 1450-nm diode laser. Cutis. 2013;92:49–53. [PubMed] [Google Scholar]
- Shah S.K., Alexis A.F. Acne in skin of color: practical approaches to treatment. J Dermatolog Treat. 2010;21:206–211. doi: 10.3109/09546630903401496. [DOI] [PubMed] [Google Scholar]
- Sheth V.M., Pandya A.G. Melasma: a comprehensive update: part I. J Am Acad Dermatol. 2011;65:689–697. doi: 10.1016/j.jaad.2010.12.046. [DOI] [PubMed] [Google Scholar]
- Sheth V.M., Pandya A.G. Melasma: a comprehensive update: part II. J Am Acad Dermatol. 2011;65:699–714. doi: 10.1016/j.jaad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Sim J.H., Park Y.L., Lee J.S., Lee S.Y., Choi W.B., Kim H.J., Lee J.H. Treatment of melasma by low-fluence 1064 nm Q-switched Nd:YAG laser. J Dermatolog Treat. 2014;25:212–217. doi: 10.3109/09546634.2012.735639. [DOI] [PubMed] [Google Scholar]
- Soung J., Cohen J., Phelps R., Cohen S. Case reports: minocycline-induced hyperpigmentation resolves during oral isotretinoin therapy. J Drugs Dermatol. 2007;6:1232–1236. [PubMed] [Google Scholar]
- Sperling L.C., Sau P. The follicular degeneration syndrome in black patients: ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68–74. [PubMed] [Google Scholar]
- Stockmeier M., Kunte C., Sander C.A., Wolff H. Kossard frontal fibrosing alopecia in a man. Hautarzt. 2002;53:409–411. doi: 10.1007/s001050100273. [DOI] [PubMed] [Google Scholar]
- Summers P., Kyei A., Bergfeld W. Central centrifugal cicatricial alopecia - an approach to diagnosis and management. Int J Dermatol. 2011;50:1457–1464. doi: 10.1111/j.1365-4632.2011.05098.x. [DOI] [PubMed] [Google Scholar]
- Swee W., Klontz K.C., Lambert L.A. A nationwide outbreak of alopecia associated with the use of a hair-relaxing formulation. Arch Dermatol. 2000;136:1104–1108. doi: 10.1001/archderm.136.9.1104. [DOI] [PubMed] [Google Scholar]
- Tan K.T., Messenger A.G. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75–79. doi: 10.1111/j.1365-2133.2008.08861.x. [DOI] [PubMed] [Google Scholar]
- Tan E., Martinka M., Ball N., Shapiro J. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25–32. doi: 10.1016/j.jaad.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Tan J., Humphrey S., Vender R., Barankin B., Gooderham M., Kerrouche N., Audibert F., Lynde C., POWER study group A treatment for severe nodular acne: a randomized investigator-blinded, controlled, noninferiority trial comparing fixed-dose adapalene/benzoyl peroxide plus doxycycline vs. oral isotretinoin. Br J Dermatol. 2014;171:1508–1516. doi: 10.1111/bjd.13191. [DOI] [PubMed] [Google Scholar]
- Tanghetti E.A., Kawata A.K., Daniels S.R., Yeomans K., Burk C.T., Callender V.D. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22–30. [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C. Skin of color: Biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46:S41–S62. doi: 10.1067/mjd.2002.120790. [DOI] [PubMed] [Google Scholar]
- Taylor S.C. Epidemiology of skin diseases in people of color. Cutis. 2003;71:271–275. [PubMed] [Google Scholar]
- Taylor S., Summers P. Acne. In: Kelly A.P., Taylor S.C., editors. Dermatology for skin of color. McGraw-Hill Companies; New York, NY: 2009. pp. 269–274. [Google Scholar]
- Taylor S.C., Torok H., Jones T., Lowe N., Rich P., Tschen E. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67–72. [PubMed] [Google Scholar]
- Taylor S., Badreshia-Bansal S., Callender V., Gathers R. Saunders; Edinburgh: 2011. Treatments for Skin of Color. [Google Scholar]
- Tirado-Sanchez A., Espindola Y.S., Ponce-Olivera R.M., Bonifaz A. Efficacy and safety of adapalene gel 0.1% and 0.3% and tretinoin gel 0.05% for acne vulgaris: results of a single-center, randomized, double-blinded, placebo-controlled clinical trial on Mexican patients (skin type III-IV) J Cosmet Dermatol. 2013;12:103–107. doi: 10.1111/jocd.12031. [DOI] [PubMed] [Google Scholar]
- Toledo-Pastrana T., Hernández M.J., Camacho Martínez F.M. Perifollicular erythema as a trichoscopy sign of progression in frontal fibrosing alopecia. Int J Trichol. 2013;5:151–153. doi: 10.4103/0974-7753.125616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok H.M. A comprehensive review of the long-term and short-term treatment of melasma with a triple combination cream. Am J Clin Dermatol. 2006;7:223–230. doi: 10.2165/00128071-200607040-00003. [DOI] [PubMed] [Google Scholar]
- Torok H.M., Jones T., Rich P. Hydroquinone, 4%, tretinoin, 0.05%, fluocinolone acetonide, 0.01%: A safe and efficacious 12-month treatment for Melasma. Cutis. 2005;75:57–62. [PubMed] [Google Scholar]
- Torok H., Taylor S., Baumann L., Jones T., Wieder J., Lowe N., Jarret M., Rich P., Pariser D., Tschen E., Martin D., Menter A., Weiss J. A large 12-month extension study of an 8-week trial to evaluate the safety and efficacy of triple combination (TC) cream in melasma patients previously treated with TC cream or one of its dyads. J Drugs Dermatol. 2005;4:592–597. [PubMed] [Google Scholar]
- Tosti A. Lonely hair sign: not specific for frontal fibrosing alopecia-reply. Arch Dermatol. 2012;148:1208–1209. doi: 10.1001/archdermatol.2012.1873. [DOI] [PubMed] [Google Scholar]
- Tosti A., Miteva M., Torres F., Vincenzi C., Romanelli P. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353–1355. doi: 10.1111/j.1365-2133.2010.09979.x. [DOI] [PubMed] [Google Scholar]
- Tosti A., Miteva M., Torres F. Lonely hair: a clue to the diagnosis of frontal fibrosing alopecia. Arch Dermatol. 2011;147:1240. doi: 10.1001/archdermatol.2011.261. [DOI] [PubMed] [Google Scholar]
- Trueb R.M. "Chignon alopecia": a distinctive type of nonmarginal traction alopecia. Cutis. 1995;55:178–179. [PubMed] [Google Scholar]
- Ullah G., Noor S.M., Bhatti Z., Ahmad M., Bangash A.R. Comparison of oral azithromycin with oral doxycycline in the treatment of acne vulgaris. Br J Dermatol. 2014;171:1508–1516. [PubMed] [Google Scholar]
- US Census Bureau Overview of Race and Hispanic Origin. 2010. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf Available at: [Accessed October 13, 2014]
- Vañó-Galván S., Molina-Ruiz A.M., Serrano-Falcón C., Arias-Santiago S., Rodrigues-Barata A.R., Garnacho-Saucedo G., Martorell-Calatayud A., Fernández-Crehuet P., Grimalt R., Aranegui B., Grillo E., Diaz-Ley B., Salido R., Pérez-Gala S., Serrano S., Moreno J.C., Jaén P., Camacho F.M. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670–678. doi: 10.1016/j.jaad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Kavanagh R., Kadekaro A.L., Terzieva S., Sturm R.A., Leachman S., Abdel-Malek Z., Ito S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006;19:154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Wang C.C., Hui C.Y., Sue Y.M., Wong W.R., Hong H.S. Intense pulsed light for the treatment of refractory melasma in Asian persons. Dermatol Surg. 2004;30:1196–1200. doi: 10.1111/j.1524-4725.2004.30371.x. [DOI] [PubMed] [Google Scholar]
- Warrier A.G., Kligman A.M., Harper R.A., Bowman J., Wickett R.R. A comparison of black and white skin using non-invasive methods. J Soc Cosmet Chem. 1996;47:229–240. [Google Scholar]
- Wattanakrai P., Mornchan R., Eimpunth S. Low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1,064 nm) laser for the treatment of facial melasma in Asians. Dermatol Surg. 2010;36:76–87. doi: 10.1111/j.1524-4725.2009.01383.x. [DOI] [PubMed] [Google Scholar]
- Wattanakrai P., Mornchan R., Eimputh S. Low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1,064 nm) laser for the treatment of facial melasma in Asians. Dermatol Surg. 2012;36:76–87. doi: 10.1111/j.1524-4725.2009.01383.x. [DOI] [PubMed] [Google Scholar]
- Weigand D., Haygood C., Gaylor J. Cell layers and density of Negro and Caucasian stratum corneum. J Invest Dermatol. 1974;62:563–568. doi: 10.1111/1523-1747.ep12679412. [DOI] [PubMed] [Google Scholar]
- Wind B.S., Kroon M.W., Meesters A.A., Beek J.F., van der Veen J.P., Nieuweboer-Krobotová L., Bos J.D., Wolkerstorfer A. Non-ablative 1,550 nm fractional laser therapy versus triple topical therapy for the treatment of melasma: a randomized controlled split-face study. Lasers Surg Med. 2010;42:607–612. doi: 10.1002/lsm.20937. [DOI] [PubMed] [Google Scholar]
- Yentzer B.A., Hick J., Reese E.L., Uhas A., Feldman S.R., Balkrishnan R. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86:94–99. [PubMed] [Google Scholar]
- Yin N.C., McMichael A.J. Acne in patients with skin of color: practical management. Am J Clin Dermatol. 2014;15:7–16. doi: 10.1007/s40257-013-0049-1. [DOI] [PubMed] [Google Scholar]
- Yoshida-Amano Y., Hachiya A., Ohuchi A., Kobinger G.P., Kitahara T., Takema Y., Fukuda M. Essential role of RAB27A in determining constitutive human skin color. PLoS One. 2012;7:e41160. doi: 10.1371/journal.pone.0041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleski L., Fabi S., Goldman M.P. Treatment of melasma and the use of intense pulsed light: a review. J Drugs Dermatol. 2012;11:1316–1320. [PubMed] [Google Scholar]