Abstract
Objectives: The aim of this study was to evaluate the effect of the supine, left lateral decubitus, and right lateral decubitus positions on autonomic nervous activity in elderly adults by using spectral analysis of heart rate variability (HRV). Method: Forty-five adults aged 73.6 ± 5.7 years were enrolled. After lying in the supine position, all participants moved to the lateral decubitus positions in a random order and maintained the positions for 10 min, while electrocardiographic data were recorded to measure HRV. Results: The lowest heart rate continued for 10 min when participants were in the left lateral decubitus position compared with the other two positions (p < .001), while the HRV indexes remained unchanged. The low-frequency HRV to high-frequency HRV ratio (LF/HF) for the right lateral decubitus position was significantly lower than that for the other positions. Discussion: The right lateral decubitus position may attenuate sympathetic nerve activity in elderly adults.
Keywords: elderly, autonomic nervous activity, lateral decubitus position, heart rate, heart rate variability
Introduction
The autonomic nervous system has an important role in controlling blood pressure (BP) and cardiac output by adjusting heart rate (HR), myocardial contractility, and systemic vascular resistance to maintain cardiovascular homeostasis in response to the internal or external environment (Kaye & Esler, 2008). It is well known that aging produces progressive attenuation of autonomic nervous activity, consisting of parasympathetic and sympathetic activities (T. B. Kuo et al., 1999; Liao et al., 1995; Moodithaya & Avadhany, 2012). In particular, parasympathetic nerve activity, as well as arterial baroreflex sensitivity, reduces with age (Almeida-Santos et al., 2016; Monahan, 2007), while basal plasma norepinephrine levels increase with age (Pfeifer et al., 1983). Based on these findings, sympathetic nervous activity is probably elevated in elderly adults, which leads to an increased incidence of cardiovascular disease, stroke, essential hypertension, and arrhythmia (Kaye et al., 1994; Lloyd-Jones et al., 2004; Meredith, Broughton, Jennings, & Esler, 1991; Schlaich et al., 2004). In fact, about 80% of mortality in individuals aged above 65 years is due to cardiovascular diseases (Karavidas, Lazaros, Tsiachris, & Pyrgakis, 2010).
Aging is also associated with the laterality of sleeping positions, and it has been reported that elderly adults tend to sleep in the right lateral decubitus position (De Koninck, Lorrain, & Gagnon, 1992). In elderly patients with coronary artery disease, higher parasympathetic nerve activity and lower sympathetic nerve activity have been observed in the right lateral decubitus position compared with the supine and left lateral decubitus positions, which may contribute to attenuated parasympathetic nerve activity and the predominance of sympathetic nerve activity in those patients (Fujita, Miyamoto, Tambara, & Budgell, 2002; C. D. Kuo, Chen, & Lo, 2000; Yang, Chen, & Kuo, 2008). Moreover, a previous study has revealed that patients with congestive heart failure due to coronary artery disease slept longer in the right lateral decubitus position than in the supine and left lateral decubitus positions (Miyamoto et al., 2001). In addition, patients with systemic lupus erythematosus have enhanced parasympathetic nerve activity and attenuated sympathetic nerve activity when lying in the right lateral decubitus position (Huang, Chen, Wu, & Kuo, 2008). Although almost all of these patients were young, their parasympathetic and sympathetic nerve activities were impaired and accelerated, respectively (Laganá et al., 1996; Thanou et al., 2016).
Based on these findings, we hypothesized that there is a relationship between the preference for sleeping in the right lateral decubitus position and autonomic nervous activity in elderly adults, because aging gradually attenuates the autonomic nervous system (T. B. Kuo et al., 1999; Liao et al., 1995; Moodithaya & Avadhany, 2012). However, it is unclear whether the autonomic nervous system activity changes when elderly adults lie in the supine, left lateral decubitus, and right lateral decubitus positions.
Spectral analysis of heart rate variability (HRV) is a reliable and noninvasive measurement of cardiac neural regulation to assess the autonomic nervous system. HRV is represented by beat-to-beat fluctuations in HR and has been accepted as a promising quantitative index that reflects cardiac autonomic balance (Vanderlei, Pastre, Hoshi, Carvalho, & Godoy, 2009). HRV mainly includes two spectral components, one of low frequency (LF) and one of high frequency (HF), and it is well accepted that the HF component of HRV reflects vagal nerve activity at the sinus node, because atropine, a parasympathetic muscarinic blocker, abolishes almost all the HF component (Pomeranz et al., 1985). The LF component of HRV is affected by baroreflex feedback loops associated with sympathetic nerve activity. Compared with the supine position, during standing, the LF component and ratio of the LF component to the HF component (LF/HF) increases, whereas the HF component decreases (Siebert, Drabik, Lango, & Szyndler, 2004). HRV analysis has been widely used to evaluate regulation between the autonomic nervous system and cardiovascular system in many studies on aging, sex difference, obesity, and several conditions such as hypertension, diabetes mellitus, congestive heart failure, myocardial infarction, sleep disorders, orthostatic dysfunction, neuropathy, sudden cardiac death, and mental stress (Huikuri & Stein, 2013; Moodithaya & Avadhany, 2012; Sin, Sloan, McKinley, & Almeida, 2016).
Therefore, the aim of this study was to elucidate the effect of the three positions on parasympathetic and sympathetic nerve activities in elderly individuals by using spectral analysis of HRV.
Materials and Method
Study Subjects
Between February 2012 and May 2012, 52 functionally and physically active volunteers aged 63 to 85 years were recruited from the general community by advertisement. The exclusion criteria were as follows: any history of respiratory and cardiac disease (New York Heart Association class ≥ II), diabetes, neurological disorder, and regular use of alpha and beta blockers, which affect autonomic nervous regulation. We included volunteers with hypertension and asymptomatic coronary artery disease (New York Heart Association class I), because no significant differences in autonomic neuronal activity were demonstrated in these volunteers compared with controls (Casolo et al., 1995; Schroeder et al., 2003). Six volunteers with diabetes and one for whom an R wave was not detected on electrocardiogram (ECG) because of a technical error were excluded from the study. This study was approved by the Ethics Committee of the Tohoku University Graduate School of Medicine (2010-173). Written informed consent was obtained from all participants.
Experimental Protocol
All data were collected in an air-conditioned, quiet laboratory with an ambient temperature of 23 ± 2°C and humidity of 45 ± 5% between 9:00 a.m. and 4:00 p.m. on a single day, to maintain the minimum circadian rhythm effect on the autonomic nervous system. All volunteers were instructed not to do excessive exercise and drink alcohol and caffeinated beverages for at least 12 h before data were recorded. They were also requested to have more than 7 h of sleep the night before and to fast for at least 2 h prior to the experiment. Four ECG electrodes were placed in the anterior and lateral thoracic regions on both sides, and a lead II ECG was recorded continuously by using a portable ECG monitor (Radarcirc™, Dainippon Sumitomo Pharmaceutical Co., LTD., Osaka, Japan). This monitor allowed us to measure ECG data precisely during the postural changes and in each position because it has robust to any noises or artifacts (Shimpuku et al., 2010).
First, all participants were placed in the supine position on a bed for 5 min of rest for stabilization. Then they maintained the left and right lateral decubitus positions at an angle of about 45° for 10 min after being in the supine position for 10 min. The order of the left and right lateral decubitus positions was randomized. The posture was changed manually by two trained researchers. A sampling time of 10 min in each position was selected because short-term assessment of HRV requires recordings of at least 5 min (Krejčí, Botek, & McKune, 2016). During the recording, participants were asked to breathe spontaneously and relax as much as possible, with their eyes open. The respiratory rate was measured visually in each position, because a respiratory rate below 9 cycles/min may affect the HRV (Sasaki & Maruyama, 2014).
Spectral Analysis of HRV
The ECG data were stored on a personal computer after analog-to-digital conversion, with a sampling frequency of 1000 Hz. The peak of the R wave in the recorded electrocardiographic signals was automatically identified and converted into consecutive R-R intervals for analysis. Sinus pause and atrial or ventricular arrhythmia data were deleted. If the percentage of deletion was over 5%, the participant was excluded from further analysis. In addition, if a respiratory rate below 9 cycles/min was recognized, these participants were not analyzed further. The power spectral analysis was conducted off-line with continuous wavelet transformation using Fluclet™ WT Ver. 4.0 (Dainippon Sumitomo Pharmaceutical Co., Ltd., Osaka, Japan), which had a high time resolution and enabled us to assess the instantaneous changes in autonomic control (Nagai & Nagata, 1996). The LF and HF components were defined as the area under the power spectral curve between 0.04 and 0.15 Hz and between 0.15 and 0.40 Hz, respectively.
Generally, the HF component has been linked to cardiac parasympathetic nerve activity at the sinus node, whereas the LF component reflects both sympathetic and parasympathetic modulation of the R-R interval and is influenced by baroreflex activity. In this study, to estimate autonomic nervous regulation in the heart, we used the HF component as an index of cardiac vagal nerve activity and the LF/HF ratio as an index of sympathetic neuronal activity and sympatho-vagal balance (Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology, 1996).
Statistical Methods
All data analyses were performed with SPSS 21.0 software (SPSS Inc., Chicago, Illinois, USA). The sample size was estimated using the following parameters: alpha = .05, power = 80%, detectable effect for LF/HF = 0.30, and standard deviation = 0.7. According to the calculations, at least 45 individuals would be needed. We decided to enroll 10% more than the estimated number to cover any loss of data. Eventually, data were missing on seven participants in this study. However, the number of volunteers was considered appropriate for the analysis. The Kolmogorov–Smirnov test was used to test the normal distribution of variables. Categorical variables are presented in absolute numbers and percentages, while continuous variables are expressed as a mean and standard deviation or median and interquartile range, as appropriate.
Chi-square or Fisher’s exact test was used to compare the categorical variables. The basic characteristics of the three groups (namely, the healthy group, hypertensive group, and coronary artery disease group) were compared using one-way ANOVA for normally distributed values and Kruskal–Wallis test for non-Gaussian data. HR and respiratory rate were compared using repeated-measures ANOVA and the Bonferroni post hoc multiple comparison test. A Friedman test was used to compare all HRV indexes between the three positions: supine, left lateral decubitus, and right lateral decubitus; we selected the nonparametric Wilcoxon signed-rank test as a post hoc test using Bonferroni correction (a p value < .0167 was considered to indicate statistical significance in this study). Univariate and multivariate stepwise logistic regression analyses were performed to determine the independent factors influencing right lateral decubitus position’s effect on HRV. Significant variables (p < .20) included sex, age, coronary artery disease, hypertension, and LF/HF, and were entered into the model (Table 1). Whether LF/HF decreased in the right lateral decubitus position or not was regarded as a dependent variable. Odds ratios were given with 95% confidence intervals (CIs). The Hosmer–Lemeshow goodness-of-fit coefficient was computed for the regression model. The relationship between changes in LF/HF and LF/HF in the supine position was assessed with Spearman’s rank correlation coefficient. The null hypothesis was rejected at p < .05.
Table 1.
Characteristics of the Participants in the Study.
| Variables | All | Healthy group | Hypertensive group | Coronary artery disease group | p value |
|---|---|---|---|---|---|
| Number | 45 | 27 | 11 | 7 | — |
| Sex (male) | 18 (40.0) | 6 (22.2) | 7 (63.6) | 5 (71.4) | .011 |
| Age (years) | 73.6 ± 5.7 | 71.7 ± 4.8 | 74.5 ± 6.2 | 79.3 ± 4.3 | .004 |
| Height (cm) | 157.4 ± 8.8 | 155.7 ± 8.5 | 159.9 ± 8.4 | 160.3 ± 10.1 | .262 |
| Weight (kg) | 56.6 ± 10.6 | 53.5 ± 10.9 | 62.4 ± 7.8 | 59.5 ± 9.8 | .045 |
| BMI (kg/m2) | 22.7 ± 2.9 | 21.9 ± 2.7 | 24.5 ± 3.3 | 23.0 ± 2.3 | .046 |
| HT | 15 (33.3) | 0 | 11 (100) | 4 (57.1) | — |
| CAD (NYHA Class I at most) | 7 (15.6) | 0 | 0 | 7 (100) | — |
| HL | 12 (26.7) | 8 (29.6) | 3 (27.3) | 1 (14.3) | .715 |
| Medication | |||||
| ARB | 11 (24.4) | 0 | 8 (72.7) | 3 (42.9) | — |
| ACE inhibitor | 1 (2.2) | 0 | 1 (9.1) | 0 | — |
| Ca blocker | 8 (17.8) | 0 | 5 (45.5) | 3 (42.9) | — |
| Statin | 11 (24.4) | 6 (22.2) | 2 (18.2) | 3 (42.9) | .451 |
| Systolic BP (mmHg) | 121.9 ± 12.1 | 121.5 ± 13.2 | 122.6 ± 10.3 | 122.3 ± 11.8 | .965 |
| Diastolic BP (mmHg) | 69.5 ± 8.4 | 70.7 ± 7.6 | 68.5 ± 9.1 | 66.7 ± 10.4 | .490 |
| RR (cycles/minute) | 13.8 ± 1.3 | 13.7 ± 1.3 | 14.2 ± 1.3 | 14.0 ± 1.0 | .326 |
| HR (bpm) | 66.1 ± 9.6 | 65.0 ± 8.9 | 68.3 ± 11.8 | 67.0 ± 9.2 | .616 |
| LF (msec2/Hz) | 11.9 (7.6–22.3) | 12.4 (8.9–22.4) | 11.8 (6.5–18.2) | 9.0 (6.7–23.8) | .875 |
| HF (msec2/Hz) | 15.7 (8.4–34.7) | 22.6 (10.0–35.6) | 11.3 (5.1–17.4) | 21.5 (12.3–36.7) | .262 |
| LF/HF | 0.90 (0.45–2.03) | 0.64 (0.39–1.40) | 1.11 (0.80–2.04) | 1.46 (0.74–2.66) | .163 |
Note. Categorical variables are presented in absolute numbers and percentages. Continuous variables are expressed as mean and standard deviation or median and interquartile range. BMI = body mass index; HT = hypertension; CAD = coronary artery disease; NYHA = New York Heart Association; HL = hyperlipidemia; ARB = angiotensin II receptor blocker; ACE = angiotensin converting enzyme; Ca blocker = calcium channel blocker; BP = blood pressure; RR = respiratory rate; HR = heart rate; LF = low frequency component; HF = high frequency component; LF/HF = the ratio of LF to HF.
Results
Basic Characteristics
A total of 45 elderly adults were finally included in the analysis. Although four of the seven participants with coronary artery disease had concomitant hypertension, none of them had symptoms or took beta blockers. The characteristics of all participants in this study are depicted in Table 1. The percentage of men in the coronary artery disease and hypertension groups was higher than that in the healthy group. Participants in the coronary artery disease group were the older compared with those in the other two groups. However, there were no significant differences in HR, respiratory rate, and all HRV indexes such as LF, HF, and LF/HF (Table 1).
HRV, Heart Rate, and Respiration in the Three Positions
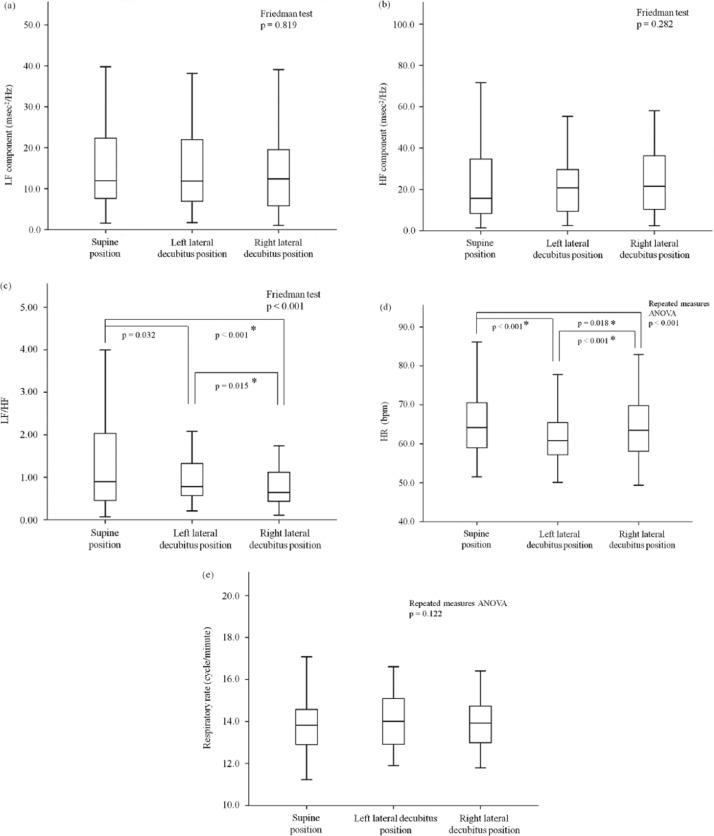
Figure 1a to 1c shows the effects of each position on HRV, HR, and respiratory rate in the 45 elderly volunteers. There were no significant changes in LF or HF; however, LF/HF significantly decreased in participants when they were lying in the right lateral decubitus position followed by those in the left lateral decubitus and those in the supine positions (0.90 [0.45-2.03], 0.78 [0.52-1.35), and 0.64 [0.43-1.13], respectively). There was a decrease in LF/HF when participants moved from the supine position to the left lateral decubitus position, although it was not statistically significant because a post hoc test adopted Bonferroni correction. The HR in the supine, left lateral decubitus, and right lateral decubitus positions was 66.1 ± 9.6 bpm, 62.2 ± 8.3 bpm, and 64.9 ± 10.2 bpm, respectively. The HR was the lowest in the left lateral decubitus position compared with the other two positions (Figure 1d). Moreover, HR remained low in the left lateral decubitus position throughout the experiment (Figure 2). The respiratory rate did not show significant differences among the three positions (Figure 1e).
Figure 1.
Comparison of indexes of HRV (a-c), HR (d) and respiratory rate (e) in the supine, left lateral decubitus, and right lateral decubitus positions.
Note. The asterisks indicate statistical significance between two positions. As for nonparametric analysis of LF/HF (c), a p value of .0167 or lower was considered statistically significant, using Bonferroni correction as a post hoc test after the Friedman test. HRV = heart rate variability; HR = heart rate; LF = low frequency; HF = high frequency; LF/HF = the ratio of LF to HF; bpm = beats per minute.
Figure 2.
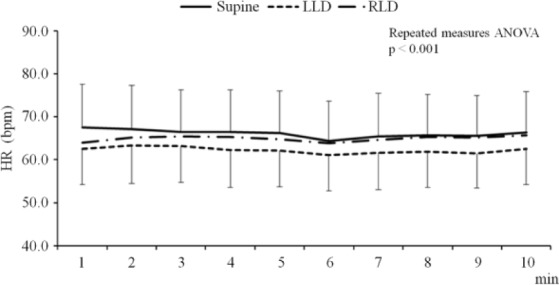
Time-course changes in HR for 10 min among the three positions.
Note. The upper, middle, and lower lines indicate the HR in the supine position, right lateral decubitus position, and left lateral decubitus position, respectively. Mean ± standard deviation. HR = heart rate; Supine = supine position; LLD = left lateral decubitus position; RLD = right lateral decubitus position; bpm = beats per minute; min = minute.
Low LF/HF in the Right Lateral Decubitus Position
To identify factors that were independently associated with decreased LF/HF in the right lateral decubitus position, we performed univariate and multivariate logistic regression analyses. As presented in Table 2, the multivariable stepwise forward regression analysis showed that only LF/HF in the supine position had a higher association with decreased LF/HF in participants lying in the right lateral decubitus position. The Hosmer-Lemeshow goodness-of-fit coefficient of this model was 0.840. Figure 3 shows the changes in the actual measurement of LF/HF from the supine position to the right lateral decubitus position. In volunteers whose LF/HF was high in the supine position, LF/HF decreased due to the postural change from the supine to the right lateral decubitus position. Thus, LF/HF in the supine position correlated significantly and negatively with decreased LF/HF, which was expressed as an index of sympathetic neuronal activity.
Table 2.
Univariate and Multivariate Analyses Regarding the Beneficial Effect of the Right Lateral Decubitus Position.
| Factors | Univariate regression analysis |
Multivariate regression analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p value | |
| Age | 1.07 | [0.932, 1.217] | 0.352 | — | — | .491 |
| Male | 2.80 | [0.510, 15.380] | 0.236 | — | — | .332 |
| HT | 5.09 | [0.573, 45.224] | 0.144 | — | — | .603 |
| CAD | 1.60 | [0.168, 15.273] | 0.683 | — | — | .753 |
| LF/HF | 1.79 | [1.090, 2.973] | 0.021 | 1.79 | [1.090, 2.973] | .021 |
Note. OR = odds ratio; CI = confidence interval; HT = hypertension; CAD = coronary artery disease; LF/HF = the ratio of low frequency component to high frequency component in the supine position.
Figure 3.
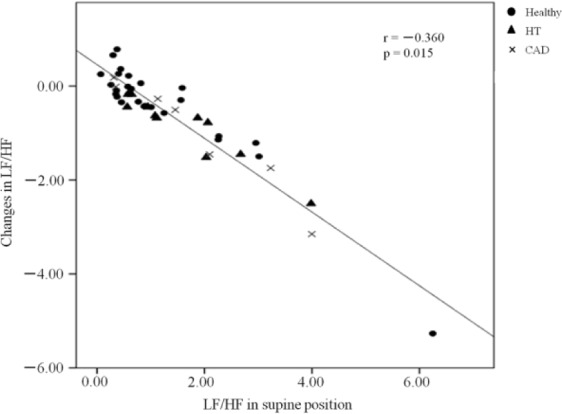
Correlation between LF/HF in the supine position and changes in LF/HF.
Note. Changes in LF/HF were determined as differences in the actual measurements between the supine and right lateral decubitus positions. Black circles, black triangles, and crosses represent the individual data of the 45 participants in the healthy, HT, and CAD groups, respectively. LF/HF = the ratio of the low frequency component to the high frequency component; HT = hypertension; CAD = coronary artery disease.
Discussion
Lateral decubitus positions possibly have beneficial effects in patients with congestive heart failure and systemic lupus erythematosus; however, whether the lateral decubitus positions affect HRV in elderly adults has not yet been well elucidated. This study was the first to clarify the quantitative changes in autonomic nervous activity in a large population aged 60 years or older among three positions: supine, left lateral decubitus, and right lateral decubitus positions.
Right Lateral Decubitus Position
Our study demonstrated that LF/HF and HR decreased when participants moved from the supine position to the right lateral decubitus position, which suggests that cardiac sympathetic neuronal activity attenuates in the right lateral decubitus position, and it could lead to better sympatho-vagal balance for elderly adults. In contrast, there was no significant difference in HF in the right lateral decubitus position, which suggests that vagal nerve activity remained almost unchanged. Our findings partly agree with some observations that have indicated that decreased cardiac sympathetic nerve activity and enhanced vagal nerve activity occurs in patients with congestive heart failure and systemic lupus erythematosus as well as in young healthy adults (Chen & Kuo, 1997; Fujita et al., 2002; Huang et al., 2008; C. D. Kuo et al., 2000; Miyamoto et al., 2001). Another researcher stated that the right lateral decubitus position influenced only vagal nerve activity in patients with coronary artery disease (Yang et al., 2008). As for the right recovery position, which is a modified right lateral decubitus position, no significant alteration in autonomic neural regulation occurred in young healthy adults (Ryan, Larsen, & Galletly, 2003). The discrepancy between our findings and these reports might be attributed to differences in subjects, kinds of lateral positions, and the spectral analysis method of HRV. In particular, fast Fourier transformation was used to analyze HRV in all of the above-mentioned studies, whereas continuous wavelet transformation was used in this study.
Based on the multivariate logistic regression analysis in our study, only the LF/HF ratio was indicated as an independent factor that is related to a beneficial effect of the right lateral decubitus position on cardiac sympathetic nerve activity and sympatho-vagal balance. In fact, the higher the cardiac sympathetic nerve activity in the supine position was, the lower the cardiac sympathetic nerve activity was when the position was changed from the supine to the right lateral decubitus position in elderly adults, regardless of the presence of hypertension and coronary artery disease. This hypothesis may also explain the contradictory results in young healthy adults when they lay on their right sides; the LF/HF of the supine position in report by Chen and Kuo (1997), that is, 2.8 was higher than that in the report by Ryan et al. (2003), that is, 1.6. Moreover, the LF/HF ratio in the supine position has been reported to be more than 5.0 in patients with heart failure (C. D. Kuo et al., 2000; Miyamoto et al., 2001). Given that sympathetic nervous system elevation is seen in almost all elderly adults, there is a potential relationship between attenuation of cardiac sympathetic nerve activity in the right lateral decubitus position and a preference for lying on the right side while sleeping. The attenuation might contribute to decreased cardiac workload and prevent the occurrence of arrhythmia, especially in patients with heart failure. The mechanism regarding attenuation of sympathetic nerve activity in the right lateral decubitus position has not been clarified; however, it is likely associated with changes in blood volume and baroreflex, because in one study, plasma norepinephrine concentrations showed the lowest value when participants were in the right lateral decubitus position (Miyamoto et al., 2004).
We measured the respiratory rate of participants in the three positions because it is known that a respiratory rate under 9 cycles/min has a complicated impact on HRV (Sasaki & Maruyama, 2014). Our findings showed that the median respiratory rate was approximately 14 cycles/min, and there was no significant difference in respiratory rate in participants among the three positions. Therefore, a change in HRV could be independent of the respiratory rate in this study.
Left Lateral Decubitus Position
In the 45 elderly adults who participated in this study, we found that they continuously had the lowest HR in the left lateral decubitus position (Figure 2) as well as decreased LF/HF (Figure 1c), indicating that this drop in HR could partly be related to suppressed sympathetic nerve activity in the sinus nodes. However, LF/HF did not have the lowest value, even though participants had the lowest HR in the left lateral decubitus position compared with the other two positions. Huang et al. studied healthy young women, and reported that the HR of participants in the left lateral decubitus position was significantly lower than that of participants in the supine and right lateral decubitus positions, while cardiac autonomic nervous activity was almost unchanged (Huang et al., 2008). Considering this finding, it is possible that a decrease in HR could depend on not only on the effect of the autonomic nervous system on the heart but also on other factors. In addition, another study that used cardiovascular magnetic resonance imaging to examine young adults has shown that cardiac output in the left lateral decubitus position significantly decreased from 6.8 ± 0.8 L/min to 6.2 ± 1.2 L/min in the supine position, accompanied with a downward trend in HR (Rossi et al., 2011). These results suggest that lying in the left lateral decubitus position may lead to cardiac hemodynamic changes; however, the alterations are within the normal range and small for healthy adults.
With regard to patients with congestive heart failure, a nocturnal polysomnographic study has demonstrated that patients with larger left ventricular end-diastolic diameter, higher pulmonary capillary wedge pressure, and lower cardiac output avoided the left lateral decubitus position, while sleeping (Leung, Bowman, Parker, Newton, & Bradley, 2003). If the left lateral decubitus position results in low cardiac output with low HR in patients with severe heart failure, spending less time in that position may be physiologically reasonable. Moreover, accelerated cardiac sympathetic nerve activity in the left lateral decubitus position likely contributes to compensatory response to a decrease in cardiac output, as a previous study speculated (Leung et al., 2003). This is why there was no significant difference in HR when patients with chronic heart failure (defined as left ventricular ejection fraction <40% and New York Heart Association class ≥ II) changed their posture from the supine position to the left lateral decubitus position (Miyamoto et al., 2002). Further studies are required to elucidate the hemodynamic differences and mechanisms of the left lateral decubitus position, which could guide adequate postural change in clinical practice.
Limitations
The present study has some limitations. First, we evaluated the effects of the left and right lateral decubitus positions on the cardiac autonomic nervous system and HR only in the daytime. Therefore, it is unclear whether the same phenomenon would be observed in elderly adults during the night. Second, we did not investigate the elderly volunteers’ preference for the right lateral decubitus position.
Conclusion
Our study indicates that postural change from the supine to the right lateral decubitus position leads to not only attenuation in cardiac sympathetic nerve activity but also improvement of sympatho-vagal balance in elderly individuals, especially those with elevated sympathetic nervous system activity, while lying in the supine position. The left lateral decubitus position may cause the lowest HR without alterations in the cardiac autonomic nervous system, although its decrement is within the normal range. These findings suggest that the two lateral decubitus positions have different effects on the autonomic nervous system and circulation in elderly adults.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Grant-in-Aid for Research Activity Start-Up from the Japan Society for the Promotion of Science (15H06053).
References
- Almeida-Santos M. A., Barreto-Filho J. A., Oliveira J. L., Reis F. P., da Cunha Oliveira C. C., Sousa A. C. (2016). Aging, heart rate variability and patterns of autonomic regulation of the heart. Archives of Gerontology and Geriatrics, 63, 1-8. [DOI] [PubMed] [Google Scholar]
- Casolo G. C., Stroder P., Sulla A., Chelucci A., Freni A., Zerauschek M. (1995). Heart rate variability and functional severity of congestive heart failure secondary to coronary artery disease. European Heart Journal, 16, 360-367. [DOI] [PubMed] [Google Scholar]
- Chen G. Y., Kuo C. D. (1997). The effect of the lateral decubitus position on vagal tone. Anaesthesia, 52, 653-657. [DOI] [PubMed] [Google Scholar]
- De Koninck J., Lorrain D., Gagnon P. (1992). Sleep positions and position shifts in five age groups: An ontogenetic picture. Sleep, 15, 143-149. [DOI] [PubMed] [Google Scholar]
- Fujita M., Miyamoto S., Tambara K., Budgell B. (2002). Trepopnea in patients with chronic heart failure. International Journal of Cardiology, 84, 115-118. [DOI] [PubMed] [Google Scholar]
- Huang S. T., Chen G. Y., Wu C. H., Kuo C. D. (2008). Effect of disease activity and position on autonomic nervous modulation in patients with systemic lupus erythematosus. Clinical Rheumatology, 27, 295-300. [DOI] [PubMed] [Google Scholar]
- Huikuri H. V., Stein P. K. (2013). Heart rate variability in risk stratification of cardiac patients. Progress in Cardiovascular Diseases, 56, 153-159. [DOI] [PubMed] [Google Scholar]
- Karavidas A., Lazaros G., Tsiachris D., Pyrgakis V. (2010). Aging and the cardiovascular system. Hellenic Journal of Cardiology, 51, 421-427. [PubMed] [Google Scholar]
- Kaye D. M., Esler M. D. (2008). Autonomic control of the aging heart. Neuromolecular Medicine, 10, 179-186. [DOI] [PubMed] [Google Scholar]
- Kaye D. M., Lambert G. W., Lefkovits J., Morris M., Jennings G., Esler M. D. (1994). Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. Journal of the American College of Cardiology, 23, 570-578. [DOI] [PubMed] [Google Scholar]
- Krejčí J., Botek M., McKune A. J. (2016). Dynamics of the heart rate variability and oxygen saturation response to acute normobaric hypoxia within the first 10 min of exposure. Clinical Physiology and Functional Imaging. Advance online publication. doi: 10.1111/cpf.12381 [DOI] [PubMed] [Google Scholar]
- Kuo C. D., Chen G. Y., Lo H. M. (2000). Effect of different recumbent positions on spectral indices of autonomic modulation of the heart during the acute phase of myocardial infarction. Critical Care Medicine, 28, 1283-1289. [DOI] [PubMed] [Google Scholar]
- Kuo T. B., Lin T., Yang C. C., Li C. L., Chen C. F., Chou P. (1999). Effect of aging on gender differences in neural control of heart rate. American Journal of Physiology, 277(6, Pt. 2), H2233-H2239. [DOI] [PubMed] [Google Scholar]
- Laganá B., Tubani L., Maffeo N., Vella C., Makk E., Baratta L., Bonomo L. (1996). Heart rate variability and cardiac autonomic function in systemic lupus erythematosus. Lupus, 5, 49-55. [DOI] [PubMed] [Google Scholar]
- Leung R. S., Bowman M. E., Parker J. D., Newton G. E., Bradley T. D. (2003). Avoidance of the left lateral decubitus position during sleep in patients with heart failure: Relationship to cardiac size and function. Journal of the American College of Cardiology, 41, 227-230. [DOI] [PubMed] [Google Scholar]
- Liao D., Barnes R. W., Chambless L. E., Simpson R. J., Jr., Sorlie P., Heiss G. (1995). Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—The ARIC study. Atherosclerosis Risk in Communities. American Journal of Cardiology, 76, 906-912. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D. M., Wang T. J., Leip E. P., Larson M. G., Levy D., Vasan R. S., Benjamin E. J. (2004). Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation, 110, 1042-1046. [DOI] [PubMed] [Google Scholar]
- Meredith I. T., Broughton A., Jennings G. L., Esler M. D. (1991). Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. New England Journal of Medicine, 325, 618-624. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Fujita M., Sekiguchi H., Okano Y., Ngaoya N., Ueda K., . . . Sasayama S. (2001). Effects of posture on cardiac autonomic nervous activity in patients with congestive heart failure. Journal of the American College of Cardiology, 37, 1788-1793. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Fujita M., Tambara K., Sekiguchi H., Eiho S., Hasegawa K., Tamaki S. (2004). Circadian variation of cardiac autonomic nervous activity is well preserved in patients with mild to moderate chronic heart failure: Effect of patient position. International Journal of Cardiology, 93, 247-252. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Tambara K., Tamaki S., Nagaya N., Hasegawa K., Nohara R., . . . Fujita M. (2002). Effects of right lateral decubitus position on plasma norepinephrine and plasma atrial natriuretic peptide levels in patients with chronic congestive heart failure. American Journal of Cardiology, 89, 240-242. [DOI] [PubMed] [Google Scholar]
- Monahan K. D. (2007). Effect of aging on baroreflex function in humans. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 293, R3-R12. [DOI] [PubMed] [Google Scholar]
- Moodithaya S., Avadhany S. T. (2012). Gender differences in age-related changes in cardiac autonomic nervous function. Journal of Aging Research, 2012, Article 679345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R., Nagata S. (1996). New algorithms for real-time, 24 hr continuous and noise-adjusted power spectral analysis of heart rate and blood pressure fluctuations in conscious rats. Japanese Journal of Pharmacology, 72, 355-364. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. A., Weinberg C. R., Cook D., Best J. D., Reenan A., Halter J. B. (1983). Differential changes of autonomic nervous system function with age in man. American Journal of Medicine, 75, 249-258. [DOI] [PubMed] [Google Scholar]
- Pomeranz B., Macaulay R. J., Caudill M. A., Kutz I., Adam D., Gordon D., . . . Benson H. (1985). Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology, 248, H151-H153. [DOI] [PubMed] [Google Scholar]
- Rossi A., Cornette J., Johnson M. R., Karamermer Y., Springeling T., Opic P., . . . van Geuns R. J. (2011). Quantitative cardiovascular magnetic resonance in pregnant women: Cross-sectional analysis of physiological parameters throughout pregnancy and the impact of the supine position. Journal of Cardiovascular Magnetic Resonance, 13, Article 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. D., Larsen P. D., Galletly D. C. (2003). Comparison of heart rate variability in supine, and left and right lateral positions. Anaesthesia, 58, 432-436. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Maruyama R. (2014). Consciously controlled breathing decreases the high-frequency component of heart rate variability by inhibiting cardiac parasympathetic nerve activity. Tohoku Journal of Experimental Medicine, 233, 155-163. [DOI] [PubMed] [Google Scholar]
- Schlaich M. P., Lambert E., Kaye D. M., Krozowski Z., Campbell D. J., Lambert G., . . . Esler M. D. (2004). Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension, 43, 169-175. [DOI] [PubMed] [Google Scholar]
- Schroeder E. B., Liao D., Chambless L. E., Prineas R. J., Evans G. W., Heiss G. (2003). Hypertension, blood pressure, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Hypertension, 42, 1106-1111. [DOI] [PubMed] [Google Scholar]
- Shimpuku G., Morimura N., Sakamoto T., Isshiki T., Nagata S., Goto T. (2010). Diagnostic performance of a new multifunctional electrocardiograph during uninterrupted chest compressions in cardiac arrest patients. Circulation Journal, 74, 1339-1345. [DOI] [PubMed] [Google Scholar]
- Siebert J., Drabik P., Lango R., Szyndler K. (2004). Stroke volume variability and heart rate power spectrum in relation to posture changes in healthy subjects. Medical Science Monitor, 10(2), MT31-MT37. [PubMed] [Google Scholar]
- Sin N. L., Sloan R. P., McKinley P. S., Almeida D. M. (2016). Linking daily stress processes and laboratory-based heart rate variability in a national sample of midlife and older adults. Psychosomatic Medicine, 78, 573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation, 93, 1043-1065. [PubMed] [Google Scholar]
- Thanou A., Stavrakis S., Dyer J. W., Munroe M. E., James J. A., Merrill J. T. (2016). Impact of heart rate variability, a marker for cardiac health, on lupus disease activity. Arthritis Research & Therapy, 18, Article 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlei L. C., Pastre C. M., Hoshi R. A., Carvalho T. D., Godoy M. F. (2009). Basic notions of heart rate variability and its clinical applicability. Revista Brasileira de Cirugia Cardiovascular, 24, 205-217. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Chen G. Y., Kuo C. D. (2008). Comparison of effect of 5 recumbent positions on autonomic nervous modulation in patients with coronary artery disease. Circulation Journal, 72, 902-908. [DOI] [PubMed] [Google Scholar]