ABSTRACT
Glycation is an important protein modification that could potentially affect bioactivity and molecular stability, and glycation of therapeutic proteins such as monoclonal antibodies should be well characterized. Glycated protein could undergo further degradation into advance glycation end (AGE) products. Here, we review the root cause of glycation during the manufacturing, storage and in vivo circulation of therapeutic antibodies, and the current analytical methods used to detect and characterize glycation and AGEs, including boronate affinity chromatography, charge-based methods, liquid chromatography-mass spectrometry and colorimetric assay. The biological effects of therapeutic protein glycation and AGEs, which ranged from no affect to loss of activity, are also discussed.
KEYWORDS: Advanced glycation end products, antibody, bioactivity, glycation, methods, modification
Introduction
Protein glycation is a non-enzymatic glycosylation on protein amine groups, primarily the alpha amine terminal and epsilon amine group on the lysine side chain.1-4 It occurs when protein is incubated with reducing sugars, including glucose, galactose5 and fructose.6 The reaction between the amino acid and reducing sugar was first described by Maillard in 1912.7 The susceptible amine group reversibly condenses with an aldehyde group of the reducing sugar, to form an unstable Schiff base intermediate, which can undergo a spontaneous multistep Amadori rearrangement to form a more stable, covalently bonded ketoamine (Fig. 1a).5 However, the ketoamine Amadori product can be reversibly driven to lose the sugar adduct under certain conditions. For a highly glycated recombinant monoclonal antibody, the total glycation level decreased from 42% to 20% when it was incubated at 37°C for 100 hours in a pH 7 phosphate buffer without glucose, which confirmed the reversible nature of the glycation.8
Figure 1.
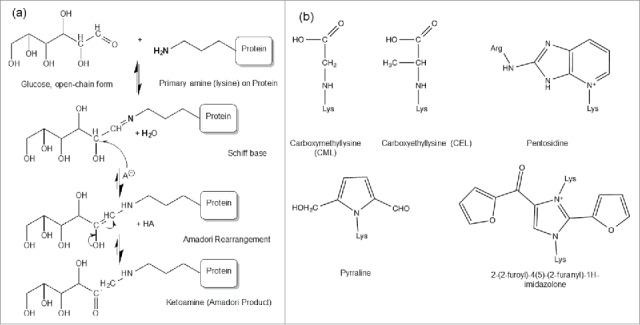
(a) Protein glycation Maillard reactions. The Maillard reaction is initiated by a deprotonated amine on the anomeric carbon of an open-chain reducing sugar to reversibly form a Schiff base species, followed by acid catalysis to rearrange into a stable Amadori product (aldosamine → ketosamine). Figure reproduced with permission from Ref.5 (b) The structure of some common AGEs including CML, CEL, pentosidine, pyrraline and imidazolone.
Although positively charged primary amines are generally located on the surface of protein molecules, only some of these accessible sites will be specifically reactive toward reducing sugar molecules. No specific sequence that signals a potential glycation site has been identified. However, some studies show that the three-dimensional local environments could affect the formation of glycation. Histidine residues or basic residues (arginines and other lysines) have been observed to correlate with glycation occurrence in some proteins with known structures (e.g., liver alcohol dehydrogenase,3 RNase A,9 DNase I,10 albumin,11 hemoglobin.12,13).5 The reactivity of the amine group depends on the localized conditions that influence both initial amine deprotonation and stabilization of the intermediate before the ketoamine Amadori product is formed. Modeling of the antigen-binding fragment of a recombinant humanized monoclonal antibody (rhuMAb) has shown that the unusually high level of glycation of lysine (K) residue 49 (near complementarity-determining region (CDR) 2 was catalyzed by an aspartic acid residue in CDR1 on the light chain.14 The catalytic effect of carboxylic acids in close proximity was also suggested as the cause of preferential glycation of K99 in the heavy chain of another mAb.15
Over time, the glycated proteins with Amadori products exposed to elevated oxidation conditions, including in vivo circulation, can slowly undergo further degradation that results in advanced glycation end products (AGEs). AGEs include structures such as carboxymethyllysine (CML), carboxyethyllysine (CEL), pentosidine, pyrraline, and imidazolone (Fig. 1b).16-19 AGEs correlate with some pathological responses observed in diabetes, arthritis, atherosclerosis, amyloidosis and aging.2024 Physiological AGEs formation correlates with oxidative and inflammatory processes, not with glucose levels, and occurs in the timeframe of months to years. In vivo, advanced glycation-modified proteins can induce AGEs-specific receptor expression, anti-AGEs protein immune response, and alterations in cellular signaling and function.25-28 AGEs-damaged proteins are highly reactive and become cross-linked, structurally altered, and ultimately aggregated, and these non-native states are toxic for many cell types (e.g., endothelial, neuronal, retinal, leukocytes) and associated with advanced diseases.29 However, in the manufacturing of therapeutic recombinant antibody products, AGEs formation has not been observed at significant amounts, even with low21 or high levels of glycation.8
For therapeutic mAbs, the potential effects of glycation, such as blocking the biologically functional site or further degradation that induces aggregation, make glycation a potential critical quality attribute (CQA). Comprehensive studies are required to characterize and understand the structure and function relationship of glycation in mAbs. Here, we review the studies of antibody glycation regarding the modification, methods and potential effects on biological function.
Causes of therapeutic antibody glycation
Due to the complexity of commercial therapeutic antibody production, glycation is not unusual, but the reaction kinetics and extent are not predictable.5 Glycation of mAbs occurs during the fermentation process, where glucose is an energy source for the mAb-producing cells. The level of glycation is strongly affected by the total sugar feed during the mammalian cell culture process.5 The temperature, pH, time, and ionic strength, which are kept close to physiological conditions, can affect the kinetics and extent of glycation.30 The types of sugars present, such as hexose sugars, for example, and the specific reactivity of the accessible amino groups affect the protein glycation, and therefore increase the heterogeneity of therapeutic proteins.31
During storage of therapeutic mAbs, glycation can be introduced by reducing sugars in the formulation. The presence of reducing sugars in the formulation has produced protein glycation even in the solid state after lyophilization.10 Although reducing sugars are not typically used in formulation, they may be produced by degradation of higher order carbohydrates like the disaccharide sucrose, which is widely used in formulation. The acidic pH and elevated temperature can cause accelerated sucrose hydrolysis, leading to the production of reducing sugars and subsequent glycation.32,33 The speed of the Maillard reaction also depends on formulation buffer salt components and the type of sugar in the formulation buffers. Nevertheless, the overall glycation rate in optimized storage conditions is minimal to none. Several studies have shown that formulation, storage temperature, and storage duration affect the rate of glycation. Stability data from studies using formulation stored at 4–5 °C for up to 21/months showed a minimal or low to no increase in the level of glycation.32,34,35 The same formulations stored at elevated temperatures from 29 to 37 °C for 1–21/months showed significant increases in glycation levels. Based on these observations, the use of accelerated stability data are of questionable value for predicting protein stability in sucrose-containing formulations stored at 2–8 °C, where little or no glycation was observed.32 The results of studies on antibody glycation during storage are summarized in Table 1.
Table 1.
Glycation generated during storage.
| References | Formulation, Sample | Glycation Study | Impact on Antibody Quality Attributes |
|---|---|---|---|
| Andya, et al.34 | Lactose, rhuMabE25 | Spray-dried rhuMabE25 formulated in lactose was detected using IEF to have glycated sites. The acidic shift increased with increased amount of lactose in the formulation. | 16 of 44 lysine residues in primary structure of rhuMabE25 were lactosylated. |
| Modification increased with increased storage temperature from 5°C to 30 °C. | |||
| Banks, et al.32,72 | Sucrose, IgG1 and IgG2 monoclonal antibodies | mAb001 at 100 mg/mL formulated with either 270 mM sucrose, 270 mM sorbitol, or no excipient and buffered at pH 5.2 with 10 mM sodium acetate and pH 4.8 with 10 mM sodium lactate | Long term mAb001 glycation in sucrose is followed by increase in the rate of high molecular weight species accumulation. The timing of mAb001 aggregation in sucrose formulation was a function of storage temperature and pH. The hydrolysis of sucrose into glucose and fructose changed the rate of MAB001 aggregation. mAb002 formation of HMWS increased significantly in comparison to non-glycated sample over time, up to 12/weeks. Conclusion that aggregation following glycation was not molecule specific, |
| mAb002 stored at 1/week at 37 °C in sodium phosphate solution buffered at pH 7.0 in the presence of 270 mM glucose or 270 mM sorbitol. | |||
| Fischer, et al.40 | Sucrose, mAb | mAb protein concentration 7.5/mg/ml, glycated by storage for 1/week at 40°C in presence of 500 mM glucose, followed by size exclusion chromatography (SEC) and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) | No difference observed between amount of HMWS between glycated mAb and control. |
| Gadgil, et al.33 | Sucrose, IgG2 | Antibody samples aged at elevated temperatures in sucrose containing formulation (9% sucrose, pH 5) or sorbitol containing formulation (5% sorbitol, pH 5). | No glycation found in sucrose containing formulations incubated at 4°C after 18/months. Glycation was observed in formulations incubated at 37°C after 1/month. Glycation sites were 10 lysine residues and the N-terminal of light chain. |
The overall glycation reaction rate is typically low for mAbs, and, since there are many potential glycation reaction sites, the identification and characterization of glycation in therapeutic mAbs can be challenging. Forced glycation of the antibody is an approach to generate higher levels of glycation at susceptible sites in order to provide guidance on glycated mAb characterization. During forced glycation, the antibody is incubated with a high concentration of reducing sugars, such as glucose or sucrose, under elevated temperatures. High temperature and low pH conditions increase the glycation reaction rates.36
Glycation of antibodies also occurs during in vivo circulation. The concentration of endogenous immunoglobulin G (IgG) in circulation in humans is ∼10 mg/mL, and the measured glycation level of endogenous IgG in healthy individuals varies widely between studies, ranging in mole to mole amounts from 0.14 to 5 glucose per IgG, which corresponds to overall glycation levels of 14% to 500%.37,38 In comparison, a therapeutic mAb in circulation is typically at ≤0.2/mg/ml, with an overall glycation level at 10–25%.5,8,33,37,39-42 The average lysine residue glycation rate is similar among the therapeutic antibodies, endogenous human IgG and human serum albumin.37 If the IgG does not contain a highly reactive glycation site, then the glycation will be spread across the whole molecule with low level glycation on all susceptible glycation sites under endogenous conditions.37
Analytical methods for antibody glycation
Boronate affinity chromatography
Boronate affinity chromatography (BAC) is a technique for isolation and enrichment of cis-diol compounds. Boronate functional groups on the stationary phase will form a tetrahedral anion under alkaline pH conditions, which can interact with the cis-1,2-diol arrays found on sugar molecules5 and separate glycated from non-glycated species. To elute the glycated species, the interactions are disrupted by lowering the pH or adding a competing source of hydroxyl groups, such as sorbitol.5 BAC has been used for the analysis of carbohydrates43 and intact proteins.44 For antibody glycation analysis, BAC is a common method of identification, quantitation and isolation of glycated antibodies because it requires minimum sample preparation and uses native running conditions.5,15,31 As an example, a normal boronate affinity chromatogram of the therapeutic antibody is shown in Fig. 2. The non-glycated peak elutes at the beginning of the gradient and the glycated species elutes at the end. There are some non-specific interactions between the antibody and column stationary phase, which can be prevented by using shielding reagents such as Tris in the mobile phase.5,45 Careful optimization of the concentration of shielding agent, pH and buffer salt composition allows the quantitation of the glycation level of the bulk-drug substance.31 BAC could also be coupled with other methods by offline fraction collection for further characterization of the glycated species. Although BAC has been widely used for enrichment of glycated species, it has some limitations. The glycosylation species also interact with the BAC surface, which may cause some non-specific binding.46,47 The quantitation is also an estimate because it cannot differentiate between proteins with a single glycation or multiple glycations.
Figure 2.
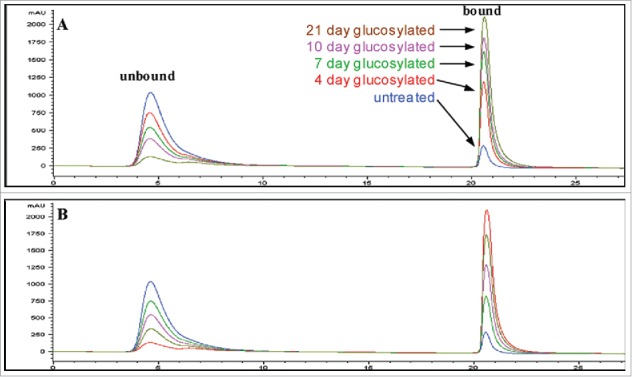
Boronate affinity chromatograms of untreated and forced-glycated antibody samples. The figure shows the chromatograms obtained for control and forced-glycated samples of antibody obtained by boronate affinity chromatography in phosphate buffer (A). Also shown are the chromatographic results obtained for a series of standards made by diluting untreated and 21-day forced-glycated antibody (both samples at the same concentration) at different ratios, where the highest absorbing unbound peak is untreated starting material and the highest bound peak is 21-day forced-glycated material, with 1:3, 1:1, and 3:1 mixtures of these samples representing the remaining three intermediate chromatograms (B). Figure reproduced with permission from Ref.31
Charge-based methods
Capillary isoelectric focusing (cIEF) or imaged capillary electric focusing (icIEF) are charge-based separation methods that can detect glycation due to the loss of positive charge on the glycation sites. There is a shift to the acidic region for fully glycated, retained boronate fractions compared with the non-glycated, non-retained boronate fraction.5 The icIEF has been known to separate species with 0.05-pI difference and can resolve a glycated antibody that theoretically has a 0.09-pI unit difference due to a blocked lysine residue.5 This charge difference separation is also observable in co-mixed glycated and non-glycated boronate fractions.5
Ion exchange chromatography (IEC) may also resolve glycated and non-glycated proteins that have surface charge difference. Analysis of glycated boronate fractions reveals a distinct acidic shift to the main peak under linear gradient conditions.5 Correspondingly, the acidic variants fractionated from the IEC also show a small enrichment in glycation.15 Quan et al.5 observed a shift to the acidic region for the fully glycated boronate-retained antibody compared to the original unfractionated antibody. The amount of shift was equivalent to ∼0.5 mM sodium chloride in the linear gradient. The IEC peaks for the boronate-retained fraction were also noticeably broadened, whereas the IEC peaks for the boronate non-retained fraction (non-glycated) were sharper than the unfractionated starting material (Fig. 3). Overall, IEC does not appear to have sufficient resolution to separate the glycation species within the starting material, which presents the combined charge effect from multiple sites of low-level glycation across the molecule. In comparison, molecules with zero, one, or two lysine residues on the carboxyl termini are thoroughly resolved from each other, apparently due to the singular and unique positional interactions with the resin.5
Figure 3.
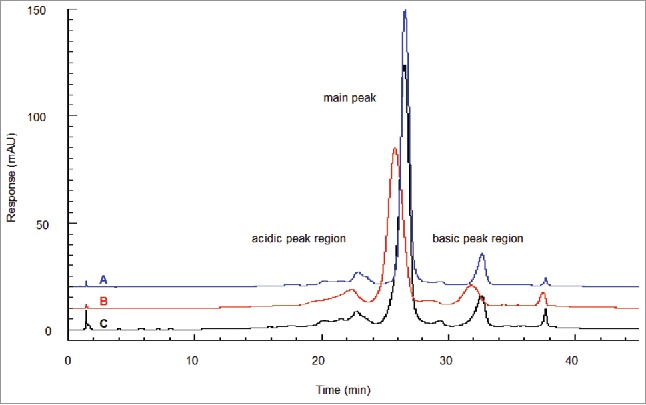
IEC of boronate affinity chromatography fractions. Shown is glycated material found throughout the IEC profile and separated in an acidic shift from non-glycated material. At the glycation level found in starting material (18%), the glycated molecules segregate to the acidic side of each peak region, giving each a broadened leading edge. (A) Boronate non-retained fraction (non-glycated). (B) Boronate-retained fraction (glycated). (C) Starting material. Figure reproduced with permission from Ref.5
Liquid chromatography-mass spectrometry methods
Top-down mass spectrometry of the intact antibody or enzyme-cleaved mAb fragments can also be used to determine glycation level,31 either by matrix-assisted laser desorption/ionization (MALDI)48 or electrospray ionization (ESI).8,15,49 As each glycation site shows a +162 Da mass shift, the top-down approach can be used as a quick estimation of glycation level in the antibody. It has been reported that, after deglycosylation and removal of C-terminal lysine, the quantification of glycation by mass spectrometry could have a limit of detection at 1.0% and a limit of quantitation at 3.0%, and there is a correlation between the BAC and mass spectrometry results.45
To locate the glycation site, a bottom-up peptide mapping approach is commonly used.8,14,15,33,50 Since trypsin is inhibited by glycation of lysine residues,33 a missed tryptic cleavage with a +162 Da mass addition indicates a glycated lysine.51 Tryptic peptide mapping of the collected BAC retained fraction or of the forced glycated sample reveals sites of glycation susceptibility across the antibody.14
An issue with traditional mass spectrometry methods is the sensitivity, particularly because of the low abundance of glycation in typical therapeutic mAb. At the peptide level, the sensitivity issue is more pronounced because glycation sites are typically distributed across multiple lysine residues. Another issue for the bottom-up approach by traditional peptide mapping is the varying degree of neutral water loss on the sugar with collision induced dissociation (CID) observed through tandem mass spectrometry (MS/MS) analysis of glycated peptides, which makes low-level detection of glycation sites even more challenging.52-55 An alternative way of fragmentation in mass spectrometry is the electron transfer dissociation (ETD). Studies on the fragmentation method comparison show ETD provides complete sequence fragmentation without any neutral loss. However, the sensitivity of low abundance protein or peptide for ETD is low because of the low fragmentation efficiency.53,54,56-58 These issues often require extra experiments to confirm or detect glycated peptides.31 Another way of improving the sensitivity and reducing the neutral loss of the glycated peptide is by using sodium borohydride or sodium cyanoborohydride reduction followed by trypsin cleavage and peptide map analysis with MS/MS detection.31,37,59 In this approach, the bond between the carbohydrate and peptide is stabilized due to the reduced glycated sugar moiety, which results in higher quality MS/MS spectra.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is also a commonly used method to study AGEs. The level of AGEs is measured by evaluating the potential glycation sites during peptide mapping. Some major AGEs, such as CML and CEL, could be quantitated by the area ratio between the modified peptides and the sum of all wild type peptides at all charge states. Since there are multiple AGEs products, each kind of AGEs product needs to be quantitated separately.16,21,60 In addition to lysine, arginine is a potential spot where AGEs modifications may occur in recombinant antibodies.17
Isotope labeling has also been applied to antibody glycation analysis. A study by Zhang et al. utilized forced glycation on an antibody with a 1:1 mixture of 12C/13C6 reducing sugars at 37 °C.55 The glycated samples and the control were analyzed by trypsin digestion and LC-MS/MS analysis. This approach simplified the detection of glycated tryptic peptide elution in the LC/MS analysis by giving a unique signature of two molecular ions with about equal intensity and differing by 6.018 Da. After identification of all the glycation sites in the forced glycated samples, finding glycated peptides in the native sample is simplified. This methodology for the identification of site-specific glycation not only significantly enhances data searching speed, but also improves sensitivity, even at levels below 0.5%. Their results showed that glycation sites were detected at low levels across the entire therapeutic antibody.55
Another isotope labeling approach includes a sodium borohydride reduction step. In this method, the samples were divided into two aliquots, sample A and B.41 Sample A and B were reduced with sodium borohydride and sodium borodeuteride, respectively, before tryptic digestion, which resulted in a 2 Da or 3 Da molecular weight increase for glycated peptides. When samples A and B were analyzed as a 1:1 mixture by LC-MS/MS, the 1 Da difference in molecular weight was used to identify peptides with glycation and to calculate the relative percent of glycation. These results demonstrated that a 1 Da molecular weight difference was sufficient for relative quantitation when stable isotope distribution was achieved and peak overlapping was taken into consideration.41
Colormetric assay
The ketoamine formed from antibody glycation can be quantitated by the nitroblue tetrazolium (NBT) reduction assay. The NBT reduction assay for measuring protein glycation was first introduced by Johnson, et al.61 in 1983.58 NBT is reduced by the ketoamine form of glycated protein, which results in a change in absorbance at 525 nm. This method has been used to measure poly-lysine62 and glycated albumin.63 NBT analysis was applied to glycated antibody by Ahmad, et al.39 in 2012.37 Recently, Butko, et al.21 reported that several AGEs found in manufactured recombinant mAb were strongly related to a color change in the antibody solution, and that AGEs are partially responsible for the color of mAb products.19 Additionally, an enzyme-linked immunosorbent assay (ELISA) format has been applied to study the AGEs in mAbs, utilizing the binding between a sample and a biotin-conjugated primary antibody that targets a specific kind of AGEs.21
Effects on biological function
Reports of antibody glycation and their potential effects on biological activities are summarized in Table 2. The effect of glycation modification on mAbs is a topic of ongoing debate because reported results are varied, likely depending on the number and position of the glycation. Glycation occurs at a number of sites and can be produced by forced stress conditions (95.4% overall glycation), and physiological cell culture antibody manufacturing conditions (10–40% glycation).5,8,37,64,65 It is evident that glycation levels increase in stress exposure conditions with increased sugar concentration in the formulation, higher storage temperature, and time in storage. The glycation sites in mAbs manufactured under normal cell culture conditions with standard physiological parameters, such as temperature and pH, with elevated glucose levels, are similar to endogenous antibodies.8,37 The effect of glycation on antibody activities ranged from no effect5,8,37,65 to loss of activity,64,66 although typically mAb glycation does not appear to substantially affect antibody binding activities. The effects of AGEs on antibodies have not been reported; however, it was reported that AGEs could potentially reduce the activity of the therapeutic protein interferon alfacon-1.67 The FcRn binding activities of mAb glycation were also studied using forced-glycation stressed samples. Both in-vivo mouse model and FcRn binding studies indicated glycation at the levels observed for mAb does not affect antibody pharmacokinetics (PK).42,68
Table 2.
Effects of glycation on biological function.
| Biological Function Impacts | Sample/Stress Condition | Analytical Methodology | Glycated Sites and Levels | |
|---|---|---|---|---|
| Miller, et al.8 | Glycation did not affect potency. Lysine mutation to arginine had no effect on potency | Forced Glycation: 1/mg/ml antibody concentration in 0.1/M phosphate buffer containing 5% glucose, Incubated at 37 °C for 91/hr. | Chromatography, Electrospray Ionization after deglycosylation, Tryptic Peptide Mapping | Lysine 98 on heavy chain, adjacent to a CDR. 40% mAb1 glycated under production conditions, 80% glycated under stress conditions |
| Quan, et al.5 | No effects to potency: | rhuMab (IgG1) | Shielded boronate affinity chromatographic method, shallow linear gradient | Four lysines in the light chain: (residues 169, 183, 188, and 190), and four lysines in the heavy chain (65, 151, 252, 292). Located mostly in the Fab portion and the heavy chain K65 is within the CDR. Overall for the drug substance, 82% non-glycated, 16% monoglycated, 1% doubly glycated, and 1% triply glycated. The glycated fraction isolated from BAC has almost 100% glycation with 10% CDR glycation. |
| Retained fraction of BAC with 100% glycation overall with 10% CDR glycation, full binding activity in comparison to starting material and controls. | Physiological cell-culture conditions | |||
| Formulated 25/mg/ml in a histidine-sucrose buffer at pH 6. | Site-level integration of parent and modified peptides at the 214/nm wavelength used to estimate amount of glycation at each ID site as well as the relative glycation reactivity order. Amount of glycation at eight identified sites ranged from 1 to 12%, | |||
| Goetze, et al.68 | No effects on binding to FcγIIIa receptors or neonatal Fc receptor (FcRn) or protein A. | Highly glycated mAb, incubated with high glucose concentrations (2/M) under otherwise physiological conditions (pH 7.4 at 37˚C) for extended periods of time (up to 23/days) | To test the effect of glycation on IgG function, highly glycated IgG1 and IgG2 were prepared containing on average 42–49 Glc molecules per IgG. | Heavy Chain Lysine 246, 274, 288, 322, 360, 392, 409, 414, 439 |
| Intact mass analysis | Within Fc region sites had variable reactivity: 0% glycation Lys 409, full glycation of Lys 246/248 | |||
| Kennedy, et al.64 | In vitro glycation can significantly lower the affinity of an antibody for its antigen, and significantly increases the rate of dissociation of the antigen-antibody complex | Whole mouse IgG glycated for 21 days at 37 °C using glucose concentrations of 0-, 15-, 50-, and 500-mM | The extent of glycation was measured using phenylboronate chromatography. | The percentage glycated IgG was 95.4% after incubation with 0.5 M glucose and 20.9% after incubation without added glucose. Glycation sites are not specified. |
| Measured association and dissociation rates in glycated and non-glycated mAb, using surface plasmon resonance and radiolabelled antigen, and ELISA procedure | ||||
| Morin, et al.65 | No biological effect: Did not detect any impairment in immunoglobulin function with a complement-fixation assay or a complement-mediated assay. | Broad spectrum of antibodies used, whole serum and purified immunoglobulins used | Compared immunoreactivity of glycated and non-glycated human immunoglobulins against infectious agents. Measured antigen-antibody binding, cell agglutination, cytotoxicity, and complement-fixation properties using Abbott Rubazyme assay, complement-fixation evaluations, and cell agglutination assay | Glycation sites are not specified |
| Dolhofer, et al.66 | Decrease in complement-fixation properties for human and rabbit IgG, observed a 50% decrease in hemolytic activity. | Incubated with relatively low concentrations of glucose for several days. | Micro complement fixation test | Glycation sites are not specified. |
| Inactivation of specific antibody was dependent on incubation time and glucose concentration. | ||||
| Yang, et al.42 | No PK effect: Clearance not affected by glycation. Glycated product is not recognized by the mannose receptor. IgG with high levels of mannose (18/mol/mol protein) did not clear faster in mice than the underivatized protein. | Mouse monoclonal IgG, 10 mg/mL in 10 mM sodium acetate, 9% sucrose, pH 5.2, stored at −80°C until use | Mouse PK study and PK parameter analysis performed using surface plasmon resonance. | Glycation distributed across lysine on the antibody, generally 10–30% by at different positions. |
| Characterization of mannosylated IgG with mass spec. analysis. | ||||
| Mironova et al.67 | The bioactivity is dropped by two fold for the protein with CML and imidazolone modifications | IFN alfacon-1 with a month prior to expiration data indicated on the drug vial | Western blots, SDS-PAGE, mass spectrometry | The CML was 1.7 AGE-BSA eq/vial and imidazolone 2.3 AGE-BSA eq/vial |
In terms of immunogenicity and safety, the constant regions of canonical therapeutic mAbs and endogenous antibodies are typically very similar, so glycation in the constant region of mAbs can be considered similar to endogenous mAbs, and therefore less of a concern. It has been hypothesized that lysine residues in the constant region of antibodies have evolved to be resistant to glycation.8,68 One of the concerns of glycation of therapeutic antibody, especially the AGEs, is in the development of immunogenicity. The AGEs are more immunogenic than normal antibody in animal models.39 The presence of AGEs was found in rheumatoid arthritis (RA) patients as the result of inflammation. AGE-damaged antibody could trigger an immune response by generating anti-IgG autoantibodies in RA patients.28 AGE-damaged IgG is a potent immunogen that appears to be a trigger for the antigen-driven induction of autoantibodies in RA.20 However, with optimal cell culture conditions, AGEs are very low or not observed for therapeutic antibodies.21
Antibody aggregation is an important CQA related to immunogenicity and safety. Reports correlating glycation levels with aggregation have been inconsistent, with one study showing a correlation,32 but another indicating that there was no significant difference in the level of aggregation observed between glycated and control mAb stored at 40°C for 1/week.40 The detailed mechanism of glycation-induced protein aggregation remains unclear.69-71 For AGEs, it was reported that AGEs occurred along with the therapeutic protein degradation and cross-linking.67
Conclusions
Glycation is a common and potentially important posttranslational modification that can occur during the manufacturing and storage of therapeutic proteins, including mAbs, and its effects should be thoroughly evaluated. BAC and LC-MS are currently the standard methods to analyze the overall glycation of antibodies. Forced glycation is useful for the identification of glycation sites, since glycation levels at individual sites are typically low. The effect of glycation on biological activity is dependent on the location of the modification, and is antibody specific. There appears to be no reports of significant effects on Fc binding or PK change due to antibody glycation. Appropriate cell culture processes, formulations, and storage conditions need to be developed in order to lower the risk of glycation and AGEs formation to ensure the safety and efficacy of therapeutic drug products.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Holmquist WR, Schroeder WA. Properties and partial characterization of adult human hemoglobin A1c. Biochim Biophys Acta 1964; 82:639-41; PMID:14148841; http://dx.doi.org/ 10.1016/0304-4165(64)90466-0 [DOI] [PubMed] [Google Scholar]
- 2.Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem 1983; 258(10):6142-6; PMID:6853480. [PubMed] [Google Scholar]
- 3.Shilton BH, Walton DJ. Sites of glycation of human and horse liver alcohol dehydrogenase in vivo. J Biol Chem 1991; 266(9):5587-92; PMID:2005099. [PubMed] [Google Scholar]
- 4.Walton DJ, Shilton BH. Site specificity of protein glycation. Amino Acids 1991; 1(2):199-203; PMID:24194104; http://dx.doi.org/ 10.1007/BF00806917 [DOI] [PubMed] [Google Scholar]
- 5.Quan C, Alcala E, Petkovska I, Matthews D, Canova-Davis E, Taticek R, Ma S. A study in glycation of a therapeutic recombinant humanized monoclonal antibody: Where it is, how it got there, and how it affects charge-based behavior. Anal Biochem 2008; 373(2):179-91; PMID:18158144; http://dx.doi.org/ 10.1016/j.ab.2007.09.027 [DOI] [PubMed] [Google Scholar]
- 6.Jairajpuri DS, Fatima S, Saleemuddin M. Immunoglobulin glycation with fructose: A comparative study. Clin Chim Acta 2007; 378(1–2):86-92; PMID:17173886; http://dx.doi.org/ 10.1016/j.cca.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 7.Maillard L. Action of amino acids on sugars. Formation of melanoidins in a methodical way. Compt. Rend 1912; 154:66. [Google Scholar]
- 8.Miller AK, Hambly DM, Kerwin BA, Treuheit MJ, Gadgil HS. Characterization of site-specific glycation during process development of a human therapeutic monoclonal antibody. J Pharm Sci 2011; 100(7):2543-50; PMID:21287557; http://dx.doi.org/ 10.1002/jps.22504 [DOI] [PubMed] [Google Scholar]
- 9.Watkins NG, Thorpe SR, Baynes JW. Glycation of amino groups in protein. Studies on the specificity of modification of RNase by glucose. J Biol Chem 1985; 260(19):10629-36; PMID:4030761. [PubMed] [Google Scholar]
- 10.Quan CP, Wu S, Dasovich N, Hsu C, Patapoff T, Canova-Davis E. Susceptibility of rhDNase I to glycation in the dry-powder state. Anal Chem 1999; 71(20):4445-54; PMID:10546528; http://dx.doi.org/ 10.1021/ac9900580 [DOI] [PubMed] [Google Scholar]
- 11.Iberg N, Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem 1986; 261(29):13542-5; PMID:3759977. [PubMed] [Google Scholar]
- 12.Shapiro R, McManus MJ, Zalut C, Bunn HF. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem 1980; 255(7):3120-7; PMID:7358733. [PubMed] [Google Scholar]
- 13.Zhang X, Medzihradszky KF, Cunningham J, Lee PD, Rognerud CL, Ou CN, Harmatz P, Witkowska HE. Characterization of glycated hemoglobin in diabetic patients: Usefulness of electrospray mass spectrometry in monitoring the extent and distribution of glycation. J Chromatogr B Biomed Sci Appl 2001; 759(1):1-15; PMID:11499613; http://dx.doi.org/ 10.1016/S0378-4347(01)00196-7 [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Yang Y, Yuk I, Pai R, McKay P, Eigenbrot C, Dennis M, Katta V, Francissen KC. Unveiling a glycation hot spot in a recombinant humanized monoclonal antibody. Anal Chem 2008; 80(7):2379-90; PMID:18307322; http://dx.doi.org/ 10.1021/ac701810q [DOI] [PubMed] [Google Scholar]
- 15.Saleem RA, Affholter BR, Deng S, Campbell PC, Matthies K, Eakin CM, Wallace A. A chemical and computational approach to comprehensive glycation characterization on antibodies. MAbs 2015; 7(4):719-31; PMID:26030340; http://dx.doi.org/ 10.1080/19420862.2015.1046663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: A review. Diabetologia 2001; 44(2):129-46; PMID:11270668; http://dx.doi.org/ 10.1007/s001250051591 [DOI] [PubMed] [Google Scholar]
- 17.Chumsae C, Gifford K, Lian W, Liu H, Radziejewski CH, Zhou ZS. Arginine modifications by methylglyoxal: Discovery in a recombinant monoclonal antibody and contribution to acidic species. Anal Chem 2013; 85(23):11401-9; PMID:24168114; http://dx.doi.org/ 10.1021/ac402384y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res 2001; 56:1-21; PMID:11237208; http://dx.doi.org/ 10.1210/rp.56.1.1 [DOI] [PubMed] [Google Scholar]
- 19.Palimeri S, Palioura E, Diamanti-Kandarakis E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab Syndr Obes 2015; 8:415-26; PMID:26366100; http://dx.doi.org/ 10.2147/DMSO.S63089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newkirk MM, Goldbach-Mansky R, Lee J, Hoxworth J, McCoy A, Yarboro C, Klippel J, El-Gabalawy HS. Advanced glycation end-product (AGE)-damaged IgG and IgM autoantibodies to IgG-AGE in patients with early synovitis. Arthritis Res Ther 2003; 5(2):R82-90; PMID:12718751; http://dx.doi.org/ 10.1186/ar622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butko M, Pallat H, Cordoba A, Yu XC. Recombinant antibody color resulting from advanced glycation end product modifications. Anal Chem 2014; 86(19):9816-23; PMID:25181536; http://dx.doi.org/ 10.1021/ac5024099 [DOI] [PubMed] [Google Scholar]
- 22.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med 1995; 46:223-34; PMID:7598459; http://dx.doi.org/ 10.1146/annurev.med.46.1.223 [DOI] [PubMed] [Google Scholar]
- 23.Wautier JL, Guillausseau PJ. Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab 2001; 27(5 Pt 1):535-42; PMID:11694852. [PubMed] [Google Scholar]
- 24.Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc Res 1998; 37(3):586-600; PMID:9659442; http://dx.doi.org/ 10.1016/S0008-6363(97)00233-2 [DOI] [PubMed] [Google Scholar]
- 25.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation 2002; 105(7):816-22; PMID:11854121; http://dx.doi.org/ 10.1161/hc0702.104183 [DOI] [PubMed] [Google Scholar]
- 26.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, et al.. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 1999; 274(44):31740-9; PMID:10531386; http://dx.doi.org/ 10.1074/jbc.274.44.31740 [DOI] [PubMed] [Google Scholar]
- 27.Virella G, Thorpe SR, Alderson NL, Stephan EM, Atchley D, Wagner F, Lopes-Virella MF, Group DER. Autoimmune response to advanced glycosylation end-products of human LDL. J Lipid Res 2003; 44(3):487-93; PMID:12562876; http://dx.doi.org/ 10.1194/jlr.M200370-JLR200 [DOI] [PubMed] [Google Scholar]
- 28.Ligier S, Fortin PR, Newkirk MM. A new antibody in rheumatoid arthritis targeting glycated IgG: IgM anti-IgG-AGE. Br J Rheumatol 1998; 37(12):1307-14; PMID:9973155; http://dx.doi.org/ 10.1093/rheumatology/37.12.1307 [DOI] [PubMed] [Google Scholar]
- 29.Bouma B, Kroon-Batenburg LM, Wu YP, Brünjes B, Posthuma G, Kranenburg O, de Groot PG, Voest EE, Gebbink MF. Glycation induces formation of amyloid cross-beta structure in albumin. J Biol Chem 2003; 278(43):41810-9; PMID:12909637; http://dx.doi.org/ 10.1074/jbc.M303925200 [DOI] [PubMed] [Google Scholar]
- 30.Yuk IH, Zhang BY, Yang Y, Dutina G, Leach KD, Vijayasankaran N, Shen AY, Andersen DC, Snedecor BR, Joly JC. Controlling glycation of recombinant antibody in fed-batch cell cultures. Biotechnol Bioeng 2011; 108(11):2600-10; PMID:21618472; http://dx.doi.org/ 10.1002/bit.23218 [DOI] [PubMed] [Google Scholar]
- 31.Brady LJ, Martinez T, Balland A. Characterization of nonenzymatic glycation on a monoclonal antibody. Anal Chem 2007; 79(24):9403-13; PMID:17985928; http://dx.doi.org/ 10.1021/ac7017469 [DOI] [PubMed] [Google Scholar]
- 32.Banks DD, Hambly DM, Scavezze JL, Siska CC, Stackhouse NL, Gadgil HS. The effect of sucrose hydrolysis on the stability of protein therapeutics during accelerated formulation studies. J Pharm Sci 2009; 98(12):4501-10; PMID:19388069; http://dx.doi.org/ 10.1002/jps.21749 [DOI] [PubMed] [Google Scholar]
- 33.Gadgil HS, Bondarenko PV, Pipes G, Rehder D, McAuley A, Perico N, Dillon T, Ricci M, Treuheit M. The LC/MS analysis of glycation of IgG molecules in sucrose containing formulations. J Pharm Sci 2007; 96(10):2607-21; PMID:17621682; http://dx.doi.org/ 10.1002/jps.20966 [DOI] [PubMed] [Google Scholar]
- 34.Andya JD, Maa YF, Costantino HR, Nguyen PA, Dasovich N, Sweeney TD, Hsu CC, Shire SJ. The effect of formulation excipients on protein stability and aerosol performance of spray-dried powders of a recombinant humanized anti-IgE monoclonal antibody. Pharm Res 1999; 16(3):350-8; PMID:10213364; http://dx.doi.org/ 10.1023/A:1018805232453 [DOI] [PubMed] [Google Scholar]
- 35.Gadgil HS, Bondarenko PV, Pipes GD, Dillon TM, Banks D, Abel J, Kleemann GR, Treuheit MJ. Identification of cysteinylation of a free cysteine in the Fab region of a recombinant monoclonal IgG1 antibody using Lys-C limited proteolysis coupled with LC/MS analysis. Anal Biochem 2006; 355(2):165-74; PMID:16828048; http://dx.doi.org/ 10.1016/j.ab.2006.05.037 [DOI] [PubMed] [Google Scholar]
- 36.Vrdoljak A, Trescec A, Benko B, Hecimovic D, Simic M. In vitro glycation of human immunoglobulin G. Clin Chim Acta 2004; 345(1–2):105-11; PMID:15193984; http://dx.doi.org/ 10.1016/j.cccn.2004.03.026 [DOI] [PubMed] [Google Scholar]
- 37.Goetze AM, Liu YD, Arroll T, Chu L, Flynn GC. Rates and impact of human antibody glycation in vivo. Glycobiology 2012; 22(2):221-34; PMID:21930650; http://dx.doi.org/ 10.1093/glycob/cwr141 [DOI] [PubMed] [Google Scholar]
- 38.Lapolla A, Tonani R, Fedele D, Garbeglio M, Senesi A, Seraglia R, Favretto D, Traldi P. Non-enzymatic glycation of IgG: An in vivo study. Horm Metab Res 2002; 34(5):260-4; PMID:12063640; http://dx.doi.org/ 10.1055/s-2002-32140 [DOI] [PubMed] [Google Scholar]
- 39.Ahmad S, Moinuddin AA. Immunological studies on glycated human IgG. Life Sci 2012; 90(25–26):980-7; PMID:22634323; http://dx.doi.org/ 10.1016/j.lfs.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Fischer S, Hoernschemeyer J, Mahler HC. Glycation during storage and administration of monoclonal antibody formulations. Eur J Pharm Biopharm 2008; 70(1):42-50; PMID:18583113; http://dx.doi.org/ 10.1016/j.ejpb.2008.04.021 [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Ponniah G, Neill A, Patel R, Andrien B. Identification and comparative quantitation of glycation by stable isotope labeling and LC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 958:90-5; PMID:24705536; http://dx.doi.org/ 10.1016/j.jchromb.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Primack R, Frohn M, Wang W, Luan P, Retter MW, Flynn GC. Impact of glycation on antibody clearance. AAPS J 2015; 17(1):237-44; PMID:25413724; http://dx.doi.org/ 10.1208/s12248-014-9694-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang W, Yan J, Springsteen G, Deeter S, Wang B. A novel type of fluorescent boronic acid that shows large fluorescence intensity changes upon binding with a carbohydrate in aqueous solution at physiological pH. Bioorg Med Chem Lett 2003; 13(6):1019-22; PMID:12643902; http://dx.doi.org/ 10.1016/S0960-894X(03)00086-6 [DOI] [PubMed] [Google Scholar]
- 44.Brena BM, Batista-Viera F, Rydén L, Porath J. Selective adsorption of immunoglobulins and glucosylated proteins on phenylboronate-agarose. J Chromatogr 1992; 604(1):109-15; PMID:1639919; http://dx.doi.org/ 10.1016/0021-9673(92)85535-2 [DOI] [PubMed] [Google Scholar]
- 45.Viski K, Gengeliczki Z, Lenkey K, Ganzler KB. Parallel development of chromatographic and mass-spectrometric methods for quantitative analysis of glycation on an IgG1 monoclonal antibody. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1032:198-204; PMID:27179993; http://dx.doi.org/ 10.1016/j.jchromb.2016.04.043 [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Lu Y, Ma Q, Guo L, Feng YQ. Boronate affinity monolith for highly selective enrichment of glycopeptides and glycoproteins. Analyst 2009; 134(10):2158-64; PMID:19768230; http://dx.doi.org/ 10.1039/b909581k [DOI] [PubMed] [Google Scholar]
- 47.Li YC, Pfuller U, Larsson EL, Jungvid H, Galaev IY, Mattiasson B. Separation of mistletoe lectins based on the degree of glycosylation using boronate affinity chromatography. J Chromatogr A 2001; 925(1–2):115-21; PMID:11519797; http://dx.doi.org/ 10.1016/S0021-9673(01)00967-0 [DOI] [PubMed] [Google Scholar]
- 48.Kislinger T, Humeny A, Peich CC, Becker CM, Pischetsrieder M. Analysis of protein glycation products by MALDI-TOF/MS. Ann N Y Acad Sci 2005; 1043:249-59; PMID:16037245; http://dx.doi.org/ 10.1196/annals.1333.030 [DOI] [PubMed] [Google Scholar]
- 49.Leblanc Y, Bihoreau N, Jube M, Andre MH, Tellier Z, Chevreux G. Glycation of polyclonal IgGs: Effect of sugar excipients during stability studies. Eur J Pharm Biopharm 2016; 102:185-90; PMID:26992291; http://dx.doi.org/ 10.1016/j.ejpb.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 50.Frolov A, Hoffmann R. Analysis of amadori peptides enriched by boronic acid affinity chromatography. Ann N Y Acad Sci 2008; 1126:253-6; PMID:18448825; http://dx.doi.org/ 10.1196/annals.1433.060 [DOI] [PubMed] [Google Scholar]
- 51.Lapolla A, Fedele D, Reitano R, Aricò NC, Seraglia R, Traldi P, Marotta E, Tonani R. Enzymatic digestion and mass spectrometry in the study of advanced glycation end products/peptides. J Am Soc Mass Spectrom 2004; 15(4):496-509; PMID:15047055; http://dx.doi.org/ 10.1016/j.jasms.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 52.Lapolla A, Fedele D, Seraglia R, Traldi P. The role of mass spectrometry in the study of non-enzymatic protein glycation in diabetes: An update. Mass Spectrom Rev 2006; 25(5):775-97; PMID:16625652; http://dx.doi.org/ 10.1002/mas.20090 [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, Tang N, Brock JW, Mottaz HM, Ames JM, Baynes JW, Smith RD, Metz TO. Enrichment and analysis of nonenzymatically glycated peptides: Boronate affinity chromatography coupled with electron-transfer dissociation mass spectrometry. J Proteome Res 2007; 6(6):2323-30; PMID:17488106; http://dx.doi.org/ 10.1021/pr070112q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q, Petyuk VA, Schepmoes AA, Orton DJ, Monroe ME, Yang F, Smith RD, Metz TO. Analysis of non-enzymatically glycated peptides: Neutral-loss-triggered MS(3) versus multi-stage activation tandem mass spectrometry. Rapid Commun Mass Spectrom 2008; 22(19):3027-34; PMID:18763275; http://dx.doi.org/ 10.1002/rcm.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Zhang T, Jiang L, Hewitt D, Huang Y, Kao YH, Katta V. Rapid identification of low level glycation sites in recombinant antibodies by isotopic labeling with 13C6-reducing sugars. Anal Chem 2012; 84(5):2313-20; PMID:22324758; http://dx.doi.org/ 10.1021/ac202995x [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q, Frolov A, Tang N, Hoffmann R, van de Goor T, Metz TO, Smith RD. Application of electron transfer dissociation mass spectrometry in analyses of non-enzymatically glycated peptides. Rapid Commun Mass Spectrom 2007; 21(5):661-6; PMID:17279487; http://dx.doi.org/ 10.1002/rcm.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q, Schepmoes AA, Brock JW, Wu S, Moore RJ, Purvine SO, Baynes JW, Smith RD, Metz TO. Improved methods for the enrichment and analysis of glycated peptides. Anal Chem 2008; 80(24):9822-9; PMID:18989935; http://dx.doi.org/ 10.1021/ac801704j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q, Tang N, Schepmoes AA, Phillips LS, Smith RD, Metz TO. Proteomic profiling of nonenzymatically glycated proteins in human plasma and erythrocyte membranes. J Proteome Res 2008; 7(5):2025-32; PMID:18396901; http://dx.doi.org/ 10.1021/pr700763r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fedorova M, Frolov A, Hoffmann R. Fragmentation behavior of Amadori-peptides obtained by non-enzymatic glycosylation of lysine residues with ADP-ribose in tandem mass spectrometry. J Mass Spectrom 2010; 45(6):664-9; PMID:20527035; http://dx.doi.org/ 10.1002/jms.1758 [DOI] [PubMed] [Google Scholar]
- 60.Chikazawa M, Otaki N, Shibata T, Miyashita H, Kawai Y, Maruyama S, Toyokuni S, Kitaura Y, Matsuda T, Uchida K. Multispecificity of immunoglobulin M antibodies raised against advanced glycation end products: Involvement of electronegative potential of antigens. J Biol Chem 2013; 288(19):13204-14; PMID:23543734; http://dx.doi.org/ 10.1074/jbc.M113.452177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson RN, Metcalf PA, Baker JR. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta 1983; 127(1):87-95; PMID:6825313; http://dx.doi.org/ 10.1016/0009-8981(83)90078-5 [DOI] [PubMed] [Google Scholar]
- 62.Ansari NA, Moinuddin AR. Physicochemical analysis of poly-L-lysine: An insight into the changes induced in lysine residues of proteins on modification with glucose. IUBMB Life 2011; 63(1):26-9; PMID:21280174; http://dx.doi.org/ 10.1002/iub.410 [DOI] [PubMed] [Google Scholar]
- 63.Mashiba S, Uchida K, Okuda S, Tomita S. Measurement of glycated albumin by the nitroblue tetrazolium colorimetric method. Clin Chim Acta 1992; 212(1–2):3-15; PMID:1486680; http://dx.doi.org/ 10.1016/0009-8981(92)90133-B [DOI] [PubMed] [Google Scholar]
- 64.Kennedy DM, Skillen AW, Self CH. Glycation of monoclonal antibodies impairs their ability to bind antigen. Clin Exp Immunol 1994; 98(2):245-51; PMID:7955529; http://dx.doi.org/ 10.1111/j.1365-2249.1994.tb06133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morin LG, Austin GE, Rodey GE, Johnson JE. Nonenzymic glycation of human immunoglobulins does not impair their immunoreactivity. Clin Chem 1989; 35(6):1039-42; PMID:2543516. [PubMed] [Google Scholar]
- 66.Dolhofer R, Siess EA, Wieland OH. Nonenzymatic glycation of immunoglobulins leads to an impairment of immunoreactivity. Biol Chem Hoppe Seyler 1985; 366(4):361-6; PMID:4026990; http://dx.doi.org/ 10.1515/bchm3.1985.366.1.361 [DOI] [PubMed] [Google Scholar]
- 67.Mironova R, Sredovska A, Ivanov I, Niwa T. Maillard reaction products in the Escherichia coli-derived therapeutic protein interferon alfacon-1. Maillard React: Recent Adv Food Biomed Sci 2008; 1126:181-84. [DOI] [PubMed] [Google Scholar]
- 68.Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011; 21(7):949-59; PMID:21421994; http://dx.doi.org/ 10.1093/glycob/cwr027 [DOI] [PubMed] [Google Scholar]
- 69.Adrover M, Marino L, Sanchis P, Pauwels K, Kraan Y, Lebrun P, Vilanova B, Munoz F, Broersen K, Donoso J. Mechanistic insights in glycation-induced protein aggregation. Biomacromolecules 2014; 15(9):3449-62; PMID:25057908; http://dx.doi.org/ 10.1021/bm501077j [DOI] [PubMed] [Google Scholar]
- 70.Wei Y, Chen L, Chen J, Ge L, He RQ. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol 2009; 10:10; PMID:19216769; http://dx.doi.org/ 10.1186/1471-2121-10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei Y, Han CS, Zhou J, Liu Y, Chen L, He RQ. D-ribose in glycation and protein aggregation. Biochim Biophys Acta 2012; 1820(4):488-94; PMID:22274132; http://dx.doi.org/ 10.1016/j.bbagen.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 72.Banks DD, Hambly DM, Scavezze JL, Siska CC, Stackhouse NL, Gadgil HS. The effect of sucrose hydrolysis on the stability of protein therapeutics during accelerated formulation studies. J Pharm Sci 2009; 98(12):4501-10; PMID:19388069; http://dx.doi.org/ 10.1002/jps.21749 [DOI] [PubMed] [Google Scholar]


