Abstract
The melanocortin 2 receptor (MC2R) accessory protein, MRAP, is one of a growing number of G protein-coupled receptor accessory proteins that have been identified in recent years that add control and complexity to G protein-coupled receptor functional expression and signal transduction. MRAP interacts directly with MC2R and is essential for its trafficking from the endoplasmic reticulum to the cell surface, where it acts as the receptor for the pituitary hormone ACTH. In addition, MRAP2, a newly described homolog of MRAP, is also able to support the cell surface expression of MC2R. Although it is clear that MRAP is required for MC2R function, the mechanism of MRAP action is only beginning to be understood. Recent work has started to reveal some of these mechanisms and the MRAP domains involved in MC2R functional expression, and new data have shown a potential role for both MRAP and MRAP2 in the regulation of the other melanocortin receptors.
The MRAP proteins are recently identified accessory proteins for certain melanocortin receptors. Evidence for specific functional domains and a unique antiparallel dimeric structure is reviewed.
Over the past decade, an emerging theme of G protein-coupled receptor (GPCR) biology is the growing number of GPCR accessory proteins, which add control and complexity to GPCR functional expression and signal transduction. GPCR accessory proteins modulate GPCR function, direct receptor trafficking and targeting, moderate signaling intensity, and modify receptor structure and ligand binding. Melanocortin 2 receptor accessory protein (MRAP), and its recently described homolog, MRAP2, are GPCR accessory proteins that function in the melanocortin system (1). The melanocortin receptors (MCRs) are a subfamily of GPCRs that act as the receptors to α-, β-, and γ-MSH and ACTH—peptide hormones originating from the proopiomelanocortin precursor peptide (2, 3). The MCRs have a diverse range of physiological functions; MC1R controls skin pigmentation, MC2R—the receptor for ACTH—plays a critical role in the hypothalamic-pituitary-adrenal axis, whereas MC3R and MC4R have essential roles in energy homeostasis and MC5R is believed to be involved in exocrine function (4).
The existence of an MC2R accessory factor had long been suspected, because unlike other melanocortin receptors, heterologous expression of MC2R does not yield a functional receptor in most cell types (5). MRAP was shown to directly interact with MC2R and is essential for the trafficking of MC2R from the endoplasmic reticulum (ER) to the cell surface (1).
In addition to the role of MRAP in the trafficking of MC2R, there is emerging evidence that MRAP and its homolog MRAP2 may have a much larger role in the melanocortin system. The tissue expression profile of both MRAP proteins extends beyond that of MC2R, suggesting that there is MC2R-independent function of the two proteins (1, 6). Recent work has shown that MRAP and MRAP2 can indeed interact with each of the human MCRs (7). It is interesting that in contrast to the effect identified with MC2R, the MRAP proteins negatively modulate the functional expression of the other MCRs at the level of ligand binding and signal transduction. MRAP and MRAP2 therefore appear to be bidirectional modulators of MCR function.
The Identification of MRAP
Melanocortin 2 receptor
MC2R is the smallest member of the GPCR superfamily of proteins (in terms of number of amino acids) and a member of the melanocortin subfamily of receptors along with the MC1, 3, 4, and 5 receptors (2, 3). The MCRs are the receptors for neuropeptides derived from proopiomelanocortin precursor peptide, which include the melanocyte-stimulating hormones (MSHs) and ACTH. MC2R is expressed primarily in the adrenal cortex, where it acts as the receptor for ACTH. The binding of ACTH to MC2R induces intracellular cAMP production and leads to adrenal glucocorticoid production. Inactivating mutations in MC2R cause the rare autosomal recessive disorder familial glucocorticoid deficiency (FGD; Online Mendelian Inheritance in Man database no. 202200) (8). FGD was first described in the 1960s, is characterized by high ACTH and low cortisol, and if untreated leads to death in early childhood, usually from profound hypoglycemia or overwhelming infection. Early reports speculated that the disease might result from defects in the receptor for ACTH. The melanocortin receptors were cloned in 1992, and the ACTH receptor was proposed to be the MC2R (2, 3). Within a short space of time, mutations in MC2R were found in FGD patients (9, 10). Around 25% of FGD patients have mutations in MC2R, implying that other genetic causes led to the same clinical phenotype (11).
Mutations in MRAP Cause FGD Type 2
In 2005, having applied a single-nucleotide polymorphism genotyping approach in highly informative families with individuals affected by FGD and a normal MC2R, Metherell et al. (1) identified a new locus on chromosome 21q22.1 in some families. Examination of the tissue distribution of the genes expressed within the critical interval revealed a single gene, C21orf61, that was expressed in the adrenal gland, but not in brain or liver. This gene encoded a small protein of unknown function that had previously been reported to be expressed in a differentiating adipocyte cell line after application of proteomic two-dimensional gel mapping and mass spectroscopy (12). At this time, this protein was named fat tissue-specific low molecular weight protein. However, the function of this protein remained speculative. DNA sequencing of C21orf61 in certain FGD families led to the identification of a number of nonsense or splice site mutations. Functional analysis of this gene led to it being re-named MRAP for reasons described in the following section.
MRAP Is Required for Functional MC2R Expression
It has been notoriously difficult to obtain functional MC2R expression in heterologous cell lines. Indeed, functional expression of MC2R in the absence of other melanocortin receptors has only been achieved in a limited number of cell types, such as the Y6 and OS3 cell lines that are derived from a mouse adrenocortical tumor and that had been selected as being ACTH resistant (13). In most cell types, transiently transfected MC2R is retained in the ER (5), whereas in Y6 or OS3 cells MC2R localizes to the cell surface, where it forms a functional ACTH-responsive receptor (14, 15) This inability of MC2R to reach the cell surface in nonadrenal cells led to the belief that an adrenal-specific accessory factor was required to facilitate MC2R trafficking. Therefore, on the identification of MRAP as one of the genes underlying FGD, it was hypothesized that MRAP was an MC2R trafficking factor.
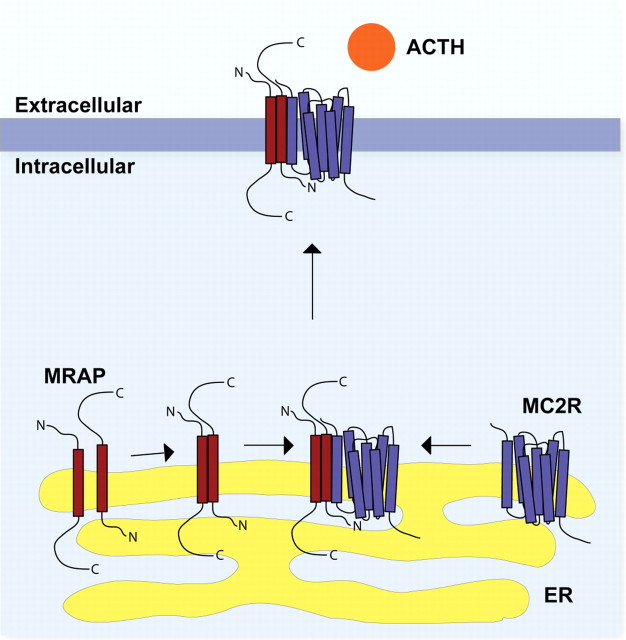
Epitope-tagged mouse MRAP was shown to localize to the ER and plasma membrane of transiently transfected cells by immunocytochemistry (1, 16, 17). MC2R localizes to the ER when expressed in most cell lines. When MRAP and MC2R were coexpressed, they were found to colocalize at the ER, and more important, a significant amount of receptor expression was seen at the plasma membrane colocalizing with MRAP. MC2R and MRAP were shown to interact by coimmunoprecipitation (1). Furthermore, cells expressing MC2R after heterologous transfection were shown to be ACTH responsive only when MRAP was cotransfected (1). These results indicate that MRAP is an accessory protein for MC2R, which is required for the trafficking of MC2R to the cell surface, allowing the formation of an ACTH-responsive receptor (Fig. 1). Mouse Y1 cells endogenously express both MC2R and MRAP and show a cAMP response to ACTH stimulation. Small interfering RNA knockdown of endogenous mMRAP resulted in a loss of responsiveness to ACTH, which was rescued through the transient expression of small interfering RNA-insensitive human MRAPα (18).
Fig. 1.
Hypothetical model of the interaction of MRAP with the MC2R. MRAP (red) forms an antiparallel homodimer within the ER. This homodimer interacts with the MC2R (purple), possibly assisting its folding into an appropriate conformation. After further posttranslational modification in the ER and Golgi, the heterotrimeric structure then traffics to the cell surface, where it is capable of recognizing and responding to the ACTH peptide.
MRAP Gene Structure and Conservation
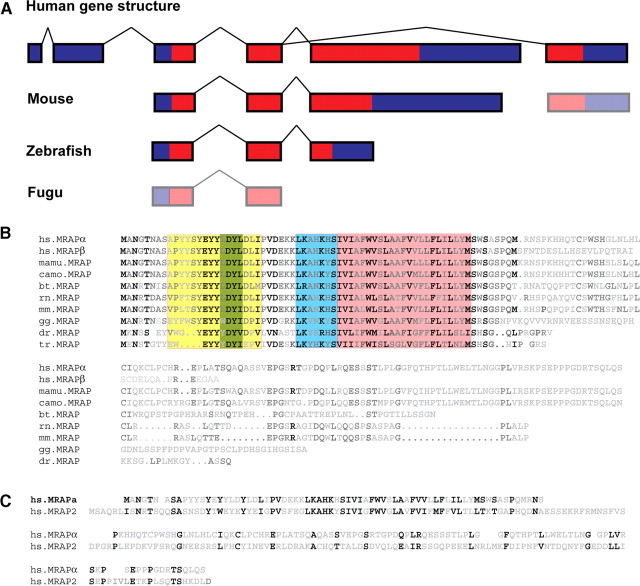
Human MRAP is a six-exon gene spanning a 23-kb region on chromosome 21q22.1 (Fig. 2A). Exons 3 to 5 encode the 172-amino-acid MRAPα. Alternative splicing of exon 4 to exon 6 results in the 102-amino-acid MRAPβ isoform. The transmembrane domain of MRAP is encoded by exon 4; therefore, the MRAPα and MRAPβ proteins have identical N-termini and transmembrane domains and differ in their C-termini. It is interesting that MRAP appears to have alternative transcriptional start sites. Our observations lead us to believe that exons 1 and 2, which consist of 177 nucleotides of 5′-UTR, are not present in MRAP mRNA transcripts in the adrenal gland. Instead, transcription begins at exon 3 with MRAPα being encoded by exons 3, 4, and 5 and MRAPβ by exons 3, 4, and 6. Furthermore, the scarcity of ESTs containing the 5′-UTR exons 1 or 2 suggests that the principal transcriptional start site is at exon 3. Exons 1 and 2 have been identified previously by rapid amplification of cDNA ends using RNA prepared from human adult brain (6). It therefore seems likely that different MRAP transcriptional start sites are used in a tissue-specific manner, although the transcriptional control of this is presently obscure.
Fig. 2.
Conservation of MRAP and MRAP2. A, MRAP gene structure in different species. The human MRAP gene consists of six exons, with exons 3 to 6 coding for the MRAP peptide. Alternative splicing of exon 4 to 5 or 4 to 6 gives rise to MRAPα and MRAPβ respectively in the human. This gene structure shows surprisingly little conservation between species. Lower mammals (represented by mouse) do not have the noncoding exons 1 and 2, whereas the presence of exon 6 is unclear at present. In lower vertebrates such as zebrafish, MRAP is encoded by three exons, which are homologous to human exons 3 to 5. However, in fugu homologous sequence can only be identified to human exons 3 and 4. B, Sequence alignments of MRAP. Alignment of human MRAPα with MRAPβ and MRAP from Macacca mulatta (marmoset; old world primate), Callicebus moloch (red-bellied titi; new world primate), Bos taurus (domestic cow), Rattus norvegicus (Norway rat), Mus musculus (mouse), Gallus gallus (chicken), Danio zerio (zebrafish), and Takifugu rubripes (fugu). MRAP shows little conservation across species, with sequence homology restricted to the N-terminus and transmembrane regions. Notable conservation lies in the surface expression domain (yellow), including the short region proposed to be essential for ligand binding by MC2R (green), the antiparallel dimerization domain (blue), and the transmembrane domain (pink). There is very little homology over the C-terminus, which varies greatly in length. C, Alignment of human MRAPα with human MRAP2. MRAPα and MRAP2 show 27% sequence identity, although again, the majority of conservation is across the N-terminus and transmembrane regions. D, Alignment of human MRAP2 with mouse MRAP2, Xenopus tropicalis MRAP2, the two zebrafish MRAP2 genes, and fugu MRAP2. As in panel B, the conserved transmembrane domain is highlighted in pink and the predicted antiparallel homodimerization domain is highlighted in blue. bt, B. taurus; camo, C. moloch; dr, zebrafish; gg, chicken; hs, H. sapiens; mamu, M. mulatta; mm, mouse; rn, R. norvegicus; tr, fugu; xtr, X. tropicalis.
Surprisingly, MRAP shows little overall conservation in either gene structure or protein sequence (Fig. 2, A and B). For example, there is only 63% sequence identity between human MRAPα and mouse MRAP, with most of the homology covering the N-terminus and transmembrane regions. The C-terminus shows negligible sequence homology between species and varies greatly in length; for example, there are 112 amino acids C-terminal to the last residue of the transmembrane domain in humans compared with 27 amino acids in zebrafish. Furthermore, sequence analysis fails to identify the two 5′-UTR exons from humans outside of primates, suggesting that in other mammals and lower vertebrates transcription starts at a homologous exon to human exon 3, whereas exons 1 and 2 are primate specific. The MRAPβ isoform may also be primate specific. There are no expression data to suggest an MRAPβ isoform in nonprimates; however, there is some sequence similarity between human exon 6 and the syntenic genomic region in lower mammals. In lower vertebrates, MRAP is only readily identified in zebrafish, where there are three exons encoding a gene homologous to MRAPα and no evidence to suggest the presence of an MRAPβ isoform. In both fugu and tetraodon, there is no annotated MRAP ortholog. Sequence comparison of the syntenic genomic sequence reveals potential MRAP sequences. Exons homologous to human exons 3 and 4 can be found in both species; however, at present it is unclear whether there are any further exons, or if these sequences are even transcribed. It may be that fugu MC2R does require MRAP to traffic to the cell surface, as functional studies have used cells in which human MC2R can be expressed, although it is not clear whether this receptor could be expressed in cells lacking MRAP (19). It is possible that if some lower vertebrates have indeed lost MRAP, it may be that MC2R functional expression is controlled by the ortholog of MRAP2 in these species.
An MRAP Ortholog
Sequence analysis of the human genome had previously identified a single MRAP homolog as a gene of unknown function, C6orf117, which has now been named MRAP2 (Fig. 2C). This gene likely resembles the ancestral MRAP gene and shows 27% sequence identity to MRAPα but much higher conservation between species than MRAP. For example, there is 87% sequence identity between human and mouse, and 44% identity between human and fugu (Fig. 2D). Interestingly, the MRAP2 gene has been duplicated in zebrafish. The two copies, one transcribed from chromosome 4 and one transcribed from chromosome 16, show 46% and 52% sequence identity to human MRAP2, respectively.
Mechanism of MRAP Action
Receptor accessory proteins
MRAP shares functional similarity with other receptor accessory proteins that have been identified in the past decade (20, 21, 22, 23, 24). The receptor accessory modifying proteins (RAMPs) are a family of three related proteins that control both the cell surface expression and the ligand selectivity of the calcitonin receptor and calcitonin-like receptors, both class II GPCR (20, 21). RAMP1 results in calcitonin-like receptors acting as the receptor for calcitonin gene-related peptide, whereas RAMP2 and 3 cause calcitonin-like receptors to act as the receptor for adrenomedullin. In the case of the calcitonin receptor, RAMP association leads to this receptor attaining high affinity for amylin (25). Additionally, the RAMPs have been shown to interact with a number of class II and class III GPCRs; however the physiological importance of these interactions remains to be determined (22).
Odorant receptors are the largest family of GPCRs. As with MC2R, it has been notoriously difficult to study these because of the difficulty in achieving functional expression of many odorant receptors in heterologous cell lines. The receptor transporting proteins (RTPs) 1 and 2 and the related receptor expression-enhancing protein (REEP) 1, along with a number of potential RTP and REEP homologs, have been shown to allow the functional cell surface expression of a number of odorant receptors, and more recently, RTP and REEP family members have been described as being able to assist the cell surface expression of taste receptors (23, 24). The RTPs and REEPs are single transmembrane domain proteins and share no sequence homology with either MRAP or the RAMPs. Unlike the RAMPs, they are found at the cell surface with their N-terminus on the inside of the cell and the C-terminus on the outside of the cell.
There is no obvious sequence homology between these different classes of accessory proteins, although certain similarities exist. All are relatively small single transmembrane domain glycoproteins that directly interact with one or more GPCRs, apparently initially in the ER, and they subsequently traffic with their partner receptor to the plasma membrane. Here they may contribute to ligand affinity. Although membrane topology differs, these proteins have the ability to homodimerize and heterodimerize with accessory proteins from the same family, although this is not necessarily a requirement for function as it is in the case of MRAP (see MRAP Forms a Unique Antiparallel Homodimer section).
MRAP Functional Domains
The available evidence suggests that the functions of MRAP include interaction with the MC2R in addition to possible chaperone-like and/or trafficking roles and potentially a ligand interaction-signal transduction role. The MRAP protein may undertake all of these roles intrinsically or may act as an “adapter” to enable other specialized proteins to conduct these functions with the MC2R. Understanding the functional domains of MRAP may help define these mechanisms (Fig. 3).
Fig. 3.
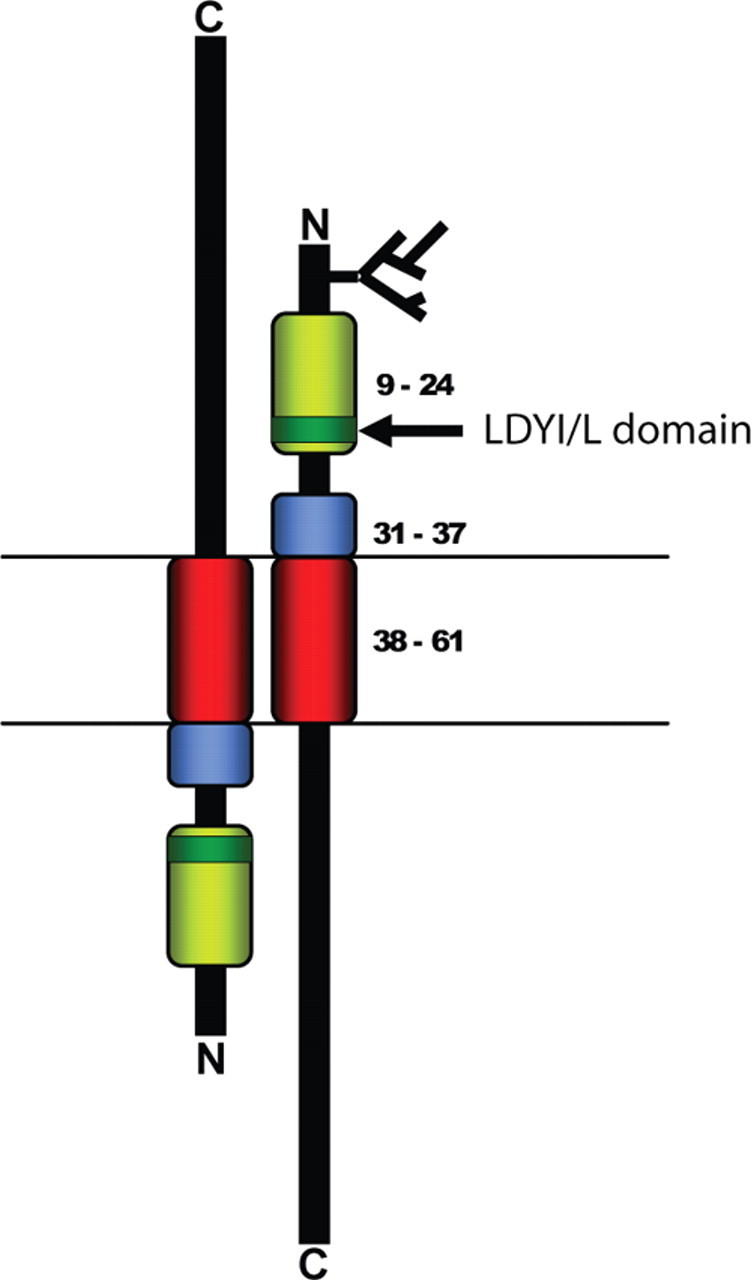
Functional domains of MRAP. Three functionally distinct domains of MRAP can be defined. The transmembrane domain (red; residues 36-61) is responsible for membrane anchorage of MRAP and for the physical interaction between MRAP and MC2R and between MRAP and its homodimeric partner. The cell-surface expression domain (yellow; residues 9-24) was shown by Webb et al. (26 ) to be required for effective trafficking of the MC2R to the plasma membrane. Sebag and Hinkle (27 ) reported that the four-amino-acid sequence LDYI between positions 18 and 21 (green) was required for effective MC2R ligand binding and signal transduction. The short sequence between positions 31 and 37 immediately N-terminal to the transmembrane (blue) domain was found to be required for adoption of the antiparallel homodimer structure unique to these proteins (27 ).
MRAP-MC2R Interaction
This domain of MRAP has proven the simplest to identify in that robust MRAP-MC2R coimmunoprecipitation assays have been used to demonstrate their interaction already. Webb et al. (26) used a deletion construct and co-immunoprecipitation (IP) approach to show that the sequence between residues 36 and 61 of hMRAPα was required for MC2R interaction. This is effectively the transmembrane domain of the protein. It is therefore likely that MRAP interacts with MC2R via hydrophobic interactions with one or more of its seven transmembrane domains. However, the ability of MRAP to interact with MC2R is not in itself sufficient to promote receptor trafficking.
MC2R Cell Surface Expression
A second key function of MRAP is that of trafficking MC2R to the cell surface. Webb et al. (26) demonstrated using confocal imaging and a cell surface assay that key elements in this function were located between residues 9 and 24 of hMRAP-α. This 16-residue domain is rich in tyrosine residues (six of 16 residues) but does not seem to be required for cell surface expression of MRAP itself, as judged by confocal imaging. Although sequence homology is not readily identifiable between the MRAP proteins and other proteins involved in GPCR function, this region has some similarities to a region within another GPCR trafficking protein. REEP1 was first identified as a transmembrane protein that is able to promote the cell surface expression of odorant receptors (23). Like the MRAP proteins, REEP1 has a tyrosine-rich region in its N-terminus. It is therefore possible that a tyrosine-rich domain may be a feature of some proteins involved in GPCR trafficking.
In contrast to Webb et al.’s (26) findings, Sebag and Hinkle (27), using mMRAP, found that residues 1-30 could be removed without any deleterious effect on this function. These findings seem to contradict each other, and, although species differences may be an explanation, only six amino acids differ over the first 37 N-terminal residues, and most of these are conservative substitutions. The assays for cell surface expression used were also relatively similar in that they both required the immunodetection of an epitope tag on the MC2R.
MRAP Forms a Unique Antiparallel Homodimer
The mMRAP protein has a predicted molecular mass of 14.1 kDa. However, by Western blot analysis against both transiently transfected epitope-tagged MRAP and using an MRAP antibody to detect endogenous protein in mouse Y1 adrenal cells, a band of more than 30 kDa has been reported (18). It was suggested that this species represents a sodium dodecyl sulfate-resistant homodimer. Immunoprecipitation and mass spectroscopic analysis of this higher molecular weight band confirmed that it contained MRAP protein consistent with the homodimer hypothesis. Furthermore, MRAP has been shown to homodimerize by transient transfection of MRAP with different epitope tags and coimmunoprecipitation (17, 18).
Since its initial identification, it has been recognized that the membrane topology of MRAP was not obvious. It lacks an N-terminal signal peptide (in contrast to the RAMPs) and in silico prediction tools suggest an even balance of N-terminal outside and C-terminal outside orientations. In elegant studies, Sebag and Hinkle (17) demonstrated that MRAP existed as a dual-topology antiparallel homodimer both by staining for N- or C-terminal epitope tags after overexpression and by using antibodies directed against the N- and C-termini of MRAP in Y1 cells that endogenously express MRAP. Engineered glycosylation sites in the C-terminus also lend support to this view (17). It also appears that this dual-topology homodimer exists in a stable complex with MC2R as suggested by coimmunoprecipitation studies. Dual topology is a unique structure in naturally occurring eukaryotic proteins.
Sebag and Hinkle (27) showed that residues 31-37 of mMRAP were required for formation of this dual-topology homodimer. When these residues were deleted, MRAP was present on the cell surface only in the N-terminus outside orientation. Furthermore, co-IP studies suggested that in the absence of these residues, MRAP would not homodimerize and was unable to assist MC2R trafficking. Perhaps the simplest model by which this domain might direct the antiparallel dimer formation would be for it to interact with a region lying C-terminal to the transmembrane domain. However, as described in the C-Terminus of MRAP section, the C-terminus is not essential for MRAP function, and thus a more complex mode of action for the 31–37 dimerization motif must be considered. Residues 31-37 are positively charged and thus likely to influence membrane orientation, positioned as they are immediately proximal to the transmembrane domain. Loss of this positively charged region alone may be sufficient to alter the balance of bidirectional orientation, thus leading to loss of antiparallel homodimerization. Other possibilities may include an interaction between this region and the receptor or with another as yet unidentified protein.
It is notable that this dimerization motif lies within the 16-residue region shown by Webb et al. (26) to be required for effective MC2R trafficking and is consistent with the view that the antiparallel homodimer is an essential requirement for receptor trafficking. Studies using bimolecular fluorescent complementation (28) suggested that the MRAP antiparallel homodimer forms in the ER (29, 30) and remains in this configuration throughout trafficking to the plasma membrane.
MC2R Signaling
Although cell surface expression of the MC2R is likely to be an essential requirement for signal generation, there is increasing evidence that the role of MRAP in these two processes may be distinct. Webb et al. (26) found that the tyrosine-rich region between residues 9 and 24 of hMRAP-α was required for both cell surface expression and a cAMP response to ACTH stimulation. Sebag and Hinkle (27) reported similar findings using mMRAP, showing that the conserved region between residues 10 and 20 was essential for ACTH signaling, and in particular the residues between 18 and 21 were required for ligand binding and signal generation.
C-Terminus of MRAP
As mentioned earlier, the C-terminal portion of MRAP is not conserved across species. In humans, MRAP is alternatively spliced to encode two proteins known as MRAPα and MRAPβ, identical in their N-terminal and transmembrane domains, but differing only in their C-terminal tail. MRAP constructs in which the entire C-terminus has been deleted appear to be able to function normally (17, 26). The C-terminus is not required for MRAP to interact with MC2R or for MRAP to traffic MC2R to the cell surface. Instead, the C-terminus of MRAP may have a regulatory effect on MC2R trafficking and function. Both MRAPα and MRAPβ have been shown to increase MC2R cell surface expression and produce an ACTH-responsive MC2R. Interestingly, MRAPβ was proposed to result in higher MC2R cell surface expression than MRAPα in both transiently transfected and stable cell lines. This observation was reflected in greater cAMP production after ACTH stimulation, although sensitivity to ACTH appears to be slightly stronger in the presence of MRAPα (16). The in vivo relevance of these observations is currently unclear. Both MRAPα and MRAPβ mRNA are expressed in the human adrenal gland at similar levels relative to glyceraldehyde-3-phosphate dehydrogenase (1); however, it is not known whether expression changes with age or on certain stresses or signals.
MRAP2, a Novel Paralog of MRAP
As discussed earlier, MRAP has a single paralog in the human genome in the form of C6orf117. This gene encodes a 205-residue single transmembrane domain protein with 27% homology to MRAPα. Recent work has identified a potential role for this paralog in the trafficking and functional expression of MC2R, and this has now been renamed MRAP2. Expression of MRAP2 was identified in human adult adrenal gland and brain by RT-PCR from a panel of cDNAs, and, using an antibody directed against the C-terminus of the orthologous mouse MRAP2, expression was confirmed in mouse adrenal gland and brain (7). In situ hybridization suggests that this gene appears to be primarily expressed in the ventromedial hypothalamus in the brain (31), a site of expression of the MC3R and MC4R (32, 33). No expression was detected in any other tissue tested.
MRAP2 appears to have a very similar ability to MRAP in the trafficking of MC2R to the cell surface (7, 27). MC2R and MRAP2 were shown to interact by coimmunoprecipitation after transient transfection into a heterologous cell line lacking endogenous melanocortin receptors and to colocalize in the ER and at the cell surface by confocal microscopy. Furthermore, MRAP and MRAP2 were able to heterodimerize in this model. It is notable that the antiparallel dimerization domain of MRAP lying between residues 31 and 37 in MRAP is almost completely conserved in MRAP2. Using a cell surface assay, significant MC2R expression at the cell surface was only found when coexpressed with MRAP2. Furthermore, when coexpressed with MRAP2, MC2R forms a functional ACTH receptor, as shown by a cAMP response following 10−6 m ACTH stimulation. Therefore, MRAP2 appears to be able to perform the same function as MRAP in the expression of MC2R in heterologous cells. In contrast, Sebag and Hinkle (30) were not able to demonstrate an MRAP-like signaling function for mMRAP2 in response to 10−7 m ACTH unless the Leucine-Aspartis Acid-Tyrosine Isoleucine (LDYI) sequence between residues 18 and 21 of mMRAP was introduced. An explanation for these discrepancies may lie in an altered dose responsiveness of the MRAP2-MC2R complex.
The physiological relevance of MRAP2 in ACTH signaling in the adrenal gland is unclear at present. The in vitro data suggest that MC2R would require MRAP or MRAP2 to traffic to the cell surface and form an ACTH-responsive receptor. However, in FGD patients with MRAP mutations, MRAP2 does not replace MRAP function. This perhaps suggests that MRAP2 expression is not found in the same regions of the adrenal gland as MRAP, or possibly is not expressed at the same stages of development. Further investigation of the temporal and spatial expression patterns of the two MRAP proteins in relation to MC2R is required to fully understand the roles of MRAP and MRAP2 in MC2R signaling. Alternatively, the MRAP2-MC2R complex may be less responsive to the concentrations of ACTH found in the circulation.
MRAP2 N-Glycosylation
As with MRAP, MRAP2 was shown to be N-glycosylated (7). With a MRAP2 mutant in which the normally glycosylated asparagine at position 9 was substituted with glutamine and which was therefore incapable of being glycosylated, MC2R trafficked to the cell surface. However, this MRAP2 mutant did not support a functional MC2R. Stimulation with ACTH did not result in cAMP generation when MC2R was coexpressed with the MRAP2 glycosylation mutant. This result, therefore, suggests that MRAP2 has two roles in the functional expression of MC2R, one in receptor trafficking and one in ligand binding or receptor signaling. These findings mirror those with MRAP, suggesting distinct trafficking and signaling functions for these accessory proteins.
MRAP Proteins as Bidirectional Modulators of Melanocortin Receptors
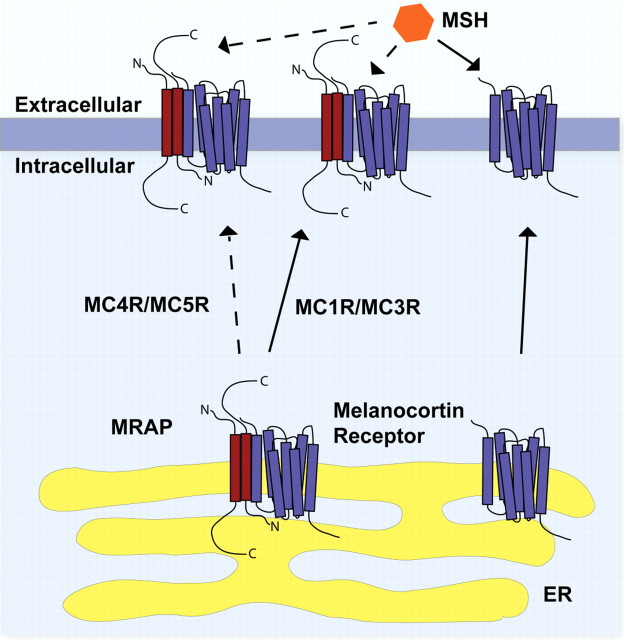
Until recently, the study of MRAP has been restricted to the mechanism of its role in the functional expression of MC2R. Recent work suggests that both MRAP and MRAP2 may have a novel role in the regulation of MCR functional expression through a combination of receptor cell surface trafficking and the modulation of signaling. Each of the MCRs has been shown to interact with both MRAP proteins in co-IP studies (7, 30). However, unlike MC2R, the other melanocortin receptors traffic reasonably efficiently to the cell surface in the absence of an MRAP. Intriguingly, it appears that MRAP and MRAP2 act as negative regulators of MCR function. Reduced signaling, shown by a reduced cAMP response to nor-leucine d-phenylalanine (NDP)-MSH stimulation was identified with all of the MCRs other than MC2R when coexpressed with either or both MRAPs (7) (Fig. 4).
Fig. 4.
Hypothetical model of the regulation of MCRs by the MRAP proteins. In the absence of MRAP (or MRAP2), the MCRs (purple) traffic to the cell surface from the ER and respond to MSH peptides. In a heterotrimeric complex with MRAP or MRAP2 (red), both MC4R and MC5R show impaired cell surface expression. MRAP or MRAP2 does not change the trafficking of MC1R and MC3R. At the cell surface, these heterotrimeric MRAP-MCR complexes show reduced signaling in response MSH.
Sebag and Hinkle (30) studied this effect on the MC5R in further detail and argued that MRAP impairs MC5R dimer-oligomer formation (but not MC2R dimerization) using either bimolecular fluorescence complementation or co-IP approaches, and they proposed that this dimer-disrupting action is the mechanism for this negative effect. Using deletion mutants of MRAP, it was apparent that the N-terminal 30 amino acids of the molecule were essential for this inhibitory action and that deletion of the 31–37 region required for dual-topology formation was without influence. Thus, antiparallel MRAP dimers are not required for the inhibitory action on MC5R.
Conclusions
The MRAPs join several other GPCR accessory proteins that have been identified in the past decade. MRAP forms a unique antiparallel homodimer in the ER and interacts with the MC2R to promote its trafficking to the plasma membrane. It is also required for the receptor to generate a signaling response to ACTH, probably by assisting binding of ligand to the receptor. In addition, MRAP2, a newly identified paralog of MRAP, is also able to interact with and support the functional expression of MC2R. Moreover, MRAP and MRAP2 can interact with the remaining melanocortin receptors and reduce receptor expression and signaling at the cell surface, probably by interfering with receptor dimer formation. These latter actions are of significant interest in view of MC3R and MC4R, which, because of their involvement in the central regulation of metabolism, appetite, and food intake are the focus of much pharmaceutical research, and thus the MRAP proteins might prove to be important future targets of therapeutic manipulation.
Footnotes
Present address for T.R.W.: Institute of Ophthalmology, University College London, London EC1V 9EL, United Kingdom.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 23, 2009
Abbreviations: ER, Endoplasmic reticulum; FGD, familial glucocorticoid deficiency; GPCR, G protein-coupled receptor; IP, immunoprecipitation; MC2R, melanocortin 2 receptor; MRAP, MC2R accessory protein; MCRs, melanocortin receptors; MSHs, melanocyte-stimulating hormones; RAMPs, receptor accessory modifying proteins; REEP, receptor expression-enhancing protein; RTP, receptor transporting proteins.
References
- 1.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJL2005. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- 2.Cone RD, Mountjoy KG, Robbins LS Nadeau JH, Johnson KR, Roselli-Rehfuss L, Mortrud MT1993. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann NY Acad Sci 680:342–363 [DOI] [PubMed] [Google Scholar]
- 3.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD1992. The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251 [DOI] [PubMed] [Google Scholar]
- 4.Cone RD2006. Studies on the physiological functions of the melanocortin system. Endocr Rev 27:736–749 [DOI] [PubMed] [Google Scholar]
- 5.Noon LA, Franklin JM, King PJ, Goulding NJ, Hunyady L, Clark AJ2002. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol 174:17–25 [DOI] [PubMed] [Google Scholar]
- 6.Gardiner K, Slavov D, Bechtel L, Davisson M2002. Annotation of human chromosome 21 for relevance to Down syndrome: gene structure and expression analysis. Genomics 79:833–843 [DOI] [PubMed] [Google Scholar]
- 7.Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertová M, Elphick MR, Cheetham ME, Metherell LA, Clark AJ2009. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA 106:6146–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AJ, Weber A1998. Adrenocorticotropin insensitivity syndromes. Endocr Rev 19:828–843 [DOI] [PubMed] [Google Scholar]
- 9.Clark AJ, McLoughlin L, Grossman A1993. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341:461–462 [DOI] [PubMed] [Google Scholar]
- 10.Tsigos C, Arai K, Hung W, Chrousos GP1993. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest 92:2458–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark AJ, Metherell LA, Cheetham ME, Huebner A2005. Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol Metab 16:451–457 [DOI] [PubMed] [Google Scholar]
- 12.Xu A, Choi KL, Wang Y, Permana PA, Xu LY, Bogardus C, Cooper GJ2002. Identification of novel putative membrane proteins selectively expressed during adipose conversion of 3T3-L1 cells. Biochem Biophys Res Commun 293:1161–1167 [DOI] [PubMed] [Google Scholar]
- 13.Schimmer BP, Kwan WK, Tsao J, Qiu R1995. Adrenocorticotropin-resistant mutants of the Y1 adrenal cell line fail to express the adrenocorticotropin receptor. J Cell Physiol 163:164–171 [DOI] [PubMed] [Google Scholar]
- 14.Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, Gantz I1997. Effects of agouti-signaling protein on melanocortin action. Mol Endocrinol 11:274–280 [DOI] [PubMed] [Google Scholar]
- 15.Elias LL, Huebner A, Pullinger GD, Mirtella A, Clark AJ1999. Functional characterization of naturally occurring mutations of the human adrenocorticotropin receptor: poor correlation of phenotype and genotype. J Clin Endocrinol Metab 84:2766–2770 [DOI] [PubMed] [Google Scholar]
- 16.Roy S, Rached M, Gallo-Payet N2007. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms α and β in isogenic human embryonic kidney 293 cells. Mol Endocrinol 21:1656–1669 [DOI] [PubMed] [Google Scholar]
- 17.Sebag JA, Hinkle PM2007. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA 104:20244–20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooray SN, Almiro Do Vale I, Leung KY, Webb TR, Chapple JP, Egertová M, Cheetham ME, Elphick MR, Clark AJ2008. The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology 149:1935–1941 [DOI] [PubMed] [Google Scholar]
- 19.Klovins J, Haitina T, Fridmanis D, Kilianova Z, Kapa I, Fredriksson R, Gallo-Payet N, Schiöth HB2004. The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol Biol Evol 21:563–579 [DOI] [PubMed] [Google Scholar]
- 20.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- 21.Hay DL, Poyner DR, Sexton PM2006. GPCR modulation by RAMPs. Pharmacol Ther 109:173–197 [DOI] [PubMed] [Google Scholar]
- 22.Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM2003. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem 278:3293–3297 [DOI] [PubMed] [Google Scholar]
- 23.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H2004. RTP family members induce functional expression of mammalian odorant receptors. Cell 119:679–691 [DOI] [PubMed] [Google Scholar]
- 24.Behrens M, Bartelt J, Reichling C, Winnig M, Kuhn C, Meyerhof W2006. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J Biol Chem 281:20650–20659 [DOI] [PubMed] [Google Scholar]
- 25.Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, Main MJ, Foord SM, Sexton PM1999. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 56:235–242 [DOI] [PubMed] [Google Scholar]
- 26.Webb TR, Chan L, Cooray SN, Cheetham ME, Chapple JP, Clark AJ2009. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 150:720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebag JA, Hinkle PM2009. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem 284:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerppola TK2006. Visualization of molecular interactions by fluorescence complementation. Nat Rev Mol Cell Biol 7:449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinkle PM, Sebag JA2009. Structure and function of the melanocortin2 receptor accessory protein. Mol Cell Endocrinol 300:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebag JA, Hinkle PM2009. Opposite effects of the melanocortin-2 receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J Biol Chem 284:22641–22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, et al.2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176 [DOI] [PubMed] [Google Scholar]
- 32.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- 33.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH2000. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26:97–102 [DOI] [PubMed] [Google Scholar]