Abstract
Previously, we demonstrated that bone marrow-derived mesenchymal stem cells (MSCs) differentiate into steroidogenic cells such as Leydig and adrenocortical cells by the introduction of steroidogenic factor-1 (SF-1) and treatment with cAMP. In this study, we employed the same approach to differentiate umbilical cord blood (UCB)-derived MSCs. Despite UCB-MSCs differentiating into steroidogenic cells, they exhibited characteristics of granulosa-luteal-like cells. We found that peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) was expressed and further induced by cAMP stimulation in UCB-MSCs. Consistent with these results, tissue-specific expression of Pgc-1α was observed in rat ovarian granulosa cells. PGC-1α binds to the NR5A family [SF-1 and liver receptor homolog-1 (LRH-1)] of proteins and markedly enhances their transcriptional activities. Reporter assays revealed that PGC-1α activated the promoter activities of SF-1 and LRH-1 target genes. Infection of KGN cells (a human cell line derived from granulosa cells) with adenoviruses expressing PGC-1α resulted in the induction of steroidogenesis-related genes and stimulation of progesterone production. PGC-1α also induced SF-1 and LRH-1, with the latter induced to a greater extent. Knockdown of Pgc-1α in cultured rat granulosa cells resulted in attenuation of gene expression as well as progesterone production. Transactivation of the NR5A family by PGC-1α was repressed by Dax-1. PGC-1α binds to the activation function 2 domain of NR5A proteins via its consensus LXXLL motif. These results indicate that PGC-1α is involved in progesterone production in ovarian granulosa cells by potentiating transcriptional activities of the NR5A family proteins.
PGC-1alpha regulates the progesterone production in ovarian granulosa cells at multiple levels.
The differentiation of ovarian granulosa cells by gonadotropins is associated with the induction of responsiveness to LH and the steroidogenic pathway (1, 2). LH stimulates ovulation by acting on the induced LH receptors in granulosa cells. Granulosa cells differentiate from predominantly estrogen-producing cells into predominantly progesterone-producing luteal cells. Although dramatic alternation in transcription of multiple genes appears to occur during this transition, the detailed mechanism by which this occurs remains unclear.
It has been reported that ovarian steroidogenesis is regulated by multiple nuclear receptors such as steroidogenic factor-1 (SF-1), also known as adrenal 4 binding protein (Ad4BP), liver receptor homolog-1 (LRH-1), and dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1 (DAX-1) (3, 4, 5, 6, 7, 8). SF-1 and LRH-1 belong to the NR5A subfamily of nuclear receptors (9). They function as monomers to regulate genes by binding to similar response elements (10, 11, 12). SF-1 is essential for normal adrenal and gonadal development given that SF-1 knockout mice exhibit adrenal and gonadal agenesis, resulting in postnatal death due to severe adrenal insufficiency (13, 14). SF-1 regulates the cell-specific expression of a variety of different genes involved in steroidogenesis including a number of steroid hydroxylases (10, 11). Aided with cAMP, SF-1 can induce the differentiation of bone marrow (BM)-derived mesenchymal stem cells (MSCs) into steroidogenic cells such as testicular Leydig cells and adrenocortical cells (15, 16). In the ovary, SF-1 is expressed in granulosa and theca cells (17). Impairment of SF-1 expression in granulosa cells can result in multiple defects such as abnormal estrous cycle, infertility, and reduction of steroidogenesis (3). LRH-1 is mainly expressed in tissues of endodermal origin, such as the liver and intestine (18, 19). Recently, elevated expression of LRH-1 has been demonstrated in gonads, suggesting the involvement of LRH-1 in steroidogenesis (4, 5, 6, 7, 20, 21). Additionally, we recently reported that LRH-1 could induce the differentiation of MSCs into steroidogenic cells, as is the case with SF-1 (22). In the ovary, LRH-1 is exclusively expressed in granulosa cells and shown to be involved in ovulation (4). Expression of LRH-1 is much higher than SF-1 expression in the corpus luteum (5).
DAX-1 has been shown to repress the transcriptional activities of both SF-1 and LRH-1 (23). The human DAX-1 gene is located on the X chromosome at p21, which, when duplicated, gives rise to XY females, a condition referred to as dosage-sensitive sex reversal. DAX-1 mutations are associated with the pathogenesis of adrenal hypoplasia congenita and hypogonadotropic hypogonadism (24, 25). In granulosa cells, DAX-1 negatively regulates steroidogenesis and is down-regulated by FSH (5, 7, 8).
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcriptional coactivator of nuclear receptors as well as some other transcription factors (26, 27). It regulates adaptive thermogenesis in brown adipose tissue (28), fiber type switching in skeletal muscle (29), and β-oxidation of fatty acids (30) and gluconeogenesis in the liver (31, 32). PGC-1α also promotes insulin resistance in the liver through peroxisome proliferator-activated receptor (PPAR)α-dependent induction of TRB-3, a fasting-inducible inhibitor of serine-threonine kinase Akt/PKB (33). It was recently reported that mice with a liver-specific heterozygous knockout of the PGC-1α gene exhibited an insulin-resistant phenotype (34). Insulin resistance is a common feature of polycystic ovary syndrome (PCOS), the most common endocrinopathy in women and the most common cause of anovulatory infertility, affecting 5–10% of the population (35, 36). Although PCOS is associated with intrinsic theca dysregulation leading to hyperandrogenism, it was also reported that some abnormalities occur in granulosa cells (37, 38).
In this study, we investigated the role of PGC-1α in the steroidogenesis of ovarian granulosa cells. We demonstrated that PGC-1α protein was expressed exclusively in granulosa cells. We also showed that PGC-1α potentiated the transcriptional activation of steroidogenesis-related genes by the NR5A family proteins. Overexpression of PGC-1α in granulosa cells induced the genes essential for progesterone synthesis, whereas knockdown of PGC-1α in granulosa cells attenuated the expression of these genes. Our results indicate that PGC-1α represents one of the important factors for progesterone production in luteinized granulosa cells.
Results
UCB-derived MSCs differentiated into granulosa-luteal-like cells
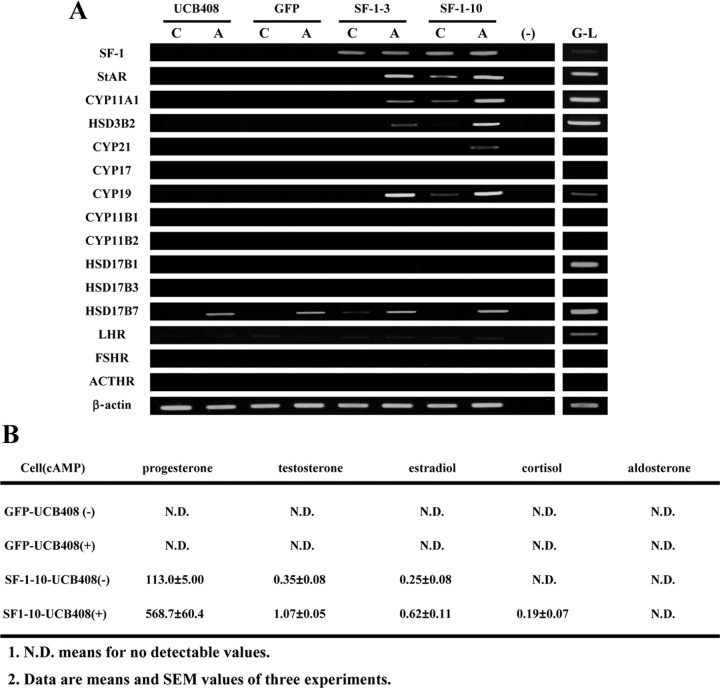
In previous studies, we have demonstrated that human BM-MSCs differentiated into adrenocortical-like cells by stable expression of SF-1 with cAMP treatment (15). In this study, we employed this method to study the differentiation of human UCB-MSCs. Green fluorescent protein (GFP) or SF-1 was ectopically expressed in UCB-MSCs (UCB408 E6E7T-33) by retrovirus-mediated transfection. SF-1 induced expression of some steroidogenesis-related genes [StAR, cytochrome P450 (CYP)11A1, and CYP19A1] and the production of progesterone (Fig. 1). Treatment with cAMP further up-regulated those genes and increased progesterone production. Although some other steroid hormones, such as testosterone and cortisol, were detectable at low levels, secretion patterns of steroid hormones from the differentiated cells were quite similar to those from granulosa-derived luteal cells (granulosa-luteal cells). The differentiated cells were also able to produce estradiol in the presence of androstenedione (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). In contrast, GFP-transduced cells did not produce steroid hormones or express any of the steroidogenesis-related genes, except hydroxysteroid dehydrogenase (HSD)17B7. These results indicate that even though UCB-MSCs could be differentiated into a steroidogenic lineage by SF-1 and cAMP, their steroidogenic properties were markedly different from BM-MSCs.
Fig. 1.
Differentiation of UCB-MSCs into steroidogenic cells by SF-1. UCB-MSCs were transduced with GFP or SF-1 by retrovirus-mediated transfection. A, RT-PCR analysis of each gene in each clone cultured with or without 8-bromo-cAMP for 2 d. Lanes G–L represent granulosa-luteal cells from women undergoing oocyte retrieval for in vitro fertilization. B, Production of steroid hormones by UCB-MSCs stably expressing GFP or SF-1 in the presence (+) or absence (−) of 8-bromo-cAMP. ACTHR, ACTH receptor; FSHR, FSH receptor; LHR, LH receptor.
PGC-1α acts as a coactivator for NR5A proteins in the transactivation of their target genes
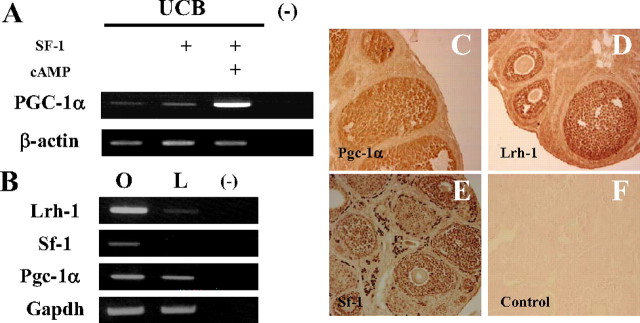
To determine difference between MSCs, we investigated and reported the fluctuation of gene expression by DNA microarray as described earlier (39). Among MSCs, PGC-1α was expressed only in UCB-MSCs but at relatively high levels (Fig. 2A). Although transduction of SF-1 had no effect on PGC-1α gene expression, it was induced by cAMP treatment. This observation may reflect the importance of PGC-1α’s involvement in the differentiation of granulosa-luteal cells.
Fig. 2.
A, PGC-1α was induced in UCB-MSCs. The mRNA levels of each gene in MSCs were analyzed by RT-PCR. B–E, The expression and localization of the NR5A protein family and PGC-1α in the murine ovary. B, The mRNA levels of each gene were analyzed by RT-PCR. Lanes O and L represent an ovary and a liver, respectively. C–E, Localization of NR5A and Pgc-1α proteins in the murine ovary. Positive staining for Pgc-1α (C) and Lrh-1 (D) in nuclei of granulosa cells, and Sf-1 (E) in nuclei of granulosa and theca/interstitial cells. Absence of any nuclear staining in a control section incubated with nonimmune IgG and counterstained with eosin (F).
To investigate the relevance of this hypothesis, ovarian expression of Pgc-1α as well as the NR5A family was investigated by RT-PCR and immunohistochemistry (Fig. 2). Pgc-1α mRNA was abundantly expressed in the ovary (Fig. 2B) as determined by quantitative RT-PCR (42.2 ± 6.90% relative to its expression in the liver; average ± sem, n = 3). Immunohistochemical studies demonstrated that Pgc-1α protein was localized in granulosa cells (Fig. 2C). Shin and Osborne (40) reported that Pgc-1α is a preferred coactivator for Lrh-1 and induces the Cyp7a1 gene in the liver. Here we showed that the Lrh-1 protein is exclusively expressed in granulosa cells (Fig. 2D). Mice lacking Lrh-1 in granulosa cells are sterile due to anovulation with a reduction in progesterone production (4). Pgc-1α may participate in the above phenomena by potentiating transcriptional activities of Lrh-1 in granulosa cells. Additionally, Lrh-1 and Sf-1 can bind to similar DNA sequences and activate transcription of the same genes including steroidogenic enzymes. In the ovary, Sf-1 is expressed in granulosa as well as theca cells, with stronger staining detectable in the latter cells (Fig. 2E). Defective Sf-1 in granulosa cells causes infertility and reduced progesterone production, similar to cases in which Lrh-1 is defective (3). Considering the results observed in UCB-MSCs, it is also possible that Pgc-1α could act via Sf-1 in granulosa cells.
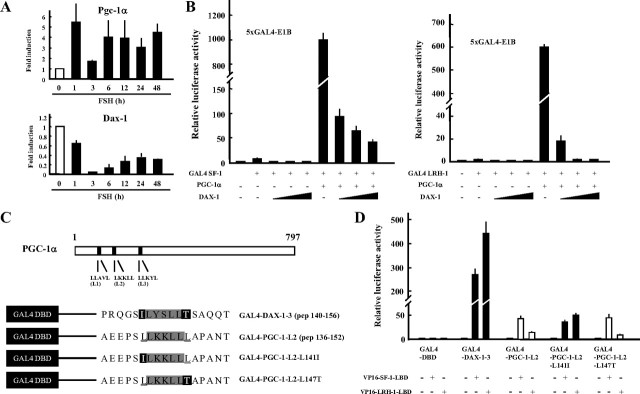
To evaluate the ability of PGC-1α to functionally interact with the NR5A family of proteins, a hybrid assay was performed using GAL4-SF-1 and GAL4-LRH-1 fusion proteins (Fig. 3A). Each fusion protein moderately activated a GAL4 reporter. The SF-1 fusion protein demonstrated a 10-fold increase from the baseline, and a 2.5-fold increase was observed with GAL4-LRH-1. Consistent with previous reports (40), GAL4-LRH-1 was markedly activated by PGC-1α in a dose-dependent manner and also activated GAL4-SF-1. Among the examined coactivators such as PGC-1β, steroid receptor coactivator (SRC)-1, and p300, PGC-1α exhibited exceptionally high coactivator activities for SF-1 and LRH-1 (Fig. 3B). These results indicate that PGC-1α acts as a powerful coactivator for LRH-1 and SF-1.
Fig. 3.
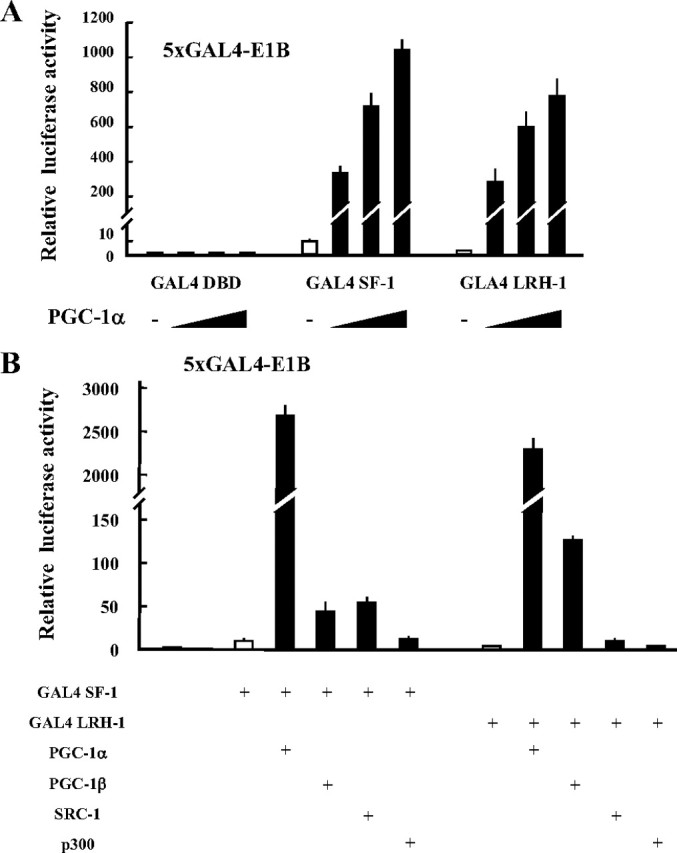
PGC-1α is a preferential coactivator for SF-1 and LRH-1. A, Dose-dependent effect of PGC-1α on the transactivation of SF-1 and LRH-1 in COS7 cells. B, Comparison of the coactivation ability between each coactivator on transactivation of SF-1 and LRH-1. COS7 cells were transfected with the 5×GAL4-E1B/Luc expression vector for 48 h. Luciferase activities were measured and relative activities were shown. Data are the mean ± sem of at least four independent experiments. DBD, DNA-binding domain.
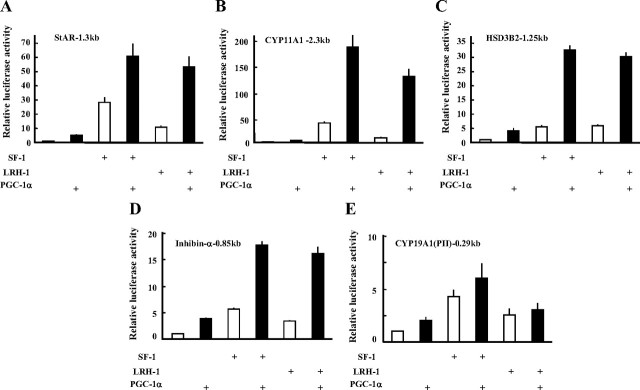
Our observations suggest that PGC-1α may regulate ovarian folliculogenesis and steroidogenesis. As shown in Fig. 4, A–C, PGC-1α potentiated the SF-1- and LRH-1-induced promoter activities of steroidogenesis-related genes such as StAR, CYP11A1, and HSD3B2. It could also activate the promoter of other SF-1- and LRH-1 target genes in folliculogenesis such as inhibin-α (Fig. 4D) and anti-Müllerian hormone (AMH) (Supplemental Fig. 2). In contrast, PGC-1α poorly activated the CYP19A1 promoter II (Fig. 4E).
Fig. 4.
PGC-1α induces SF-1- and LRH-1-induced promoter activities of steroidogenic genes (A–C) and Inhibin-α (D), but not the CYP19A1 gene (E). HEK293 cells were transiently transfected with each reporter and expression vector for 48 h. Luciferase activities were measured, and relative activities are shown. Data are expressed as the mean ± sem of at least four independent experiments.
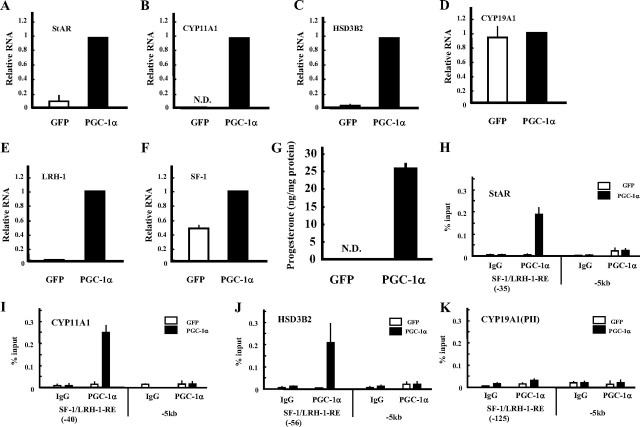
To confirm the above results, KGN cells (a human cell line derived from a granulosa cell tumor) were infected with adenoviruses expressing GFP or PGC-1α (Fig. 5). PGC-1α induced StAR, CYP11A1, and HSD3B2 mRNAs and progesterone production (Fig. 5, A–C and G). CYP19A1 was not induced by PGC-1α (Fig. 5D). The chromatin immunoprecipitation (ChIP) assay showed that PGC-1α binds to the promoter region of StAR, CYP11A1, and HSD3B2 where SF-1- and LRH-1-binding sites exist (Fig. 5, H–J). Consistent with the above results, the specific binding of PGC-1α was barely detectable in the CYP19A1 promoter region (Fig. 5K). Surprisingly, expression of PGC-1α also induced expression of SF-1 and LRH-1 mRNA, with the latter induced at much higher levels (Fig. 5, E and F). These results indicate that PGC-1α is a key factor for the differentiation of granulosa cells into progesterone-producing luteal cells via the NR5A family proteins.
Fig. 5.
PGC-1α induces steroidogenic genes and progesterone production in granulosa-like tumor KGN cells. KGN cells were infected with adenoviruses expressing GFP or PGC-1α. Gene expression of StAR (A), CYP11A1 (B), HSD3B2 (C), CYP19A1 (D), LRH-1 (E), and SF-1 (F) was measured by real-time PCR and normalized to 36B4 expression. Data are the mean ± sem of at least three independent experiments. G, Progesterone levels of each group in the medium were measured using ELISA. H–K, ChIP assays demonstrating the occupancy of PGC-1α on the promoters of StAR, CYP11A1, HSD3B2, and CYP19 genes. Assays were performed on GFP- (white column) or PGC-1α- (black column) infected KGN cell extracts using control IgG or specific antibody. Recovered chromatin was subjected to quantitative PCR analysis using primers encompassing proximal SF-1 and LRH-1 binding sites, 5 kb from the transcription initiation site of each gene. Data are the mean ± sem values of at least three independent experiments.
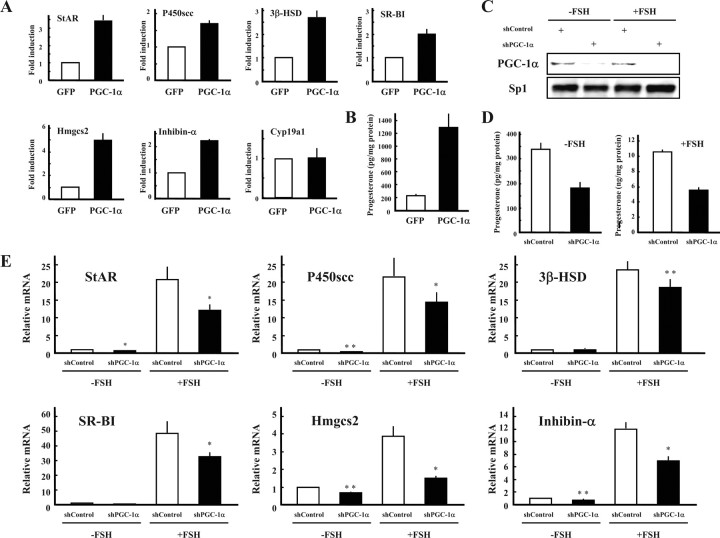
Similar to the results obtained in KGN cells, infection of immature rat granulosa cells with adenoviruses expressing Pgc-1α induced expression of steroidogenesis-related genes as well as progesterone production (Fig. 6, A and B). Additionally, it markedly up-regulated cholesterogenic 3-hydroxy-3-methylglutaryl-coenzyme A synthase-2 (Hmgcs2), another SF-1 and LRH-1 target gene in gonads (our unpublished data). It also induced inhibin-α mRNA as per the results of the promoter assay. Again, Cyp19a1 was not induced in these cells.
Fig. 6.
Pgc-1α is involved in the progesterone production of granulosa cells isolated from immature diethylstilbestrol-primed rats. Granulosa cells were infected with adenoviruses expressing GFP or Pgc-1α (A and B). A, Gene expression of StAR, P450scc, 3β-HSD, Hmgcs2, SR-BI, Inhibin-α, and Cyp19a was measured by quantitative PCR and normalized to 36B4 expression. Data are the mean ± sem of at least three independent experiments. B, Progesterone levels of each group in the medium were measured using ELISA. Granulosa cells were infected with adenoviruses expressing control and Pgc-1α short hairpin RNA, and cultured with or without FSH (C–E). C, Nuclear extracts from cells of each treatments were subjected to SDS-PAGE, and Western blot analysis was performed using each antibody. D, Progesterone levels of each group in the medium were measured using ELISA. E, Gene expression of StAR, P450scc, 3β-HSD, Hmgcs2, and inhibin-α was measured by real-time PCR and normalized to 36B4 expression. Data are the mean ± sem of at least two independent experiments. *, P < 0.05; **, P < 0.01.
Knockdown experiments were performed using adenoviruses expressing control and Pgc-1α short hairpin RNA (Fig. 6, C–E) to reveal the role(s) of endogenous Pgc-1α in granulosa cells during progesterone production. Pgc-1α knockdown resulted in an approximate 60% decrease of progesterone production levels, in the presence or absence of FSH (Fig. 6D). Expression of steroidogenesis-related genes was also down-regulated (Fig. 6E). Consistent with the results of PGC-1α overexpression, knockdown of Pgc-1α decreased expression of inhibin-α and Hmgcs2 genes. Taken together, it is conceivable that endogenous Pgc-1α plays a vital role in progesterone production within granulosa cells.
DAX-1 inhibits PGC-1α/NR5A transactivation
In granulosa cells, the expression of Pgc-1α mRNA and protein was relatively high even before FSH stimulation, with the effects of FSH stimulation only marginally apparent after 24 h (Fig. 6C and Fig. 7A). We previously reported that Dax-1 was expressed in unstimulated granulosa cells and inhibited Sf-1-mediated transactivation of target genes (8). Upon FSH stimulation, Dax-1 expression was markedly decreased (Fig. 7A), and consequently Sf-1 target genes including steroidogenic enzymes were up-regulated. We hypothesized that Dax-1 inhibits the Pgc-1α-dependent coactivation of the NR5A family of proteins. In a reporter assay, Dax-1 inhibited not only the transactivation of NR5A proteins, but also Pgc-1α-dependent coactivation in a dose-dependent manner (Fig. 7B). Coactivation of Lrh-1 was completely repressed by equal amounts of Dax-1 plasmid.
Fig. 7.
Inhibition of Pgc-1α activity by Dax-1. A, Granulosa cells were isolated from immature diethylstilbestrol-primed rats and treated with FSH for the indicated times. Gene expression of each gene was measured by quantitative PCR. B and C, HEK293 cells were transiently transfected with each reporter and expression vector for 48 h. Luciferase activities were measured, and relative activities are shown. Data are the mean ± sem of at least four independent experiments. C, Schematic structure of PGC-1α and LXXLL constructs for mammalian two-hybrid interaction assays. D, Interaction of LXXLL-related peptides of DAX-1, PGC-1α, and its amino acid-substituted forms with SF-1 and LRH-1. Data are the mean ± sem of at least four independent experiments. DBD, DNA-binding domain.
DAX-1 interacts with SF-1 and LRH-1 through three LXXLL-related motifs that are essential for hydrophobic interactions with the nuclear receptors (23). PGC-1α also has three LXXLL-like motifs at its N terminus (Fig. 7C and Supplemental Fig. 3A). To characterize the interaction through each LXXLL-related motif to the NR5A family, mammalian two-hybrid assays were performed. In the mammalian two-hybrid assays, expression of a reporter gene (pGL3-5xGAL4-E1B) indicates the interaction between bait and prey molecules. Consistent with previous studies (41, 42), transcriptional activation of VP16-SF-1 and VP16-LRH-1 was observed only when a L2 consensus LXXLL peptide construct was used as bait (Supplemental Fig. 3B). Immunoprecipitation and reporter assays indicated that the L2 motif was responsible for the interaction with the activation function-2 domain of the NR5A proteins (Supplemental Fig. 3, C and D). Although both NR5A proteins exhibited a significant interaction with the L2 peptide, SF-1 demonstrated a greater degree of interaction (Supplemental Fig. 3B and Fig. 7D). LRH-1 showed greater interaction with the Dax-1-3 consensus LXXLL motif (Fig. 7D). Sablin et al. (41) reported similar phenomena between the PGC-1α L2 and SRC peptides. They suggested from structural studies that the presence of leucine residues flanking the LXXLL motif might be responsible for the high affinity of the PGC-1α L2 peptide to SF-1 ligand-binding domain (LBD). To test this hypothesis, plasmids were constructed to express GAL4-PGC-1α L2 peptides in which each leucine residue was replaced by a corresponding amino acid of the GAL4-Dax-1-3 peptide. The L147T substitution had marginal effects on the interaction with VP16-SF-1 and VP-16-LRH-1. The substitution of L141I barely affected interaction with VP16-SF-1, yet remarkably enhanced interaction with VP16-LRH-1. The L141I peptide interacted with VP16-SF-1 and VP16-LRH-1 in a fashion similar to the Dax-1-3 peptide interactions, despite the latter demonstrating better interaction with the NR5A family proteins. These results indicate that the leucine residue located just before the LXXLL motif of the PGC-1α L2 peptide may be important for the determination of specificity of interactions with NR5A proteins.
Discussion
PGC-1α has been shown to be a key regulator of various metabolic pathways in skeletal muscle, fat, and liver. In this study, we reported that ovarian Pgc-1α is associated with progesterone production in granulosa cells via Sf-1 and Lrh-1. Recently, Grasfeder at al. (43) also reported that Pgc-1α regulates fasting-induced hepatic dehydroepiandrosterone production via estrogen-related receptor α (ERRα) and hepatocyte nuclear factor 4α (HNF4α). Both results raised the possibility that PGC-1α may play a vital role in steroidogenesis in various tissues. However, they also reported that PGC-1α did not stimulate the expression of steroidogenesis-related genes in extrahepatic cells including H295R cells of adrenocortical origin. Therefore, tissue specificity may be present with respect to PGC-1α-associated steroidogenesis.
MSCs represent multipotent somatic stem cells with the potential for application in regenerative medicine. Although they were originally identified in BM (44, 45), similar populations have been reported in many other tissues such as adipose tissue, UCB, and the placenta (46, 47, 48). Whatever the source, they generally have a common potential to differentiate into bone, fat, and cartilage. We have demonstrated that BM-MSCs differentiate into steroidogenic cells in the presence of cAMP and the NR5A family of proteins (15, 16, 22). In this study, we showed that UCB-MSCs could also differentiate into steroidogenic cells using SF-1 and cAMP, but they exhibited markedly different steroidogenic profiles when compared with those derived form BM-MSCs. Gondo et al. (49, 50) reported that forced expression of Sf-1 in mouse BM- and AT-MSCs caused differentiation with markedly different steroidogenic profiles. Umezawa and his colleagues (51) reported that UCB-MSCs have a higher cardiomyogenic differentiation potential than BM-MSCs. Steroidogenic profiles after MSC differentiation may be dependent on their ontogenic sources, even though they share multiple common characteristics. Consistent with the above observations, Wagner et al. (52) showed that there are significant differences in the global gene expression pattern of MSCs from BM, AT, and UCB, and such differences could reflect the characteristics of each MSC. It is conceivable from our study that PGC-1α might represent one of the important factors that contribute to the different steroidogenic phenotypes between MSCs. Recently, it was demonstrated that PGC-1α induction by BMP7 in MSCs is important for the differentiation of the cells into brown adipocytes, whereas it was not induced during their commitment to white adipocytes (53). Thus, the steroidogenic potency of MSCs should be determined not only by their origin, but also by their developmental and culture conditions. In addition to PGC-1α, other factors are also likely to dictate the granulosa-luteal-like phenotype of SF-1-transduced UCB-MSCs because PGC-1α is not involved in estrogen production that is prominent in granulosa-luteal cells.
Although our results demonstrated that Pgc-1α is important for the differentiation of granulosa-luteal cells, it was reported that Pgc-1α-deficient mice were fertile (54). It is conceivable that other coactivators compensated for its deficiency in the mouse model above, with Pgc-1β one of the most plausible candidates. Pgc-1β is also expressed in granulosa cells and moderately transactivates Lrh-1, even though its activity is much lower than Pgc-1α (our unpublished data). It was shown in the heart of double-knockout mice that Pgc-1α and Pgc-1β controlled overlapping programs required for prenatal maturation, whereas each gene-deficient mouse appeared normal under unstressed conditions (55). Because the double-knockout mice died shortly after birth, it was impossible to investigate the effects of those deficiencies on folliculogenesis. Hence, generation and characterization of conditional knockout mice deficient for the Pgc-1 proteins in granulosa cells will be necessary for resolving these problems.
To date, it has been demonstrated that various transcriptional coactivators interact with SF-1 and LRH-1 and are involved in steroidogenesis (56, 57, 58, 59). Among them, SRC-1 and CBP/p300 are able to form complexes with PGC-1α (60). Although PGC-1α itself has low inherent transcriptional activity, it recruits SRC-1 and CBP/p300 to activate transcription when PGC-1α binds to various transcription factors. Therefore, it is plausible that progesterone production stimulated by SRC-1 or CBP/p300 may be mediated through PGC-1α in granulosa cells and vice versa. In contrast to progesterone-producing enzyme genes, PGC-1α did not enhance the promoter activity of CYP19A1, one of the target genes of SF-1 and LRH-1 (21). It is probable that there are some selective effects of PGC-1α between SF-1/LRH-1 target genes. These phenomena were also reported in PPARγ and HNF4α target genes by Spiegelman and co-workers (26, 28, 61). Similar to PPARγ and HNF4α, additional components might be necessary to control PGC-1α specificity between CYP19A1 and other steroidogenesis-related genes.
In addition to steroidogenesis- and folliculogenesis-related genes, PGC-1α also induced the expression of SF-1 and LRH-1. Thus, PGC-1α could induce the differentiation of granulosa-luteal cells not only by coactivating the NR5A family proteins, but also by directly inducing the NR5A proteins themselves. The multiple roles of PGC-1α in a biological system were also reported with respect to oxidative phosphorylation (OXPHOS) via Errα and Gabpa/b genes in skeletal muscle (62). In this model, up-regulation of Pgc-1α results in the increased transcriptional activity of Errα and Gabpa/b with their own promoters, leading to a stable increase in the expression of these two factors. These two factors then work together with Pgc-1α to induce downstream target genes of OXPHOS. This model is likely applicable to our results in granulosa cells. There is no report regarding regulatory mechanisms for SF-1 and LRH-1 gene expression in the ovary. Morohashi and colleagues (63) reported that Sf-1/Ad4bp binds to its own intron 4 region to maintain the expression in fetal adrenal. This autoregulation is repressed by Dax-1 in the future adult zone and is completely lost after birth. In future studies, it will be necessary to determine whether Sf-1 and Lrh-1 possess autoregulatory mechanisms in granulosa cells.
Granulosa cells in early-stage follicles express both Sf-1 and Lrh-1, and both transcription factors are involved in fertility and progesterone production; therefore their distinctive roles for early folliculogenesis are unclear. Sf-1 deficiency at the earliest stages of folliculogenesis results in multiple abnormalities (3, 4). Consistent with our observations, Peng et al. (5) reported that LRH-1 is highly expressed in the human corpus luteum and SF-1 is expressed at very low levels. Although PGC-1α induced expression of both genes, LRH-1 expression was at a much higher level. Taken together, PGC-1α may be a key factor that promotes differentiation of granulosa cells into progesterone-producing luteal cells by acting on LRH-1 at multiple steps.
Despite lower expression of steroidogenesis-related genes in granulosa cells before differentiation, Pgc-1α levels were already high in these cells. This may indicate that the coactivator activity of Pgc-1α must be repressed by some factor(s) in these cells. We demonstrated that Dax-1 is the most plausible candidate, because it is down-regulated by FSH (10). The persistent expression of Dax-1 in granulosa cells, even after FSH stimulation, inhibits transcriptional activity of Sf-1 and steroidogenesis (9, 10). Because both PGC-1α and DAX-1 (23) can bind to SF-1 and LRH-1 via a LXXLL-related motif, it is conceivable that both proteins compete for interaction with NR5A proteins to control target gene expression positively or negatively. Our data suggest that a leucine residue just before the LXXLL motif of PGC-1α L2 may be an important determinant for the specificity of the interactions with SF-1, because substitution of isoleucine for a leucine residue in PGC-1α L2 rendered the motif more specific to LRH-1 (Fig. 7D). In addition to this residue, differences in other amino acid residue(s) might result in a higher affinity of the DAX-1 LXXLL motif to NR5A proteins.
In summary, we have demonstrated that ovarian PGC-1α is an important factor for the differentiation of granulosa cells into progesterone-producing luteal cells by acting on the NR5A family of proteins at multiple biological steps. In other organs, PGC-1α is a well-known metabolic regulator. It is implicated in insulin resistance in the liver and skeletal muscle (33). Metabolic dysfunction including insulin resistance is a common feature of PCOS. Under pathological conditions, PGC-1α might be one of the candidate factors to connect the metabolic and reproductive consequences of PCOS. This might be supported by the fact that PGC-1α regulates the promoter activity or expression of the inhibin-α and AMH genes, the abnormal production of which is often reported in PCOS patients (38, 64).
Materials and Methods
Animals, cell culture, transfection, and luciferase assay
Immature C57BL/6J mice (21–28 d of age) were purchased from Charles River Laboratory (Wilmington, MA). Granulosa cells were obtained from immature Sprague Dawley female rats (21 d of age) that received an injection of 2 mg diethylstilbestrol in 0.2 ml sesame oil once daily over four consecutive days. Granulosa cell culture was performed as described previously (10). At all times, the animals were treated according to NIH guidelines. Granulosa cells were cultured in DMEM/Ham’s F-12 supplemented with 0.1% BSA on collagen-coated plates.
COS7, human embryonic kidney (HEK)293, Phoenix, and UCB408E6E7T-33 (44) cells were cultured in DMEM with 10% fetal calf serum. KGN cells [kindly provided by Dr. Toshihiko Yanase, Fukuoka University, Fukuoka, Japan (65)] and granulosa-luteal cells from women undergoing transvaginal oocyte retrieval for in vitro fertilization were cultured in DMEM/Ham’s F-12 medium with 10% fetal calf serum. COS7 and HEK293 cells were transfected using Lipofectamine plus (Invitrogen, Carlsbad, CA). Luciferase assays were performed as described previously (10). Each data point represents the mean of at least four independent experiments.
Retrovirus preparation and infection
Retrovirus preparation was performed as described previously (22). Briefly, the packaging cell line, Phoenix, was transfected transiently with the retroviral plasmids using FuGENE 6 (Roche, Indianapolis, IN). The supernatant was concentrated by centrifugation, and the virus solution was stored at −80 C until use. MSCs were infected with the retrovirus in the presence of 8 μg/ml Polybrene (Sigma, St. Louis, MO) for 48 h. Cells were replated and selected by puromycin.
Plasmids
CYP11A1, HSD3B2, CYP19, AMH, and inhibin-α promoter were amplified by PCR and cloned into pGL3 Basic (Promega Corp., Madison, WI). pGL2-StAR1.3kb, pcDNA3.1/PGC-1α, pcDNA3/PGC-1β, pCR3.1/SRC-1, CMVβ/p300, pSG424, and pHK3NVP16 vectors were kindly provided by Dr. Teruo Sugawara (Hokkaido University Graduate School of Medicine, Sapporo, Japan), Dr. Daniel P. Kelly (Center for Cardiovascular Research, Washington University School of Medicine, St. Louis, MO), Dr. Anatasha Kralli (Biozentrum of the University of Basel, Basel, Switzerland), Dr. Ming-Jer Tsai (Baylor College of Medicine, Houston, TX), Dr. David Livingston (Dana Farber Institute, Boston, MA), Dr. R. Stein (Vanderbilt University, Nashville, TN), and Dr. Tony Kouzarides (Wellcome/CRC Institute and Department of Pathology, Cambridge University, Cambridge, UK), respectively. pSG424-SF-1 (amino acids 123-461) and pSG424-LRH-1 (amino acids 161-541) were constructed by inserting the corresponding cDNAs of human SF-1 and LRH-1. pSG424-LxxVL, pSG-LxxLL, and pSG-LxxYL were constructed by inserting double-stranded synthetic oligonucleotides encoding the corresponding amino acid sequences into pSG424 cleaved with EcoRI and SalI. pHK3N-VP16-SF-1-LBD and pHK3N-VP16-LRH-1-LBD were constructed by inserting the corresponding cDNAs as described above. Truncations in pHK3N-SF-1-LBDΔAF2C and pHK3N-VP16-LRH-1-LBDΔAF2C were introduced by PCR-mediated mutagenesis using primers containing the mutations and were verified by DNA sequencing. Mutation of the LxxLL motif in the PGC-1α expression vector was created by the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Expression vectors for SF-1, LRH-1, and DAX-1 have been described previously (8, 22). Flag-tagged SF-1, Flag-tagged LRH-1, and myc-tagged PGC-1α plasmids were made by inserting each cDNA into pCMV-tag2 or pCMV-tag3 (Stratagene).
RT-PCR and quantitative PCR
Total RNA from the tissues and the cultured cells was extracted using the Trizol reagent (Invitrogen). RT-PCR and quantitative PCR was performed as described previously (15). The RT-PCR products were electrophoresed on a 1.5% (wt/vol) agarose gel, and the resulting bands were visualized by staining with ethidium bromide. The primers used were described elsewhere (15) and are outlined in Supplemental Table 1.
Immunohistochemistry
Immunohistochemistry was performed as described previously (16, 22). Briefly, murine ovaries were fixed in 4% paraformaldehyde solution, dehydrated in a graded ethanol series, and embedded in paraffin wax. Sections of 7 μm thickness were subjected to an antigen-retrieval technique with HistoVT One (Nacalai Tesque, Inc., Kyoto, Japan) and treated with normal rabbit IgG, anti-LRH-1 (Abcam, Inc., Cambridge, MA), anti-Ad4BP/SF-1 (kindly given by Dr. Ken-ichirou Morohashi, University of Kyushu, Fukuoka, Japan), or anti-PGC-1α (Chemicon International, Inc., Temecula, CA) antibodies. They were developed using a Vectastain Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA).
ChIP assay
The ChIP assay was performed as described previously (22). Briefly, GFP- or PGC-1α-infected KGN cells were cross-linked with 1% formaldehyde, rinsed with PBS, and resuspended in sodium dodecyl sulfate lysis buffer. Cell lysates were sonicated and immunoprecipitated with normal IgG or an antibody using Dynabeads Protein G (VERITAS Software Corp., Tokyo, Japan). Immunoprecipitated complexes were eluted with elution buffer. Cross-links were then reversed and DNA fragments were purified for analysis by real-time PCR, using the primers described in Supplemental Table 1.
Immunoprecipitation
Immunoprecipitation and Western blotting were performed as described previously (8). Briefly, HEK 293 cells were transfected with each plasmid and harvested after 48 h. The Flag-tagged SF-1 or LRH-1 was immunoprecipitated using ANTI-FLAG M2 Affinity Gel (Sigma) and eluted with Laemmli sample buffer. They were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Western blot analysis of FLAG-tagged protein and PGC-1α was performed with anti-FLAG M2-Peroxidase (Sigma) and anti-PGC-1α antibodies. Enhanced chemiluminescence Western blot reagents (GE Biotech, Piscataway, NJ) were used for detection.
Adenovirus production and infection
Adenovirus vectors were prepared using Adeno-X Expression System 1 and knockout Adenoviral RNAi System 1 (Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s instructions and a previous study (33). Using these vectors, replication-defective recombinant adenoviruses were propagated and titrated in HEK293 cells. KGN and granulosa cells were then infected with the recombinant adenoviruses. Infected cells were processed for RNA or protein extraction at 48 h after infection. Culture media were collected for the measurement of progesterone production by ELISA (Cayman Chemical Co., Ann Arbor, MI) as described previously (16, 22).
Statistical analysis
Statistical significance (P < 0.05 and P < 0.01) was assessed by Student’s t test.
Acknowledgments
We thank Drs. K. Morohashi (University of Kyushu, Fukuoka, Japan), T. Sugawara (Hokkaido University Graduate School of Medicine, Sapporo, Japan), D. P. Kelly (Center for Cardiovascular Research, Washington University School of Medicine, St. Louis, Missouri), A. Kralli (Biozentrum of the University of Basel, Basel, Switzerland), M-J. Tsai (Baylor College of Medicine, Houston, Texas), D. Livingston (Dana Farber Institute, Boston, Massachusetts), R. Stein (Vanderbilt University, Nashville, Tennessee), T. Kouzarides (Wellcome/CRC Institute and Department of Pathology, Cambridge University, Cambridge, UK), and T. Yanase (Fukuoka University, Fukuoka, Japan), for providing reagents. We thank Dr. T. Sekiguchi for critical reading of the manuscript. We also thank Ms. Y. Inoue, K. Matsuura, and H. Fujii for technical assistance.
NURSA Molecule Pages:
Coregulators: PGC-1;
Ligands: Progesterone;
Nuclear Receptors: DAX1 | LRH-1 | SF-1.
Footnotes
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (21790269).
Disclosure Summary: All authors have nothing to disclose.
First Published Online February 4, 2010
Abbreviations: Ad4BP, Adrenal 4 binding protein; AMH, anti-Müllerian hormone; BM, bone marrow; ChIP, chromatin immunoprecipitation; CYP, cytochrome P450; ERR, estrogen-related receptor; GFP, green fluorescent protein; HEK, human embryonic kidney; HNF4α, hepatocyte nuclear factor 4α; HSD, hydroxysteroid dehydrogenase; LRH-1, liver receptor homolog-1; MSC, mesenchymal stem cell; PCOS, polycystic ovary syndrome; PGC, PPAR coactivator; PPAR, peroxisome proliferator-activated receptor; SF-1, steroidogenic factor-1; SRC, steroid receptor coactivator; UCB, umbilical cord blood.
References
- 1.Stocco C, Telleria C, Gibori G2007. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- 2.Murphy BD2000. Models of luteinization. Biol Reprod 63:2–11 [DOI] [PubMed] [Google Scholar]
- 3.Pelusi C, Ikeda Y, Zubair M, Parker KL2008. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod 79:1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K2008. Liver receptor homolog 1 is essential for ovulation. Genes Dev 22:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng N, Kim JW, Rainey WE, Carr BR, Attia GR2003. The role of the orphan nuclear receptor, liver receptor homologue-1, in the regulation of human corpus luteum 3β-hydroxysteroid dehydrogenase type II. J Clin Endocrinol Metab 88:6020–6028 [DOI] [PubMed] [Google Scholar]
- 6.Saxena D, Safi R, Little-Ihrig L, Zeleznik AJ2004. Liver receptor homolog-1 stimulates the progesterone biosynthetic pathway during follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 145:3821–3829 [DOI] [PubMed] [Google Scholar]
- 7.Saxena D, Escamilla-Hernandez R, Little-Ihrig L, Zeleznik AJ2007. Liver receptor homolog-1 and steroidogenic factor-1 have similar actions on rat granulosa cell steroidogenesis. Endocrinology 148:726–734 [DOI] [PubMed] [Google Scholar]
- 8.Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Miyamoto K2003. Involvement of cyclic adenosine 5′-monophosphate response element-binding protein, steroidogenic factor 1, and Dax-1 in the regulation of gonadotropin-inducible ovarian transcription factor 1 gene expression by follicle-stimulating hormone in ovarian granulosa cells. Endocrinology 144:1920–1930 [DOI] [PubMed] [Google Scholar]
- 9.Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA2005. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- 10.Lala DS, Rice DA, Parker KL1992. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol 6:1249–1258 [DOI] [PubMed] [Google Scholar]
- 11.Morohashi K, Honda S, Inomata Y, Handa H, Omura T1992. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 [PubMed] [Google Scholar]
- 12.Galarneau L, Paré JF, Allard D, Hamel D, Levesque L, Tugwood JD, Green S, Bélanger L1996. The α1-fetoprotein locus is activated by a nuclear receptor of the Drosophila FTZ-F1 family. Mol Cell Biol 16:3853–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo X, Ikeda Y, Parker KL1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 14.Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K2006. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocinology 147:4104–4111 [DOI] [PubMed] [Google Scholar]
- 16.Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A, Miyamoto K2008. Cyp11b1 is induced in the murine gonad by luteinizing hormone/ human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen; conservation and evolution of androgen metabolic pathway. Endocrinology 149:1786–1792 [DOI] [PubMed] [Google Scholar]
- 17.Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K1999. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol 13:1267–1284 [DOI] [PubMed] [Google Scholar]
- 18.Fayard E, Auwerx J, Schoonjans K2004. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol 4:23–34 [DOI] [PubMed] [Google Scholar]
- 19.Lee YK, Moore DD2008. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci 13:5950–5958 [DOI] [PubMed] [Google Scholar]
- 20.Volle DH, Duggavathi R, Magnier BC, Houten SM, Cummins CL, Lobaccaro JM, Verhoeven G, Schoonjans K, Auwerx J2007. The small heterodimer partner is a gonadal gatekeeper of sexual maturation in male mice. Genes Dev 21:303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezzi V, Sirianni R, Chimento A, Maggiolini M, Bourguiba S, Delalande C, Carreau S, Andò S, Simpson ER, Clyne CD2004. Differential expression of steroidogenic factor-1/adrenal 4 binding protein and liver receptor homolog-1 (LRH-1)/fetoprotein transcription factor in the rat testis: LRH-1 as a potential regulator of testicular aromatase expression. Endocrinology 145:2186–2196 [DOI] [PubMed] [Google Scholar]
- 22.Yazawa T, Inanoka Y, Mizutani T, Kuribayashi M, Umezawa A, Miyamoto K2009. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology 150:3885–3893 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Kasahara M, Yoshioka H, Morohashi K, Umesono K2003. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol 23:238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, Meitinger T, Monaco AP, Sassone-Corsi P, Camerino G1994. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641 [DOI] [PubMed] [Google Scholar]
- 25.Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Schwarz HP, Kaplan J-C, Camerino G, Meitinger T, Monaco AP1994. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672–676 [DOI] [PubMed] [Google Scholar]
- 26.Handschin C, Spiegelman BM2006. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735 [DOI] [PubMed] [Google Scholar]
- 27.Lin JD2009. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol 23:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839 [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelmen BM2002. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418:797–801 [DOI] [PubMed] [Google Scholar]
- 30.Vega RB, Huss JM, Kelly DP2000. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- 32.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- 33.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M2004. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat Med 10:530–534 [DOI] [PubMed] [Google Scholar]
- 34.Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM2009. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic PGC-1α expression. Diabetes 58:1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunaif A1997. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- 36.De Leo V, la Marca A, Petraglia F2003. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev 24:633–667 [DOI] [PubMed] [Google Scholar]
- 37.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A2008. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab 93:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N2008. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:4456–4461 [DOI] [PubMed] [Google Scholar]
- 39.Inaoka Y, Yazawa T, Mizutani T, Kokame K, Kangawa K, Uesaka M, Umezawa A, Miyamoto K2008. Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production. Reprod Biol Endocrinol 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin DJ, Osborne TF2008. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. J Biol Chem 283:15089–15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, Williams JD, Fletterick RJ, Ingraham HA2009. Structure of SF-1 bound by different phospholipids: evidence for regulatory ligands. Mol Endocrinol 23:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Choi M, Suino K, Kovach A, Daugherty J, Kliewer SA, Xu HE2005. tructural and biochemical basis for selective repression of the orphan nuclear receptor liver receptor homolog 1 by small heterodimer partner. Proc Natl Acad Sci USA 102:9505–9510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasfeder LL, Gaillard S, Hammes SR, Ilkayeva O, Newgard CB, Hochberg RB, Dwyer MA, Chang CY, McDonnell DP2009. Fasting-induced hepatic production of DHEA is regulated by PGC-1α, ERRα and HNF4α. Mol Endocrinol 23:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedenstein AJ, Gorskaja JF, Kulagina NN1976. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267–274 [PubMed] [Google Scholar]
- 45.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F1998. Muscle regeneration by bone marrow- derived myogenic progenitors. Science 279:1528–1530 [DOI] [PubMed] [Google Scholar]
- 46.Terai M, Uyama T, Sugiki T, Li XK, Umezawa A, Kiyono T2005. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell 16:1491–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH2003. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109 [DOI] [PubMed] [Google Scholar]
- 48.Okamoto K, Miyoshi S, Toyoda M, Hida N, Ikegami Y, Makino H, Nishiyama N, Tsuji H, Cui CH, Segawa K, Uyama T, Kami D, Miyado K, Asada H, Matsumoto K, Saito H, Yoshimura Y, Ogawa S, Aeba R, Yozu R, Umezawa A2007. ‘Working’ cardiomyocytes exhibiting plateau action potentials from human placenta-derived extraembryonic mesodermal cells. Exp Cell Res 313:2550–2562 [DOI] [PubMed] [Google Scholar]
- 49.Gondo S, Yanase T, Okabe T, Tanaka T, Morinaga H, Nomura M, Goto K, Nawata H2004. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells 9:1239–1247 [DOI] [PubMed] [Google Scholar]
- 50.Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T2008. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology 149:4717–4725 [DOI] [PubMed] [Google Scholar]
- 51.Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, Ikegami Y, Miyado K, Segawa K, Terai M, Sakamoto M, Ogawa S, Umezawa A2007. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells 25:2017–2024 [DOI] [PubMed] [Google Scholar]
- 52.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD2005. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33:1402–1416 [DOI] [PubMed] [Google Scholar]
- 53.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM2004. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- 55.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP2008. Transcriptional coactivators PGC-1α and PGC-lβ control overlapping programs required for perinatal maturation of the heart. Genes Dev 22:1948–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gizard F, Lavallee B, DeWitte F, Teissier E, Staels B, Hum DW2002. The transcriptional regulating protein of 132 kDa (TReP-132) enhances P450scc gene transcription through interaction with steroidogenic factor-1 in human adrenal cells. J Biol Chem 277:39144–39155 [DOI] [PubMed] [Google Scholar]
- 57.Dammer EB, Leon A, Sewer MB2007. Coregulator exchange and sphingosine-sensitive cooperativity of steroidogenic factor-1, general control nonderepressed 5, p54, and p160 coactivators regulate cyclic adenosine 3′,5′-monophosphate-dependent cytochrome P450c17 transcription rate. Mol Endocrinol 21:415–438 [DOI] [PubMed] [Google Scholar]
- 58.Xu PL, Liu YQ, Shan SF, Kong YY, Zhou Q, Li M, Ding JP, Xie YH, Wang Y2004. Molecular mechanism for the potentiation of the transcriptional activity of human liver receptor homolog 1 by steroid receptor coactivator-1. Mol Endocrinol 2004:1887–1905 [DOI] [PubMed] [Google Scholar]
- 59.Weck J, Mayo KE2006. Switching of NR5A proteins associated with the inhibin α-subunit gene promoter after activation of the gene in granulosa cells. Mol Endocrinol 20:1090–1103 [DOI] [PubMed] [Google Scholar]
- 60.Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM1999. Activation of PPARγ coactivator-1 through transcription factor docking. Science 286:1368–1371 [DOI] [PubMed] [Google Scholar]
- 61.Rhee J, Ge H, Yang W, Fan M, Handschin C, Cooper M, Lin J, Li C, Spiegelman BM2006. Partnership of PGC-1α and HNF4α in the regulation of lipoprotein metabolism. J Biol Chem 281:14683–14690 [DOI] [PubMed] [Google Scholar]
- 62.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM2004. Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101:6570–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zubair M, Parker KL, Morohashi K2008. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol 7:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirshfeld-Cytron J, Barnes RB, Ehrmann DA, Caruso A, Mortensen MM, Rosenfield RL2009. Characterization of functionally typical and atypical types of polycystic ovary syndrome. J Clin Endocrinol Metab 94:1587–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H2001. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 142:437–445 [DOI] [PubMed] [Google Scholar]