Abstract
Purpose
This study investigated the effect of goji (Lycium chinense Mill.) on erectile dysfunction in old-aged rats.
Materials and Methods
Twenty-four 18-month-old male Sprague-Dawley rats (defined as old-aged rats) were used. Treatment groups contained eight rats each: a control group, goji extract of 150 mg/kg/day group, and goji extract of 300 mg/kg/day group. Treatment was by orogastric tube once daily for 6 weeks. After 6 weeks of treatment, testes weight, serum testosterone, superoxide dismutase, nitric oxide (NO)-cyclic guanosine monophosphate (cGMP)-related parameters, intracavernous pressure/mean arterial pressure, and histological changes were examined.
Results
Treatments with goji extracts increased serum testosterone level, increased the expression of endothelial NO synthase, neuronal NO synthase, and cGMP, improved the oxidative stress marker, and decreased corporal fibrosis.
Conclusions
Our results indicate that goji extract may have a positive effect on erectile dysfunction via its antioxidant effects.
Keywords: Antioxidants, Erectile dysfunction, Testosterone
INTRODUCTION
Age-associated erectile dysfunction (ED) is characterized histologically within the cavernosa by progressive apoptosis or loss of corporal smooth muscle cells and replacement with collagen [1,2,3]. It is hypothesized that the high output of nitric oxide (NO), produced intracellularly by inducible nitric oxide synthase (iNOS), can act in this setting as an anti-apoptotic and anti-fibrotic factor [4,5,6]. The anti-fibrotic effect of iNOS plays an important role in age-related ED [3,4,5,6,7,8]. Oxidative stress has been linked with ED due to excessive generation of free radicals in the cavernosal tissues [9]. Antioxidants are capable of reducing oxidative stress by scavenging free radicals.
Nutraceuticals are substances that offer nutritional (food), health, or medicinal benefits, including prevention and/or treatment of diseases [10]. For example, ginger (Zingiber officinale Roscoe) has been shown to enhance iNOS activity in an in vitro study [11,12]. We previously demonstrated that an herbal formulation successfully improved ED caused by peripheral neuropathy in diabetic rats [13]. A traditional product, goji (Lycium chinense Mill.) is an herb that has antioxidant effects. Experimental studies have demonstrated that goji has a role in anti-aging, immune modulation, anti-fatigue, anti-tumor, and male fertility-enhancing effects [14,15,16]. Luo et al [17] demonstrated that goji might have protective effects against heat-induced damage of rat testes and positive effects on sexual behavior in hemicastrated rats. Dursun et al [18] demonstrated that ischemic injury with testes torsion was decreased by the goji extract treatment via peritoneal injection. Several studies reported that goji extracts have an antioxidant effect on the reproductive system of male rats [17,19,20].
This study aimed to evaluate the effects of goji (L. chinense Mill.) on erectile function in old-aged rats, as well as the protective effects via antioxidant effects.
MATERIALS AND METHODS
1. Goji (L. chinense Mill.) extract preparation
Goji (L. chinense Mill.) was obtained from Cheongyang, Korea. The test medication was manufactured by Biomix Inc., Goyang, Korea. The crude drug mixture was decocted with 30% EtOH for 8 hours after filtration and dried. The extract was administered orally to Sprague-Dawley (SD) rats at a 150 mg/kg/day or 300 mg/kg/day for 6 weeks.
2. Animal groups and study design
Experimental animals were obtained from Samtaco Bio. Co., Osan, Korea. SD male rats (age, 18 weeks) were randomly separated into 3 groups of 8 animals each (24 total): 1) aging control, 2) aging group treated with goji extracts at 150 mg/kg/day, and 3) aging group treated with goji extracts at 300 mg/kg/day. In each group, oral feeding with either distilled water or the goji extracts was continued for 6 weeks using an orogastric feeding tube once daily. None of the rats died during the experimental period. Treatment protocols were performed according to the Guide for Care and Use of Laboratory Animals. This study was approved by the Institutional Animal Care and Use Committee of the Catholic University of Korea, Seoul St Mary's Hospital (institutional review board approval number: CUMC-2015-0035-01). After 6 weeks of distilled water or goji extract administration, erectile function was evaluated by measuring intracavernosal pressure (ICP) and ICP/mean arterial pressure (ICP/MAP). Testes, epididymides, penile tissues, and blood samples were obtained from all sacrificed animals.
3. Serum testosterone level measurement
Venous blood samples were collected from the inferior vena cava. The serum testosterone level was analyzed with an enzyme-linked immunosorbent assay (ELISA) testosterone detection kit (BioVendor, Brno City, Czech Republic) as previously described [21].
4. Measurement of intracavernosal pressure
The ICP and ICP/MAP ratio was measured as previously described [22]. Rats were anaesthetized with an intraperitoneal injection of 0.2 mL tiletamine (Zoletil®, Virbac, France). The penis was dissected and the corpus cavernosum and crus were exposed in the supine position. A low-midline incision was made to access the pelvis, and the pelvic ganglion lateral to the right prostate was exposed. The penile skin was degloved and the corpus cavernosum identified. To measure ICP, a heparinized 23-gauge butterfly needle was inserted in the corpus cavernosum of the proximal portion. A bipolar electrical stimulator was placed on the ganglion to stimulate the cavernosal nerve for 50 seconds at 10 V and 2.4 mA for 0.5 milliseconds. The cavernosal nerve stimulation was conducted 3 times and the interval between stimulations was maintained for more than 10 minutes. Both MAP and ICP were continuously monitored during electrical stimulation.
5. Expression of endothelial nitric oxide synthase, enzymatic nitric oxide synthase, cyclic guanosine monophosphate by Western blot
Harvested tissues were cut into small pieces (20 mg wet weight). Two-hundred and Fifty microliter radioimmunoprecipitation assay buffer (25 mmol/L tris(hydroxymethyl) aminomethane-HCI [pH 7.6]), 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], protease inhibitor cocktail was added to the tissues. The ice was added to the homogenized buffer solution and incubated at 4℃ for 16 hours. Samples were centrifuged at 13,000 revolution per minute at 4℃ for 15 minutes and the supernatant decanted into new tubes. The supernatant solution was quantified by Bradford protein assay (Bio-Rad, Hercules, CA, USA), and stored at −70℃. Fifty microgram of quantified proteins were electrophoresed on 8% and 15% SDS-polyacrylamide gels at 100 V for 1.5 hours. Proteins were then transferred to polyvinyl difluoride membranes (Millipore, Billerica, MA, USA) at 70 V for 2 hours and blocked with 5% skim milk solution for 1 hour. Proteins were reacted with endothelial nitric oxide synthase (eNOS, 1:500; BD Pharmingen, San Diego, CA, USA), enzymatic NOS (nNOS, 1:4,000; BD Pharmingen, San Diego, CA, USA), cyclic guanosine monophosphate (cGMP, 1:200; Santa Cruz, Dallas, TX, USA), and beta-actin (1:10,000, Santa Cruz) at 4℃ for 16 hours, washed 3 times with tris buffered saline with tween (TBST) solution (Affymetrix, Santa Clara, CA, USA), and reacted with anti- mouse immunoglobulin G-horseradish peroxidase (IgG-HRP) (1:2,000, 1:5,000; Invitrogen, Carlsbad, CA, USA) and anti-rabbit IgG-HRP (1:2,000; Invitrogen) at room temperature for 1 hour. Proteins were washed 3 times with TBST solution for 10 minutes and reacted with enhanced chemiluminescence plus solution (GE Healthcare, Chalfont St. Giles, UK) for 1 minute. We compared the intensity of bands and the expression of proteins by film exposure.
6. Measurement of oxidative stress
Oxidative stress in cavernosal tissues was assessed quantitatively by measuring 8-hydroxy-2-deoxyguanosine (8-OHdG) and superoxide dismutase (SOD) levels. Total DNA was extracted from the cavernosal tissue using a DNeasy Blood & Tissue kit (Qiagen, Valencia, CA, USA). The 8-OHdG levels were measured with a DNA oxidation kit (Highly Sensitive 8-OHdG Check ELISA; Japan Institute for the Control of Aging, Fukuroi, Japan). Absorbance was measured at 450 nm after the final color was developed with the addition of 3, 3′, 5, 5′-tetramethylbenzidine. Tissue sample concentration was calculated from a standard curve and corrected for DNA concentration. SOD activity (CuZn-SOD and Mn SOD) in tissue was measured using a SOD Assay Kit-WST (Dojindo, Rockville, MD, USA), monitoring the decrease in the rate of superoxide-mediated reduction of nitroblue tetrazolium at 450 nm using a spectrophotometer [23].
7. Immunohistochemistry staining
The skin-denuded middle part of the penile shafts were fixed overnight in 10% formalin, washed, and stored in 70% alcohol at 4℃ until processing for paraffin-embedded tissue sectioning (5 µm). The cavernosal tissue was obtained for the Masson trichrome staining. After staining, the color distribution of the muscle tissue was approximated by using Adobe Photoshop CS 8.0 (Adobe Systems Incorporated, San Jose, CA, USA). The entire color distribution of the image was calculated, and we selected the muscle tissue distribution (expressed as the red color). There were somewhat standard deviations in our calculations because of color overlays and ambiguity of the color spectrum of the muscle tissues.
8. Statistical analysis
Data were analyzed with the SPSS Statistics software ver. 16.0 (IPSS Inc., Chicago, IL, USA). All data were presented as mean±standard devaition. Statistical significance was analyzed by Scheffe test and p<0.05 were considered to be significant.
RESULTS
1. General features
Table 1 describes body weight, testes weight, and testosterone level. The control group and goji groups (150 mg/kg/day and 300 mg/kg/day) showed no significant differences in body and testes weight. The mean serum testosterone level was 1.86±0.03 pmol/L in the control group, 2.07±0.06 pmol/L in the goji 150 mg/kg/day group, and 2.39±0.08 pmol/L in the goji 300 mg/kg/day group (Table 1). The serum testosterone level of the both treatment group was increased (p<0.05).
Table 1. Comparison of body weight, testes weight, and serum testosterone among groups at 6 weeks.
| Variable | Controla | Goji (150 mg/kg)b | Goji (300 mg/kg)c |
|---|---|---|---|
| Body weight (g) | 778.50±21.20 | 780.40±45.30 | 783.50±36.30 |
| Testis weight (g) | 1.74±0.20 | 1.75±0.15 | 1.83±0.16 |
| Serum testosterone (pmol/L) | 1.86±0.03 | 2.07±0.06* | 2.39±0.08* |
Values are presented as mean±standard deviation. Eight rats were selected for analysis from each group. *Versus control groups (p<0.05).
aNormal control group. bGoji extract at 150 mg/kg/day group. cGoji extract at 300 mg/kg/day group.
2. Measurement of intracavernosal pressure/mean arterial pressure
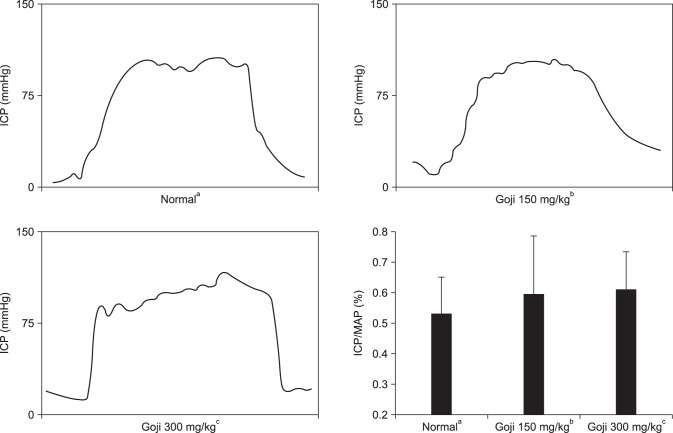
The maximum ICP and ICP/MAP ratios increased in both goji extract groups compared to the control group, but the increase was not statistically significant (Fig. 1).
Fig. 1. Intracavernous pressure (ICP) in response to electrical stimulation of the cavernous nerve in rats from each experimental group. ICP/MAP ratio. MAP: mean arterial pressure. aNormal control group (n=8), bgoji extract at 150 mg/kg/day group (n=8), and cgoji extract at 300 mg/kg/day group (n=8).
3. Western blot
In all groups, the expression of cGMP against actin was different, so we corrected to compare the expression of cGMP. We corrected the expression of cGMP against actin as 100. The expression of cGMP, eNOS, and cGMP proteins were increased according to Western blot analysis in both goji treatment groups compared with the control group. The penile expression level of cGMP activities was significantly increased in both goji treatment groups compared with the control group (Fig. 2, 3).
Fig. 2. (A) Western blot analysis of endothelial nitric oxide synthase (eNOS), and β-actin in corporal tissue. (B) Cyclic guanosine monophosphate (cGMP) concentration in corporal tissue. *Significant difference between the control and goji groups (p<0.05). aNormal control group (n=8), bgoji extract at 150 mg/kg/day group (n=8), and cgoji extract at 300 mg/kg/day group (n=8).
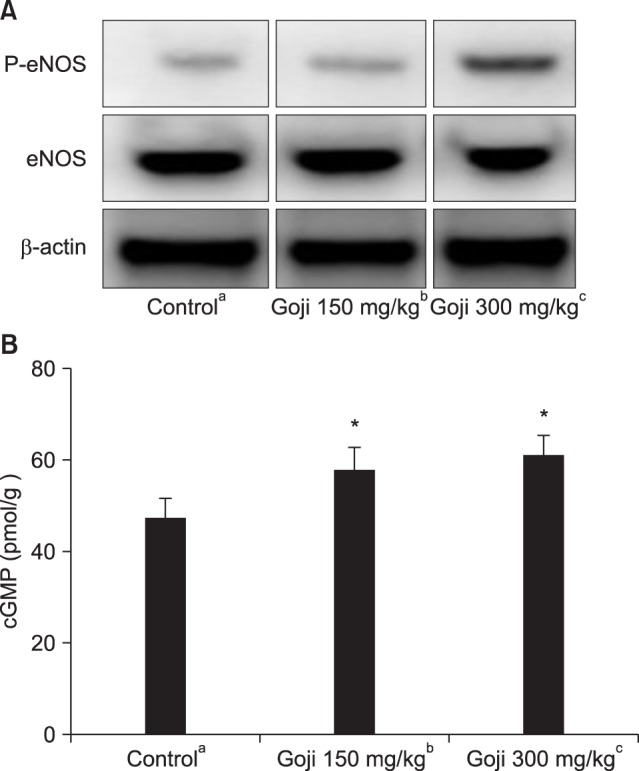
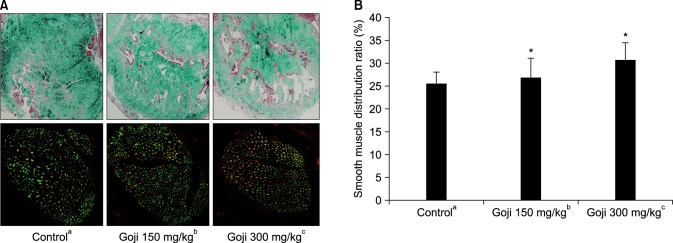
Fig. 3. (A) Masson trichrome staining for collagen (blue) and smooth muscle (red) in corporal tissue. Enzymatic nitric oxide synthase (nNOS) (red) and dorsal nerve (green) (×100). (B) Smooth muscle distribution ratio. Data are expressed as mean±standard deviation. *Significant difference between the control and goji groups (p<0.05). aNormal control group (n=8), bgoji extract at 150 mg/kg/day group (n=8), and cgoji extract at 300 mg/kg/day group (n=8).
4. Comparison of oxidative stress in cavernosal tissue
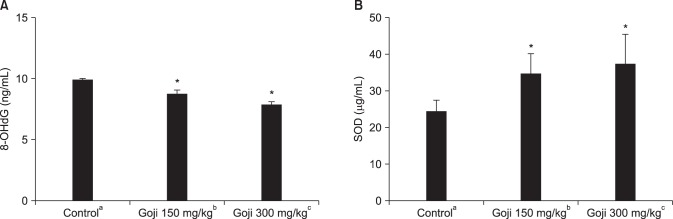
Oxidative stress in cavernosal tissues was evaluated quantitatively by measuring 8-OHdG in cavernosal tissue by ELISA. The 8-OHdG level was 9.85±0.07 ng/mL in the control group, 8.80±0.17 ng/mL in the goji 150 mg/kg/day group, and 7.90±0.12 ng/mL in the goji 300 mg/kg/day group. Both goji group showed a lower oxidative stress than the control group (p<0.05). SOD activities in the both goji group were increased compared with the control group (p<0.05). SOD activity level was 24.45±3.19 µg/mL in the control group, 34.58±5.62 µg/mL in the goji 150 mg/kg/day group, and 37.32±8.37 µg/mL in the goji 300 mg/kg/day group (Fig. 4).
Fig. 4. Comparison of the expression levels of superoxide dismutase (SOD) and 8-hydroxy-2-deoxyguanosine (8-OHdG). *Significant difference between the control and goji groups (p<0.05). aNormal control group (n=8), bgoji extract at 150 mg/kg/day group (n=8), and cgoji extract at 300 mg/kg/day group (n=8).
5. Immunohistochemistry staining and histological changes
Collagen deposition was increased in the both goji group, and smooth muscle contents were decreased in the control group. The muscle/collagen ratio was 25.75%±2.50% in the control group, 27.16%±4.02% in the goji 150 mg/kg/day group, and 30.83%±3.81% in the goji 300 mg/kg/day group, which was a significant increase in muscle/collagen ratio for both goji groups (Fig. 3).
DISCUSSION
L. chinense Mill. (goji) is a well-known, traditional medicine in Asian countries, which all have different common names. The traditional therapeutic effects of goji have made the plantefic experiments. Several clinical studies, in vivo animal, and in vitro cell studies have reported the efficacy of goji (antioxidant effects) fore [14,15,16,17,18,19,20,24].
Causes of ED have been classified as psychogenic and organic (vasculogenic, endocrinologic, neurogenic). Aging may affect all erectile function processes, including nerve, artery, vein, cavernosal tissue, and hormones. These conditions interfere on the expression of vascular growth factors and receptors causing disturbance in endothelial function [25]. Serum testosterone decreases with the process of aging. There are four different mechanisms of age-related low serum androgen levels: primary testicular changes, altered neuroendocrine regulation of Leydig cell function, increase of plasma sex hormone-binding globulin binding capacity, and decreased adrenal androgen secretion [26]. Traish and Guay [27] reported that androgens have critical roles for regulation of erectile function and penile tissue development, growth, and maintenance of function. As age advances, the gonadal steroid hormones and testosterone decrease, nerve conduction slows, the efficiency of the vascular microcirculation of the penis is reduced, and the smooth muscle/collagen ratio in the copora cavernosum decreases [28]. Yu et al [29] demonstrated that chronic hypoxia might cause ED due to the decrease in the quantity of nNOS nerve fibers and expression on eNOS.
In this study, old-aged rats were used as the model to investigate the therapeutic effect of goji extracts on erectile dysfunction. The key findings of this study were:
(1) Following treatment of goji extract, the levels of serum testosterone did not decrease.
(2) The expression of eNOS and nNOS in cavernosal tissue of goji extract treated groups increased.
(3) Oxidative stress markers were improved in goji extract treated groups.
(4) Muscle/collagen ratios in the goji extract treated groups were elevated in comparison with the control group.
In this study, administration of goji extract (at 150 mg/kg/day and 300 mg/kg/day) showed an improvement of serum testosterone levels in old-aged rats. It is hypothesized that goji extract prevents the decrease of serum testosterone due to antioxidant effects, rather than increase testosterone. The role of testosterone supplementation for sexual dysfunction is controversial [30]. Gojies are not a direct supplement of testosterone, soefor late onset hypogonadism.
We observed a reduction of 8-OHdG, and increased expression of eNOS, nNOS, and cGMP with goji extract administration. Ies results in synthesis of cGMP, subsequently inhibiting apoptosis in muscle cells and preventing collagen deposition. These results demonstrate that goji extracts can improve sexual function in an old-aged rat model by increasing the level of eNOS, nNOS and testosterone. Due to the limitation of the rat serum sampling, we were unable to compare the serum testosterone levels before and after administration of the goji extract. A composition analysis of goji extract has not been published, therefore effects of other components of the extract on our results cannot be excluded. Further experimental and clinical studies are needed to understand safety and efficacy of goji.
CONCLUSIONS
The current study suggests that goji extracts may have a positive effect to improve erectile function in an old-aged rat model. Treatment with goji extract may prevent decrease of serum testosterone levels, minimizing oxidative stress to erection-related endothelial function in an old aged rat model. Goji extract could be resistant to oxidative stress, which increases therapeutic potential for improving erectile function in human males.
ACKNOWLEDGEMENTS
This work was supported by the Technological Innovation R&D Program (S2198495) funded by the Small and Medium Business Administration (SMBA, Korea)
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Garbán H, Vernet D, Freedman A, Rajfer J, González-Cadavid N. Effect of aging on nitric oxide-mediated penile erection in rats. Am J Physiol. 1995;268:H467–H475. doi: 10.1152/ajpheart.1995.268.1.H467. [DOI] [PubMed] [Google Scholar]
- 2.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, Lue TF. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–738. [PubMed] [Google Scholar]
- 3.Ferrini M, Magee TR, Vernet D, Rajfer J, González-Cadavid NF. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod. 2001;64:974–982. doi: 10.1095/biolreprod64.3.974. [DOI] [PubMed] [Google Scholar]
- 4.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–244. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Davila HH, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Gene transfer of inducible nitric oxide synthase complementary DNA regresses the fibrotic plaque in an animal model of Peyronie's disease. Biol Reprod. 2004;71:1568–1577. doi: 10.1095/biolreprod.104.030833. [DOI] [PubMed] [Google Scholar]
- 6.Ferrini MG, Kovanecz I, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int. 2006;97:625–633. doi: 10.1111/j.1464-410X.2006.05955.x. [DOI] [PubMed] [Google Scholar]
- 7.Nehra A, Goldstein I, Pabby A, Nugent M, Huang YH, de las Morenas A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996;156:1320–1329. doi: 10.1016/s0022-5347(01)65578-2. [DOI] [PubMed] [Google Scholar]
- 8.Ferrini MG, Kovanecz I, Sanchez S, Vernet D, Davila HH, Rajfer J, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod. 2007;76:915–923. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 9.Jeremy JY, Angelini GD, Khan M, Mikhailidis DP, Morgan RJ, Thompson CS, et al. Platelets, oxidant stress and erectile dysfunction: an hypothesis. Cardiovasc Res. 2000;46:50–54. doi: 10.1016/s0008-6363(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 10.Position of the American Dietetic Association: phytochemicals and functional foods. J Am Diet Assoc. 1995;95:493–496. doi: 10.1016/s0002-8223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 11.Imanishi N, Mantani N, Sakai S, Sato M, Katada Y, Ueda K, et al. Inducible activity of ginger rhizome (Zingiber offifinale Rosc.) on the mRNA expression of macrophage- inducible nitric oxide (NO) synthase and NO production in a macrophage cell line, RAW264.7 cells. Am J Chin Med. 2004;32:727–735. doi: 10.1142/S0192415X04002302. [DOI] [PubMed] [Google Scholar]
- 12.Liao H, Banbury LK, Leach DN. Elucidation of danzhixiaoyao wan and its constituent herbs on antioxidant activity and inhibition of nitric oxide production. Evid Based Complement Alternat Med. 2007;4:425–430. doi: 10.1093/ecam/nel091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park CS, Ryu SD, Hwang SY. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of aged and diabetic rats. J Ethnopharmacol. 2004;94:85–92. doi: 10.1016/j.jep.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Lin CL, Wang CC, Chang SC, Inbaraj BS, Chen BH. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int J Biol Macromol. 2009;45:146–151. doi: 10.1016/j.ijbiomac.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Li XM, Ma YL, Liu XJ. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J Ethnopharmacol. 2007;111:504–511. doi: 10.1016/j.jep.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Luo Q, Li Z, Huang X, Yan J, Zhang S, Cai YZ. Lycium barbarum polysaccharides: Protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006;79:613–621. doi: 10.1016/j.lfs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Dursun R, Zengin Y, Gündüz E, İçer M, Durgun HM, Dağgulli M, et al. The protective effect of goji berry extract in ischemic reperfusion in testis torsion. Int J Clin Exp Med. 2015;8:2727–2733. [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Q, Cui X, Yan J, Yang M, Liu J, Jiang Y, et al. Antagonistic effects of Lycium barbarum polysaccharides on the impaired reproductive system of male rats induced by local subchronic exposure to 60Co-γ irradiation. Phytother Res. 2011;25:694–701. doi: 10.1002/ptr.3314. [DOI] [PubMed] [Google Scholar]
- 20.Xin YF, You ZQ, Gao HY, Zhou GL, Chen YX, Yu J, et al. Protective effect of Lycium barbarum polysaccharides against doxorubicin-induced testicular toxicity in rats. Phytother Res. 2012;26:716–721. doi: 10.1002/ptr.3633. [DOI] [PubMed] [Google Scholar]
- 21.Bae WJ, Ha US, Choi JB, Kim KS, Kim SJ, Cho HJ, et al. Protective Effects of KH-204 in the Bladder of Androgen-Deprived Rats. World J Mens Health. 2015;33:73–80. doi: 10.5534/wjmh.2015.33.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ha US, Koh JS, Kim HS, Woo JC, Kim SJ, Jang H, et al. Cyanidin-3-O-β-D-glucopyranoside concentrated materials from mulberry fruit have a potency to protect erectile function by minimizing oxidative stress in a rat model of diabetic erectile dysfunction. Urol Int. 2012;88:470–476. doi: 10.1159/000336136. [DOI] [PubMed] [Google Scholar]
- 23.Bae WJ, Ha US, Kim KS, Kim SJ, Cho HJ, Hong SH, et al. Effects of KH-204 on the expression of heat shock protein 70 and germ cell apoptosis in infertility rat models. BMC Complement Altern Med. 2014;14:367. doi: 10.1186/1472-6882-14-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Yang M, Wu X, Yan J. Study on protective action of lycium barbarum polysaccharides on DNA imparments of testicle cells in mice. Wei Sheng Yan Jiu. 2003;32:599–601. [PubMed] [Google Scholar]
- 25.Fonseca J, Tomada N, Magalhães A, Rodrigues AR, Gouveia AM, Neves D. Effect of aging and cardiovascular risk factors on receptor Tie1 expression in human erectile tissue. J Sex Med. 2015;12:876–886. doi: 10.1111/jsm.12794. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 27.Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006;3:382–404. doi: 10.1111/j.1743-6109.2006.00245.x. discussion 404-7. [DOI] [PubMed] [Google Scholar]
- 28.Shen ZJ, Jin XD, Chen ZD, Shi YH. Effect of aging on penile ultrastructure. Asian J Androl. 2001;3:281–284. [PubMed] [Google Scholar]
- 29.Yu DP, Liu XH, Wei AY. Effect of chronic hypoxia on penile erectile function in rats. Genet Mol Res. 2015;14:10482–10489. doi: 10.4238/2015.September.8.9. [DOI] [PubMed] [Google Scholar]
- 30.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]