Abstract
The IGF-I receptor (IGF-IR) was identified as a tumor progression factor, but its role in invasion and metastasis has been the subject of some controversy. Previously we reported that in murine lung carcinoma M-27 cells, overexpression of IGF-IR increased the synthesis and activation of matrix metalloproteinase (MMP)-2 via Akt/phosphatidylinositol 3-kinase signaling. In contrast, we show here that in these and other cells, IGF-IR overexpression reduced the constitutive and phorbol 12-myristate 13-acetate (PMA)-inducible expression of three protein kinase C (PKC)-regulated metalloproteinases, MMP-3, MMP-9, and MMP-13, in cultured cells as well as in vivo in sc tumors. To elucidate the underlying mechanism, we analyzed the effect of IGF-IR on PKC expression and activity using wild-type and IGF-IR-overexpressing (M-27IGFIR) tumor cells. Our results show that overexpression and activation of IGF-IR reduced PKC-α expression, PKC activity, and downstream ERK1/2 signaling, and these effects were reversed in cells expressing kinase (Y1131,1135,1136F) or C-terminal (Y1250/51F) domain mutants of IGF-IR. This reduction was due to transcriptional down-regulation of PKC-α as evidenced by reduced PKC-α mRNA expression in a phosphatidylinositol 3-kinase-dependent manner and a blockade of PKC-α promoter activation as revealed by a reporter gene assay. Finally, reconstitution of PKC-α levels could restore MMP-9 expression levels in these cells. Collectively, these results show that IGF-IR can inhibit PKC-α gene transcription and thereby block the synthesis of PMA-regulated MMPs, suggesting that within the same cells, IGF-IR can act as both a positive and negative regulator of MMP expression and function.
The IGF-I receptor acts as a negative regulator of PKC-α transcription and thereby an inhibitor of PMA-inducible MMP-3, MMP-9 and MMP-13 synthesis.
The IGF-I receptor (IGF-IR) is a heterotetramer consisting of two 130- to 135-kDa α-chains and two 90- to 95-kDa β-chains, with several disulfide bridges between subunits α-α and α-β. The ligand-binding domain is located on the extracellular α-subunit. The kinase domain is located on the intracellular region of the β-subunit as are also binding sites for receptor substrates and docking proteins. Among them, C-terminal tyrosines 1250, 1251, and serine 1248 were identified as important for the transmission of ligand-induced signals (1, 2, 3).
The IGF-IR ligands are IGF-I, IGF-II, and insulin, but IGF-I binds with the highest affinity. Ligand-induced activation of the intrinsic IGF-IR tyrosine kinase can initiate several signaling mechanisms including the Ras/Raf/ERK pathway implicated in receptor-mediated mitogenesis and transformation, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway implicated in the transmission of cell survival signals and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling regulating inflammatory responses (2, 4, 5). Although up-regulated expression of IGF-IR has been noted in many tumor types, and it has been recognized as a promoter of tumor progression and a therapeutic target (6, 7), there has been some controversy regarding the specific role that the IGF-IR axis plays in tumor invasion and the acquisition of a metastatic phenotype (2). This stems from conflicting results obtained in different tumor models where IGF-IR expression could be shown to correlate with enhanced or decreased tumor invasion and metastasis. The data suggest that the cell/tissue context may be important in determining the ultimate effect of IGF-IR on the invasive/metastatic phenotype (8, 9) (reviewed in Ref. 2).
The matrix metalloproteinases (MMPs) play diverse and important roles in tumor progression by facilitating tumor cell invasion, tumor cell communication with the microenvironment, angiogenesis, and metastasis (10, 11, 12, 13, 14, 15, 16). Among the 24 known MMPs, the gelatinases MMP-2 and MMP-9 are major mediators of basement membrane type IV collagen degradation, a critical step in tumor invasion (12, 14, 17). Recent findings implicate these proteins in the degradation of non-extracellular matrix (non-ECM) proteins including the cleavage/activation of growth factors such as TGF-β, receptors such as fibroblast growth factor receptor-1 and proinflammatory cytokines and chemokines such as IL-1 and TNF-α (reviewed in Ref. 13).
The transcriptional regulation of MMP-2 and MMP-9 are distinct. MMP-9 belongs to a subgroup of MMPs whose promoters contain a TATA box and several cis-acting elements, including an AP-1 binding site and a PEA3 site that appear to cooperate for maximal induction of gene expression by growth factors, or the protein kinase C (PKC) activating 12-O-tetradecanoyl-phorbol-13-acetate (TPA) (reviewed in Refs. 18 and 19). This subgroup also includes MMP-1, MMP-3, and MMP-13 (reviewed in Ref. 19). MMP-2 belongs to a subgroup of MMPs (with MMP-14 and MMP-28) that lack a TATA box and an AP-1 site in the promoter and respond more weakly to growth factor-induced signaling.
The PKC family has been implicated in intracellular signaling pathways that are associated with transformation and tumor progression. The PKCs comprise a family of at least 12 different serine/threonine kinases with substantial homology in conserved (C) protein domains that are interspersed by isoform-specific variable (V) regions. They are the conventional calcium- and diacylglycerol (DAG)-dependent, phorbol 12-myristate 13-acetate (PMA)-inducible PKCs (α, βI, βII, and γ), the novel calcium-independent and DAG-dependent PKCs (δ, ε, η, and θ), and the atypical calcium- or DAG-independent PKCs (λ, ι, μ, and ξ) (reviewed in Refs. 20 and 21). Individual PKCs differ in their tissue distribution, subcellular localization, and substrate specificity and can phosphorylate/activate different substrates in a cell-context-dependent manner and trigger different signaling pathways including activation of the Raf/MAPK kinase/MAPK cascade, RhoA and Rac1, glycogen synthase kinase-3β (GSK-3β), and nuclear factor-κB (NFκB) (21). PKC-α is ubiquitously expressed and can be activated by different stimuli including phorbol esters, growth factor receptors, physical stress, and cell-cell contact. PKC-α can play diverse and sometime opposing roles in cell cycle control, cell proliferation, differentiation, survival, and motility depending on the signaling pathways it intercepts or activates (22). Up-regulation of this PKC has been reported in different tumor types, and its expression was shown to correlate with invasion and metastasis in different tumors such as breast and ovarian carcinomas (23, 24). PKC-α activation involves maturation of the enzyme through phosphorylation of specific sites in the catalytic domain, recruitment of the cofactors calcium and DAG or the DAG substitute PMA, altered conformation, and translocation of the activated form from the cytosolic (soluble) fraction to the particulate (membrane) fraction, a step that involves an association with scaffolding proteins, such as the receptor for activated C-kinase (RACK-1) (25). Among the proteins whose transcription is regulated downstream of PMA-activated PKC-α and the Raf/MAPK pathway are the metalloproteinases MMP-3, MMP-9, and MMP-13 that commonly have an AP-1 binding site in their promoter region (26, 27, 28).
In previous studies on the effect of IGF-IR overexpression on invasion and metastasis, we identified the IGF-IR as a positive regulator of MMP-2 and MMP-14 synthesis in Lewis lung carcinoma tumor sublines with divergent metastatic potentials (29, 30, 31, 32). In contrast, the present data show that the constitutive and PMA-inducible expression of MMP-3, MMP-9, and MMP-13 are down-regulated in IGF-IR-overexpressing cells and suggest that this is due, at least in part, to transcriptional down-regulation of PKC-α.
Results
Altered MMP expression profile in M-27 cells overexpressing the IGF-IR
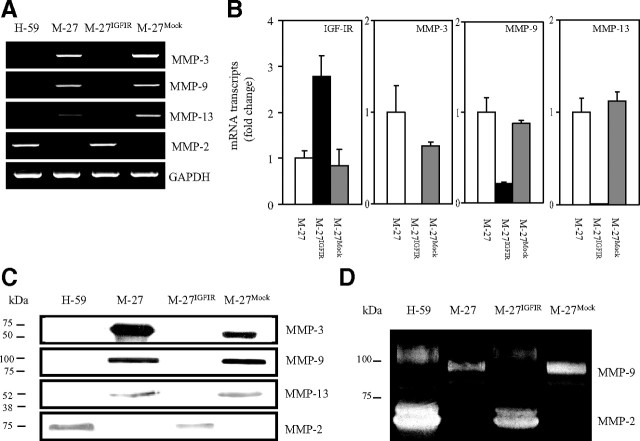
In previous studies, we identified the IGF-IR as a positive regulator of MMP-2 and MMP-14 expression in Lewis lung carcinoma sublines M-27 and H-59 cells that have different IGF-IR expression levels and distinct metastatic phenotypes. We documented an increase in MMP-2 production in H-59 cells that express high endogenous IGF-IR levels and in M-27 cells that were stably transfected with recombinant human (rh)IGF-IR cDNA (M-27IGFIR) and showed that this regulation was PI3K/Akt dependent (29, 31, 33). Subsequent chip microarray analysis performed on these cells revealed, however, that in contrast to these MMPs, IGF-IR expression levels were inversely correlated with the expression of the metalloproteinases MMP-3, MMP-9, and MMP-13, and this was confirmed by RT-PCR (Fig. 1A) and further quantified by quantitative RT-PCR (qRT-PCR) (Fig. 1B). The changes in mRNA expression levels resulted in reduced protein production as revealed by Western blotting performed on serum-free conditioned media (Fig. 1C) and by gelatin zymography performed to detect enzymatically active MMP-9 and MMP-2 (Fig. 1D).
Fig. 1.
Altered MMP synthesis in cells with high IGF-IR expression levels. MMP expression was analyzed by RT-PCR (A), qRT-PCR (B), Western blotting (C), and gelatin zymography (D) performed on total RNA (A and B) or concentrated conditioned media (C and D) derived from the indicated cells. H-59 cells were used as a positive control in some of the analyses because of their relatively high endogenous IGF-IR levels. For Western blotting (C), 100× concentrated conditioned media were used, and 15, 75, and 150 μg protein were loaded per lane for the detection of MMP-3, MMP-9 (and MMP-2), and MMP-13, respectively. For gelatin zymography, 200 μg protein were loaded per lane for the detection of MMP-2 and MMP-9 activity. A, C, and D, Representative results of three experiments performed. Where applicable, the positions of molecular weight markers are indicated on the left. B, Means and sd of three separate analyses expressed as a ratio to GAPDH, relative to M-27 cells that were assigned a value of 1. There was no significant difference (P > 0.05) between the mean ratios calculated for M-27 and M-27Mock cells in any of the qRT-PCR analyses performed.
Increased IGF-IR expression inhibits PMA-induced MMP synthesis
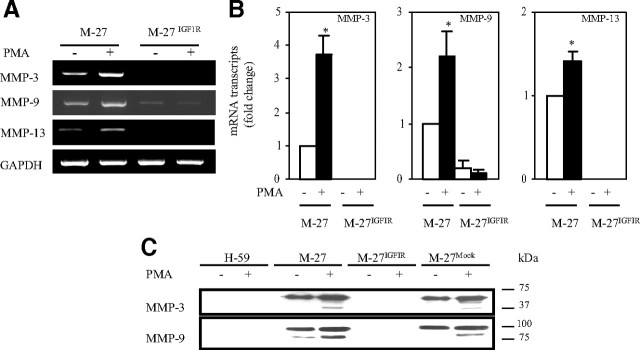
MMP-3, -9, and -13 are inducible by phorbol esters (26, 27, 28). We therefore asked whether IGF-IR overexpression interfered with the induction of these enzymes and measured their expression after PMA stimulation by RT-PCR and Western blotting. We found that whereas in wild-type and mock-transfected M-27 cells, PMA treatment increased the expression of these MMPs, as measured by RT-PCR (Fig. 2A) and quantified by qRT-PCR (Fig. 2B), there was no increase in their expression in M-27IGFIR cells. Western blot analysis revealed an increase in the production of both MMP-3 and MMP-9 after PMA stimulation of M-27 cells, but no increase could be observed when M-27IGFIR cells were treated with up to 500 nm PMA (Fig. 2C). Interestingly, in conditioned media harvested from PMA-stimulated M-27 cells, we observed an increase in the levels of both the proenzyme and processed forms of MMP-3 and -9 (Fig. 2C). This effect was not specific to the M-27 cells because IGF-IR overexpression also suppressed basal and PMA-inducible MMP-9 expression in HT-1080 fibrosarcoma cells (34) (supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), whereas in H-59 cells, the suppression of IGF-IR by antisense RNA (35) increased MMP-9 expression by 2- to 4-fold (supplemental Fig. 1B).
Fig. 2.
Loss of PMA-mediated MMP induction in cells expressing increased IGF-IR levels. Serum-starved cells were stimulated with 12.5 nm PMA for 8 h before RNA extraction (A and B) or with 500 nm PMA for 18 h before harvesting conditioned media (C). Shown are the results of RT-PCR (A), qRT-PCR (B), and Western blot (C) analyses performed on total RNA or concentrated conditioned media (C) derived from the indicated cells. Results in A are of a representative RT-PCR analysis of a total of three performed. Results in B are expressed as the means and sd of the ratios to GAPDH relative to M-27 cells that were assigned a value of 1 in three independent qRT-PCR analyses. Shown in C is a representative Western blot of four performed with each of the indicated antibodies revealing the proenzyme and activated forms of the respective MMPs. The position of the molecular weight markers is shown on the right. *, P < 0.05 as compared with untreated M-27 cells.
Reduced PKC-α mRNA and protein synthesis in M-27IGFIR cells
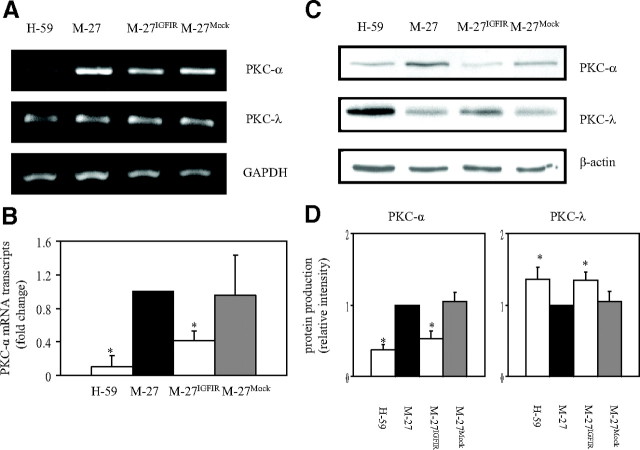
Conventional and novel PKCs are inducible by, and mediate the effects of, PMA. The failure of PMA to induce MMP-3, -9, and -13 in M-27IGFIR cells was therefore indicative of suppressed PKC-mediated signaling in these cells. To elucidate the role of IGF-IR in the regulation of PKC activity, we first analyzed PKC expression levels in M-27IGFIR and wild-type cells. Western blot analysis (not shown) identified PKC-α and PKC-λ as the two major PKC isoforms expressed in M-27 cells. Their expression levels in M-27IGFIR cells were therefore investigated. RT-PCR analysis showed a significant reduction in PKC-α mRNA levels in M-27IGFIR, as compared with M-27 (or mock-transfected) cells (Fig. 3A), and this was confirmed by qRT-PCR that revealed a 2.5-fold reduction in PKC-α mRNA levels in M-27IGFIR as compared with wild-type cells (Fig. 3B). This reduced expression was also reflected in a 2-fold decrease in protein production, as revealed by Western blotting (Fig. 3, C and D) and was specific to PKC-α because PKC-λ mRNA and protein levels did not decrease and even slightly increased as a consequence of IGF-IR overexpression (Fig. 3, A, C, and D). Reduced PKC-α levels were also observed in H-59 cells, consistent with their low MMP-3, -9, and -13 expression levels (Fig. 3, A–D).
Fig. 3.
Reduced basal PKC-α levels in cells overexpressing IGF-IR. PKC-α expression levels were measured using RT-PCR (A), qRT-PCR (B), and Western blotting (C and D) performed on total RNA (A and B) or cell lysate proteins (C) derived from the indicated cells. The results in A are of a representative analysis of a total of three performed. The results in B are expressed as the means and sd of three qRT-PCR analyses. Shown in C are representative results of three Western blots. Results of densitometry performed on the protein bands are depicted in the bar graph (D) and are expressed as the ratios of PKC-α or PKC-λ to β-actin relative to M-27 cells that were assigned a value of 1 (n = 3). *, P < 0.05 as compared with M-27 cells.
Reduced PKC activity and downstream signaling in cells overexpressing IGF-IR
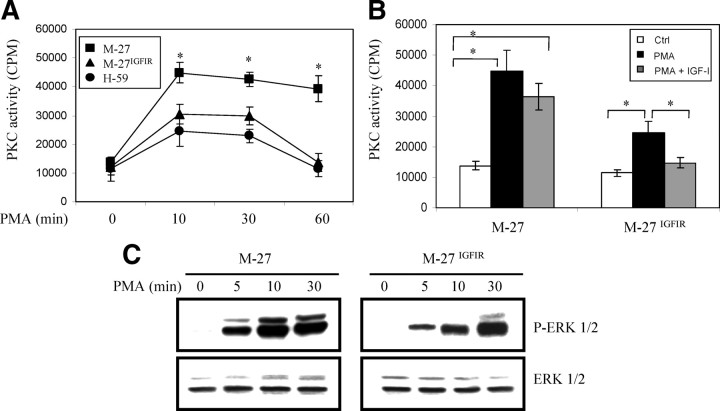
We next analyzed the effect of reduced PKC-α levels on the total, PMA-inducible PKC activity in the tumor cells. In all cells, the basal, PKC activity, as measured using the in vitro kinase assay with myelin basic protein as substrate, was low. PMA stimulation increased PKC activity levels in both M-27 and M-27IGFIR cells, but the magnitude of the response was significantly weaker (>2-fold reduction) in the latter, as also seen in H-59 cells (Fig. 4A). Pretreatment of M-27IGF-IR cells with 10 ng/ml IGF-I reduced the PKC activity levels by a further 40% but had no significant effect on M-27 cells (Fig. 4B). The reduction in PMA-induced PKC activity resulted in reduced PKC signaling. Whereas in M-27 cells, PMA induced a rapid phosphorylation of ERK1/2 that was evident as early as 5 min after stimulation, the response was both delayed and weaker in M-27IGFIR cells (Fig. 4C).
Fig. 4.
Reduced total PKC activity and downstream signaling in cells with increased IGF-IR expression. Serum-starved tumor cells were stimulated with 500 nm PMA for the indicated time intervals (A and C) or pretreated (or not) with 10 ng/ml IGF-I for 10 min and then with PMA for 60 min (B). The cells were then lysed, and total PKC activity was measured using the in vitro kinase activity assay (A and B) or cell lysate proteins analyzed by Western blotting using the indicated antibodies (C). The results in A and B are expressed as means and sd of triplicate samples in a representative experiment of two performed. Results in C are representative of three Western blots performed. *, P < 0.05 relative to M-27IGFIR and H-59 cells (A) or relative to the indicated comparison group (B). Ctrl, Control; P-ERK 1/2, phospho-ERK 1/2.
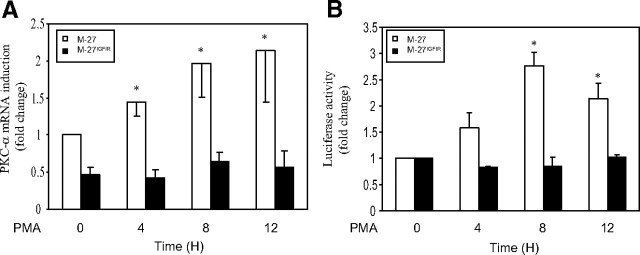
Transcriptional activation of PKC-α by PMA is blocked in cells overexpressing IGF-IR
It has been shown that PKC-α transcription can be autoregulated in an AP-2-dependent and phorbol ester-responsive manner (36). To assess whether the effect of IGF-IR on PKC-α expression was at the transcriptional level, we used PMA stimulation as means of activating PKC-α transcription and measured the increase in PKC-α mRNA levels using RT-PCR. In M-27 cells, we observed a 2.1-fold increase in PKC-α mRNA expression levels in response to PMA, but in M-27IGFIR cells, PKC-α transcription was not significantly altered (Fig. 5A). Moreover, when cells were transiently transfected with a pGL3 basic vector expressing an 1800-bp fragment of the PKC-α promoter (nucleotides −1573 to +227) upstream of a firefly luciferase reporter gene, and the dual-luciferase assay was used to measure PKC-α promoter activation in response to PMA, a 3-fold increase in promoter activity was observed in M-27 cells but none in M-27IGFIR cells (Fig. 5B), confirming that IGF-IR expression blocked transcriptional activation of PKC-α.
Fig. 5.
Transcriptional activation of PKC-α is inhibited by IGF-IR. A, Serum-starved cells were stimulated with 1 μm PMA in the presence of 1% FCS for the indicated time intervals, and RNA was extracted and analyzed by RT-PCR. The results are based on five separate analyses and expressed as means (and sd) of fold change relative to untreated M-27 cells that were assigned a value of 1. B, Cells were cotransfected with the pGL3-basic vector expressing the firefly luciferase gene downstream of an 1800-bp mouse PKC-α promoter fragment and the pRL-SV-40 plasmid expressing the renilla luciferase gene, serum starved overnight, and then stimulated with PMA as above and lysed at the indicated time intervals. The results are expressed as means (and sd) of the ratios of firefly to renilla luciferase activities relative to the respective untreated cells (n = 4). *, P < 0.05 relative to unstimulated cells.
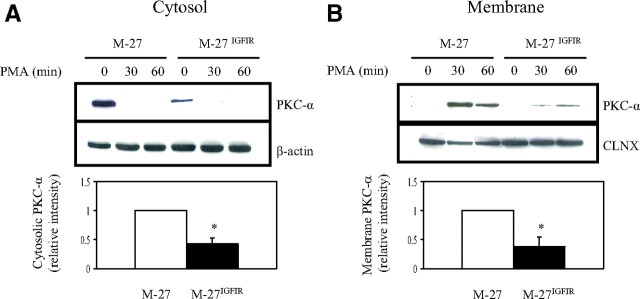
IGF-IR overexpression does not affect phorbol ester-induced PKC-α translocation
The results described thus far established that PKC-α expression, activation, and signaling were impaired in cells overexpressing IGF-IR. PMA-induced activation of conventional PKCs involves a translocation of the enzyme from the cytosol to specialized subcellular compartments, where they phosphorylate the appropriate substrates to initiate signaling. It was therefore of interest to determine whether IGF-IR interfered with the translocation of PKC-α in response to phorbol ester. Western blot analysis showed that under basal conditions, PKC-α was detectable mainly in the cytosolic fractions of both M-27 and M-27IGFIR cells. After a 30-min stimulation with PMA, a marked reduction in PKC-α protein levels in the cytosolic fraction (Fig. 6A) and a concomitant increase in its plasma membrane levels (Fig. 6B) were observed in both M-27 and M-27IGFIR cells. This suggested that PMA-induced PKC-α translocation per se was not altered in IGF-IR-overexpressing cells, although the total amount of membrane-translocated PKC-α was obviously reduced (∼70%) in M-27IGFIR cells leading us to conclude that reduced membrane-associated PKC-α dosage rather than translocation efficiency per se was responsible for reduced PKC-α activity and MMP synthesis.
Fig. 6.
IGF-IR overexpression does not affect PKC-α translocation. Serum-starved cells were stimulated with 500 nm PMA for the indicated time intervals, the cells were lysed, and the cytosolic and membrane fractions were separated as detailed in the Materials and Methods. Western blotting was performed on the separated cytosolic (A) and membranous (B) fractions. The nitrocellulose membranes were probed consecutively with antibodies to PKC-α and β-actin (A) or calnexin (B). Shown are representative results of three analyses. The results of densitometry are shown in the bar graphs. They are expressed as the ratios of PKC-α to β-actin before (A) and PKC-α to calnexin (CLNX) 30 min after (B) PMA stimulation relative to the respective M-27 cells that were assigned a value of 1 (n = 3). *, P < 0.05 as compared with M-27 cells.
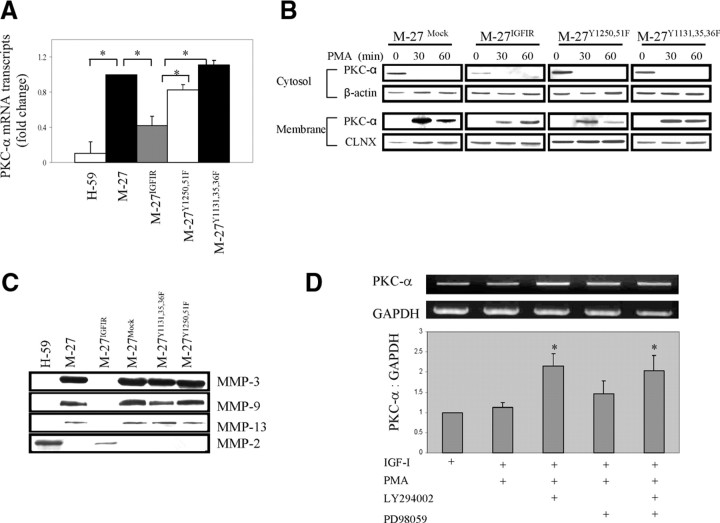
Reduced PKC-α expression and activity are reversed in cells expressing IGF-IR mutants
We have previously shown that the positive regulation of MMP-2 gene transcription by IGF-IR was abolished in cells expressing an IGF-IR with tyrosine-phenylalanine substitutions in the kinase or C-terminal domains (33). Here we tested whether the negative regulation of PKC-α synthesis and thereby of PKC-associated MMPs was also dependent on signals from both the kinase domain (tyrosines 1131, 1135, and 1136) and C-terminal tyrosines 1250 and 1251, known to mediate protein-protein interactions required for IGF-IR signaling, migration, and survival, particularly in adherent cells (1, 3). Results of qRT-PCR analyses (Fig. 7A) showed that in M-27 cells overexpressing the Y1131,1135,1136F or Y1250,1251F IGF-IR mutants, PKC-α down-regulation was reversed and mRNA levels were restored to those seen in wild-type cells. As a consequence, the enzyme levels that translocated to the membrane in response to PMA were significantly increased (Fig. 7B), resulting in the restoration of PMA-induced MMP-3, -9, and -13 synthesis in these cells (Fig. 7C). This suggested that similarly to IGF-IR-induced transcriptional activation of MMP-2, the suppressive effect on PKC-α transcription was also dependent on receptor kinase activity as well as signaling initiated via C-terminal domain residues 1250/51. To identify more directly the signaling pathways involved in suppression of PKC-α transcription, M-27IGFIR cells were stimulated with IGF-I in the presence of the PI3K and MAPK kinase inhibitors LY294002 and PD98059, respectively, before cell treatment with PMA to induce PKC-α transcription. We found that inhibition of the PI3K pathway completely reversed the suppressive effect of activated IGF-IR, and as a result, PKC-α mRNA expression in response to PMA increased 2.2-fold to levels comparable to M-27 cells, as revealed by RT-PCR analysis. Cell treatment with PD98059 had only a minor effect on PKC-α transcription (1.4-fold increase in mRNA expression, P = 0.06), whereas a combination of the two inhibitors increased PKC-α transcription to a level not exceeding that of LY294002 alone. Taken together, these data identify PI3K signaling as the major pathway transmitting the PKC-α suppressive effect of IGF-IR.
Fig. 7.
Reduced PKC-α and MMP expression is reversed in cells expressing kinase and C-terminal domain mutants of the IGF-IR or pretreated with a PI3K inhibitor. PKC-α expression levels were measured by qRT-PCR performed on total RNA (A), and PKC-α translocation (B) was analyzed after stimulation of the cells with 500 nm PMA for the indicated time intervals, as described in the legend to Fig. 6. MMP expression (C) was measured by Western blotting using concentrated conditioned media. The effect of signal transduction inhibitors on PKC-α transcription (D) was measured by RT-PCR after pretreatment of M-27IGFIR cells for 5 h with 20 μm LY294002 and/or PD98059, and the addition of 10 ng/ml IGF-I and 1 μm PMA for an additional 8 h. Shown in A are the mean values (and sd) obtained in three experiments and expressed relative to M-27 cells that were assigned a value of 1. Shown in B are the results of a representative Western blot of a total of three performed. Shown in C are the results of a representative experiment of three performed using the indicated antibodies and the experimental conditions described in the legend to Fig. 1, and shown in D are results of a representative RT-PCR of four performed (top panel) and mean values (and sd) obtained in four experiments and expressed relative to M-27IGFIR cells treated with IGF-I only that were assigned a value of 1. *, P < 0.05 relative to the indicated comparison group.
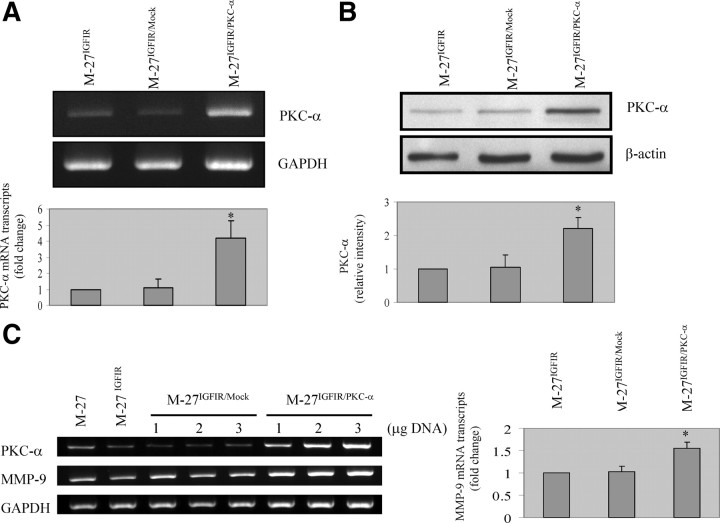
Reconstitution of PKC-α expression in M-27IGFIR cells restores MMP-9 expression
To obtain more direct evidence that PKC-α expression levels were the (critical) limiting factor regulating MMP expression levels in our cells, we transiently transfected M-27IGFIR cells with a plasmid vector expressing full-length murine PKC-α cDNA and assessed the effect of the transfection on MMP expression levels. In M-27IGFIR cells transfected with the PKC-α vector, PKC-α mRNA levels increased by up to 4.2-fold and protein levels by up to 2.4-fold relative to controls, as assessed by RT-PCR (Fig. 8A) and Western blotting (Fig. 8B), respectively. Concomitantly, we found in these cells, but not in mock-transfected cells, an increase in MMP-9 expression levels that was proportional to the increase in PKC-α levels (Fig. 8C), confirming that suppression of PKC-α expression by IGF-IR altered MMP-9 expression in these cells. Interestingly, however, MMP-3 and -13 levels did not significantly increase in response to elevated PKC-α levels (not shown), suggesting that in addition to reduced PKC-α expression, other factors may also be involved in the down-regulation of these enzymes in M-27IGFIR cells.
Fig. 8.
Increased MMP-9 expression levels in cells with reconstituted PKC-α levels. M-27IGFIR cells were transiently transfected with 2 μg (A and B) or the indicated concentration (C) of the pCMV-SPORT plasmid vector (M-27IGFIR/Mock) or the same vector expressing a full-length murine PKC-α cDNA (M-27IGFIR/PKC-α). RNA was extracted or the cells were lysed 48 h later and analyzed by RT-PCR (A and C) and Western blotting (B), respectively. Shown in A–C are representative results of three different experiments performed for each of the analyses. Shown in the bar graphs are results of densitometry expressed as the means (and sd) of the ratios of PKC-α (Α) and MMP-9 (C) to GAPDH and of PKC-α (B) to β-actin relative to untransfected M-27IGFIR cells that were assigned a value of 1 (n = 3). Values shown in C are for cells transfected with 3 μg plasmid DNA. *, P < 0.05 as compared with untransfected M-27IGFIR cells.
Reduced MMP expression levels in sc M-27IGF-IR tumors
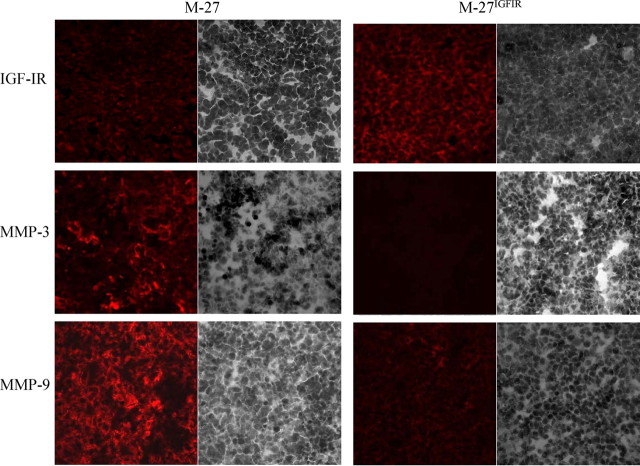
Finally, we wished to ascertain that the differential expression of PKC-α-regulated MMPs observed in the cultured tumor cells was relevant to their growth in vivo. We therefore inoculated the tumor cells sc into syngeneic mice and analyzed frozen sections derived from the sc tumors by immunohistochemistry using antibodies to the IGF-IR and the metalloproteinases MMP-3 and -9 that are both highly expressed in M-27 cells. Results shown in Fig. 9 confirmed that IGF-IR levels were increased in M-27IGFIR as compared with M-27 tumors and showed that similarly to our observations in vitro, MMP-3 and MMP-9 levels in these tumors were markedly reduced relative to those of wild-type cells, essentially reproducing the phenotype change observed in vitro.
Fig. 9.
Reduced MMP synthesis in M-27IGFIR tumors growing in vivo. Subcutaneous tumors were generated by injection of 105 tumor cells in the right flank. Tumors were excised when their mean diameter was 1.7 cm and snap frozen. Eight-micrometer cryostat sections were processed for immunohistochemistry, stained with the indicated primary antibodies and Alexa Fluor 568 secondary antibodies, and counterstained with hematoxylin. Five sections per tumor were analyzed for each antibody, and representative images of immunostained (left) and hematoxylin-stained (right) fields acquired with a ×40 objective are shown.
Discussion
Taken together with our previous findings, these results show that within the same cell context, the IGF-IR can play dual and opposing roles in the regulation of MMP synthesis and ECM degradation. Namely, in our cells, IGF-IR overexpression, although increasing MMP-2 and MMP-14 production (31, 32) (Figs. 1 and 2), reduced the expression of the PKC-α-dependent MMP-3, -9, and -13, thereby playing both a positive and a negative role in MMP regulation. These divergent effects may be controlled, at least in part, by the balance between signaling mediated through the PKC/ERK/AP-1 arm on one hand and the PI3K pathway on the other (31) (reviewed in Ref. 2). Previously, we have already shown that preferential activation of ERK by IGF-I can attenuate the PI3K-dependent induction of MMP-2 (31). In contrast, the present results suggest that ERK activation downstream of PKC induces the synthesis of MMP-3, -9, and -13, and this is attenuated through overexpression and/or activation of IGF-IR.
We observed that in response to PMA, M-27 cells produced increased levels of both the precursor and active forms of MMP-3 and MMP-9. This is consistent with reports that identified MMP-3 as a major activator of the MMP-9 precursor (37) and suggests that in these cells, MMP-3 and -9 may be engaged in a proteolytic cascade that could result in increased MMP-9 activity (10, 38).
Relatively little is known about the role of IGF-I in MMP-3 and MMP-13 regulation. This was studied mainly in chondrocytes, where a negative correlation between IGF-I and MMP-3 expression was reported in one study (39), but an indirect, positive regulatory role for IGF-I was described in another (40). Also, in agreement with our results, a recent study identified IGF-I as a negative regulator of both basal and fibronectin/IL-1β-induced MMP-13 expression in human chondrocytes (41). The regulatory link between IGF-IR and MMP-9 was more extensively studied, but the results are conflicting. Consistent with our results, increased IGF-I expression has been shown to coincide with reduced MMP-9 production in some cells (42). Also, in a recent study, the binding of IGF-II to the IGF-IIR was shown to induce a time-dependent increase in MMP-9 expression and activity (43). This is consistent with a negative regulatory role for IGF-IR in MMP-9 synthesis, because the main effect of IGF-IIR binding is to reduce ligand bioavailability and thereby attenuate IGF-IR activation and signaling (2). However, a positive correlation between IGF-IR expression levels and MMP-9 production has also been documented (44, 45). It is also noteworthy that MMP-3 and MMP-9 have been implicated in the proteolytic processing of some of the IGF-binding proteins (IGFBP) such as IGFBP-1 and IGFBP-3 (46, 47) and may therefore be involved in an autoregulatory loop by controlling IGF-I bioavailability and ligand-induced receptor activation. Collectively, these findings suggest that the role of the IGF-IR/IGF axis in transcriptional regulation of MMPs may be cell-context dependent and may be regulated by expression levels and type of PKCs as well as by the contributions of the IGF-IIR and IGFBP that could together determine the type and intensity of the signal elicited by engagement of the IGF-IR.
Interestingly, we observed that reconstitution of PKC-α levels in M-27IGFIR cells, although it restored MMP-9 expression to levels comparable to wild-type M-27 cells, failed to significantly alter basal MMP-3 and MMP-13 mRNA levels. The activation of MMP transcription depends on the coordinated activation of several cis-acting elements on their promoters (19). Although MMP-3, -9, and -13 all have AP-1 and PEA3 binding sites on their promoters, an NF-κB binding site has been identified only on the MMP-9 promoter (19). NF-κB as well as AP-1 can be activated downstream of PKC-α signaling (48), and therefore, the reconstitution of PKC-α may be sufficient to engage the critical cis-acting elements on the MMP-9 promoter, leading to transcription. Our results suggest that in addition to PKC-α, other factors, yet to be identified, that are required for full transcriptional activation of MMP-3 and -13 may also be down-regulated or inactivated by IGF-IR overexpression.
The cross talk between the IGF-IR axis and PKC signaling is complex and not fully resolved. Both pathways were implicated in similar cellular processes such as cell proliferation, differentiation, transformation, and malignant progression, but the extent of their interdependence, reciprocal regulation, or cooperative signaling appear to be cell type and PKC isoform specific. For example, PMA-induced PKC activation was reported to antagonize (49, 50) or promote (51) IGF signaling. In turn, IGF-IR activation was also shown to suppress (52) or induce (53) PKC signaling, and in many cell types, transcriptional activation downstream of IGF-IR signaling was shown to depend, either partially or exclusively, on the activation of PKCs (53, 54, 55). These seemingly conflicting reports may be due to the multiplicity and variability of PKC isoforms expressed in any given cell and the prevalent use of broad, isoform-nonspecific PKC inhibitors in many of the reported studies. Together, these factors preclude definitive conclusions on the cross talk between the IGF axis and specific PKC isoforms. Moreover, many of the studies that identified IGF-IR as a regulator of PKC activity were based on analysis of normal cells (51, 56), and the possibility that the reciprocal cross talk between the IGF-IR axis and specific PKC isoforms is altered as a consequence of malignant transformation and IGF-IR overexpression cannot be ruled out.
In our own cells, a differential effect of IGF-IR on PKC expression was also evident as IGF-IR overexpression decreased PKC-α while elevating PKC-λ levels. This is consistent with published reports that identified distinct transcriptional elements in the PKC-α and PKC-λ promoters. Namely, AP-2 and Sp1 binding sites were implicated in transcriptional regulation of PKC-α (36, 57, 58), but TFII-I and Elk1 sites were implicated in the transcription of PKC-λ (59). It is relevant to note in this regard that the Sp1 binding site on the PKC-α promoter was implicated in its transcriptional repression (58), and IGF-IR was identified as an activator of Sp1 (60), suggesting that this transcription factor may be involved in transmitting the inhibitory effect of IGF-IR. Moreover, our data strongly implicate the PI3K signaling pathway in IGF-IR-mediated transcriptional repression of PKC-α, and this is in line with several reports that have in fact identified a requirement for PI3K signaling for Sp1-mediated regulation of gene transcription (61, 62, 63, 64). Although the molecular mechanism mediating transcriptional down-regulation of PKC-α in the M-27IGFIR cells remains to be fully resolved, the evidence is consistent with the interpretation that PI3K-mediated regulation of Sp1 activity plays a role in this suppression.
In previous studies, we have shown that IGF-IR overexpression in M-27 cells resulted in increased invasion as measured in Matrigel and in the acquisition of a liver-metastasizing potential (33). The reason for the relatively low invasive potential of M-27 cells in Matrigel (33) despite the production of MMP-9 is not immediately clear but may be related to their high endogenous levels of the MMP-9 inhibitor, tissue inhibitor of metalloproteinases-1 (TIMP-1) (Brodt, P., unpublished observation).
The MMP profiles of tumor cells have previously been linked to their site of growth and metastasis (65, 66). High MMP-3 expression has, in fact, been reported in human lung adenocarcinomas, and both MMP-3 and 13 were identified as negative prognostic factors for non-small-cell lung carcinoma (67, 68, 69). Immunohistochemistry-based data implicated MMP-3 in the generalized growth and expansion of the neoplastic cell mass in the lung and MMP-9 and 13 in neoangiogenesis (70), identifying these enzymes as facilitators of lung carcinoma cell growth in the orthotopic site. Our results are consistent with these findings and also suggest that MMP-3, -9, and -13 expression was not critical for the liver-colonizing ability of the M-27IGFIR cells.
Taken together with previous data, the present study provides a possible mechanistic explanation for the conflicting data on the role of the IGF-IR in invasion and metastasis (2, 9, 71). They suggest that the consequences of loss or acquisition of IGF-IR expression may depend on the tumor cell type and may also be affected by the unique properties of the anatomic site of primary and/or metastatic tumor growth. Namely, it is possible that in cells where invasion, angiogenesis, and metastasis are driven mainly by MMPs that are regulated downstream of the PKC/ERK/AP-1 signaling pathway, the consequences of increased IGF-IR expression (or conversely, its suppression) may be distinct from those seen in cells that use PKC-independent MMPs such as MMP-2 and MMP-14 for ECM remodeling. This, in turn, may be further regulated by the requirements for tumor expansion/growth imposed by the specific host organ microenvironment. The opposing effects that altered IGF-IR expression may have on MMP synthesis, and thereby invasion and metastasis, provide an additional rationale for the careful selection of tumors for treatment with IGF-IR-targeting drugs.
Materials and Methods
Cells
The origin and invasive/metastatic phenotypes of Lewis lung carcinoma sublines H-59 and M-27 were described in detail previously (72). M-27IGFIR cells were produced by stable transfection of M-27 cells with the pCI-neo vector expressing a full-length human IGF-IR cDNA. Increased cell surface receptor levels, the acquisition of IGF-I responsiveness, and the altered metastatic properties of these cells were previously documented (33). Mock-transfected M-27 cells were generated by transfection with the pCI-neo vector. H-59IGFIRAS cells were produced by transduction of H-59 cells with a VSVG-pseudotyped retroviral vector expressing the first 309 bp of the human IGF-IR RNA in the antisense orientation (35). The HT-1080 fibrosarcoma cells were a kind gift from Dr. Richard Béliveau (Université du Québec a Montréal, Montreal, Quebec, Canada) (73). All the cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and antibiotics.
Plasmids and transfection
The pCI-neo vector expressing full-length human IGF-IR cDNA (33) was used to generate transiently transfected HT1080IGFIR cells and a pCMV-SPORT-β-Gal plasmid expressing full-length mouse PKC-α cDNA (Open Biosystems, Huntsville, AL) was used to generate the transiently transfected M-27IGFIR-PKCα cells. All transfections were performed using Lipofectamine, as per the manufacturer’s instruction.
Antibodies and reagents
The polyclonal anti-IGF-IR rabbit antibody [IGF-IRα (N-20)] and the polyclonal anti-MMP-9 goat antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal anti-MMP-2 was from Fuji for Medicorp (Montreal, Quebec, Canada). The monoclonal anti-MMP-3 antibody was from R&D Systems (Minneapolis, MN), the rabbit anti-MMP-13 antibody (no. 2557) was produced as described elsewhere (74), the monoclonal anti-PKC-α and anti-PKC-λ antibodies were from BD Transduction Laboratories (Mississauga, Ontario, Canada), the polyclonal anti-phospho-ERK1/ERK2 and anti-ERK1/ERK2 antibodies were from Cell Signal Technology (Beverly, MA), the monoclonal anti-β-actin antibody and PMA were from Sigma Chemical Co. (St. Louis, MO), the anti-calnexin antibody was a kind gift from Dr. Eric Chevet (University of Bordeaux, Bordeaux, France), and the Alexa Fluor 568 goat antimouse and goat antirabbit IgG were from Invitrogen (Eugene, OR). The rhIGF-I was from United States Biological (Swampscott, MA), the inhibitors LY294002 and PD98059 were from Calbiochem (San Diego, CA), and the protease inhibitor cocktail tablets were from Roche Diagnostics (Indianapolis, IN).
PMA stimulation
Optimal PMA concentrations for induction of MMP expression were established based on preliminary dose-response analyses. For all the experiments, the cells were serum starved overnight and then stimulated with the indicated PMA concentrations for 8 or 18 h before mRNA extraction or the collection of conditioned media for protein analysis, respectively. For transcriptional activation of PKC-α, the cells were incubated with 1 μm PMA in the presence of 1% FCS, as specified in the text. Where indicated, cells were pretreated for 5 h with 20 μm LY294002 or PD98059 and then incubated for 10 min with 10 ng/ml IGF-I before stimulation with PMA.
RT-PCR
Total cellular RNA was extracted using Trizol (Life Technologies, Burlington, Ontario, Canada). RT-PCR was performed using the Moloney murine leukemia virus reverse transcriptase and Taq DNA polymerase (both from Invitrogen, Carlsbad, CA) and specific primers as detailed in supplemental information. The cDNA was amplified using 30 cycles for MMP-2, MMP-3, MMP-9, and PKC-α; 38 cycles for MMP-13 and PKC-λ; and 24 cycles for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), each for 30 sec at 94 C, 30 sec at 58 C, and 30 sec at 72 C, and this was followed by a 10 min incubation at 72 C. The amplified DNA fragments were analyzed by electrophoresis on a 1.5% agarose gel.
Real-time quantitative PCR
PCR was performed with the LightCycler FastStart DNA Master SYBR Green I (Roche Applied Science, Laval, Quebec, Canada) in a standard PCR mixture containing 0.5 μm of each primer (described above), 3 mm MgCl2, and 2 μl cDNA. Amplification and detection were performed in a LightCycler instrument (Roche), essentially as we described previously, using 20 μl reaction mixture and 45 cycles of denaturation (95 C, 10 sec, ramp rate 20 C/sec), annealing (58 C, 5 sec, ramp rate 20 C/sec), and extension (72 C, 22 sec ramp rate 20 C/sec) (75). A single fluorescence reading was taken at each extension step. The crossing points, marking the cycle when the fluorescence of a given sample significantly exceeded the baseline signal, were recorded and expressed as a function of the cycle number. The crossing points were plotted against known concentrations determined on the basis of a preestablished standard curve.
Western blot analysis
Western blotting was performed essentially as we previously described (31). Serum-free conditioned media (for MMP-3, -9, and -13 analyses) were harvested from tumor cell monolayers that were cultured at 37 C in serum-free RPMI for 18–24 h and then concentrated 100-fold. Proteins were separated on 10% SDS-polyacrylamide gels using 15, 75, and 150 μg protein per lane for MMP-3, MMP-9 (and MMP-2), and MMP-13 (that is produced at relatively low levels), respectively. After transfer, the nitrocellulose membranes were probed with the respective antibodies diluted 1:500 (MMP-9 and MMP-2) or 1:5000 (MMP-3 and MMP-13). Total cell lysates (for ERK and PKC analysis) were obtained as we described previously (31), and proteins (50 μg/lane) were separated on 10% SDS-polyacrylamide gels, transferred, and probed consecutively with antibodies to phospho-ERK and ERK (diluted 1:1000) or to PKC-α (diluted 1:1000), PKC-λ (diluted 1:500), and β-actin (diluted 1:10,000). To analyze PMA-induced membrane translocation of PKC-α, the cells were lysed and the cytosolic and membrane proteins separated as we previously described (33). Proteins (50 μg/lane) were resolved by PAGE, transferred, and probed with an anti-PKC-α antibody. Horseradish peroxidase-conjugated antibodies were used as secondary antibodies for all the blots and the bands visualized using the Lumi-Light Western blotting substrate (Roche Diagnostics). Band intensities were measured using an Alpha Imager (Alpha Innotech, San Leandro, CA).
Zymography
The gelatinolytic activity of MMP-2 and MMP-9 was analyzed by gelatin zymography, as we previously described (24). Briefly, proteins in concentrated conditioned media (100×) were separated by electrophoresis on a 10% SDS-polyacrylamide gel containing 1 mg/ml gelatin. The gels were stained with Coomassie Blue R250 and destained with 10% acetic acid-20% methanol until the desired color intensity was obtained. The gelatinolytic activity was seen as a clear zone on the blue background.
In vitro kinase assay
Serum-starved cells were stimulated with the indicated concentrations of PMA and/or IGF-I and lysed as described (31). PKC activity was measured using the PKC assay kit (Upstate Biotechnology, Lake Placid, NY) as per the manufacturer’s instruction and essentially as we previously described (33).
Dual-luciferase reporter assay
Cells were cotransfected with the pGL3-basic vector expressing the firefly luciferase gene downstream of an 1800-bp mouse PKC-α promoter sequence (nucleotides −1573 to +227) (36) and the pRL-SV-40 plasmid expressing the renilla luciferase gene (Promega, Madison, WI), using the Lipofectamine Plus reagents (Invitrogen, Carlsbad, CA). The transfected cells were incubated for another 5 h in complete medium and then in serum-depleted RPMI medium for 24 h. RPMI containing 1 μm PMA and 1% FCS were added, and the cells were incubated for the indicated time interval. Firefly luciferase and renilla luciferase activities were analyzed by the dual-luciferase reporter assay system using the Glomax 20/20 luminometer (both from Promega).
Immunohistochemistry
Eight-micrometer cryostat sections were incubated first in a blocking solution (5% normal goat serum and 2% BSA in PBS) for 60 min and then overnight at 4 C with the primary antibodies diluted 1:50 and for 2 h at room temperature with the appropriate Alexa Fluor 568 goat antisera diluted 1:200. The sections were mounted in Prolong Gold antifade reagent (Invitrogen, Molecular Probes, Eugene, OR) and confocal images captured with a Zeiss LSM 510 microscope and processed with the Zeiss LSM 510 software.
Statistical analysis
The Student’s t test was used for data analysis.
Acknowledgments
We thank Drs. Eric Chevet, John Hiscott, and Frédéric Delom for their helpful suggestions and shared reagents.
Footnotes
This project was supported mainly by Grant MOP-81201 from the Canadian Institute for Health Research and Grant 11423 (a Terry Fox New Frontiers Initiative grant) from the National Cancer Institute of Canada (to P.B.) and also by Grant 8530 from the Shriners of North America (to E.R.L.).
Current address for D.Z.: Department of Biochemistry, McGill University, Montreal, Quebec, Canada.
Disclosure Summary: The authors have no conflict of interest to declare.
First Published Online October 23, 2009
S.L. and D.Z. contributed equally to the work described in this manuscript.
Abbreviations: DAG, Diacylglycerol; ECM, extracellular matrix; FCS, fetal calf serum; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IGFBP, IGF-binding protein; IGF-IR, IGF-I receptor; MMP, matrix metalloproteinase; NFκB, nuclear factor-κB; PI3K phosphatidylinositol 3-kinase; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate; qRT-PCR, quantitative RT-PCR; rh, recombinant human.
References
- 1.Kiely PA, Sant A, O'Connor R2002. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J Biol Chem 277:22581–22589 [DOI] [PubMed] [Google Scholar]
- 2.Samani AA, Yakar S, LeRoith D, Brodt P2007. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47 [DOI] [PubMed] [Google Scholar]
- 3.Kiely PA, Leahy M, O'Gorman D, O'Connor R 2005 RACK1-mediated integration of adhesion and insulin-like growth factor I (IGF-I) signaling and cell migration are defective in cells expressing an IGF-I receptor mutated at tyrosines 1250. and 1251. J Biol Chem 280:7624–7633 [DOI] [PubMed] [Google Scholar]
- 4.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM2008. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res 14:6364–6370 [DOI] [PubMed] [Google Scholar]
- 5.Himpe E, Kooijman R2009. Insulin-like growth factor-I receptor signal transduction and the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway. Biofactors 35:76–81 [DOI] [PubMed] [Google Scholar]
- 6.Baserga R2009. Customizing the targeting of IGF-1 receptor. Future Oncol 5:43–50 [DOI] [PubMed] [Google Scholar]
- 7.Weroha SJ, Haluska P2008. IGF-1 Receptor Inhibitors in Clinical Trials—Early Lessons. J Mammary Gland Biol Neoplasia 13:471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM1999. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res 59:2203–2209 [PubMed] [Google Scholar]
- 9.Lopez T, Hanahan D2002. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell 1:339–353 [DOI] [PubMed] [Google Scholar]
- 10.Nagase H, Woessner Jr JF1999. Matrix metalloproteinases. J Biol Chem 274:21491–21494 [DOI] [PubMed] [Google Scholar]
- 11.Overall CM, López-Otín C2002. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2:657–672 [DOI] [PubMed] [Google Scholar]
- 12.McCawley LJ, Matrisian LM2000. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 6:149–156 [DOI] [PubMed] [Google Scholar]
- 13.McCawley LJ, Matrisian LM2001. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol 13:534–540 [DOI] [PubMed] [Google Scholar]
- 14.Stetler-Stevenson WG, Yu AE2001. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol 11:143–152 [DOI] [PubMed] [Google Scholar]
- 15.Gorden DL, Fingleton B, Crawford HC, Jansen DE, Lepage M, Matrisian LM2007. Resident stromal cell-derived MMP-9 promotes the growth of colorectal metastases in the liver microenvironment. Int J Cancer 121:495–500 [DOI] [PubMed] [Google Scholar]
- 16.Kenny HA, Lengyel E2009. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 8:683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fingleton B2006. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci 11:479–491 [DOI] [PubMed] [Google Scholar]
- 18.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T2003. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253:269–285 [DOI] [PubMed] [Google Scholar]
- 19.Yan C, Boyd DD2007. Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26 [DOI] [PubMed] [Google Scholar]
- 20.Newton AC2001. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101:2353–2364 [DOI] [PubMed] [Google Scholar]
- 21.Griner EM, Kazanietz MG2007. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 7:281–294 [DOI] [PubMed] [Google Scholar]
- 22.Michie AM, Nakagawa R2005. The link between PKCα regulation and cellular transformation. Immunol Lett 96:155–162 [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, Xu Y2005. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase Cα. Clin Exp Metastasis 22:369–376 [DOI] [PubMed] [Google Scholar]
- 24.Tan M, Li P, Sun M, Yin G, Yu D2006. Upregulation and activation of PKCα by ErbB2 through Src promotes breast cancer cell invasion that can be blocked by combined treatment with PKCα and Src inhibitors. Oncogene 25:3286–3295 [DOI] [PubMed] [Google Scholar]
- 25.Mochly-Rosen D, Gordon AS1998. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J 12:35–42 [PubMed] [Google Scholar]
- 26.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW1993. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor α, interleukin 1 and phorbol ester. Biochem J 296(Pt 3):803–809 [DOI] [PMC free article] [PubMed]
- 27.Hattori Y, Nerusu KC, Bhagavathula N, Brennan M, Hattori N, Murphy HS, Su LD, Wang TS, Johnson TM, Varani J2003. Vascular expression of matrix metalloproteinase-13 (collagenase-3) in basal cell carcinoma. Exp Mol Pathol 74:230–237 [DOI] [PubMed] [Google Scholar]
- 28.Mackay AR, Ballin M, Pelina MD, Farina AR, Nason AM, Hartzler JL, Thorgeirsson UP1992. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis 12:168–184 [PubMed] [Google Scholar]
- 29.Long L, Navab R, Brodt P1998. Regulation of the Mr 72,000 type IV collagenase by the type I insulin-like growth factor receptor. Cancer Res 58:3243–3247 [PubMed] [Google Scholar]
- 30.Long L, Rubin R, Baserga R, Brodt P1995. Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res 55:1006–1009 [PubMed] [Google Scholar]
- 31.Zhang D, Bar-Eli M, Meloche S, Brodt P2004. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem 279:19683–19690 [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Brodt P2003. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene 22:974–982 [DOI] [PubMed] [Google Scholar]
- 33.Brodt P, Fallavollita L, Khatib AM, Samani AA, Zhang D2001. Cooperative regulation of the invasive and metastatic phenotypes by different domains of the type I insulin-like growth factor receptor β subunit. J Biol Chem 276:33608–33615 [DOI] [PubMed] [Google Scholar]
- 34.Huhtala P, Tuuttila A, Chow LT, Lohi J, Keski-Oja J, Tryggvason K1991. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem 266:16485–16490 [PubMed] [Google Scholar]
- 35.Samani AA, Fallavollita L, Jaalouk DE, Galipeau J, Brodt P2001. Inhibition of carcinoma cell growth and metastasis by a vesicular stomatitis virus G-pseudotyped retrovector expressing type I insulin-like growth factor receptor antisense. Hum Gene Ther 12:1969–1977 [DOI] [PubMed] [Google Scholar]
- 36.Clark JH, Haridasse V, Glazer RI2002. Modulation of the human protein kinase Cα gene promoter by activator protein-2. Biochemistry 41:11847–11856 [DOI] [PubMed] [Google Scholar]
- 37.Ogata Y, Enghild JJ, Nagase H1992. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 267:3581–3584 [PubMed] [Google Scholar]
- 38.Nagase H, Enghild JJ, Suzuki K, Salvesen G1990. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry 29:5783–5789 [DOI] [PubMed] [Google Scholar]
- 39.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD2005. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J 5:14–23 [DOI] [PubMed] [Google Scholar]
- 40.Fortier LA, Deak MM, Semevolos SA, Cerione RA2004. Insulin-like growth factor-I diminishes the activation status and expression of the small GTPase Cdc42 in articular chondrocytes. J Orthop Res 22:436–445 [DOI] [PubMed] [Google Scholar]
- 41.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF2003. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1β-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem 278:25386–25394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ1999. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes 48:1638–1644 [DOI] [PubMed] [Google Scholar]
- 43.Chang MH, Kuo WW, Chen RJ, Lu MC, Tsai FJ, Kuo WH, Chen LY, Wu WJ, Huang CY, Chu CH2008. IGF-II/mannose 6-phosphate receptor activation induces metalloproteinase-9 matrix activity and increases plasminogen activator expression in H9c2 cardiomyoblast cells. J Mol Endocrinol 41:65–74 [DOI] [PubMed] [Google Scholar]
- 44.Ma Z, Dong A, Kong M, Qian J2007. Silencing of the type 1 insulin-like growth factor receptor increases the sensitivity to apoptosis and inhibits invasion in human lung adenocarcinoma A549 cells. Cell Mol Biol Lett 12:556–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian J, Dong A, Kong M, Ma Z, Fan J, Jiang G2007. Suppression of type 1 Insulin-like growth factor receptor expression by small interfering RNA inhibits A549 human lung cancer cell invasion in vitro and metastasis in xenograft nude mice. Acta Biochim Biophys Sin (Shanghai) 39:137–147 [DOI] [PubMed] [Google Scholar]
- 46.Coppock HA, White A, Aplin JD, Westwood M2004. Matrix metalloprotease-3 and -9 proteolyze insulin-like growth factor-binding protein-1. Biol Reprod 71:438–443 [DOI] [PubMed] [Google Scholar]
- 47.Fowlkes JL, Enghild JJ, Suzuki K, Nagase H1994. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem 269:25742–25746 [PubMed] [Google Scholar]
- 48.Kang DW, Park MH, Lee YJ, Kim HS, Kwon TK, Park WS, Min do S2008. Phorbol ester up-regulates phospholipase D1 but not phospholipase D2 expression through a PKC/Ras/ERK/NFκB-dependent pathway and enhances matrix metalloproteinase-9 secretion in colon cancer cells. J Biol Chem 283:4094–4104 [DOI] [PubMed] [Google Scholar]
- 49.Zheng WH, Kar S, Quirion R2000. Stimulation of protein kinase C modulates insulin-like growth factor-1-induced Akt activation in PC12 cells. J Biol Chem 275:13377–13385 [DOI] [PubMed] [Google Scholar]
- 50.Zheng WH, Quirion R2006. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci 7:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yano K, Bauchat JR, Liimatta MB, Clemmons DR, Duan C1999. Down-regulation of protein kinase C inhibits insulin-like growth factor I-induced vascular smooth muscle cell proliferation, migration, and gene expression. Endocrinology 140:4622–4632 [DOI] [PubMed] [Google Scholar]
- 52.Oh CD, Chun JS2003. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem 278:36563–36571 [DOI] [PubMed] [Google Scholar]
- 53.Cao Z, Liu LZ, Dixon DA, Zheng JZ, Chandran B, Jiang BH2007. Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal 19:1542–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maniar R, Pecherskaya A, Ila R, Solem M2005. PKC α-dependent regulation of the IGF1 receptor in adult and embryonic rat cardiomyocytes. Mol Cell Biochem 275:15–24 [DOI] [PubMed] [Google Scholar]
- 55.Czifra G, Tóth IB, Marincsák R, Juhász I, Kovács I, Acs P, Kovács L, Blumberg PM, Bíró T2006. Insulin-like growth factor-I-coupled mitogenic signaling in primary cultured human skeletal muscle cells and in C2C12 myoblasts. A central role of protein kinase Cδ. Cell Signal 18:1461–1472 [DOI] [PubMed] [Google Scholar]
- 56.Tranque PA, Calle R, Naftolin F, Robbins R1992. Involvement of protein kinase-C in the mitogenic effect of insulin-like growth factor-I on rat astrocytes. Endocrinology 131:1948–1954 [DOI] [PubMed] [Google Scholar]
- 57.Desai DS, Hirai S, Karnes Jr WE, Niles RM, Ohno S1999. Cloning and characterization of the murine PKCα promoter: identification of a retinoic acid response element. Biochem Biophys Res Commun 263:28–34 [DOI] [PubMed] [Google Scholar]
- 58.Zhan M, Yu D, Liu J, Glazer RI, Hannay J, Pollock RE2005. Transcriptional repression of protein kinase Cα via Sp1 by wild type p53 is involved in inhibition of multidrug resistance 1 P-glycoprotein phosphorylation. J Biol Chem 280:4825–4833 [DOI] [PubMed] [Google Scholar]
- 59.Gustafson WC, Ray S, Jamieson L, Thompson EA, Brasier AR, Fields AP2004. Bcr-Abl regulates protein kinase Cι (PKCι) transcription via an Elk1 site in the PKCι promoter. J Biol Chem 279:9400–9408 [DOI] [PubMed] [Google Scholar]
- 60.Copland JA, Pardini AW, Wood TG, Yin D, Green A, Bodenburg YH, Urban RJ, Stuart CA2007. IGF-1 controls GLUT3 expression in muscle via the transcriptional factor Sp1. Biochim Biophys Acta 1769:631–640 [DOI] [PubMed] [Google Scholar]
- 61.Harris SM, Harvey EJ, Hughes TR, Ramji DP2008. The interferon-γ-mediated inhibition of lipoprotein lipase gene transcription in macrophages involves casein kinase 2- and phosphoinositide-3-kinase-mediated regulation of transcription factors Sp1 and Sp3. Cell Signal 20:2296–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qureshi HY, Ahmad R, Sylvester J, Zafarullah M2007. Requirement of phosphatidylinositol 3-kinase/Akt signaling pathway for regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-β in human chondrocytes. Cell Signal 19:1643–1651 [DOI] [PubMed] [Google Scholar]
- 63.Shen J, Jiang J, Wei Y, Zhou L, Liu D, Zhou J, Gu J2007. Two specific inhibitors of the phosphatidylinositol 3-kinase LY294002 and wortmannin up-regulate β1,4-galactosyltransferase I and thus sensitize SMMC-7721 human hepatocarcinoma cells to cycloheximide-induced apoptosis. Mol Cell Biochem 304:361–367 [DOI] [PubMed] [Google Scholar]
- 64.Sroka IC, Nagle RB, Bowden GT2007. Membrane-type 1 matrix metalloproteinase is regulated by sp1 through the differential activation of AKT, JNK, and ERK pathways in human prostate tumor cells. Neoplasia 9:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J2005. Genes that mediate breast cancer metastasis to lung. Nature 436:518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massagué J2007. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446:765–770 [DOI] [PubMed] [Google Scholar]
- 67.Hsu CP, Shen GH, Ko JL2006. Matrix metalloproteinase-13 expression is associated with bone marrow microinvolvement and prognosis in non-small cell lung cancer. Lung Cancer 52:349–357 [DOI] [PubMed] [Google Scholar]
- 68.Michael M, Babic B, Khokha R, Tsao M, Ho J, Pintilie M, Leco K, Chamberlain D, Shepherd FA1999. Expression and prognostic significance of metalloproteinases and their tissue inhibitors in patients with small-cell lung cancer. J Clin Oncol 17:1802–1808 [DOI] [PubMed] [Google Scholar]
- 69.Thomas P, Khokha R, Shepherd FA, Feld R, Tsao MS2000. Differential expression of matrix metalloproteinases and their inhibitors in non-small cell lung cancer. J Pathol 190:150–156 [DOI] [PubMed] [Google Scholar]
- 70.Bodey B, Bodey Jr B, Gröger AM, Siegel SE, Kaiser HE2001. Invasion and metastasis: the expression and significance of matrix metalloproteinases in carcinomas of the lung. In Vivo 15:175–180 [PubMed] [Google Scholar]
- 71.Sutherland BW, Knoblaugh SE, Kaplan-Lefko PJ, Wang F, Holzenberger M, Greenberg NM2008. Conditional deletion of insulin-like growth factor-I receptor in prostate epithelium. Cancer Res 68:3495–3504 [DOI] [PubMed] [Google Scholar]
- 72.Brodt P1986. Characterization of two highly metastatic variants of Lewis lung carcinoma with different organ specificities. Cancer Res 46:2442–2448 [PubMed] [Google Scholar]
- 73.Annabi B, Bouzeghrane M, Currie JC, Dulude H, Daigneault L, Garde S, Rabbani SA, Panchal C, Wu JJ, Béliveau R2006. Inhibition of MMP-9 secretion by the anti-metastatic PSP94-derived peptide PCK3145 requires cell surface laminin receptor signaling. Anticancer Drugs 17:429–438 [DOI] [PubMed] [Google Scholar]
- 74.Lee ER2006. Proteolytic enzymes in skeletal development: histochemical methods adapted to the study of matrix lysis during the transformation of a “cartilage model” into bone. Front Biosci 11:2538–2553 [DOI] [PubMed] [Google Scholar]
- 75.Wang N, Thuraisingam T, Fallavollita L, Ding A, Radzioch D, Brodt P2006. The secretory leukocyte protease inhibitor is a type 1 insulin-like growth factor receptor-regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res 66:3062–3070 [DOI] [PubMed] [Google Scholar]