Abstract
Distribution of phytochrome (as Pfr) among membranes from soybean hypocotyls (Glycine max L. cv. Wayne) was determined by the combined techniques of cell fractionation, difference spectrometry, and electron microscopic morphometry. More than 90% of the phytochrome was found in the soluble fraction. With homogenates prepared in the presence or absence of Mg2+, the portion associated with membrane was only 6.5% and 1%, respectively. In the presence of Mg2+, the content of particulate phytochrome correlated with the amount of endoplasmic reticulum with attached ribosomes in the fractions but not with mitochondria or other membranes (including endoplasmic reticulum membranes from which the ribosomes may have been lost during cell fractionation). In the absence of Mg2+, phytochrome was associated with a “heavy” plasma membrane fraction. The phytochrome content was sufficiently low to be accounted for by a contamination of less than 10% by rough-surfaced fragments of endoplasmic reticulum. The findings show association of phytochrome with a particulate fraction enriched in rough-surfaced fragments of endoplasmic reticulum but do not rule out cosedimentation of some unknown or unspecific phytochrome aggregate with this fraction.
Full text
PDF

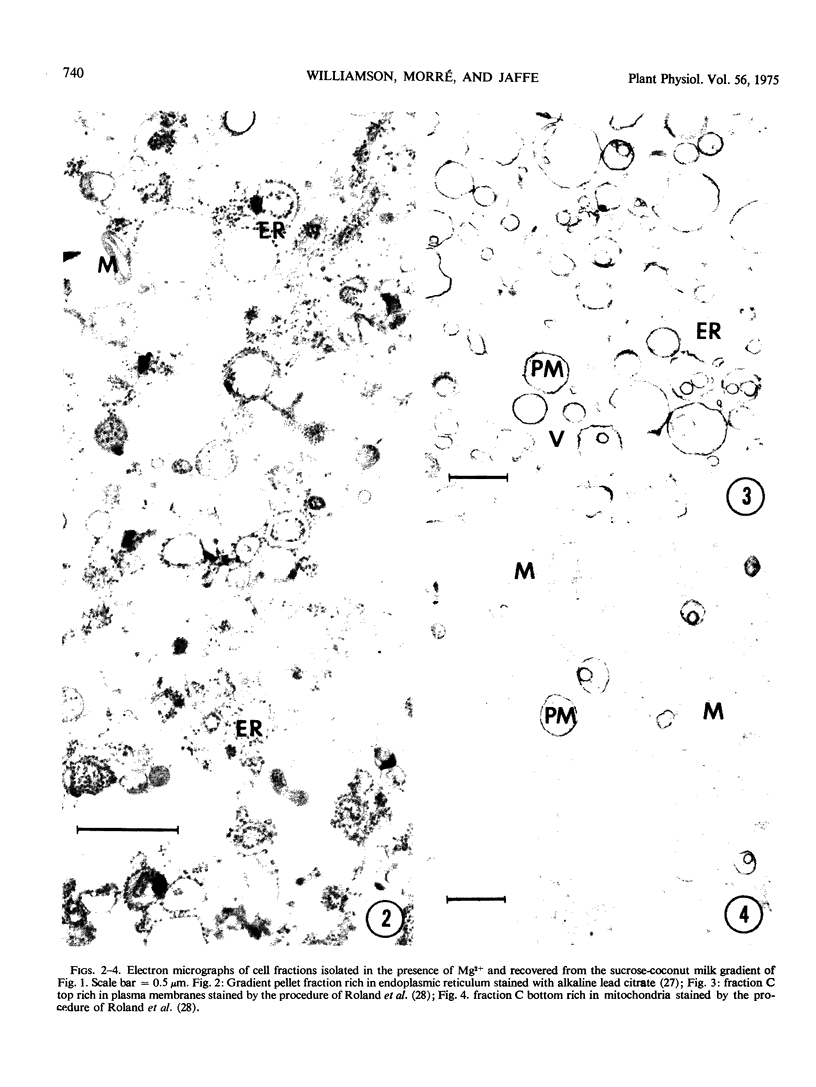

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coleman R. A., Pratt L. H. Electron microscopic localization of phytochrome in plants using an indirect antibody-labeling method. J Histochem Cytochem. 1974 Nov;22(11):1039–1047. doi: 10.1177/22.11.1039. [DOI] [PubMed] [Google Scholar]
- Crotty W. J., Ledbetter M. C. Membrane continuities involving chloroplasts and other organelles in plant cells. Science. 1973 Nov 23;182(4114):839–841. doi: 10.1126/science.182.4114.839. [DOI] [PubMed] [Google Scholar]
- Dallner G., Ernster L. Subfractionation and composition of microsomal membranes: a review. J Histochem Cytochem. 1968 Oct;16(10):611–632. doi: 10.1177/16.10.611. [DOI] [PubMed] [Google Scholar]
- GORDON S. A., SURREY K. Red and far-red action on oxidative phosphorylation. Radiat Res. 1960 Apr;12:325–339. [PubMed] [Google Scholar]
- Galston A. W. Microspectrophotometric evidence for phytochrome in plant nuclei. Proc Natl Acad Sci U S A. 1968 Oct;61(2):454–460. doi: 10.1073/pnas.61.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J. Phytochrome-mediated bioelectric potentials in mung bean seedlings. Science. 1968 Nov 29;162(3857):1016–1017. doi: 10.1126/science.162.3857.1016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmé D., Boisard J., Briggs W. R. Binding properties in vitro of phytochrome to a membrane fraction. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3861–3865. doi: 10.1073/pnas.70.12.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Merritt W. D., Lembi C. A. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73(1):43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Pine K., Klein A. O. Regulation of polysome formation in etiolated bean leaves by light. Dev Biol. 1972 May;28(1):280–289. doi: 10.1016/0012-1606(72)90144-3. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Coleman R. A. Immunocytochemical localization of phytochrome. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2431–2435. doi: 10.1073/pnas.68.10.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H., Marmé D., Schäfer E. Particle-bound phytochrome from maize and pumpkin. Nat New Biol. 1973 Oct 10;245(145):189–191. doi: 10.1038/newbio245189a0. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Schäfer E. Particle-bound phytochrome: a function of light dose and steady-state level of the far-red-absorbing form. J Membr Biol. 1974;15(4):393–404. doi: 10.1007/BF01870097. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Rubinstein B., Drury K. S., Park R. B. Evidence for bound phytochrome in oat seedlings. Plant Physiol. 1969 Jan;44(1):105–109. doi: 10.1104/pp.44.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada T. A rapid photoreversible response of barley root tips in the presence of 3-indoleacetic Acid. Proc Natl Acad Sci U S A. 1968 Feb;59(2):376–380. doi: 10.1073/pnas.59.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Key J. L., Ross C. W. Activation of 80S Maize Ribosomes by Red Light Treatment of Dark-grown Seedlings. Plant Physiol. 1974 Jan;53(1):28–31. doi: 10.1104/pp.53.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunghans H., Jaffe M. J. Rapid Respiratory Changes Due to Red Light or Acetylcholine during the Early Events of Phytochrome-mediated Photomorphogenesis. Plant Physiol. 1972 Jan;49(1):1–7. doi: 10.1104/pp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]