Abstract
In situ estrogen production by aromatase conversion from androgens plays an important role in breast tumor promotion. Here, we show that 17β-estradiol (E2) can rapidly enhance aromatase enzymatic activity through an increase of aromatase protein phosphorylation in breast cancer cell lines. In vivo labeling experiments and site-directed mutagenesis studies demonstrated that phosphorylation of the 361-tyrosine residue is crucial in the up-regulation of aromatase activity under E2 exposure. Our results demonstrated a direct involvement of nonreceptor tyrosine-kinase c-Src in E2-stimulated aromatase activity because inhibition of its signaling abrogated the up-regulatory effects induced by E2 on aromatase activity as well as phosphorylation of aromatase protein. In addition, from our data it emerges that aromatase is a target of cross talk between growth factor receptors and estrogen receptor α signaling. These findings show, for the first time, that tyrosine phosphorylation processes play a key role in the rapid changes induced by E2 in aromatase enzymatic activity, revealing the existence of a short nongenomic autocrine loop between E2 and aromatase in breast cancer cells.
Estradiol induces rapid up-regulatory effects on aromatase activity in MCF-7 breast cancer cells through an enhanced tyrosine phosphorylation of the enzyme mediated by c-Src kinase.
Estrogens play a crucial role in the development and progression of breast cancer. The biosynthesis of estrogens from androgens is catalyzed by the enzyme complex termed “aromatase,” which is composed of two polypeptides, an ubiquitous nonspecific flavoprotein, reduced nicotinamide adenine dinucleotide phosphate-cytochrome P450 reductase, and a specific microsomial form of cytochrome P450arom encoded by the cytochrome P450 (CYP)19 gene (1).
Aromatase expression in breast cancer tissue as well as in breast cancer cell lines has been shown by enzyme activity measurement, immunocytochemistry, and RT-PCR analysis (2, 3, 4). Cell culture (5) and nude mouse experiments (6) using aromatase-transfected MCF-7 cells have shown that aromatase expressed in breast cancer cells can promote tumor growth in both an autocrine and a paracrine manner. In addition, overexpression of aromatase in mammary gland of transgenic mice causes premalignant lesions, such as atypical ductal hyperplasia (7, 8). P450arom is found to be expressed at higher levels in cancer than in normal breast tissue (9, 10). Thus, induction of aromatase within the breast tumor can result in high levels of 17β-estradiol (E2) production that, in turn, stimulate tumor growth. Indeed, intratumoral aromatase of breast carcinoma has been extensively studied for its potential clinical significance as a target for endocrine therapy using aromatase inhibitors (11, 12).
It is well known that aromatase is regulated at the transcriptional level through the alternative use of tissue-specific promoters (13, 14), whereas posttranscriptional regulation of this protein remains poorly understood. Balthazart et al. (15, 16) demonstrated that phosphatases modulate the activity of brain aromatase and that the phosphorylation status of the enzyme is critical for its activity. In addition, several studies have suggested that aromatase activity could be modulated at the posttranslation level in different cell types upon the addition of growth factors and kinase inhibitors (17, 18, 19, 20). Recently, Miller et al. (21) demonstrated that aromatase serine (S) 118 is a potential phosphorylation site in mammalian cells, and mutation of S118 blocked phosphorylation and increased aromatase activity.
The classic effects of estrogens are mediated through binding to estrogen receptors (ERα and ERβ) and stimulation of transcription at nuclear levels. Recently, the nongenomic actions of estrogens have been reported through binding to membrane-associated ER (22, 23), which resides in or near the cell membrane and cross talks with the signal transduction pathways, including the c-Src/Ras/MAPK and cAMP pathway (24, 25, 26). Signaling from membrane ER induces posttranslational modification of many proteins. This includes the phosphorylation and regulation of enzymes, such as kinases or phosphatases, that impact cell physiology (27).
In the present study we demonstrated, in estrogen-dependent MCF-7 breast cancer epithelial cells, that 17β-estradiol (E2) is able to rapidly up-regulate aromatase enzymatic activity, and this may occur through an enhanced tyrosine phosphorylation levels of aromatase protein. Our results provide a new insight into the regulation of aromatase through posttranscriptional modulation in human breast cancer cells.
Results
Rapid increase of aromatase activity induced by E2 treatment
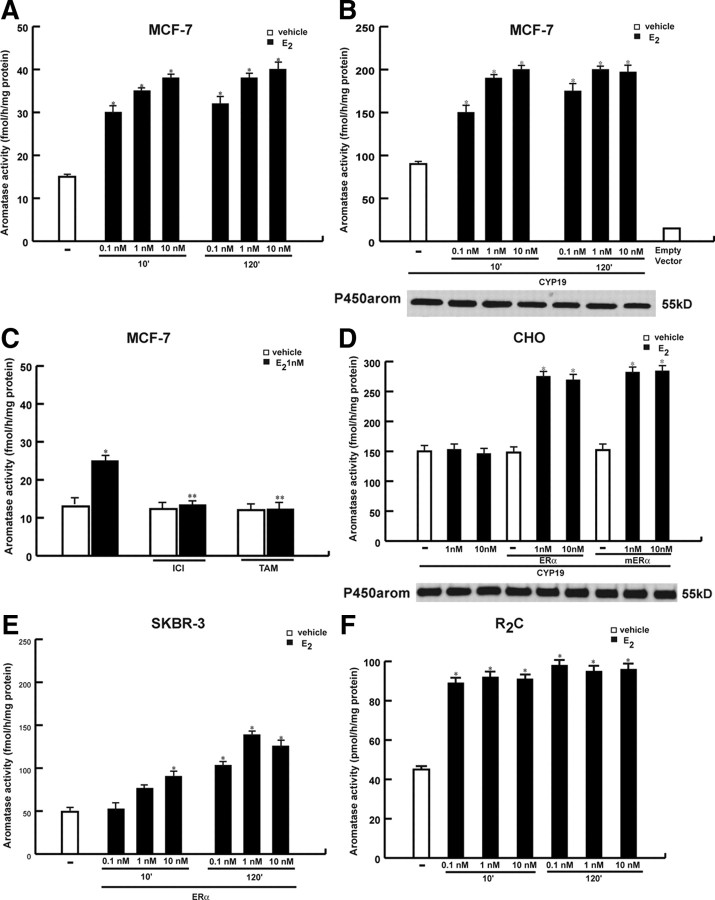
We first aimed to evaluate the effects of estrogens on aromatase activity by tritiated water assay in MCF-7 cells incubated for 10 and 120 min in the presence of 0.1, 1, and 10 nm of E2. As reported in Fig. 1A, E2 enhanced enzymatic activity at both times and doses investigated, even though to a higher extent under 1 and 10 nm E2. The E2 induction was also observed in MCF-7 cells transiently transfected with the aromatase gene (CYP19), that displayed a 6-fold increase in enzymatic activity (95.36 ± 0.92 fmol/h · mg protein) compared with parental MCF-7 cells (15.16 ± 0.47 fmol/h · mg protein) (Fig. 1B). To evaluate whether the E2 effects on aromatase activity were transient, MCF-7 cells were treated with E2 1 nm for different times (10 min; 6, 12, and 24 h; and 2, 4, and 6 d). We found that aromatase activity doubled upon E2 exposure ranging from 10 min to 12 h and remained moderately high up to 6 d (data not shown). The ER antagonists, ICI 182,780 (ICI) and tamoxifen (TAM) were able to abrogate the up-regulation induced by E2, whereas these treatments alone had no agonist activity (Fig. 1C). This suggests that estrogens can increase aromatase activity by binding to ERs.
Fig. 1.
Rapid effects of E2 on aromatase activity. MCF-7 (A) or MCF-7 cells transiently transfected with CYP19 vector (B) were treated with vehicle (-) or 0.1, 1, and 10 nm E2 for 10 and 120 min. Western blotting shows the expression of CYP19 vector used in the experiment. C, MCF-7 cells were pretreated with 1 μm ICI 182,780 (ICI) and 1 μm TAM for 30 min and then exposed or not to 1 nm E2 for 10 min. D, CHO cells were transiently transfected with CYP19 vector and ERα wt or mERα or empty vector and treated with 1 and 10 nm E2 for 10 min. Western blotting shows the expression of CYP19 vector used in the experiment. E, SKBR3 cells transiently transfected with ERα wt, and R2C cells (F) cells were treated as reported. Aromatase activity was performed as described in Materials and Methods. Empty vector: aromatase activity measured in cells transfected with pUC19 vector. The values represent the means ± se of three different experiments, each performed with triplicate samples. *, P < 0.01 compared with vehicle; **, P < 0.01 compared with E2-treated samples.
It has been shown that the rapid actions of estrogen could be mediated by membrane-associated ER (22, 23). Thus, we cotransfected in ER-negative Chinese hamster ovary (CHO) cells CYP19 vector with ERα wild-type (wt) plasmid or membrane ERα (mERα) construct. The mERα construct consists solely of the AF-2/ligand binding domain (E) of ERα cloned into the membrane-enhanced cyan fluorescent protein (Mem-ECFP) vector that encodes a fusion protein called GAP-43 (N-terminal 20 amino acids of neuromodulin) containing a signal that targets this portion of the receptor to the plasma membrane (28, 29). This construct is a well-established mutant ERα able to discriminate the nongenomic to the genomic actions of E2. As reported by Razandi et al. (30), expression of the E domain of ERα to the plasma membrane allowed the activation of ERK but did not result in the transactivation of an estrogen response element/luciferase reporter by E2 treatment. As revealed in Fig. 1D, 1 and 10 nm E2 for 10 min up-regulated enzymatic aromatase activity in CHO ectopically expressing mERα as well as ERα wt plasmids, suggesting that the expression of mERα is sufficient for E2 induction.
We also evaluated the effects of E2 on aromatase activity in ER-negative breast cancer cell line SKBR3 and in R2C rat Leydig tumor cells that express ERα and high levels of aromatase protein. No changes were observed in SKBR3 parental cells whereas E2 treatment enhanced aromatase activity both in SKBR3, ectopically expressing ERα, and in R2C cells (Fig. 1, E and F). R2C cells displayed an elevated aromatase activity that is 1 order of magnitude higher than that detected in the other cell types investigated. This may probably explain the lack of E2 dose-dependent stimulation of aromatase activity in the E2 range concentration tested.
Because we observed an enhanced aromatase activity under E2 treatment in a number of different cell lines, it could suggest that this regulation may underlie a general mechanism not related to cell specificity. However, this effect assumes a great importance in breast cancer cells, which are strongly dependent on estrogens for their growth.
E2 increases tyrosine phosphorylation levels of aromatase protein
One mechanism by which E2 might increase aromatase activity would be an enhancement in the transcription of aromatase mRNA and thus in the concentration of the enzyme. We performed RT-PCR and Western blotting analysis in MCF-7 cells treated with 1 nm E2 for 10 and 120 min. We did not observe any change on aromatase mRNA and protein level compared with the control (supplemental Fig. 1, A and B, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). These results suggest that rapid changes in E2-induced aromatase enzymatic activity are due to ERα action at the nongenomic level.
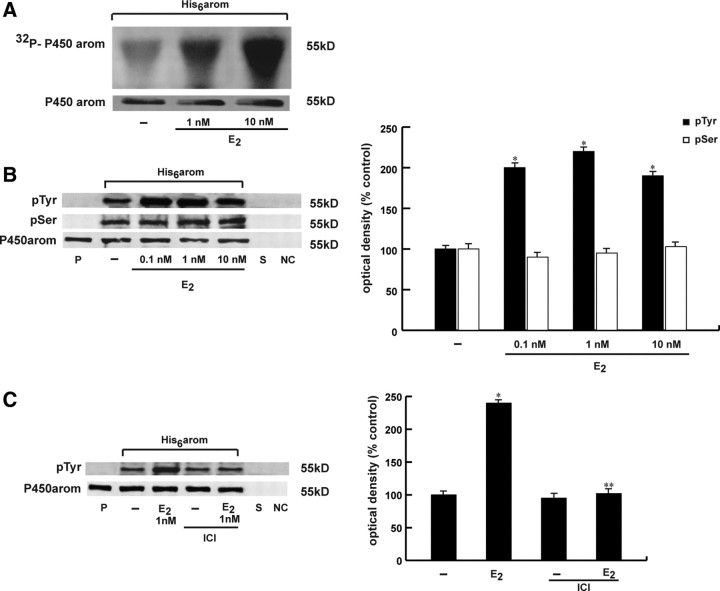
It is well known that the activity of many enzymes can be modulated rapidly by phosphorylation processes inducing conformational changes in the enzyme molecule. Previous analyses of the aromatase gene in a variety of mammalian and avian species demonstrated several consensus sites of phosphorylation on aromatase cDNA and deduced amino acid sequence (31, 32, 33). Thus, to evaluate the phosphorylation status of aromatase protein, we performed in vivo labeling experiment in MCF-7 cells transiently transfected with His6-arom, a plasmid coding for the entire human aromatase sequence with six tandem histidine residues on the carboxyl terminus, as described in Materials and Methods. The His6-tagged protein had the advantage to allow a higher yield of purified aromatase due to the specificity of Ni-NTA (nitrilotriacetic acid) agarose beads and avoid interference with the band of 55 kDa from heavy chains of antibodies used for immunoprecipitation. MCF-7 cells were transiently transfected with His6-arom, metabolically labeled with radioactive orthophosphate and then treated with 1 and 10 nm E2 for 10 min. Equal amounts of proteins were incubated with Ni-NTA agarose beads for isolation of recombinant P450arom, and the eluates were run on SDS-PAGE. Autoradiography of the membrane revealed that aromatase protein was efficiently phosphorylated in vivo upon E2 treatment (Fig. 2A). The membrane then was probed with an antiaromatase antibody to visualize the input levels of the samples.
Fig. 2.
Tyrosine phosphorylation levels of aromatase protein is enhanced by E2. A, MCF-7 cells were transiently transfected with His6-arom, labeled with [32P]orthophosphate, and then treated with vehicle (-) or 1 and 10 nm E2 for 10 min. Aromatase was purified using Ni-NTA agarose beads after which the complexes were resolved in SDS-PAGE. The top panel shows autoradiography of the SDS-PAGE, and the bottom panel shows immunoblot analysis with antiaromatase antibody (P450arom) as a control for expressed protein. B, MCF-7 cells transiently transfected with His6-arom were treated with vehicle (-) or 0.1, 1, and 10 nm E2 for 10 min. Aromatase was purified using Ni-NTA agarose beads after which the complexes were resolved in SDS-PAGE. Immunoblotting was performed using the antiphosphotyrosine (pTyr) and antiphosphoserine (pSer) antibodies. C, MCF-7 cells transiently transfected with His6-arom were pretreated with 1 μm ICI and then exposed or not to 1 nm E2 for 10 min. To verify equal loading, the membrane was probed with antiaromatase antibody. Microsomal extracts from placenta (P) were used as positive control. As negative controls we used the supernatant removed after incubation with Ni-NTA agarose beads (S) and vector-transfected MCF-7 cell lysates incubated with Ni-NTA agarose beads (NC). The side histograms represent the means ± se of three separate experiments in which band intensities were evaluated in terms of OD density arbitrary units and expressed as percentages of the control, which was assumed to be 100%. *, P < 0.01 compared with vehicle; **, P < 0.01 compared with E2-treated samples. kD, Kilodaltons.
To determine which type of amino acid is phosphorylated, we performed Western blotting analysis with antibodies directed against phosphotyrosine and phosphoserine residues using cell lysates from MCF-7 cells transfected with His6-arom and treated with 0.1, 1, and 10 nm E2 for 10 min. Our results showed that E2 was able to increase phosphotyrosine levels of purified aromatase protein, whereas no changes were detectable on serine phosphorylation status (Fig. 2B). This enhancement on tyrosine phosphorylation of aromatase was ERα-dependent because pretreatment with ICI reduced the E2-associated tyrosine phosphorylation (Fig. 2C). We obtained similar results after pretreatment with TAM (data not shown). Moreover, in the presence of a specific inhibitor of tyrosine phosphatases, sodium orthovanadate, we observed an increase of aromatase enzymatic activity as well as enhanced phosphotyrosine levels of purified aromatase protein, which were slightly increased by E2 cotreatment (data not show).
All these data indicate that E2 exposure is able to rapidly phosphorylate in vivo aromatase protein and increase tyrosine phosphorylation status of the enzyme.
Identification of tyrosine residue involved in the E2 activation
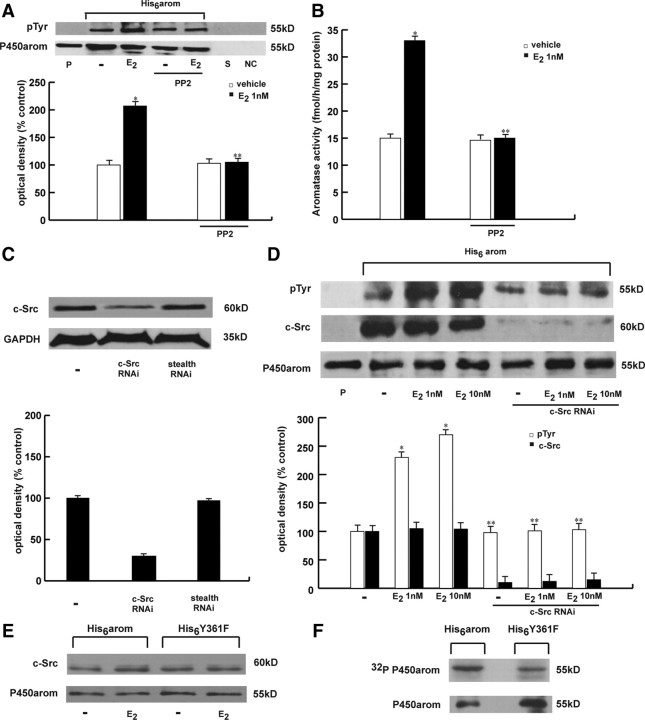
Consensus phosphorylation sites of human aromatase protein were analyzed using the public domain software (netphos 2.0 server) available on the web site of the Center for Biological Sequence Analysis at http://www.cbs.dtu.dk. Based on a deduced amino acid sequence and on a previously encoded database of potential phosphorylation sites, this program identifies all serine, threonine, and tyrosine residues in the protein that could potentially be phosphorylated (34). The program also provides for each residue a phosphorylation score ranging from 0 to 1.0 the value of which was proportional to the probability that the residue could, in fact, be phosphorylated in vivo. A score equal or larger to 0.5 was considered to predict a likely phosphorylation consensus site (35). The netphos 2.0 program identified four of the 17 tyrosine residues. The residues located at positions 184 and 361 of the human aromatase sequence have the highest consensus scores (0.992 and 0.976, respectively). The position at 361 corresponds to the residue present in the steroid-binding domain, an important functional domain of human aromatase, and notably this residue and its immediate environment are well conserved across species (Fig. 3A). Thus, to address the location of the potential phosphorylation site within the aromatase protein, we mutated the conserved tyrosine (Y) at residue 361 as well as the one at 184 to phenylalanine (F) to create two different constructs, Y184F and Y361F. These plasmids were used in an aromatase enzymatic assay. We found that Y361F and Y184F mutation didn’t affect the basal levels of aromatase activity. E2 increased aromatase activity in cells transfected with Y184F mutant expression vector but had no effect in the presence of Y361F expression plasmid (Fig. 3B). To further confirm these results, we performed an in vivo labeling experiment in MCF-7 cells transiently transfected with either His6-arom or His6-Y361F constructs. We found that the His6-Y361F mutated construct was not efficiently phosphorylated in vivo upon E2 treatment (Fig. 3C). These data directly prove that phosphorylation of the 361 tyrosine residue is crucial in the up-regulation of aromatase activity and its phosphorylation under E2 stimulation. This last result led us to question which specific cellular kinase might be responsible for phosphorylation of aromatase at the Y361 site. The research for consensus sequences corresponding to the protein kinases pointed to the Y361 site as potentially phosphorylated by c-Src tyrosine kinase (with a consensus score higher than the critical value of 0.5). Taking into account that estrogen stimulation of breast cancer cells led to an immediate tyrosine phosphorylation and activation of the c-Src kinase (26, 36), we sought to determine whether c-Src might be the tyrosine kinase involved in the E2 activation of aromatase protein. Thus, we performed Western blot analysis using the c-Src inhibitor, 4-amino-5-(4-chlorophenyl)-7-(t.butyl)pyrazolo(3,4-d)pyramidine(PP2), and as shown in Fig. 4A, PP2 reduced the E2-associated tyrosine phosphorylation of the purified aromatase. In addition, PP2 treatment was able to abrogate the E2-induced increase on aromatase enzymatic activity (Fig. 4B).
Fig. 3.
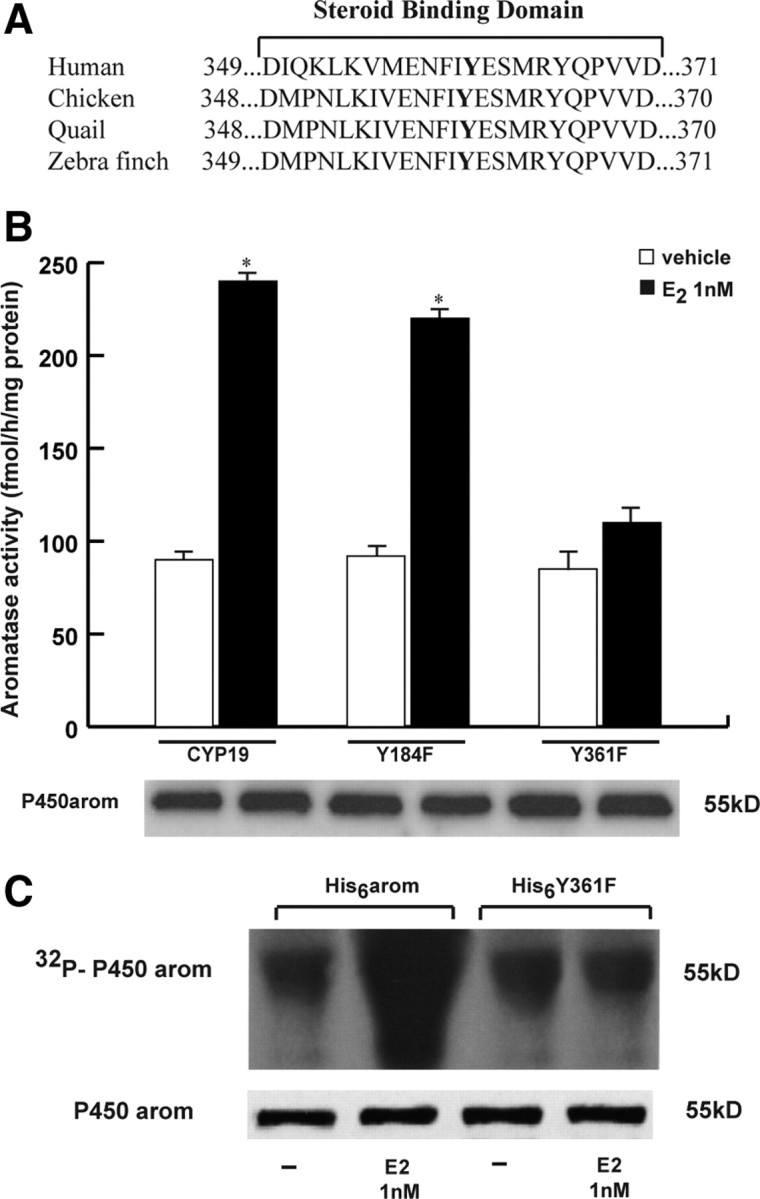
Specific tyrosine residue involved in aromatase activation. A, Comparison of the amino acid sequences of steroid binding domain of aromatase in human, chicken, quail, and zebra finch. B, MCF-7 cells were transfected with CYP19 vector or Y184F or Y361F mutants, treated with vehicle or 1 nm E2 for 10 min after which aromatase activity was performed. The values represent the means ± se of three different experiments, each performed with triplicate samples. *, P < 0.01 compared with vehicle. Western blotting shows the expression of DNA vectors used in the experiments. C, MCF-7 cells were transiently transfected with His6-arom or His6-Y361F, labeled with [32P]orthophosphate, and then treated with vehicle (−) or 1 nm E2 for 10 min. Aromatase was purified using Ni-NTA agarose beads after which the complexes were resolved in SDS-PAGE. The top panel shows autoradiography of the SDS-PAGE, and the bottom panel shows immunoblot analysis with antiaromatase antibody (P450arom) as a control for expressed protein.
Fig. 4.
c-Src signaling mediates E2-induced aromatase activity. A, MCF-7 cells transiently transfected with His6-arom were treated with vehicle (-) or 1 nm E2 for 10 min, with or without pretreatment of PP2 (3 μm). The membrane was probed with antiphosphotyrosine (pTyr) antibody. To verify equal loading, the membrane was probed with antiaromatase antibody. Microsomal extracts from placenta (P) were used as positive control. As negative controls we used the supernatant removed after incubation with Ni-NTA agarose beads (S) and vector-transfected MCF-7 cell lysates incubated with Ni-NTA agarose beads (NC). The histograms on the bottom represent the means ± se of three separate experiments in which band intensities were evaluated in terms of OD arbitrary units and expressed as percentages of the control, which was assumed to be 100%. *, P < 0.01 compared with vehicle; **, P < 0.01 compared with E2-treated cells. B, MCF-7 cells were pretreated with or without PP2 (3 μm) before E2 (1 nm) stimulation for 10 min, after which aromatase activity was performed. The values represent the means ± se from triplicate assays. *, P < 0.01compared with vehicle; **, P < 0.01 compared with E2-treated samples. C, c-Src protein expression (evaluated by Western blotting) in MCF-7 cells not transfected (-) or transfected with RNAi targeted human c-Src mRNA or with a stealth RNAi control as reported in Materials and Methods. Glyceraldehyde-3-phosphate dehydrogenase was used as loading control. The histograms represent the means ± se of three separate experiments in which band intensities were evaluated in terms of OD arbitrary units and expressed as percentages of the control, which was assumed to be 100%. D, MCF-7 cells were not transfected or transfected with c-Src RNAi, then transfected with His6-arom, and exposed to 1 and 10 nm E2 for 10 min. The membrane was probed with antiphosphotyrosine (pTyr) or anti-c-Src antibodies. To verify equal loading, the membrane was probed with antiaromatase antibody. Microsomal extracts from placenta (P) were used as positive control. The histograms represent the means ± se of three separate experiments in which band intensities were evaluated in terms of OD arbitrary units and expressed as percentages of the control, which was assumed to be 100%. *, P < 0.01 compared with vehicle; **, P < 0.01 compared with E2-treated cells. E, MCF-7 cells were transfected with His6-arom or His6-Y361F vectors and then treated with E2. Aromatase protein was purified using Ni-NTA agarose beads after which the complexes were resolved in SDS-PAGE. Immunoblotting was performed using the anti-c-Src and anti-aromatase (P450arom) antibodies. F, MCF-7 cells were transfected with His6-arom or His6-Y361F vectors. In vitro c-Src kinase assay was performed on aromatase protein purified using Ni-NTA agarose beads using recombinant full-length human Src kinase. Autoradiography is shown in the upper panel, and input aromatase is shown in the bottom panel. kD, Kilodaltons.
To further support the crucial role of c-Src, we examined whether knockdown of the c-Src gene would similarly reduce tyrosine aromatase phosphorylation. Transfection with pool of two small interfering RNA (siRNA) duplex specifically direct against human c-Src, reduced the expression of this protein (Fig. 4C). As shown in Fig. 4D, silencing of c-Src significantly decreased tyrosine phosphorylation of the purified aromatase induced by E2.
We next examined the physical association between c-Src kinase and either wt or Y361F mutant aromatase proteins. To test this possibility, we transiently transfected MCF-7 cells with either His6-arom or His6-Y361F constructs. Equal amounts of proteins were incubated with Ni-NTA agarose beads for isolation of recombinant P450arom proteins followed by immunoblot for c-Src and P450arom. Results obtained showed that both wt and Y361F mutant aromatase proteins were able to bind c-Src tyrosine kinase (Fig. 4E). We confirmed the formation of this protein complex by immunoprecipitation of c-Src and then detection of aromatase on Western blotting (data not shown).
Using in vitro recombinant c-Src kinase, we found that wt aromatase was more efficiently phosphorylated than Y361F mutant aromatase protein (Fig. 4F), addressing the importance of this residue of aromatase protein as phosphorylation substrate of c-Src.
Evidence that ERα, growth factor receptors (GF-Rs), c-Src, and aromatase interact in a multiprotein complex
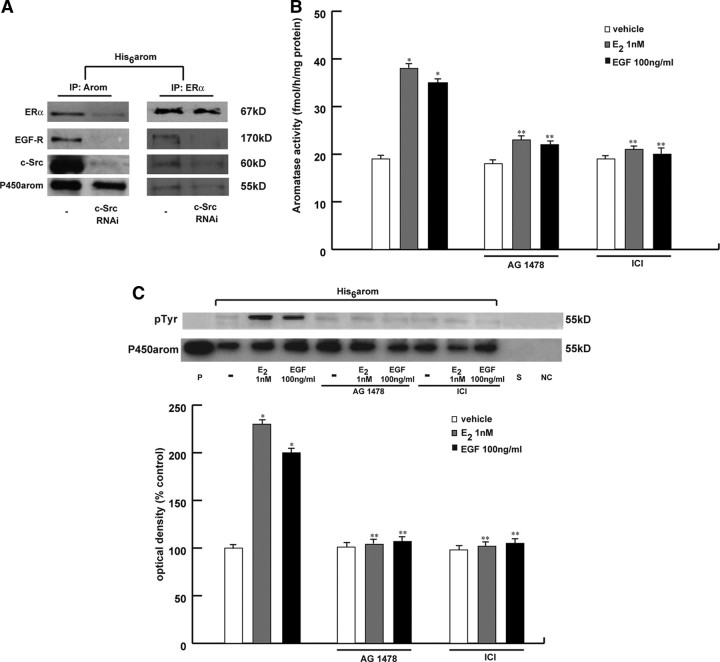
Studies in breast cancer culture have highlighted an intimate cross talk between the endogenous membrane ER and GF-Rs signaling pathways (37, 38, 39). This process may involve the sequential activation of the cellular tyrosine kinase c-Src (26, 36, 40, 41). We wondered whether the cross talk between ERα and GF-Rs, through c-Src, could be involved in the rapid modulation of E2-induced aromatase activity in breast cancer cells. To evaluate a direct protein-protein interaction among ERα, epidermal growth factor (EGF) receptor (EGFR), and aromatase, we performed coimmunoprecipitation studies. Particularly, MCF-7 cells transiently transfected with His6-arom were lysated after which protein extracts were incubated with Ni-NTA agarose beads for isolation of recombinant P450arom (Fig. 5A, left panel). Equal amounts of the lysates were immunoprecipitated with ERα-specific antibody (Fig. 5A, right panel). The membranes were probed with anti-ERα, anti-EGFR, anti-c-Src, or antiaromatase antibodies. The results showed that ERα, EGFR, c-Src, and aromatase were in a multiprotein complex. Notably, the presence of c-Src is required for this complex formation because silencing of c-Src reduced the interaction of aromatase with ERα and EGFR (Fig. 5A).
Fig. 5.
Interaction between ERα and EGFR/c-Src in the aromatase activity induction. A, MCF-7 cells were not transfected (-) or transfected with c-Src RNAi and then transfected with His6-arom vector. Aromatase protein was purified using Ni-NTA agarose beads after which the complexes were resolved in SDS-PAGE. In another set of experiments the same amount of cell lysate was immunoprecipitated (IP) with ERα antibody. Immunoblotting was performed using the anti-ERα, anti-EGFR, anti-c-Src, and antiaromatase antibodies. B, MCF-7 were pretreated with 10 μm AG1478 or 1 μm ICI for 30 min and then exposed to 1 nm E2 or 100 ng/ml EGF. After 10 min, aromatase activity was performed. The values represent the means ± se from triplicate assays. *, P < 0.01 compared with vehicle; **, P < 0.01 compared with E2- or EGF-treated samples. C, MCF-7 cells transiently transfected with His6-arom were pretreated with or without 10 μm AG1478 or 1 μm ICI for 30 min and then exposed to 1 nm E2 or 100 ng/ml EGF for 10 min. The membrane was probed with antiphosphotyrosine (pTyr) antibody. To verify equal loading, the membrane was probed with antiaromatase antibody. P, Microsomal extracts from placenta. As negative controls we used the supernatant removed after incubation with Ni-NTA agarose beads (S) and vector-transfected MCF-7 cell lysates incubated with Ni-NTA agarose beads (NC). The histograms represent the means ± se of three separate experiments in which band intensities were evaluated in terms of OD arbitrary units and expressed as percentages of the control, which was assumed to be 100%. *, P < 0.01 compared with vehicle; **, P < 0.01 compared with E2- or EGF-treated samples. kD, Kilodaltons.
Next, to determine whether EGFR stimulation leads to an increased production of E2 via an up-regulation of aromatase enzymatic activity, MCF-7 cells were treated with 100 ng/ml EGF for 10 min. Our data demonstrated that EGF is able to enhance aromatase enzymatic activity as well as the tyrosine-phosphorylated status in the His6-tagged purified aromatase protein to the same extent as E2. Pretreatment with AG1478, a specific EGFR inhibitor, or ICI completely abrogated these effects (Fig. 5, B and C). The same results were obtained under treatment with IGF and AG1024, a monoclonal antibody specific to IGF-1R (data not shown).
These data indicate that the induction of aromatase enzymatic activity may involve the cross talk between E2/ER and GF-Rs signaling.
Discussion
In the present study we demonstrate that short exposure to E2 induces an increase of aromatase enzymatic activity, through an enhanced tyrosine phosphorylation level of the enzyme, in estrogen-dependent MCF-7 breast cancer epithelial cells.
Our results showed that the rapid effect induced by E2 in enhancing aromatase activity was specifically mediated by the interaction of E2 with ERα, because it was abrogated in the presence of ER antagonists, such as TAM and ICI. Moreover, when in ER-negative CHO cells overexpressing aromatase, we transfected the membrane ERα construct yielding the ligand-binding domain of the receptor exclusively localized to the cytoplasmic face of the membrane, we also reproduced the stimulatory effects of E2 on aromatase activity. This underlines the ability of membrane ERα, which is unable to generate genomic response, in modulating aromatase activity. We also reproduced similar results in ER-negative breast cancer cells SKBR3, ectopically expressing ERα, and in R2C rat Leydig tumor cells, which display high aromatase expression. These latter results suggest that the rapid changes in aromatase activity may represent a general mechanism not related to cell specificity even though it assumes more relevance in breast cancer in which growth and progression are strongly estrogen dependent. Our data appear opposite to previous findings demonstrating that E2 treatment reduced aromatase activity in breast cancer cells (42, 43). However, it is worthwhile to point out that they come from a different experimental design performed after a long-term E2 exposure of MCF-7 cells either cultured long term in estrogen-deprived medium or stably transfected with the aromatase gene (MCF-7aro). For instance, Pasqualini and Chetrite (42) observed the maximal inhibition in aromatase activity (evaluated by quantification of 3H-estradiol from cell incubated with [3H]testosterone) at the nonphysiological dose of 50 μm in MCF-7aro. Instead, in our study, we evaluated aromatase activity by tritiated water release assay using as substrate [1β-3H] androst-4-ene-3,17-dione (Δ4) in parental or transiently expressing aromatase MCF-7 cells. We demonstrated the maximal increase in aromatase enzymatic activity after a short time of exposure with low physiological doses of E2.
The E2 induced up-regulation of aromatase activity in MCF-7 cells was not correlated with any increase in the levels of aromatase mRNA and protein content, suggesting a posttranslational modulation of aromatase protein.
Posttranslational modification of enzymatic protein has been demonstrated for different members of the P450 enzyme family in vertebrates. For instance, cAMP-dependent protein kinase was essential for the activation of human and rat cholesterol 7α-hydroxylase (CYP7A) (44) as well as for phosphorylation of serine and threonine residues in human P450c17 (CYP17) (45, 46). Bovine P450scc (CYP11A1) has been identified as an active form phosphorylated by a protein kinase C (47), and similar activation of P450s through phosphorylation has been found in human liver enzymes such as CYP2E1 and CYP2B1 (48). Recently, phosphorylation of the cytochrome P450 aromatase has been proposed, even though the specific kinases involved in this process are yet not well specified (16, 21). We demonstrated in MCF-7 cells that E2 up-regulatory effects on aromatase activity resulted from a direct phosphorylation of enzymatic protein itself. Indeed, our in vivo labeling experiments showed, after E2 treatment, a significant increase in phosphorylation of aromatase protein purified by Ni-NTA agarose beads. Particularly, we observed a specific enhancement of tyrosine phosphorylation levels of aromatase protein after E2 exposure, whereas serine phosphorylation status remains unchanged. This suggests that the rapid nongenomic effects of the hormone specifically target tyrosine residues.
Site-directed mutagenesis experiments revealed that phosphorylation of the specific tyrosine residue, located at position 361 in the steroid-binding domain of aromatase protein, is crucial in the up-regulation of enzymatic activity after E2 treatment. The 361-tyrosine residue of aromatase sequence is well conserved across species and represents a potential consensus site of phosphorylation by an important nonreceptor tyrosine kinase c-Src (with a consensus score higher than the critical value of 0.5).
c-Src mediates signal transduction pathways implicated in proliferation, survival, cell adhesion, and migration (49). This kinase can be activated by many cell surface receptors and represents a crucial molecule downstream of ERα triggering estrogen rapid action (26, 36). In our study we demonstrated a direct interaction between c-Src and aromatase protein, and the involvement of this kinase in E2-stimulated aromatase activity because blockade of c-Src activity completely reversed the E2-induced increase of aromatase activity as well as reduced tyrosine phosphorylation of aromatase purified protein. Moreover, in vitro kinase activity assay using pure c-Src protein demonstrated that this kinase directly phosphorylates aromatase, and tyrosine located at the 361 site is involved in this event. Thus, we identified the phosphorylation of the critical residue 361 by c-Src kinase as a novel mechanism for regulating enzymatic activity and function of aromatase.
It is well known that ERα and GF-Rs utilize signaling pathways that intersect and directly interact at many levels. Estrogens have been shown to activate IGF-I receptor and EGFR (37, 38, 39), and it has been reported that E2 up-regulates aromatase expression via cross talk between ER and GF in breast cancer cells (17). A number of proteins, such as c-Src, Fak (focal adhesion kinase) MNAR (50), are reported to form a complex with ERs and to be involved in extranuclear functions of ERα. Our study shows the existence of a multipartite complex involving ERα, EGFR, c-Src, and aromatase. Silencing of c-Src by siRNA reduced the interaction of ERα and EGFR with aromatase, suggesting a key role of kinase active c-Src in the formation of this complex. In addition, treatment of MCF-7 cells with IGF-I and EGF increased, in a short time, aromatase activity as well as the tyrosine phosphorylation status of aromatase protein. Treatments with specific tyrosine kinase inhibitors of GF-Rs or with the antiestrogen ICI abrogated the GFs as well as the E2 induction of aromatase activity. It has been largely demonstrated that c-Src is critical component of the bidirectional cross talk between ERα and GF-Rs (reviewed in Ref. 40). Thus, this may explain why, in the presence of antiestrogen or inhibitors of both GF-Rs tested, the up-regulatory effects on aromatase activity are not longer noticeable.
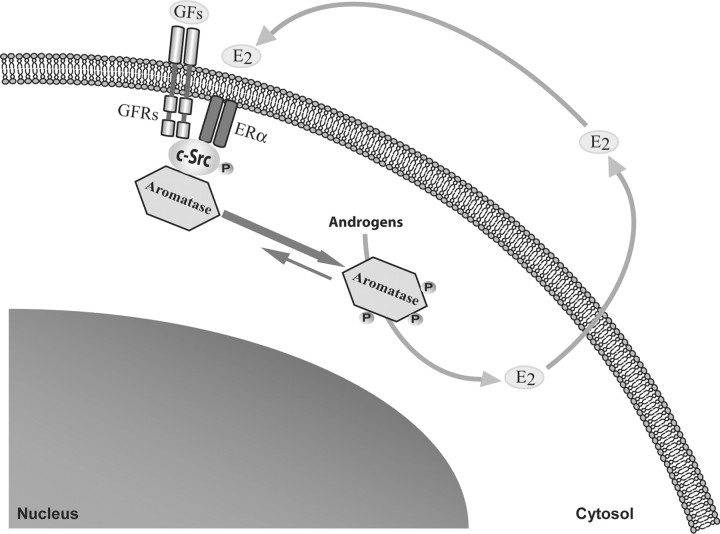
In summary, this study shows, for the first time, a new molecular mechanism by which E2 rapidly increases aromatase activity through an enhanced tyrosine phosphorylation of the enzyme. We hypothesized that E2, through an enhanced cross talk between GF-Rs, c-Src, and ER signaling, can phosphorylate and thus activate aromatase, resulting in a positive nongenomic autocrine loop between E2 and aromatase in MCF-7 breast cancer cells (Fig. 6). All these data demonstrate that aromatase may be activated by different membrane cell signaling, which should be targeted in the novel therapeutic strategies for breast cancer treatment.
Fig. 6.
Hypothetical model of the potential signaling transductional pathways through which E2 and GFs (growth factors) may rapidly enhance aromatase activity in MCF-7 breast cancer cells. P, Placenta.
Materials and Methods
Cell cultures
MCF-7 and CHO cells were cultured in DMEM/F-12 medium containing 5% calf serum or 10% fetal bovine serum (FBS), respectively (Eurobio, Les Ullis Cedex, France). SKBR3 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. R2C cells were cultured in Ham/F-10 supplemented with 15% HS, 2.5% FBS.
His6-arom plasmid construction
His6-arom plasmid construct was used to express the C-terminal 6×His-tagged form of human aromatase. The plasmid pUC19-arom containing the full-length of human aromatase gene (CYP19) was used as template. The 6×His epitope tag was inserted by two PCRs using the following primers: forward (5′-ATATAAGCTTATGGTTTTGGAAATGCTGA-3′) and two reverse (5′-ATGATGATGGTGTTCCAGACACCT-3′), (5′-ATATTCTAGACTAATGATGATGATGATGATGGTGTTCCAGA-3′). PCR product was subcloned into HindIII/XbaI sites of pcDNA3.1, and Hys6-arom sequence was confirmed by nucleotide sequence analysis. We proved that the enzymatic activity of polyhistidine-containing recombinant protein was well preserved by measuring aromatase activity in MCF-7 cells transiently transfected with Hys6-arom vector.
Site-directed mutagenesis
This step was performed with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to manufacturer’s standard method. The templates and the specific oligonucleotides used are summarized in Table 1.
Table 1.
Oligonucleotide Primers Used for Mutagenesis Studies
| Mutant | Template | Primers | Sequence |
|---|---|---|---|
| Y184F | CYP19 | Forward | 5′-CCAATGAATCGGGCtttGTGGACGTGTTGACCC-3′ |
| Reverse | 5′-GGGTCAACACGTCCACaaaGCCCGATTCATTGG-3′ | ||
| Y361F | CYP19 | Forward | 5′-GAAAACTTCATTtttGAGAGCATGCGGTACCAGCCTGTCG-3′ |
| Reverse | 5′-CGACAGGCTGGTACCGCAGCTCTCaaaAATGAAGTTTTCC-3′ | ||
| His6-Y361F | His6-arom | Forward | 5′-GAAAACTTCATTtttGAGAGCATGCGGTACCAGCCTGTCG-3′ |
| Reverse | 5′-CGACAGGCTGGTACCGCAGCTCTCaaaAATGAAGTTTTCC-3′ |
Transient transfection
Transient transfection was performed using the FuGENE 6 (Roche, Indianapolis, IN) reagent with the mixture containing 1 μg/well of CYP19 or Y184F, Y361F mutants. A set of experiments was performed cotransfecting 1 μg/well of CYP19 and 1 μg/well of the membrane ERα construct (mERα) or ERα wt.
Aromatase activity assay
The aromatase activity in subconfluent MCF-7, SKBR3, CHO, and R2C cells culture medium was measured by the tritiated water release assay using 0.5 μm [1β-3H]androst-4-ene-3,17-dione as substrate (51). The incubations were performed at 37 C for 3 h under an air-CO2 (5%) atmosphere. The results obtained were expressed as fentomoles per h (MCF-7, CHO, and SKBR3) or picomoles per h (R2C) and normalized to mg of protein.
RT-PCR assay
Total cellular RNA was extracted from MCF-7 cells using TRIzol (Invitrogen, Carlsbad, CA). Aromatase mRNA was analyzed by the RT-PCR method as previously described (52).
Immunoblotting and immunoprecipitation analysis
Whole-cell lysates were prepared in lysing buffer [50 mmol/liter HEPES (pH 7.5), 150 mmol/liter NaCl, 1.5 mmol/liter MgCl2, 1 mmol/liter EGTA, 10% glycerol, 1% Triton X-100, protease inhibitors (Sigma, Italy)]. Equal amounts of total protein were resolved on 11% SDS-PAGE, transferred onto a nitrocellulose membrane, and probed with antiserum against the human placental P450arom (Hauptman-Woodward Medical Research Institute, Buffalo, NY) or glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For immunoprecipitation studies, 300 μg of protein extracts was incubated with 1 μg of anti-ERα antibody (Santa Cruz) and 20 μl of protein A/G (Santa Cruz). The immunoprecipitated proteins were then subjected to Western blot analysis. Whole-cell lysates were used as input controls.
Detection of His6-tagged aromatase protein by immunoblotting analysis
MCF7 cells were transiently transfected with His6-arom or His6-Y361F vectors and exposed to different treatments before lysis. Cellular proteins (300 μg) were incubated with Ni-NTA agarose beads (Invitrogen). Ni-NTA resin was used to isolate P450 aromatase tagged with six tandem histidine residues from cellular lysates. The beads containing bound proteins were washed in PBS buffer added with a mixture of protease inhibitors and analyzed by Western blot. Membrane was probed with a antibodies against human cytochrome P450arom (Serotec, Oxford, UK) or phosphotyrosine-containing proteins (pY99, Santa Cruz) or phosphoserine-containing proteins. For coimmunoprecipitation studies, membranes were probed with ERα, EGFR, and c-Src antibodies (Santa Cruz). Two set of controls were done in parallel: surnatant removed after the first centrifugation was added to one control and vector-transfected cell lysates plus Ni-NTA agarose beads was included in the other control. Microsomal extracts from placenta were used as positive control.
In vivo phosphorylation experiments
MCF-7 cells were transiently transfected with His6-arom or His6-Y361F construct, labeled for 2 h with [32P]orthophosphate (PerkinElmer, Boston, MA) (0.5 mCi/ml in Krebs Ringer buffer, pH 7.4, containing 1% BSA), treated with E2, washed with PBS, and immunoprecipitated with Ni-NTA agarose beads as described above. The supernatants were resolved onto 10% SDS-PAGE gel and transferred onto nitrocellulose membrane. Phosphorylated aromatase-purified protein was detected by autoradiography, and the aromatase protein level was determined by immunoblot of the same membrane with antiaromatase antibody.
In vitro c-Src kinase activity assay
C-Src kinase activity was measured by phosphorylation of aromatase protein specifically purified from lysates of MCF-7 cells transiently transfected with His6-arom or His6-Y361F constructs, as previously described. The washed Ni-NTA beads containing bound aromatase proteins were incubated with recombinant full-length human Src kinase (Cell Signaling Technology, Danvers, MA) in the presence of 10 μCi of [γ-32P]ATP and 10 nmol/liter ATP in 40 μl kinase buffer at 30 C for 30 min. The reactions were stopped by the addition of sodium dodecyl sulfate loading buffer, and the samples were resolved by 10% SDS-PAGE. The phosphorylated aromatase protein bands were visualized by autoradiography.
c-Src knockdown by siRNA
MCF-7 cells were transfected with validated stealth RNA interference (RNAi) DuoPak (Invitrogen) targeted human c-Src or with a stealth RNAi control to a final concentration of 100 nm using Lipofectamine 2000 (Invitrogen) as recommended by the manufacturer. After 5 h the transfection medium was changed with serum free medium, transfected with His6-arom vector, and then exposed to E2. These transfected cells were used to immunoblotting analysis.
Statistical analysis
Each datum point represents the mean ± se of three different experiments. Statistical analysis was performed using ANOVA followed by Newman-Keuls testing to determine differences in means. P < 0.05 was considered as statistically significant.
Acknowledgments
We thank Dr. E. R. Simpson and Dr. C. D. Clyne (Prince Henry’s Institute of Medical Research, Clayton, Australia) and Dr. E. R. Levin (University of California, Irvine) for generously providing pUC19-arom and mERα plasmids, respectively.
NURSA Molecule Pages:
Ligands: 17β-estradiol | Fulvestrant | Tamoxifen.
Footnotes
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) grants in 2007 and 2008.
Disclosure Summary: All authors have nothing to declare.
First Published Online June 25, 2009
S.C. and I.B. contributed equally to this work.
Abbreviations: CHO, Chinese hamster ovary; CYP, cytochrome P450; E2, 17β-estradiol; EGF, epidermal growth factor; EGFR, EGF receptor; ER, estrogen receptor; FBS, fetal bovine serum; GF-R, growth factor receptor; ICI, ICI 182,780; Mem-ECFP, membrane-enhanced cyan fluorescent protein; mERα, membrane ERα; NTA, nitrilotriacetic acid; PP2, 4-amino-5-(4-chlorophenyl)-7-(t.butyl)pyrazolo(3,4-d)pyramidine; RNAi, RNA interference; siRNA, small interfering RNA; TAM, tamoxifen; wt, wild type.
References
- 1.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE1994. Aromatase cytocrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15:342–355 [DOI] [PubMed] [Google Scholar]
- 2.Sasano H, Nagura H, Harada N, Goukon Y, Kimura M1994. Immunolocalization of aromatase and other steroidogenic enzyme in human breast disorders. Hum Pathol 25:530–535 [DOI] [PubMed] [Google Scholar]
- 3.Sourdaine P, Mullen P, White R, Telford J, Parker MG, Miller WR1996. Aromatase activity and CYP19 gene expression in breast cancers. J Steroid Biochem Mol Biol 59:191–198 [DOI] [PubMed] [Google Scholar]
- 4.Maggiolini M, Carpino A, Bonofiglio D, Pezzi V, Rago V, Marsico S, Picard D, Andò S2001. The direct proliferative stimulus of dehydroepiandrosterone on MCF-7 breast cancer cells is potentiated by overexpression of aromatase. Mol Cell Endocrinol 184:163–171 [DOI] [PubMed] [Google Scholar]
- 5.Sun XZ, Zhou D, Chen S1997. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. J Steroid Biochem Mol Biol 63:29–36 [DOI] [PubMed] [Google Scholar]
- 6.Yue W, Zhou D, Chen S, Brodie A1994. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res 54:5092–5095 [PubMed] [Google Scholar]
- 7.Tekmal RR, Ramachandra N, Gubba S, Durgam VR, Mantione J, Toda K, Shizuta Y, Dillehay DL1996. Overexpression of int-5/aromatase in mammary glands of transgenic mice results in the induction of hyperplasia and nuclear abnormalities. Cancer Res 56:3180–3185 [PubMed] [Google Scholar]
- 8.Gill K, Kirma N, Tekmal RR2001. Overexpression of aromatase in transgenic male mice in the induction of gynecomastia and other biochemical changes in mammary glands. J Steroid Biochem Mol Biol 77:13–18 [DOI] [PubMed] [Google Scholar]
- 9.Harada N1997. Aberrant expression of aromatase in breast cancer tissues. J Steroid Biochem Mol Biol 61:175–184 [PubMed] [Google Scholar]
- 10.Miller WR1997. Uptake and synthesis of steroid hormones by the breast. Endocr Relat Cancer 4:307–311 [Google Scholar]
- 11.Santen RJ, Yue W, Naftolin F, Mor G, Berstein L1999. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer 6:235–243 [DOI] [PubMed] [Google Scholar]
- 12.Altundag K, Ibrahim NK2006. Aromatase inhibitors in breast cancer: an overview. Oncologist 11:553–562 [DOI] [PubMed] [Google Scholar]
- 13.Simpson ER, Michael MD, Agarwal VR, Hinshelwood MM, Bulun SE, Zhao Y1997. Expression of the CYP19 (aromatase) gene: an unusual case of alternative promoter usage. FASEB J 11:29–36 [DOI] [PubMed] [Google Scholar]
- 14.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M2003. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 86:219–224 [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Baillien M, Ball GF2001. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol 79:261–277 [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, Baillien M, Ball GF2006. Rapid control of brain aromatase activity by glutamatergic imputs. Endocrinology 147:359–366 [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita Y, Chen S2003. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor α. Cancer Res 63:3546–3555 [PubMed] [Google Scholar]
- 18.Richards JA, Petrel TA, Brueggemeier RW2002. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol 80:203–212 [DOI] [PubMed] [Google Scholar]
- 19.Shozu M, Sumitani H, Murakami K, Segawa T, Yang HJ, Inoue M2001. Regulation of aromatase activity in bone-derived cells: possible role of mitogen-activated protein kinase. J Steroid Biochem Mol Biol 79:61–65 [DOI] [PubMed] [Google Scholar]
- 20.Yue W, Wang JP, Conaway MR, Li Y, Santen RJ2003. Adaptive hypersensitivity following long-term estrogen deprivation: involvement of multiple signal pathways. J Steroid Biochem Mol Biol 86:65–274 [DOI] [PubMed] [Google Scholar]
- 21.Miller WT, Shin I, Kagawa N, Evans DB, Waterman MR, Arteaga CL2008. Aromatase is phosphorylated in situ at Serine-118. J Steroid Biochem Mol Biol 112:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razandi M, Pedram A, Park ST, Levin ER2003. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 24:2701–2712 [DOI] [PubMed] [Google Scholar]
- 23.Hammes SR, Levin ER2007. Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494 [DOI] [PubMed] [Google Scholar]
- 25.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F1996. Tyrosine kinase/p21 ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 15:1292–1300 [PMC free article] [PubMed] [Google Scholar]
- 26.Song RX, Zhang Z, Santen RJ2005. Estrogen rapid action via protein complex formation involving ERα and Src. Trends Endocrinol Metab 16:347–353 [DOI] [PubMed] [Google Scholar]
- 27.Levin ER2005. Integration of the extra-nuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC2001. Nongenotropic, sex-nonspecific signalling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- 29.Razandi M, Pedram A, Rosen EM, Levin ER2004. BRCA 1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol 24:5900–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER2002. ERs associate with and regulate the production of caveolin: implications for signalling and cellular actions. Mol Endocrinol 16:100–115 [DOI] [PubMed] [Google Scholar]
- 31.Harada N1988. Cloning of a complete cDNA encoding human aromatase: immunochemical identification and sequence analysis. Biochem Biophys Res Commun 156:725–732 [DOI] [PubMed] [Google Scholar]
- 32.McPhaul MJ, Noble JF, Simpson ER, Mendelson CR, Wilson JD1988. The expression of a functional cDNA encoding the chicken cytochrome P-450arom (aromatase) that catalyzes the formation of estrogen from androgen. J Biol Chem 263:16358–16363 [PubMed] [Google Scholar]
- 33.Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT1994. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol Brain Res 24:227–237 [DOI] [PubMed] [Google Scholar]
- 34.Kennelly PJ, Krebs EG1991. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266:15555–15558 [PubMed] [Google Scholar]
- 35.Blom N, Gammeltoft S, Brunak S1999. Sequence and structure based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294:1351–1362 [DOI] [PubMed] [Google Scholar]
- 36.Migliaccio A, Castoria G, Di Domenico M, De Falco A, Bilancio A, Auricchio F2002. Src is an initial target of sex steroid hormone action. Ann NY Acad Sci 963:185–190 [DOI] [PubMed] [Google Scholar]
- 37.Lee AV, Cui X, Oesterreich S2001. Cross-talk among estrogen receptor, epidermal growth factor, and insulin like growth factor signaling in breast cancer. Clin Cancer Res 7:4429s–4435s; discussion 4411s–4412s [PubMed] [Google Scholar]
- 38.Stoll BA2002. Oestrogen/insulin-like growth factor-I receptor interaction in early breast cancer: clinical implications. Ann Oncol 13:191–196 [DOI] [PubMed] [Google Scholar]
- 39.Levin ER2003. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 17:309–317 [DOI] [PubMed] [Google Scholar]
- 40.Ishizawar R, Parsons SJ2004. c-Src and cooperating partners in human cancer. Cancer Cell 6:209–214 [DOI] [PubMed] [Google Scholar]
- 41.Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, de Falco A, Bilancio A, Varricchio L, Ciociola A, Auricchio F2005. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res 65:10585–10593 [DOI] [PubMed] [Google Scholar]
- 42.Pasqualini JR, Chetrite GS2006. Estradiol as an anti-aromatase agent in human breast cancer cells. J Steroid Biochem Mol Biol 98:12–17 [DOI] [PubMed] [Google Scholar]
- 43.Yue W, Berstein LM, Wang JP, Clark GM, Hamilton CJ, Demers LM, Santen RJ2001. The potential role of estrogen in aromatase regulation in the breast. J Steroid Biochem Mol Biol 79:157–164 [DOI] [PubMed] [Google Scholar]
- 44.Nguyen LB, Shefer S, Salen G, Chiang JY, Patel M1996. Cholesterol 7α-hydroxylase activities from human and rat liver are modulated in vitro post-translationally by phosphorylation-dephosphorylation. Hepatology 24:1468–1474 [DOI] [PubMed] [Google Scholar]
- 45.Biason-Lauber A, Kempken B, Werder E, Forest MG, Einaudi S, Ranke MB, Matsuo N, Brunelli V, Schönle EJ, Zachmann M2000. 17α-Hydroxylase/17,20-lyase deficiency as a model to study enzymatic activity regulation: role of phosphorylation. J Clin Endocrinol Metab 85:1226–1231 [DOI] [PubMed] [Google Scholar]
- 46.Zhang LH, Rodriguez H, Ohno S, Miller WL1995. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Defaye G, Monnier N, Guidicelli C, Chambaz EM1982. Phosphorylation of purified mitochondrial cytochromes P450 (cholesterol desmolase and 11 β-hydroxylase) from bovine adrenal cortex. Mol Cell Endocrinol 27:157–168 [DOI] [PubMed] [Google Scholar]
- 48.Oesch-Bartlomowicz B, Padma PR, Becker R, Richter B, Hengstler JG, Freeman JE, Wolf CR, Oesch F1998. Differential modulation of CYP2E1 activity by cAMP-dependent protein kinase upon Ser129 replacement. Exp Cell Res 242:294–302 [DOI] [PubMed] [Google Scholar]
- 49.Thomas SM, Brugge JS1997. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13:513–609 [DOI] [PubMed] [Google Scholar]
- 50.Wong CW, McNally C, NicKbarg E, Komm BS, Cheskis BJ2002. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:4783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Lephart ED, Simpson ER1991. Assay of aromatase activity. Methods Enzymol 206:477–483 [DOI] [PubMed] [Google Scholar]
- 52.Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Andò S2003. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem 278:28668–28676 [DOI] [PubMed] [Google Scholar]