Abstract
Cell cycle regulation by differentiation signals is critical for eukaryote development. We investigated the roles of bone morphogenetic protein (BMP)-4, an important stimulator of osteoblast differentiation and bone formation, in regulating cell cycle distribution in four osteoblast-like cell lines and mouse primary osteoblasts, and the underlying mechanisms. In all cells used, BMP-4 induced G0/G1 arrest. The molecular basis of the BMP-4 effect was analyzed, and the presentation on molecular mechanism is focused on human MG63 cells. BMP-4 induced p21CIP1 and p27KIP1 expressions and hence cell differentiation but had no effects on the expressions of cyclins A, B1, D1, and E, cyclin-dependent protein kinase-2, -4, and -6. Using specific small interfering RNA (siRNA), we found that BMP-4-induced G0/G1 arrest, and p21CIP1 and p27KIP1 expressions were mediated by BMP receptor type IA (BMPRIA)-specific Sma- and Mad-related protein (Smad)1/5. BMP-4 induced transient phosphorylations of ERK; transfection of MG63 cells with ERK2, but not ERK1, -specific siRNA inhibited the BMP-4-induced responses in MG63 cells. Pretreatment of MG63 cells with Arg-Gly-Asp-Ser, which blocks the cell-extracellular matrix interaction, or transfection with β3 integrin-specific siRNA inhibited BMP-4-induced ERK and Smad1/5 phosphorylations. BMP-4 induced transient increases in associations of β3-integrin with focal adhesion kinase and Shc, the dominant-negative mutants of which inhibited BMP-4-induced ERK and Smad1/5 phosphorylations. Our results indicate that BMP-4 induces G0/G1 arrest and hence differentiation in osteoblast-like cells through increased expressions of p21CIP1 and p27KIP1, which are mediated by BMPRIA-specific Smad1/5. The extracellular matrix/β3 integrin/ focal adhesion kinase/Shc/ERK2 signaling pathway is involved in these BMP-4-induced responses in osteoblast-like cells.
ECM/β3 integrin/FAK/Shc and the downstream ERK2 are involved in BMP-4-induced G0/G1 arrest and hence differentiation in osteoblast-like cells through BMPRIA/Smad-inductions of p21CIP1 and p27KIP1.
Osteogenesis, including proliferation and differentiation of osteoblasts, is a complex process that begins in the embryo and continues in the adult to maintain the balance between bone formation and resorption. Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, were originally identified by their unique ability to induce ectopic bone and cartilage formation in vivo (1, 2). BMP-4 synthesized by osteoblasts is one of the most potent inducers of bone formation through its stimulation of osteoblast differentiation. BMP-4 transduces signals by means of heteromeric complex formation of cognate type II and type I (i.e. BMPRIA or BMPRIB) serine/threonine kinase receptors, leading to phosphorylation of receptor-regulated Sma- and Mad-related proteins (Smads) (R-Smads, i.e. Smad1/5/8), which play essential roles in mesenchymal lineage cell differentiation (3, 4, 5, 6, 7). In addition to Smads, BMPs have been shown to activate MAPKs, including ERK, which is known to regulate gene expression and cellular functions (8, 9, 10). However, the correlation between Smad- and ERK-signaling pathways in osteoblasts in response to BMP-4 remains unclear.
The proliferation of eukaryotic cells depends on their progression through the cell cycle, and cell cycle arrest at the G0/G1 phase is thought to be a prerequisite for cell differentiation (11). Cell cycle is controlled by its regulatory proteins, including cyclin-dependent protein kinases (Cdks) and their regulatory subunits cyclins, as well as inhibitors such as p21CIP1 and p27KIP1 (12). Recent studies demonstrated that p21CIP1 and p27KIP1 inhibit the activities of all Cdks and regulate cell proliferation and differentiation (12, 13). Halevy et al. (14) demonstrated that myogenic differentiation antigen (MyoD), a skeletal muscle-specific transcription regulator, induces cell cycle arrest during skeletal muscle differentiation by increasing the expression of p21CIP1. By using osteoprogenitor cells derived from the bone marrow of p27−/− mice, Drissi et al. (15) demonstrated that p27KIP1 plays a key role in regulating osteoblast differentiation by controlling proliferation-related events. Although p21CIP1 and p27KIP1 have been shown to play a role in regulating cell proliferation and differentiation, the functional significance of p21CIP1 and p27KIP1 in modulating osteoblast responses to BMP-4 has not been reported. Moreover, the mechanisms that regulate the expression of cell cycle-regulatory proteins in osteoblasts in responses to BMP-4 have not been fully clarified.
Integrins, as the main receptors that connect the cytoskeleton and the extracellular matrix (ECM), have been shown to play important roles in modulating gene expression and cellular functions in a wide variety of cells seeded on the ECM (16, 17, 18). In several systems including endothelial cells, activation of integrins leads to increases in their association with focal adhesion kinase (FAK) and Shc, an adaptor protein containing a C-terminal Src homology domain-2 (SH2) domain, which subsequently activate several intracellular signaling cascades, including MAPKs (19). Shc may associate with activated FAK and function downstream from FAK in integrin-eliciting signaling events (20). Osteoblasts express several types of integrins, including αvβ3 and those containing β1-subunit (dimerized with α-subunits, including α1, α3, α5, and α6) (21, 22, 23), which have been shown to play important roles in osteoblast differentiation and commitment (24). Administration of RGD (Arg-Gly-Asp) peptide, which is a nonspecific inhibitor of integrins, has been shown to inhibit bone formation and resorption of fetal rat parietal bones (25). Interaction between integrins and fibronectin has been shown to be required for calvarial osteoblast differentiation (26, 27). Recent studies using function-perturbing antibodies against integrins showed that αvβ- and αβ1-integrins play an essential role in BMP-2-induction of osteoblast differentiation (24). It is probable that integrins may be cooperative with BMP receptors to mediate the BMP-eliciting signaling pathways in osteoblasts and hence modulate their gene expression and cellular functions.
In the present study, we investigated the regulatory effects of BMP-4 on cell cycle distribution in four osteoblast-like cell lines [i.e. human MG63 and Saos2 cells, human fetal osteoblasts (hFOBs) 1.19, and mouse MC3T3-E1 cells] and mouse primary osteoblasts, and the mechanism underlying these BMP-4 effects. We found that BMP-4 induces G0/G1 cell cycle arrest and cell differentiation in osteoblast-like cells by increasing their expressions of p21CIP1 and p27KIP1, which are mediated by BMPRIA-specific Smad1 and/or Smad5. The ECM/β3 integrin/FAK/Shc and the downstream ERK2 are involved in these BMP-4-induced signaling and cellular responses. Our findings generate insights into the mechanisms by which BMP-4 mediates cell cycle distribution and differentiation in osteoblast-like cells.
Results
BMP-4 induces G0/G1 arrest and increased expressions of p21CIP1 and p27KIP1 in osteoblast-like cells
Human MG63, Saos2, and mouse MC3T3-E1 cells, and mouse primary osteoblasts were kept as controls or treated with BMP-4 (25 ng/ml) for 24, 48, 72, or 96 h, and their cell cycle distributions were analyzed by flow cytometry. Incubation of human MG63 and Saos2 and mouse MC3T3-E1 cells under static conditions for 48, 72, or 96 h led to increases in cell percentage in G0/G1 phases and decreases in synthetic and/or G2/M phases (Table 1). Treatment of these three types of cell lines and mouse primary osteoblasts with BMP-4 caused significant increases in cell percentage in the G0/G1 phases and decreases in the synthetic and/or G2/M phases compared with the untreated control cells for the same periods. These results suggest that BMP-4 induces G0/G1 arrest in these cells.
Table 1.
BMP-4 induces G0/G1 arrest in osteoblast-like cells
| Cell type | Control % Cells (mean ± sem) | BMP4 % Cells (mean ± sem) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G0/G1 | Synthetic | G2/M | G0/G1 | Synthetic | G2/M | ||||||
| MG63 | |||||||||||
| 0 h | 43.6 ± 2.5 | 30.0 ± 1.5 | 26.4 ± 4.0 | – | – | – | |||||
| 24 h | 62.9 ± 2.31 | 22.6 ± 2.0 | 14.5 ± 4.2 | 61.9 ± 1.1 | 24.9 ± 3.5 | 13.2 ± 2.4 | |||||
| 48 h | 67.6 ± 1.21 | 16.0 ± 0.11 | 16.4 ± 1.3 | 80.4 ± 2.62 | 9.9 ± 1.12 | 9.7 ± 1.52 | |||||
| 72 h | 76.8 ± 2.11 | 11.9 ± 0.81 | 11.3 ± 1.31 | 91.4 ± 1.02 | 4.7 ± 1.32 | 3.9 ± 0.42 | |||||
| 96 h | 79.2 ± 1.61 | 11.3 ± 0.11 | 9.5 ± 1.61 | 94.3 ± 1.22 | 2.6 ± 0.82 | 3.1 ± 0.42 | |||||
| MC3T3-E1 | |||||||||||
| 0 h | 49.8 ± 3.4 | 32.6 ± 4.9 | 17.6 ± 1.5 | – | – | – | |||||
| 24 h | 55.5 ± 1.8 | 25.1 ± 3.8 | 19.4 ± 2.1 | 60.2 ± 2.5 | 19.6 ± 0.7 | 20.2 ± 1.8 | |||||
| 48 h | 66.4 ± 1.01 | 17.7 ± 2.21 | 15.9 ± 1.2 | 77.7 ± 3.52 | 9.7 ± 0.72 | 12.6 ± 2.8 | |||||
| 72 h | 74.8 ± 0.81 | 14.4 ± 1.71 | 10.8 ± 0.91 | 90.1 ± 0.22 | 5.8 ± 0.52 | 4.1 ± 0.32 | |||||
| 96 h | 80.3 ± 0.91 | 10.6 ± 1.01 | 9.1 ± 1.91 | 95.4 ± 1.82 | 2.2 ± 0.72 | 2.4 ± 1.12 | |||||
| Saos2 | |||||||||||
| 0 h | 65.4 ± 2.5 | 27.4 ± 3.2 | 7.2 ± 2.9 | – | – | – | |||||
| 72 h | 74.6 ± 3.31 | 15.1 ± 1.81 | 10.3 ± 1.5 | 84.9 ± 2.52 | 5.9 ± 0.72 | 9.2 ± 1.1 | |||||
| mOsteoblast | |||||||||||
| 0 h | 80.9 ± 2.0 | 6.7 ± 2.1 | 12.4 ± 1.4 | – | – | – | |||||
| 72 h | 80.2 ± 1.8 | 9.1 ± 2.3 | 10.7 ± 2.0 | 88.3 ± 1.52 | 2.1 ± 0.42 | 9.6 ± 1.8 | |||||
Three osteoblast-like cell lines, i.e. human MG63 and Saos2 and mouse MC3T3-E1 cells and mouse primary osteoblasts (mOsteoblast) were kept as controls or treated with BMP-4 (25 ng/ml) for 24 h, 48 h, 72 h, or 96 h. The cells were stained with propidium iodide and analyzed for DNA content by flow cytometry to show percentages of cells in G0/G1, synthetic, or G2/M phases of the cell cycle. Data are mean ± sem from three to five independent experiments. % Cells, Percentage of cells.
P < 0.05 vs. unstimulated control cells at 0 h;
P < 0.05 vs. unstimulated control cells at the corresponding time; –, no sample.
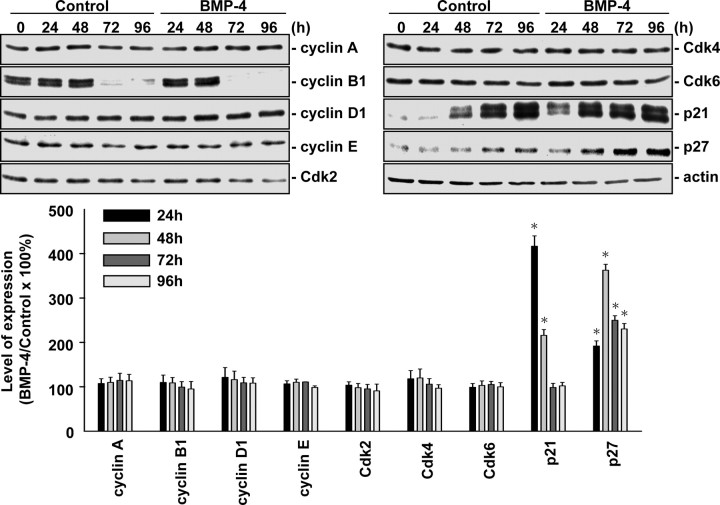
We investigated the molecular basis of this BMP-4 effect, and the presentation is focused on human MG63 osteoblast-like cells. Treatment of MG63 cells with BMP-4 (25 ng/ml) for 24, 48, 72, or 96 h resulted in increases in p21CIP1 and p27KIP1 expressions in MG63 cells (Fig. 1). In contrast, BMP-4 did not have effects on the expressions of cyclins A, B1, D1, and E, Cdk-2, -4, and -6 in these cells. BMP-4 stimulation also induced increases in p21CIP1 and p27KIP1 expressions in mouse primary osteoblasts (supplemental Fig. S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Fig. 1.
BMP-4 regulates cell cycle-regulatory protein expression in MG63 cells. MG63 cells were kept as controls or stimulated with BMP-4 (25 ng/ml) for 24, 48, 72, and 96 h. Expression of cell cycle-regulatory proteins was determined by Western blot analysis. Data are mean ± sem from three independent experiments. *, P < 0.05 vs. unstimulated control cells.
BMP-4-induced G0/G1 arrest and differentiation in MG63 cells are mediated by p21CIP1 and p27KIP
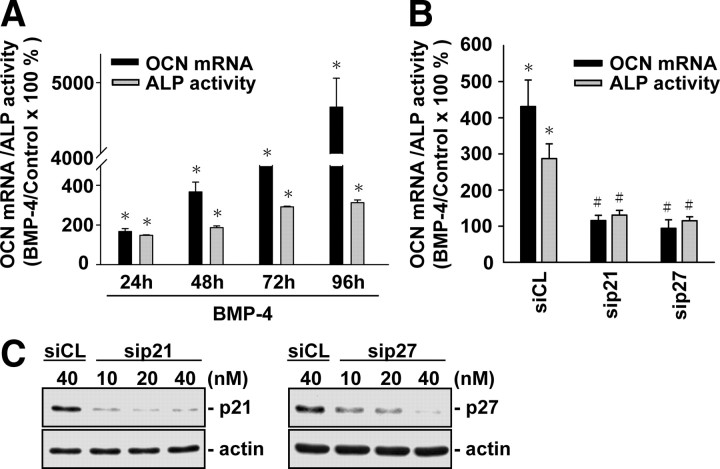
Because cell cycle regulator-led inhibition of G0/G1-to-synthetic phase transition has been shown to be critical for cell differentiation (13) and we have demonstrated that BMP-4 induces G0/G1 arrest in MG63 cells, with concomitant increases in p21CIP1 and p27KIP1 expressions, we investigated whether BMP-4-induced G0/G1 arrest and differentiation in MG63 cells are mediated by p21CIP1 and p27KIP1. MG63 cells were kept as controls or treated with BMP-4 (25 ng/ml) for 24, 48, 72, and 96 h, and their expression or activity of differentiation markers, i.e. osteocalcin (OCN) and alkaline phosphatase (ALP), were examined. Treatment with BMP-4 for 24, 48, 72, and 96 h resulted in significant increases in OCN gene expression and ALP activity in MG63 cells (Fig. 2A). These BMP-4-induced increases in OCN expression and ALP activity were abolished by transfections of cells with p21CIP1- and p27KIP1-specific small interfering RNAs (siRNAs; 40 nm for each) (Fig. 2B), which had 80–90% blocking effects on their respective protein expression (Fig. 2C). For unstimulated cells, transfection with p21CIP1- and p27KIP1-specific siRNA (compared with control siRNA) did not alter their cell cycle distribution (Table 2). After BMP-4 treatment, MG63 cells transfected with either p21CIP1- or p27KIP1-specific siRNA had a significantly lower percentage of cells in G0/G1 phases and higher percentages in synthetic and G2/M phases, when compared with cells transfected with control siRNA (Table 2; the remaining data will be discussed below). These results suggest that BMP-4-induced G0/G1 arrest and differentiation in MG63 cells are mediated by p21CIP1 and p27KIP1.
Fig. 2.
BMP-4-induced MG63 cell differentiation is mediated by p21CIP1 and p27KIP1. A, MG63 cells were kept as controls or stimulated with BMP-4 (25 ng/ml) for 24, 48, 72, and 96 h, and their OCN mRNA expression and ALP activity were determined by quantitative real-time PCR and ALP activity assay, respectively. B, MG63 cells were transfected with control siRNA (siCL) or a specific siRNA of p21CIP1 (sip21) or p27KIP1 (sip27) (40 nm for each) for 48 h, and then treated with BMP-4 for 72 h. C, MG63 cells were transfected with p21- or p27-specific siRNA at indicated concentrations for 48 h, and their p21CIP1 or p27KIP1 protein expressions were determined by Western blot analysis. Data in panels A and B are mean ± sem from three to four independent experiments. Results in panel C are representative of three independent experiments with similar results. *, P < 0.05 vs. unstimulated control cells. #, P < 0.05 vs. cells transfected with control siRNA.
Table 2.
The role of BMPR, ERK2, Smad1/5, p21CIP1, and p27KIP1 in BMP4-induced G0/G1 arrest in MG63 cells
| siRNA | Control (72 h) % Cells (mean ± sem) | BMP-4 (72 h) % Cells (mean ± sem) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0/G1 | Synthetic | G2/M | G0/G1 | Synthetic | G2/M | |||||
| siCL | 74.4 ± 1.5 | 14.6 ± 0.6 | 11.0 ± 0.9 | 88.9 ± 1.11 | 5.6 ± 0.51 | 5.5 ± 0.61 | ||||
| sip21 | 74.0 ± 0.4 | 15.8 ± 0.4 | 10.2 ± 0.1 | 72.9 ± 1.52 | 16.6 ± 1.22 | 10.5 ± 0.32 | ||||
| sip27 | 70.0 ± 0.8 | 18.0 ± 2.4 | 12.0 ± 1.6 | 74.9 ± 1.92 | 14.8 ± 1.62 | 10.3 ± 0.32 | ||||
| siSmad1 | 69.5 ± 1.1 | 14.8 ± 0.5 | 15.7 ± 0.6 | 74.2 ± 3.02 | 12.0 ± 0.52 | 13.8 ± 2.52 | ||||
| siERK2 | 73.0 ± 1.3 | 10.5 ± 0.3 | 16.5 ± 1.6 | 71.2 ± 3.32 | 11.8 ± 2.42 | 17.0 ± 0.82 | ||||
| siCL | 78.9 ± 1.7 | 12.9 ± 0.6 | 8.2 ± 1.1 | 91.5 ± 1.01 | 4.3 ± 0.31 | 4.2 ± 0.71 | ||||
| siRIA | 75.0 ± 3.5 | 11.8 ± 1.6 | 13.2 ± 1.9 | 76.5 ± 3.82 | 12.5 ± 1.32 | 11.0 ± 2.52 | ||||
| siSmad5 | 80.3 ± 0.6 | 10.7 ± 1.1 | 9.0 ± 1.6 | 78.0 ± 0.82 | 11.9 ± 0.82 | 10.1 ± 1.62 | ||||
MG63 cells were transfected with control siRNA (siCL) or specific siRNA of p21CIP1 (sip21), p27KIP1 (sip27), Smad1 (siSamd1), Smad5 (siSamd5), ERK2 (siERK2), or BMPRIA (siRIA) (40 nm for each) for 48 h, and then were kept as controls or treated with BMP-4 (25 ng/ml) for 72 h. The cells were stained with propidium iodide and analyzed for DNA content by flow cytometry to show percentages of cells in G0/G1, synthetic, or G2/M phases of the cell cycle. Data are mean ± sem from three independent experiments. % Cells, Percentage of cells.
P < 0.05 vs. unstimulated control cells;
P < 0.05 vs. cells transfected with control siRNA.
BMP-4-induced p21CIP1 and p27KIP1 expressions and G0/G1 arrest in MG63 cells are mediated by BMPRIA through Smad1 and/or Smad5
Treatment of MG63 cells with BMP-4 (25 ng/ml) induced a rapid increase (within 30 min) in the Smad1/5 phosphorylation, which reached a maximal level approximately 5 times untreated controls within 1 h, and then declined but remained elevated after 24 h of treatment (Fig. 3A). Transfection of MG63 cells with BMPRIA-specific siRNA (40 nm, compared with control siRNA) abolished the BMP-4-induced Smad1/5 phosphorylation (Fig. 3B). In contrast, BMPRIB-specific siRNA did not have inhibitory effects on BMP-4-induced Smad1/5 phosphorylation. BMPRIA- and BMPRIB-specific siRNAs almost totally abolished their respective receptor protein expression. These results suggest that BMP-4-induced Smad1/5 activation is mediated by BMPRIA, but not BMPRIB, in MG63 cells. This differential role of BMPRIA vs. BMPRIB in regulating BMP-4-induced Smad1/5 activation was also found in hFOB 1.19 osteoblastic cells (supplemental Fig. S2A).
Fig. 3.
BMP-4-induced p21CIP1 and p27KIP1 expressions in MG63 cells are mediated by BMPRIA through Smad1 and/or Smad5. A, MG63 cells were kept as controls (C) or treated with BMP-4 (25 ng/ml) for 10 min (10′), 30 min (30′), 1 h, 3 h, 6 h, and 24 h, and their Smad1/5 phosphorylation was determined by Western blot analysis. B, MG63 cells were transfected with control siRNA (siCL) or specific siRNA of BMPRIA (siRIA) or BMPRIB (siRIB) (40 nm) for 48 h and then were kept as controls or treated with BMP-4 for 10 or 30 min. C, MG63 cells were transfected with control siRNA or specific siRNA of Smad1 (siSmad1) or Smad5 (siSmad5) for 48 h (40 nm) and then were kept as controls (C) or treated with BMP-4 (B) for 48 h. Data in panels A and C are mean ± sem from three independent experiments and are presented as percentage changes in band density from control cells normalized to Smad1/5 (A) or actin (C) protein levels. Results in panel B are representative of three independent experiments with similar results. *, P < 0.05 vs. unstimulated control cells. #, P < 0.05 vs. cells transfected with control siRNA.
To investigate the role of Smad1/5 in BMP-4-modulation of cell cycle-regulatory protein expression in MG63 cells, MG63 cells were transfected with Smad1- or Smad5-specific siRNA (40 nm), which reduced the expressions of corresponding Smad proteins by approximately 80% of that with control siRNA (Fig. 3C), and then kept as controls or treated with BMP-4 for 48 h. Transfection with Smad5-specific siRNA (compared with control siRNA) resulted in significant inhibitions of the BMP-4-induced up-regulation of p21CIP1 and p27KIP1 expressions (Fig. 3C). In contrast, transfection with Smad1-specific siRNA only inhibited the BMP-4-induced up-regulation of p27KIP1 expression. For cells stimulated with BMP-4, transfection with BMPRIA-, Smad1-, or Smad5-specific siRNA (compared with control siRNA; 40 nm for each) resulted in a significantly lower cell percentage in G0/G1 phases and higher cell percentages in synthetic and G2/M phases (Table 2). Taken together, these results suggest that the BMP-4-induced p21CIP1 and p27KIP1 expressions and G0/G1 arrest in MG63 cells are mediated by BMPRIA through Smad1/5.
ERK2, but not ERK1, is involved in BMP-4-induced Smad1/5 phosphorylation in MG63 cells
Stimulation of MG63 cells with BMP-4 (25 ng/ml) induced a rapid increase in the ERK phosphorylation within 10 min (Fig. 4A). This increased level of phosphorylation decreased to nearly the basal level 30 min after BMP-4 stimulation. BMP-4 also induced transient increases in ERK phosphorylation in human Saos2 and mouse MC3T3-E1 cells and mouse primary osteoblasts (supplemental Fig. S3). Transfection with BMPRIA- and BMPRIB-specific siRNA alone or simultaneously did not inhibit BMP-4-induced ERK phosphorylation in MG63 cells (Fig. 4B) and hFOB 1.19 osteoblasts (supplemental Fig. S2A), indicating that BMP-4-induced ERK activation is not mediated through BMPRIA and BMPRIB. To determine whether ERK is involved in BMP-4-induced Smad1/5 phosphorylation, MG63 cells were incubated with PD98059 (30 μm), a specific inhibitor for MAPK kinase (MEK) that is an upstream kinase of ERK, for 1 h before and during treatment with BMP-4. Treatment of MG63 cells with PD98059 inhibited the BMP-4-induced Smad1/5 phosphorylation (Fig. 4C). Transfection of MG63 cells with an expression plasmid encoding MEK1 induced ERK and Smad1/5 phosphorylations, with the level of Smad1/5 phosphorylation lower than that induced by BMP-4 (Fig. 4D). Transfecting MG63 cells with ERK2-specific siRNA (compared with control siRNA; 40 nm), which almost totally abolished the ERK2 protein expression, resulted in significant inhibition in BMP-4-induced Smad1/5 phosphorylation (Fig. 4E). In contrast, transfection with ERK1-specific siRNA, which caused 80–90% reductions in the ERK1 protein expression, had no effect on BMP-4-induced Smad1/5 phosphorylation. These results suggest that ERK2, but not ERK1, is involved in BMP-4-induced Smad1/5 phosphorylation in MG63 cells. This differential role of ERK2 vs. ERK1 in modulating BMP-4-induced Smad1/5 phosphorylation was also found in hFOB 1.19 osteoblastic cells (supplemental Fig. S2B).
Fig. 4.
ERK2, but not ERK1, is involved in BMP-4-induced Smad1/5 activation in MG63 cells. A, MG63 cells were kept as controls (C) or treated with BMP-4 (25 ng/ml) for 10 min (10′), 30 min (30′), and 1 h, and their ERK phosphorylation was determined by Western blot analysis. Data are mean ± sem from three independent experiments and are presented as percentage changes in band density from control cells normalized to ERK2 protein levels. *, P < 0.05 vs. unstimulated control cells. B–E, MG63 cells were transfected with control siRNA (siCL), specific siRNA of BMPRIA (siRIA) and BMPRIB (siRIB) alone or simultaneously (siRIA/B) (B), or ERK1 (siERK1) and ERK2 (siERK2) (E) (40 nm for each) for 48 h. In some experiments, MG63 cells were treated with PD98059 (PD; 30 μm) for 1 h (C) or transfected with control empty vector pcDNA3 or an expression plasmid encoding MEK1 (D) (1 μg) for 48 h. The cells were then kept as controls (C) or treated with BMP-4 (B) for 10 min. The phosphorylations of ERK (B and D) or Smad1/5 (C–E) were determined by Western blot analysis. Results are representative of three independent experiments with similar results. DMSO, Dimethylsulfoxide.
ERK2 is involved in BMP-4-induced p21CIP1 and p27KIP1 expressions, G0/G1 arrest, and differentiation in MG63 cells
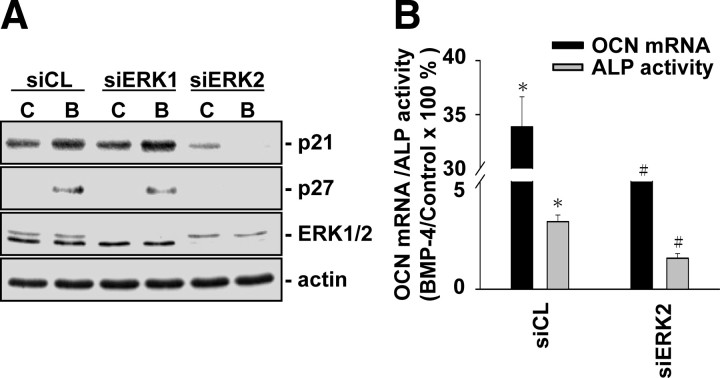
Given our findings that ERK2 is involved in BMP-4-induced Smad1/5 activation, we investigated whether ERK2 is involved in BMP-4-induced p21CIP1 and p27KIP1 expressions, G0/G1 arrest, and differentiation in MG63 cells. Transfection of MG63 cells with ERK2-specific, but not ERK1-specific, siRNA (40 nm for each) inhibited the BMP-4-induced increases in p21CIP1 and p27KIP1 expressions, as compared with the cells transfected with control siRNA (Fig. 5A). For unstimulated cells, transfection with ERK2-specific siRNA (compared with control siRNA) did not alter their cell cycle distribution (Table 2). After BMP-4 treatment, MG63 cells transfected with ERK2-specific siRNA had a significantly lower cell percentage in G0/G1 phases and higher cell percentages in synthetic and G2/M phases, when compared with cells transfected with control siRNA. These inhibitions in BMP-4-induced p21CIP1 and p27KIP1 expressions and cell cycle arrest in MG63 cells by transfection with ERK2-specific siRNA were accompanied by the inhibition in BMP-4-induced increases in OCN gene expression and ALP activity (Fig. 5B). These results suggest that ERK2 is involved in BMP-4-induced Smad1/5 activation, which can consequently regulate the BMP-4-induced p21CIP1 and p27KIP1 expressions, cell cycle arrest, and differentiation in MG63 cells.
Fig. 5.
ERK2 is involved in BMP-4-induced p21CIP1 and p27KIP1 expressions and differentiation in MG63 cells. MG63 cells were transfected with control siRNA or specific siRNA of ERK1 (siERK1) (A) or ERK2 (siERK2) (A and B) (40 nm) for 48 h and then were kept as controls (C) or treated BMP-4 (B; 25 ng/ml) for 48 h (A) or 72 h (B). The cells were lysed, and their p21CIP1 and p27KIP1 protein expression (A) or OCN mRNA expression and ALP activity (B) were determined. Results in panel A are representative of two independent experiments with similar results. Data in panel B are mean ± sem from three to four independent experiments. *, P < 0.05 vs. unstimulated control cells. #, P < 0.05 vs. cells transfected with control siRNA.
β3-Integrin/FAK/Shc are involved in BMP-4-induced ERK and Smad phosphorylations in MG63 cells
To elucidate the role of β1- and β3-integrins in BMP-4-induced ERK and Smad1/5 activations in MG63 cells, MG63 cells were transfected with β1- and β3-specific siRNAs (40 nm for each), which almost totally abolished their respective integrin expression (Fig. 6A), and then kept as controls or stimulated with BMP-4 (25 ng/ml) for 10 and 30 min. The BMP-4-induced ERK and Smad1/5 phosphorylations were inhibited by transfections of MG63 cells with β3-specific, but not β1-specific, siRNAs (compared with control siRNA), suggesting that β3- , but not β1-integrin, is involved in BMP-4-induced ERK and Smad1/5 activations (Fig. 6A). The involvement of β3-integrin in BMP-4-induced ERK and Smad1/5 activations was also found in hFOB 1.19 osteoblastic cells (supplemental Fig. S2C). We further investigated the interaction between β3-integrin and BMP receptors under unstimulated control condition or in response to BMP-4. MG63 cells were kept as controls or treated with BMP-4 (25 ng/ml) for 10 min, and their extracts were immunoprecipitated with an antibody against BMPRIA or BMPRIB, followed by Western blot analysis with an antibody against β3-integrin. In unstimulated cells, β3-integrin had constitutive associations with BMPRIA and BMPRIB (Fig. 6B). BMP-4 stimulation resulted in significant decreases in the association of BMPRIB with β3-integrin. In contrast, BMP-4 did not affect the association of BMPRIA with β3-integrin.
Fig. 6.
BMP-4-induced ERK and Smad1/5 activations in MG63 cells are mediated by β3-integrin through FAK and Shc. MG63 cells were transfected with control siRNA (siCL) or specific siRNA of β1 (siβ1) or β3 (siβ3) integrin (40 nm for each) for 48 h, and then were kept as controls (C) or treated with BMP-4 (25 ng/ml) for 10 (10′) or 30 (30′) min (A). In some experiments, MG63 cells were transfected with control empty vector pcDNA3 or dominant-negative mutant of FAK [FAK(F397Y); mFAK] or Shc (Shc-SH2; mShc) (1 μg) for 48 h and then treated with BMP-4 (B) for 10 min (D). The phosphorylations of ERK and Smad1/5 were determined by Western blot analysis. B, MG63 cells were kept as controls (C) or treated with BMP-4 for 10 min. The associations of BMPRIA and BMPRIB with β3-integrin were determined by immunoprecipitation assay and Western blot analysis, as described in Materials and Methods. C, The associations of β3-integrin with FAK and Shc were determined by using an antibody against β3-integrin for immunoprecipitation, followed by Western blot analysis with an antibody against FAK or Shc. Results in panels A and C are representative of three independent experiments with similar results. Data in panels B and D are mean ± sem from three to four independent experiments. *, P < 0.05 vs. unstimulated control cells. #, P < 0.05 vs. cells transfected with control pcDNA3 (D). IP, Immunoprecipitation.
Because FAK and Shc have been shown to play important roles in integrin-elicited signaling, and their associations with integrins are critical for the activation of several intracellular signaling cascades, including MAPKs, we investigated whether BMP-4 induces associations of β3 integrin with FAK and Shc. MG63 cells were kept as controls or treated with BMP-4 (25 ng/ml) for 10 and 30 min, and their extracts were immunoprecipitated with an antibody against β3-integrin, followed by Western blot analysis with an antibody against FAK or Shc. BMP-4 induced rapid increases (significant within 10 min) in the association of β3-integrin with FAK and Shc, as shown by their coimmunoprecipitation, in comparison with the unstimulated controls (Fig. 6C). These increases in associations of β3-integrin with FAK and Shc decreased to nearly the basal level 30 min after BMP-4 stimulation. Transfection of MG63 cells with dominant-negative mutants of FAK and Shc [i.e. FAK (F397Y) and Shc-SH2; compared with control pcDNA3 parental plasmid] inhibited the BMP-4-induced ERK and Smad1/5 phosphorylations (Fig. 6D), suggesting that FAK and Shc are involved in BMP-4-induced ERK- and Smad-signaling events.
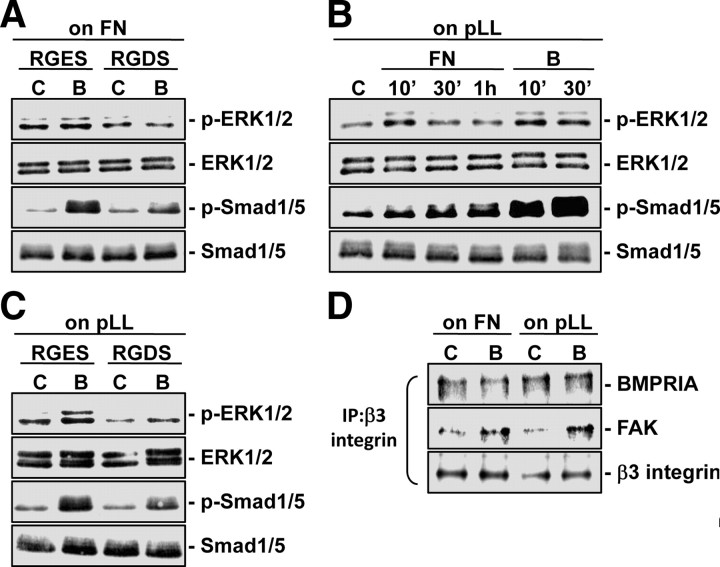
ECM plays a role in modulating BMP-4-induced ERK and Smad phosphorylations in MG63 cells
ECM is thought to be a prerequisite in integrin-mediating signaling events. To investigate whether ECM is involved in BMP-4-induced ERK and Smad phosphorylations in MG63 cells, MG63 cells were pretreated with RGDS (Arg-Gly-Asp-Ser), which blocks the cell-ECM interaction mediated by the integrin-recognition sequence RGD (Arg-Gly-Asp) on ECM proteins, and then kept as controls or stimulated with BMP-4 (25 ng/ml) for 10 min. As shown in Fig. 7A, pretreating MG63 cells with RGDS inhibited BMP-4-induced ERK and Smad1/5 phosphorylations in comparison with the cells pretreated with control RGES (Arg-Gly-Glu-Ser). Stimulation of MG63 cells seeded on poly-l-lysine with soluble fibronectin (40 μg/ml) induced ERK and Smad1/5 phosphorylations within 10 min (Fig. 7B). This suggests that stimulation of β3-integrin via fibronectin can activate the ERK and Smad1/5 intracellular signaling cascades. However, the levels of Smad1/5 phosphorylation induced by fibronectin were much lower than those induced by BMP-4 for the same periods (Fig. 7B). The BMP-4-induced increases in ERK and Smad1/5 phosphorylations in MG63 cells seeded on poly-l-lysine were inhibited by pretreating the cells with RGDS (compared with control RGES) (Fig. 7C), suggesting that autocrine-secreted ECM may be involved in these BMP-4-induced signaling activations. Coimmunoprecipitation assays showed that BMP-4 induces increases in the association of β3-integrin with FAK and did not affect the constitutive association of BMPRIA with β3-integrin in MG63 cells seeded on either fibronectin or poly-l-lysine (Fig. 7D).
Fig. 7.
ECM plays a role in modulating BMP-4-induced ERK and Smad phosphorylations in MG63 cells. MG63 cells seeded on fibronectin (FN) (A) or poly-l-lysine (pLL) (C) (40 μg/ml) were pretreated with RGDS or RGES peptides (500 μg/ml) for 2 h, and then kept as controls (C) or stimulated with BMP-4 (B; 25 ng/ml) for 10 min. B, MG63 cells seeded on poly-l-lysine were kept as controls or treated with soluble fibronectin (FN; 40 μg/ml) or BMP-4 for the indicated times. The phosphorylations of ERK and Smad1/5 were determined by Western blot analysis. D, The associations of β3 integrin with BMPRIA and FAK in MG63 cells seeded on fibronectin or poly-l-lysine under static conditions or in response to BMP-4 (10 min) were examined by coimmunoprecipitation assays. Results are representative of triplicate experiments with similar results. IP, Immunoprecipitation.
Discussion
The aim of the present study was to investigate the role of BMP-4 in cell cycle distribution in osteoblast-like cells and the mechanism underlying the BMP-4 effect. In a series of systematic studies, we have characterized the mechanisms by which BMP-4 regulates cell cycle and hence differentiation in osteoblast-like cells through specific BMP receptors, integrins, MAPKs, and downstream Smad and cell cycle-regulatory proteins, as summarized in Fig. 8. Our study has generated the following findings. First, this work shows that BMP-4 induces G0/G1 arrest in osteoblast-like cells, with concomitant increases in the expressions of Cdk inhibitors p21CIP1 and p27KIP1. These BMP-4-inductions of p21CIP1 and p27KIP1 expressions are prerequisite for the BMP-4-induced G0/G1 arrest and differentiation in these cells. Second, BMP-4-induced G0/G1 arrest, and p21CIP1 and p27KIP1 expressions are mediated by BMPRIA, but not BMPRIB, through Smad1 and/or Smad5. Third, β3 integrin and its association with FAK and Shc and the downstream ERK2 pathway are involved in BMP-4-induced Smad1/5 activation, with the existence of β3-BMPRIA heteromeric complex in BMP-4-stimulated cells. Finally, the ECM proteins such as fibronectin play a role in modulating the BMP-4-induced signaling activation. Thus, our findings provide insights into the molecular mechanisms by which BMP-4 regulates cell cycle and differentiation in osteoblast-like cells.
Fig. 8.
Schematic representation of the signaling pathways regulating BMP-4-induced cell cycle arrest and differentiation in osteoblast-like cells. BMP-4 induces G0/G1 cell cycle arrest and differentiation in osteoblast-like cells by increasing their expressions of Cdk inhibitors p21CIP1 and p27KIP1, which are mediated by BMPRIA through Smad1 and/or Smad5. ECM, β3 integrin and its association with FAK and Shc, and the downstream ERK2 are involved in these BMP-4-induced signaling and cellular responses. Shc is shown to be associated with FAK and function downstream from FAK, as previously reported (20 ) ↑, Up-regulation by BMP-4. P, Phosphorylation.
It is known that entry into and progression through the cell cycle is regulated by different cell cycle-regulatory proteins, including cyclin-Cdk complexes and Cdk inhibitors, which facilitate the transition between different phases of the cell cycle (12). The role of BMP-4 in regulating cell cycle-regulatory protein expression in osteoblasts remains unclear. Wong et al. (28) demonstrated that BMP-2 inhibits proliferation of human aortic smooth muscle cells via increased expression of p21CIP1. Franzen and Heldin (29) demonstrated that BMP-7 induces cell cycle arrest in anaplastic thyroid carcinoma cells via increased expressions of p21CIP and p27KIP and decreased activities of Cdk2 and Cdk6. A recent study by Jeffery et al. (30) demonstrated that BMP-4 inhibits proliferation of human fetal lung fibroblasts and promotes their differentiation to myofibroblasts through Smad1 and p21CIP1. These results suggest that p21CIP1 and p27KIP1 may serve as downstream targets of BMP signaling and contribute to the antiproliferative and prodifferentiation effects of BMPs. In the present study, we found that BMP-4 induces increased expressions of p21CIP1 and p27KIP1 in MG63 cells (and also in mouse primary osteoblasts; supplemental Fig. S1). Further support for the involvement of p21CIP1 and p27KIP1 in the prodifferentiation effect of BMP-4 was provided by the findings that transfections of cells with p21CIP1- and p27KIP1-specific siRNAs inhibited the BMP-4-induced G0/G1 arrest and expression or activity of differentiation markers OCN and ALP. Moreover, our results showed that the BMP-4-induced expressions of both p21CIP1 and p27KIP1 were mediated by Smad5, whereas Smad1 only regulated the BMP-4-induced p27KIP1 expression. It is noted that incubation of MG63 cells under static conditions for 48 h, 72 h, or 96 h also led to G0/G1 arrest (Table 1). This cell contact-induced G0/G1 arrest was also accompanied by the increases in p21CIP1 and p27KIP1 expressions in unstimulated control cells (Fig. 1). Thus, p21CIP1 and p27KIP1 may also be involved in the cell contact-induced cell cycle arrest in unstimulated control cells. Our findings indicate the importance of p21CIP1 and p27KIP1 in regulating cell cycle in osteoblast-like cells under unstimulated control conditions or in response to BMP-4.
BMPs exert their effects via binding to two types of serine/threonine kinase receptors; the type II receptor is a constitutively active kinase, which trans-phosphorylates type I receptors (i.e. BMPRIA and BMPRIB) upon ligand binding, leading to activation of downstream signals Smads (3). BMPRIA and BMPRIB have been shown to exert differential biological effects (4, 5). It has been reported that BMPRIA is critical for BMP-2-induced adipocyte differentiation, whereas BMPRIB is responsible for BMP-2-induced osteoblast differentiation and apoptosis (5, 6). However, additional studies found that there is no functional difference between BMPRIA and BMPRIB in modulating BMP-induced osteoblast differentiation (4). By using BMPRIA- and BMPRIB-specific siRNAs, our results showed that BMP-4-induced cell cycle arrest via Smad activation in MG63 cells (and also in hFOB 1.19 osteoblasts; supplemental Fig. S2A) is mediated by BMPRIA, but not BMPRIB. The discrepancy in biological effects of BMPRIA and BMPRIB between our present results and previous studies may be attributable to the different cell types used in various experimental systems.
It has been reported that osteoblasts express β1- and β3-containing integrins at the focal adhesion sites, and these integrins play important roles in regulating signaling and functions in osteoblasts (21, 22, 23, 24). Numerous mechanisms have been proposed to contribute to the regulatory effects of integrins on osteoblasts (31, 32). However, the mechanism by which integrins regulate the BMP-eliciting signaling in osteoblasts remains unclear. There is evidence that integrins may be cooperative with receptors of several growth factors, including insulin receptor and platelet-derived growth factor-β receptor, to form integrin-receptor heteromeric complexes in mediating downstream signaling cascades (33). Recent study by Lai and Cheng (24) showed that β1-integrin interacts with BMP-2 receptors in BMP-2-stimulated osteoblasts and may serve as an anchor for BMP-2 receptors to facilitate the activation of downstream signaling cascades. Schneider et al. (34) showed that β3-integrin modulates the expression of bone sialoprotein, a bone-specific matrix, and the β3 integrin-bone sialoprotein interaction plays important roles in the process of osteoblast differentiation and mineralization. Using specific siRNA, our present study demonstrated that β3, but not β1, integrin is involved in BMP-4-induced Smad signaling in MG63 cells (and also in hFOB 1.19 osteoblasts; supplemental Fig. S2C). Our coimmunoprecipitation experiments using an antibody against BMPRIA or BMPRIB, followed by Western blot analysis using an antibody against β3-integrin, further demonstrated that β3-integrin can form heteromeric complexes with BMPRIA and BMPRIB in the unstimulated MG63 cells. However, BMP-4 stimulation resulted in a significant inhibition in the formation of β3-BMPRIB complex but did not influence the formation of β3-BMPRIA complex. The existence of β3-BMPRIA heteromeric complex in BMP-4-stimulated cells provides the possibility for close cross talk between β3-integrin and BMPRIA, which may exert synergistic effects at the levels of downstream signaling pathway and gene expression in response to BMP-4.
In addition to Smad, our present results showed that BMP-4 induces a transient activation of ERK, a member of MAPK family activated by upstream MEK, in MG63 cells. This BMP-4-induced transient activation of ERK was also observed in human Saos2 and mouse MC3T3-E1 cells and mouse primary osteoblasts (supplemental Fig. S3). These results are in agreement with the result by Lou et al. (10) but are in contrast to the result by Zanotti et al. (35), which failed to detect either transient or sustained effects of BMP-2 on ERK activation in murine stromal cells and calvarial osteoblasts. The discrepancy in the results obtained regarding the effect of BMP on ERK activation may be due to the different cell lines and culture conditions used in diverse experimental protocols (35). The role of ERK in osteogenesis and bone homeostasis is controversial. It has been reported that activation of ERK is involved in BMP-2-induced differentiation of mesenchymal cells to osteoblasts (10). Using a transgenic approach, Ge et al. (36) demonstrated that ERK activation achieved by selective expression of constitutively active MEK1 in osteoblasts promotes in vitro differentiation of calvarial cells, as well as in vivo bone development, whereas dominant-negative MEK1 is inhibitory. However, activation of ERK upon stimulation with epidermal growth factor was shown to phosphorylate Smad proteins at the linker region and inhibit nuclear accumulation and transcriptional activity of Smads (37, 38). In the present study, we found that ERK2, but not ERK1, is involved in BMP-4-induced Smad1/5 activation in both MG63 and hFOB 1.19 cells, as evidenced by the inhibition of BMP-4-induced Smad1/5 phosphorylation by transfections of cells with ERK2-specific, but not ERK1-specific, siRNA, which consequently resulted in inhibitions in BMP-4-induced G0/G1 arrest and differentiation in MG63 cells. In addition, transfecting MG63 cells with an expression plasmid encoding MEK1 resulted in increases in Smad1/5 phosphorylation. It is possible that the differential effect of activated ERK on inhibiting or inducing Smad phosphorylation is attributable to the different regulatory sites (e.g. the linker region or the receptor-dependent C terminus) of Smads phosphorylated by the activated ERK. The precise molecular mechanisms underlying the involvement of ERK2 in BMP-4-induced Smad1/5 signaling and cellular responses remain an important question that warrants further investigation.
The detailed mechanisms by which BMP-4 induces ERK phosphorylation in MG63 cells remain to be clarified. The failure to suppress BMP-4-induced ERK phosphorylation by depleting the BMPRIA and BMPRIB expressions individually or simultaneously using the respective specific siRNA did not completely rule out the role of BMP receptors in BMP-4-induced ERK activation, because other BMP receptors may be involved. Recent studies demonstrated that the ERK pathway is activated by integrins and consequently contributes to the integrin-mediated osteoblast differentiation (8, 9, 10, 39). In the present study, the role of integrins in BMP-4-induced ERK and hence Smad1/5 activations was evidenced by the blockage of BMP-4-induced ERK and Smad1/5 phosphorylations by transfections of cells with β3-specific siRNA. Our results further showed that BMP-4-activation of ERK in MG63 cells is mediated by β3-integrin through FAK and Shc, inasmuch as 1) BMP-4 induces associations of β3 integrin with FAK and Shc and 2) dominant-negative mutants of FAK and Shc inhibited the BMP-4-induced ERK and Smad1/5 phosphorylations. Thus, our findings demonstrated that BMP-4 induces Smad1/5 phosphorylation through BMPRIA, and that β3-integrin/FAK/Shc and the downstream ERK2 are involved in this BMP-4-induced Smad1/5 phosphorylation.
Osteoblasts are responsible for synthesizing bone ECM, which is composed of collagenous and noncollagenous matrix molecules such as fibronectin (8). Induction of osteoblast function requires interactions between osteoblasts and the ECM proteins they secrete (40). Previous studies have shown that signals from ECM, transduced by integrins, play critical roles in regulating gene expression, differentiation, and survival of osteoblasts (41). In the present study, we found that both BMP-4 and soluble fibronectin induce ERK and Smad1/5 phosphorylations in MG63 cells seeded on poly-l-lysine, with the former having stronger effects. The involvement of ECM in BMP-4-induced signaling activation was demonstrated by the blockage of BMP-4-induced ERK and Smad1/5 phosphorylations in MG63 cells seeded on either fibronectin or poly-l-lysine by pretreating the cells with RGDS. These results are in agreement with the results by Xiao et al. (8), which indicate that ECM promotes the BMP responsiveness of osteoblasts. Our findings that BMP-4 induced increases in β3-integrin-FAK association and ERK and Smad1/5 phosphorylations in MG63 cells seeded on poly-l-lysine and that these BMP-4-induced signaling activations were inhibited by RGDS suggest that osteoblast-like cells may secrete ECM, which may be involved in BMP-4-induced signaling cascades.
In summary, our present study demonstrated that BMP-4 induces G0/G1 cell cycle arrest and hence cell differentiation in osteoblast-like cells, with concomitant increases in the expressions of p21CIP1 and p27KIP1. These BMP-4-induced responses are mediated by BMPRIA, but not BMPRIB, through Smad1/5, the activation of which involves ECM/β3 integrin/FAK/Shc and the downstream ERK2. Our findings provide a molecular basis for the mechanisms by which BMP-4 mediates cell cycle distribution in the differentiation commitment process in osteoblast-like cells.
Materials and Methods
Materials
Mouse monoclonal antibodies (mAbs) against cyclin E (sc-25303), Cdk2 (sc-748), ERK2 (sc-1647), phospho-ERK1/2 (sc-7383), and β3 integrin (sc-46655), and goat polyclonal antibody (pAb) against Smad1/5 (sc-6031) were purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA). Mouse mAbs against ERK1/2, cyclins A (no. 4656), B1 (no. 4135), and D1 (no. 2926), Cdk-4 (no. 2906) and -6 (no. 3136), and p21CIP1 (no. 2946), and rabbit pAbs against p27KIP1 (no. 2552) and phospho-Smad1/5 (no. 9511) were purchased from Cell Signaling Technology (Beverly, MA). Mouse mAb against β1 integrin (MAB2253) was purchased from Chemicon (Temecula, CA). Mouse mAb against FAK was purchased from BD Biosciences (Bedford, MA). Rabbit pAb against Shc was obtained from Upstate Biotechnology (Lake Placid, NY). The control siRNA and specific siRNAs of BMPRIA, BMPRIB, Smad1, Smad5, p21CIP1, p27KIP1, ERK1, ERK2, and β1- and β3-integrins were purchased from Invitrogen (Carlsbad, CA). The plasmids MEK1, FAK(F397Y), and Shc-SH2 were previously described (42, 43, 44). Recombinant human BMP-4 was purchased from R & D Systems (Minneapolis, MN). PD98059 was purchased from Calbiochem (La Jolla, San Diego, CA). All other chemicals of reagent grade were obtained from Sigma (St. Louis, MO), unless otherwise noted.
Cell cultures
Human MG63, Saos2, and hFOB 1.19, and mouse MC3T3-E1 osteoblast-like cells were obtained from American Type Culture Collection (Manassas, VA) and grown in Petri dishes precoated with fibronectin (40 μg/ml). The human MG63 and Saos2 and mouse MC3T3-E1 cells were grown in the DMEM (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Inc.) and 1% penicillin/streptomycin at 37 C, whereas the hFOB 1.19 cells were grown in a medium consisting of DMEM and Ham’s F-12 medium (1:1) supplemented with FBS and antibiotics at 34 C. Mouse primary osteoblasts were gifts from Dr. Jui-Sheng Sun (National Taiwan University Hospital, Taiwan). Briefly, the cells were isolated from sequential digestion from calvaria of newborn ICR mice by using a modification of the methods previously described (45) and then were cultured in α-MEM supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), 10% FBS, ascorbic acid (50 mg/ml), and β-glycerophosphoric acid disodium salt (5 mm), with change of new medium every 2 d. The culture medium was exchanged with a medium that was identical except that it contained only 0.5% FBS, and the cells were further incubated for 24 h before treating with BMP-4.
Flow cytometric analysis
The cells were harvested in PBS containing 2 mm EDTA, washed once with PBS, and fixed for 30 min in cold ethanol (70%). Fixed cells were washed and permeabilized with 0.1% Triton X-100 in PBS. They were then stained with 50 μg/ml of propidium iodide (Roche, Basel, Switzerland) and 1 mg/ml ribonuclease A for 30 min. Stained cells were analyzed with a fluorescence-activated cell sorter Calibur (Becton-Dickinson, Franklin Lakes, NJ), and the data were analyzed using a mod-fit cell cycle analysis program.
ALP activity assay
Cell extract was prepared with 0.1% Triton X-100. Cellular ALP activity was assayed at the end of the incubation with 10 mm p-nitrophenyl phosphate in 0.15 m sodium carbonate buffer (pH 10.3) and 1 mm MgCl2 as previously described (17) and was normalized against cellular protein determined by the Bio-Rad protein assay.
RNA isolation and quantitative real-time PCR
The total RNA was isolated by the guanidium isothiocyanate/phenochloroform method and converted to cDNA. The cDNA was amplified through PCR on a LightCycler (Roche Diagnostics, East Sussex, UK) using LightCycler FastStart DNA MasterPlus SYBR Green I (Roche Diagnostics) with 0.5 μm primers of OCN (sense: 5′-TGAGAGCCCT CACACTCCTC-3′; antisense: 5′-ACCTTTGCTGGACTCTGCAC-3′; product length, 98 bp) and β-actin (sense: 5′-AAATCGTCCGTGACATCAAG-3′; antisense: 5′-GGAAGGAAGGCTGGAAGAGA-3′; product length, 180 bp) genes. PCR was performed in triplicate at 95 C for 10 min followed by 45 cycles of denaturation at 95 C for 10 sec, annealing at 60 C for 5 sec, extension at 72 C for 8 sec, and single signal acquisition for 10 sec. The β-actin gene expression was used as an internal control. The PCR conditions were optimized to obtain a PCR product with a single peak on melting curve analysis on the LightCycler. Raw data collected from the LightCycler were analyzed using LightCycler Software Version 3.5 (Roche Diagnostics). The OCN gene expression level was normalized with β-actin gene expression level in the same sample.
Western blot analysis
The cells were collected by scraping and lysed with a buffer containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and a protease inhibitor mixture (phenylmethylsulfonylfluoride, aprotinin, and sodium orthovanadate). The total cell lysate (100 μg of protein) was separated by SDS-PAGE (10% running, 4% stacking) and transferred onto a polyvinylidene fluoride membrane (Immobilon P; 0.45-μm pore size). The membrane was then incubated with the designated antibodies. Immunodetection was performed by using the Western-Light chemiluminescent detection system (Applied Biosystems, Foster City, CA).
Immunoprecipitation
The cells were scraped and lysed with a buffer containing 25 mm HEPES (pH 7.4), 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 0.125 m NaCl, 5 mm EDTA, 50 mm NaF, 1 mm Na3VO4, 1 mm phenylmethylsulfonylfluoride, 10 mg/ml leupeptin, and 2 mm β-blycerophosphate. The cells were disrupted on ice by repeated aspiration through a 21-gauge needle. The same amount of protein from each sample was incubated with a designated antibody for 2 h at 4 C with gentle shaking. The immune complex was then incubated with protein A/G plus agarose for 1 h and collected by centrifugation. These agarose-bound immunoprecipitates were washed and incubated with boiling sample buffer containing 62 mm Tris-HCl (pH 6.7), 1.25% (wt/vol) SDS, 10% (vol/vol) glycerol, 3.75% (vol/vol) mercaptoethanol, and 0.05% (wt/vol) bromphenol blue. The samples were then subjected to SDS-PAGE and Western blotting.
Treatment with RGD peptides
The ECM contains the integrin-recognition tripeptide RGD (Arg-Gly-Asp) sequence (46). To block specific integrin-ECM interactions, the cells were preincubated with the tetrapeptide RGDS (Arg-Gly-Asp-Ser; 500 μg/ml), which blocks cell adhesion through the RGD sequence on ECM proteins, for 2 h before seeding onto the Petri dishes precoated with fibronectin or poly-l-lysine (40 μg/ml).
DNA plasmids, siRNA, and transfection
DNA plasmids at a concentration of 1 μg/ml were transfected into MG63 cells at 60% confluence by using lipofectamine (Life Technologies, Inc.). The pSV-β-galactosidase plasmid was cotransfected to normalize the transfection efficiency. The cells were kept as static controls or treated with BMP-4 48 h after transfection. For siRNA transfection, MG63 cells at 70–80% confluence were transfected with the designated siRNA at various concentrations (10–40 nm) for 48 h using RNAiMAX transfection kit (Invitrogen) before the BMP-4 treatment.
Statistical analysis
Results are expressed as mean ± sem. Statistical analysis was performed by using an independent Student’s t test for two groups of data and ANOVA followed by Scheffe’s test for multiple comparisons. A P value < 0.05 was considered significant.
Footnotes
This work was supported by National Science Council (Taiwan) Grants 97-3112-B-400-006, 96-2628-B-400-002-MY3 (to J.-J.C.), 97-2627-B-010-002 (to C.-K.C.), and 95-2113-M-009-025 (to C.A.C.); and National Health Research Institutes (Taiwan) Grant ME-097-PP-06 (to J.-J.C.).
Disclosure Summary: The authors have no conflict of interest to disclose.
First Published Online October 9, 2009
Abbreviations: ALP, Alkaline phosphatase; BMP-4, bone morphogenetic protein-4; BMPR, BMP receptor; Cdk, cyclin-dependent protein kinase; ECM, extracellular matrix; FAK, focal adhesion kinase; FBS, fetal bovine serum; hFOB, human fetal osteoblast; mAb, monoclonal antibody; MEK, MAPK kinase; OCN, osteocalcin; pAb, polyclonal antibody; RGD, Arg-Gly-Asp; RGDS, Arg-Gly-Asp-Ser; SDS, sodium dodecyl sulfate; SH2, Src homology domain-2; siRNA, small interfering RNA; Smad, Sma- and Mad-related protein.
References
- 1.Urist MR1965. Bone: formation by autoinduction. Science 150:893–899 [DOI] [PubMed] [Google Scholar]
- 2.Luyten FP, Cunningham NS, Ma S, Muthukumaran N, Hammonds RG, Nevins WB, Woods WI, Reddi AH1989. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem 264:13377–13380 [PubMed] [Google Scholar]
- 3.Yamaguchi A, Komori T, Suda T2000. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev 21:393–411 [DOI] [PubMed] [Google Scholar]
- 4.Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K1999. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell 10:3801–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE1998. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haÿ E, Lemonnier J, Fromigué O, Guénou H, Marie PJ2004. Bone morphogenetic protein receptor IB signaling mediates apoptosis independently of differentiation in osteoblastic cells. J Biol Chem 279:1650–1658 [DOI] [PubMed] [Google Scholar]
- 7.Akiyama S, Katagiri T, Namiki M, Yamaji N, Yamamoto N, Miyama K, Shibuya H, Ueno N, Wozney JM, Suda T1997. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp Cell Res 235:362–369 [DOI] [PubMed] [Google Scholar]
- 8.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT2002. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res 17:101–110 [DOI] [PubMed] [Google Scholar]
- 9.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S2001. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 28:491–498 [DOI] [PubMed] [Google Scholar]
- 10.Lou J, Tu Y, Li S, Manske PR2000. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun 268:757–762 [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ1994. G1 phase progression: cycling on cue. Cell 79:551–555 [DOI] [PubMed] [Google Scholar]
- 12.Graña X, Reddy EP1995. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 11:211–219 [PubMed] [Google Scholar]
- 13.Zavitz KH, Zipursky SL1997. Controlling cell proliferation in differentiating tissues: genetic analysis of negative regulators of G1→S-phase progression. Curr Opin Cell Biol 9:773–781 [DOI] [PubMed] [Google Scholar]
- 14.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB1995. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267:1018–1021 [DOI] [PubMed] [Google Scholar]
- 15.Drissi H, Hushka D, Aslam F, Nguyen Q, Buffone E, Koff A, van Wijnen A, Lian JB, Stein JL, Stein GS1999. The cell cycle regulator p27kip1 contributes to growth and differentiation of osteoblasts. Cancer Res 59:3705–3711 [PubMed] [Google Scholar]
- 16.Hynes RO1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25 [DOI] [PubMed] [Google Scholar]
- 17.Chang SF, Chang CA, Lee DY, Lee PL, Yeh YM, Yeh CR, Cheng CK, Chien S, Chiu JJ2008. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc Natl Acad Sci USA 105:3927–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DY, Yeh CR, Chang SF, Lee PL, Chien S, Cheng CK, Chiu JJ2008. Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress: roles of extracellular matrix, Shc, and mitogen-activated protein kinase. J Bone Miner Res 23:1140–1149 [DOI] [PubMed] [Google Scholar]
- 19.Shyy JY, Chien S2002. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91:769–775 [DOI] [PubMed] [Google Scholar]
- 20.Schlaepfer DD, Hauck CR, Sieg DJ1999. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 71:435–478 [DOI] [PubMed] [Google Scholar]
- 21.Clover J, Dodds RA, Gowen M1992. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci 103:267–271 [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ1997. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res 12:1189–1197 [DOI] [PubMed] [Google Scholar]
- 23.Hughes DE, Salter DM, Dedhar S, Simpson R1993. Integrin expression in human bone. J Bone Miner Res 8:527–533 [DOI] [PubMed] [Google Scholar]
- 24.Lai CF, Cheng SL2005. αvβ Integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Miner Res 20:330–340 [DOI] [PubMed] [Google Scholar]
- 25.Gronowicz GA, Derome ME1994. Synthetic peptide containing Arg-Gly-Asp inhibits bone formation and resorption in a mineralizing organ culture system of fetal rat parietal bones. J Bone Miner Res 9:193–201 [DOI] [PubMed] [Google Scholar]
- 26.Moursi AM, Damsky CH, Lull J, Zimmerman D, Doty SB, Aota S, Globus RK1996. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci 109:1369–1380 [DOI] [PubMed] [Google Scholar]
- 27.Moursi AM, Globus RK, Damsky CH1997. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci 110:2187–2196 [DOI] [PubMed] [Google Scholar]
- 28.Wong GA, Tang V, El-Sabeawy F, Weiss RH2003. BMP-2 inhibits proliferation of human aortic smooth muscle cells via p21Cip1/Waf1. Am J Physiol Endocrinol Metab 284:E972–E979 [DOI] [PubMed]
- 29.Franzén A, Heldin NE2001. BMP-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1). Biochem Biophys Res Commun 285:773–781 [DOI] [PubMed] [Google Scholar]
- 30.Jeffery TK, Upton PD, Trembath RC, Morrell NW2005. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am J Physiol Lung Cell Mol Physiol 288:L370–L378 [DOI] [PubMed]
- 31.Tamura Y, Takeuchi Y, Suzawa M, Fukumoto S, Kato M, Miyazono K, Fujita T2001. Focal adhesion kinase activity is required for bone morphogenetic protein–Smad1 signaling and osteoblastic differentiation in murine MC3T3-E1 cells. J Bone Miner Res 16:1772–1779 [DOI] [PubMed] [Google Scholar]
- 32.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT1998. Role of the α2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem 273:32988–32994 [DOI] [PubMed] [Google Scholar]
- 33.Schneller M, Vuori K, Ruoslahti E1997. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J 16:5600–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider GB, Whitson SW, Cooper LF1999. Restricted and coordinated expression of β3-integrin and bone sialoprotein during cultured osteoblast differentiation. Bone 24:321–327 [DOI] [PubMed] [Google Scholar]
- 35.Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Canalis E2008. Activation of the ERK pathway in osteoblastic cells, role of gremlin and BMP-2. J Cell Biochem 104:1421–1426 [DOI] [PubMed] [Google Scholar]
- 36.Ge C, Xiao G, Jiang D, Franceschi RT2007. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol 176:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verheyen EM2007. Opposing effects of Wnt and MAPK on BMP/Smad signal duration. Dev Cell 13:755–756 [DOI] [PubMed] [Google Scholar]
- 38.Kretzschmar M, Doody J, Massagué J1997. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature 389:618–622 [DOI] [PubMed] [Google Scholar]
- 39.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT2000. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem 275:4453–4459 [DOI] [PubMed] [Google Scholar]
- 40.Franceschi RT1999. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med 10:40–57 [DOI] [PubMed] [Google Scholar]
- 41.Damsky CH1999. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 25:95–96 [DOI] [PubMed] [Google Scholar]
- 42.Chiu JJ, Wung BS, Hsieh HJ, Lo LW, Wang DL1999. Nitric oxide regulates shear stress-induced early growth response-1. Expression via the extracellular signal-regulated kinase pathway in endothelial cells. Circ Res 85:238–246 [DOI] [PubMed] [Google Scholar]
- 43.Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S2002. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA 99:3546–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY1999. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem 274:18393–18400 [DOI] [PubMed] [Google Scholar]
- 45.Chang WH, Chen LT, Sun JS, Lin FH2004. Effect of pulse-burst electromagnetic field stimulation on osteoblast cell activities. Bioelectromagnetics 25:457–465 [DOI] [PubMed] [Google Scholar]
- 46.Ruoslahti E1996. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715 [DOI] [PubMed] [Google Scholar]