Abstract
Retinoic acid (RA) signaling is mediated by the retinoic acid receptor (RAR), belonging to the nuclear hormone receptor superfamily. In addition to its classical transcriptional actions, RAR also mediates rapid transcription-independent (nongenomic) actions, consisting in the activation of signal transduction pathways, as the phosphatidyl-inositol-3-kinase or the ERK MAPK-signaling pathways. RA-induced rapid transcription-independent actions play a role in different physiological contexts. As an effort toward understanding the functions of those rapid actions on signaling elicited by RA, we have identified nuclear proteins the phosphorylation state of which is rapidly modified by RA treatment in neuroblastoma cells, using a proteomic approach. Our results show that RA treatment led to changes in the phosphorylation patterns in two families of proteins: 1) those related to chromatin dynamics in relation to transcriptional activation, and 2) those related to mRNA processing and, in particular, mRNA splicing. We show that treatment of neuroblastoma cells with RA leads to alteration of the regulation of pre-mRNA splicing and mRNA translation. Thus, our results underscore novel functions for the rapid signaling elicited by RAR in the regulation of mRNA processing. We conclude that RA activation of signaling pathways can indeed regulate mRNA processing as part of a cellular response orchestrated by the nuclear receptor RAR.
Treatment of neuroblastoma cells with RA leads to alteration of the regulation of premRNA splicing and mRNA translation, via the activation of signaling pathways.
Retinoic acid (RA), the biologically active form of vitamin A, is an important molecular signal for the early embryonic development and the differentiation of many cell types (1, 2, 3). RA induces the differentiation of neuroblastoma cells when added in vitro (4, 5), and a therapeutic effect for RA has been demonstrated in a multicenter clinical assay with high-risk neuroblastoma patients (6).
The actions of RA are mediated by two types of receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs), belonging to the nuclear hormone receptor superfamily, which encompasses receptors for steroid and thyroid hormones, vitamins A and D, and other signaling molecules. Members of this superfamily act as ligand-gated transcriptional regulators (7), although rapid, transcription-independent (also called nongenomic) actions on signal transduction pathways have also been reported (8). In addition to its well-characterized action as transcriptional regulator, RA administration to neuroblastoma cells activates a transcription-independent signaling response, which includes the rapid activation of important signaling pathways as the phosphatidyl-inositol-3-kinase (PI3K)/Akt and the ERK MAPK pathways (9, 10). The activation of PI3K by RA is required for neural differentiation of neuroblastoma cells, because blocking PI3K pathway with a specific inhibitor abolishes RA-induced differentiation of SH-SY5Y cells (9). As a first approach to understand the physiological role of the transcription-independent signaling response in the context of the induction of neural differentiation by RA, we wanted to identify the proteins that become rapidly phosphorylated as a consequence of RA treatment. We have restricted our study to nuclear proteins, because our interest is to confirm whether the rapid signaling response would play a role on the gene expression changes occurring during RA-induced differentiation. For this purpose, nuclear phosphoproteins from control and RA-treated neuroblastoma cells were enriched by affinity chromatography. The differences between the two phosphoprotein populations were investigated using comparative [mass spectrometry (MS) identification of differential spots in two-dimensional electrophoresis (2DE)-gels] and quantitative [isolated tags for relative and absolute quantification (iTRAQ) assay] proteomic approaches. The results obtained show that the changes in phosphorylation induced by RA occurred mainly in two families of nuclear proteins: 1) those related to chromatin dynamics involved in transcriptional regulation and 2) those related to mRNA processing and, in particular, mRNA splicing. The involvement of rapid signaling response in chromatin remodeling and transcriptional regulation of steroid receptor target genes was reported previously (11, 12, 13), and participation of rapid transcription-independent actions in nuclear receptor phosphorylation and cofactor recruitment has been recently reported also for the RAR (14). However, our results underscore novel functions for the rapid transcription-independent signaling elicited by RAR in the regulation of mRNA processing. Indeed, we show experimental evidence supporting that RA treatment of neuroblastoma cells results in alteration of the regulation of mRNA splicing and mRNA translation. In conclusion, we put forward the idea that RA, through the rapid activation of signaling pathways, could regulate mRNA processing, as part of a cellular response orchestrated by the nuclear receptor RAR.
Results
RA treatment of neuroblastoma cells results in activation of downstream components of the PI3K-signaling pathway
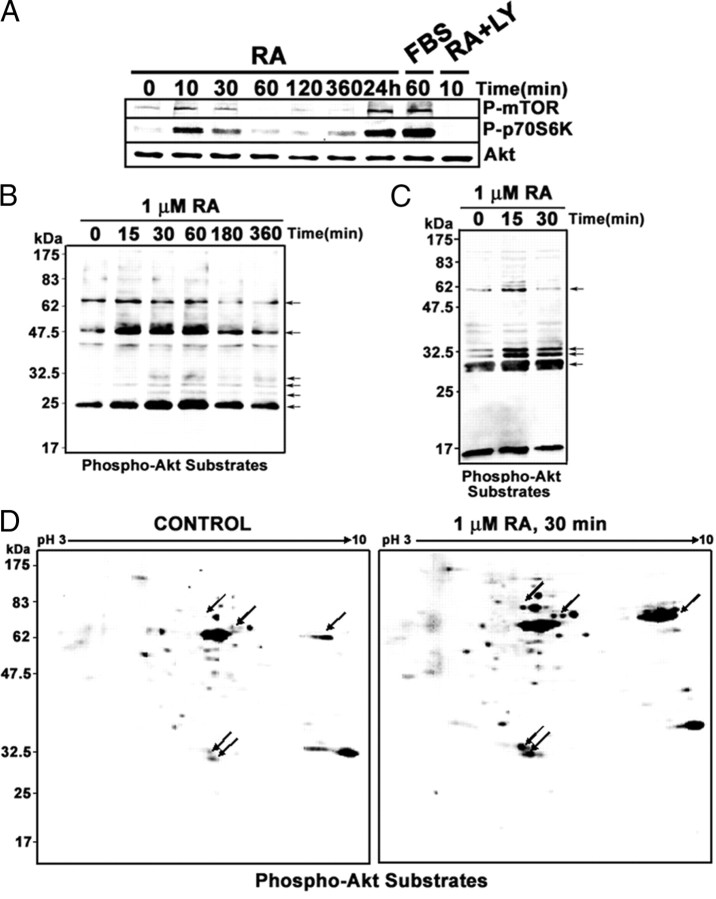
Treatment of SH-SY5Y neuroblastoma cells with RA in vitro results in the rapid activation of the PI3K/Akt signal transduction pathway (9, 10). Activation of PI3K/Akt pathway downstream targets is also rapidly induced by RA treatment, as shown in Fig. 1A. A rapid and transient activation of mammalian target of rapamycin (mTOR) and p70S6 kinases was detectable after 10–30 min of RA treatment. A second, long-term activation wave could be detected after 24 h of treatment. Activation levels obtained by RA treatment are comparable to those obtained by serum stimulation [30 min, 10% fetal bovine serum (FBS)] of neuroblastoma cells. Activation of mTOR and p70S6 kinases by RA appeared to be dependent on PI3K activation because it was abolished by simultaneous administration of RA and the PI3K inhibitor LY294002.
Fig. 1.
RA treatment of neuroblastoma cells results in activation of downstream components of the PI3K-signaling pathway. A, RA-induced activation of mTOR and p70S6 kinases. SH-SY5Y neuroblastoma cells were treated with RA (1 μm) for the times indicated, and total cell extracts were prepared. For comparison, we included an experiment in which cells were serum starved for 18 h and stimulated with 10% FBS for 30 min (lane labeled “FBS”). In addition, the extract from cells treated simultaneously with LY294002 (10 μm) and RA for 10 min was also included. The phosphorylation state of mTOR and p70S6 kinases was analyzed by Western blot with specific antibodies against the phosphorylated forms of the kinases (P-mTOR and P-p70S6). The filter was reprobed finally with antibodies against total Akt (AKT). Each lane contains 30 μg of total protein. B, RA-induced phosphorylation of Akt substrates. SH-SY5Y neuroblastoma cells were treated with RA (1 μm) for the times indicated, and total cell extracts were prepared. Each lane contains 30 μg protein from whole-cell extracts. The phosphorylation state of Akt substrates was analyzed by Western blot with specific antibodies against phosphorylated Akt recognition site. Arrows show several RA-induced bands. C, RA-induced phosphorylation of Akt substrates in the nucleus. SH-SY5Y neuroblastoma cells were treated with RA (1 μm) for the times indicated in the figure, and nuclear extracts were prepared. Each lane contains 30 μg protein from nuclear extracts. The phosphorylation state of Akt substrates was analyzed by Western blot with specific antibodies against phosphorylated Akt recognition site. Arrows show several RA-induced bands. D, RA-induced phosphorylation of nuclear Akt substrates in 2DE-Western blots. Each gel contains 30 μg nuclear protein from cells treated with RA (30 min, 1 μm) or vehicle (Control). The phosphorylation state of Akt substrates was analyzed by 2DE-Western blot with specific antibodies against phosphorylated Akt recognition site. Arrows show prominent spots the signal of which is increased by RA treatment. LY, LY294002.
Use of a specific antibody against phosphoserine/threonine residues within Akt kinase phosphorylation consensus site (RXRXXS/T) revealed that RA treatment increased rapidly the phosphorylation of several Akt substrate proteins, detectable in whole-cell extracts (Fig. 1B; proteins of approximately molecular mass 70, 48, and 24 kDa and minor bands at 25–30 kDa) as well as in nuclear extract (Fig. 1C; proteins of approximately molecular mass 60, 32, 30, and 27 kDa). 2-DE Western blots demonstrate that RA treatment leads to an increase in the number and intensity of the spots corresponding to phosphorylated Akt substrates in nuclear extracts from neuroblastoma cells. In addition, new spots with significant displacement toward the positive electrode could be detected for some proteins (Fig. 1D).
A proteomic approach to the identification of RA-induced phosphorylation changes in nuclear proteins
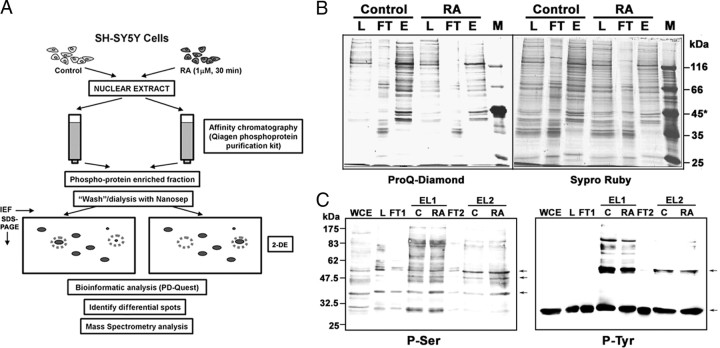
As an attempt to understand the functions of the rapid transcription-independent actions mediated by the RAR nuclear receptor in the context of the cellular response to RA, we wanted to identify the protein targets of the activated signaling pathways. By comparing the nuclear phosphoproteomes from control and RA-treated cells, we aimed to identify changes in the phosphorylation state of nuclear proteins elicited by RA, which could give us insight into the cellular function of the activated signaling pathways. We assume that the overall levels of the proteins remain unchanged during the short-term RA treatment, and therefore the changes in the levels of phosphoproteins will reflect changes in the stoichiometry of phosphorylation. The study was restricted to nuclear proteins because our interest was to confirm whether the rapid signaling response would play a role on the gene expression changes occurring during RA-induced differentiation. In addition, a reduction in the complexity of the protein samples would make the comparison easier.
The general strategy followed is depicted in Fig. 2A. Phosphoproteins from crude nuclear lysates from control and RA-treated (30 min, 1 μm) neuroblastoma cells were obtained with phosphoprotein columns (QIAGEN, Hilden, Germany; 2.5 mg nuclear protein as starting material). As shown in Fig. 2B, specific staining of phosphoproteins by ProQ Diamond stain (15) showed an enrichment of phosphoproteins in the eluate fraction, as compared with the total protein staining with SyproRuby. Similarly, Western blot with Phospho-Ser- and Phospho-Tyr-specific antibodies demonstrated the enrichment in proteins containing these phosphorylated amino acids in the eluted fractions (Fig. 2, C and D; lanes labeled “EL1”). In this experiment, the flow-through fraction from a first phosphoprotein purification column was loaded to a second column. As shown in the figure, most of the phosphorylated proteins were present in the eluate of the first column, and only a minimal fraction of phosphorylated proteins was captured in the second purification step, corresponding to apparently redundant protein species (Fig. 2, C and D; lanes labeled “EL2”). Therefore, a single-column enrichment protocol was used for the subsequent experiments.
Fig. 2.
Proteomic approach to the identification of RA-induced phosphorylation changes in nuclear proteins. A, General strategy. Neuroblastoma cells were treated with RA or vehicle for 30 min, and nuclear protein extracts were prepared. Phosphoprotein-enriched fractions were obtained by affinity chromatography, dialyzed with Nanosep spin columns, and loaded onto 2DE-gels (Isoelectrofocusing/SDS-PAGE). After electrophoresis, the gels were stained with CBB and compared with the help of a conventional bioinformatic package (Bio-Rad PD-Quest), and the differential spots were detected. The individual spots were isolated and digested with trypsin, and the proteins were identified by MS (peptide sequencing by CID-MS/MS and/or MALDI-TOF/TOF peptide fingerprinting). B, Enrichment in phosphoproteins shown by phosphoprotein-specific staining. The different fractions from the purification columns were run on SDS-PAGE gels, and the phosphoproteins were stained with ProQ-Diamond dye (left). After documentation, the gel was destained and total proteins were stained with SyproRuby (right). Total nuclear lysate (load, L), flow-through (FT), and phosphoprotein eluate (E) from control and RA-treated cells are shown (4 μg protein per lane). *, Molecular mass markers included ovalbumin (45 kDa, two phosphorylated residues). C, Enrichment in phosphoproteins shown by immunodetection of phosphorylated amino acid residues. The different fractions from the purification columns were run on SDS-PAGE gels, transferred to nitrocellulose, and incubated sequentially with specific antibodies against phosphoserine and phosphotyrosine. Whole-cell extract (WCE), nuclear lysate (load, L), flow-through (FT1), and phosphoprotein eluate [EL1, from Control (C) and RA-treated cells (RA)]. In this experiment the flow-through fractions from the affinity purification were loaded to a second phosphoprotein purification column and the flow-through fraction (FT2) and eluates [EL2, from Control (C), and RA-treated cells (RA) from this second column are shown]. Each lane contains 25 μg protein. For simplification only the WCE, L, FT1, and FT2 fractions from control cells are shown. The arrows point to protein bands that are present in the eluates from the second phosphoprotein purification column (lanes labeled “EL2”).
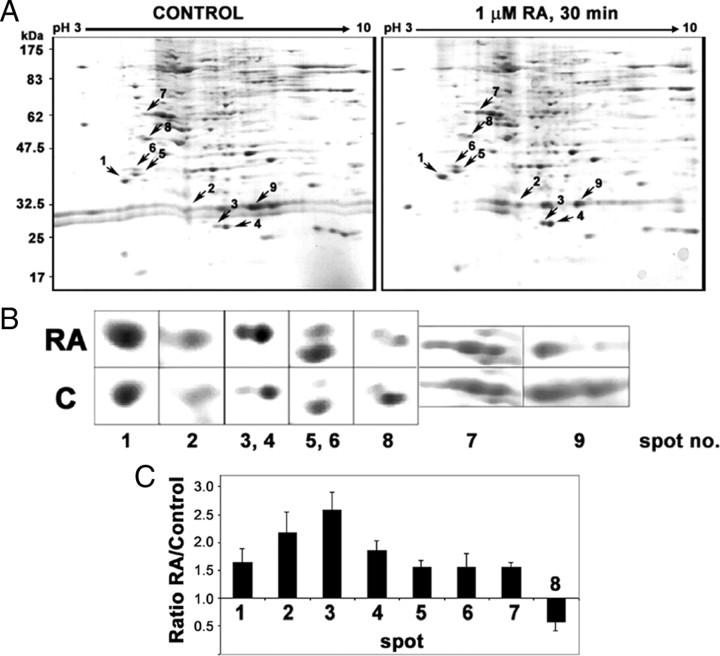
Phosphoprotein-enriched fractions from control cells and from cells treated with RA for 30 min were analyzed by 2DE, run with isoelectrofocusing (pH 3–10) as first dimension and SDS-PAGE (12.5%) as the second. Five pairs of 2DE-gels (control and RA-treated extracts from independent experiments) were run, stained with Coomassie brilliant blue (CBB), and compared with the aid of a software package. A representative gel pair is shown in Fig. 3A. Those spots showing consistently a robust change trend (RA-control ratio >1.5-fold for increased spots; <0.7-fold for decreased spots) in at least three of the five experiments were selected. In addition, those spots having a significant displacement toward the positive electrode were included. Nine spots were selected in total (seven of increased intensity, one showing displacement toward acidic pH, and only one the intensity of which decreased as the effect of RA treatment. The average values for fold change in intensity for the different spots are shown in Fig. 3C. After tryptic digestion, we have followed two MS-based protocols to identify the proteins present in each spot. First we have used nanoelectrospray ionization using a Q-Trap mass spectrometer, in which selected double- or triple-charged ions were fragmented [collision-induced dissociation-tandem MS (MS/MS)] and the sequences of the peptides deduced from the fragmentation spectra were obtained. The results are shown in Table 1. In addition, peptide fingerprints were obtained by matrix-assisted laser desorption ionization (MALDI)-TOF (time of flight)/TOF for some of the spots with a 4700 Proteomic Analyzer, and the identity of the protein was deduced with the MASCOT package (Table 2). All nine spots were identified.
Fig. 3.
Comparison of nuclear phosphoprotein patterns from control and RA-treated cells by 2DE. A, RA-induced changes in the patterns of nuclear phosphorylated proteins. Representative 2DE gel pair with nuclear phosphorylated protein samples from untreated (control) or RA-treated cells (1 μm, 30 min). Each gel was loaded with 125 μg nuclear phosphorylated protein. Isoelectrofocusing (pH 3–10) was run as first dimension and SDS-PAGE (12.5%) was run as second dimension. The gels were stained with CBB. Arrows mark the selected spots that appeared increased (1–7), decreased (8), or displaced toward lower pH (9) as a consequence of the RA treatment. B, Zoom-in detail of the regions including the selected differentially expressed spots. C, Quantitative analysis of the differentially expressed spots. Optical density was measured in five matched gel pairs from control and RA-treated nuclear proteins for the selected spots (1–8). After normalization for the total protein density, the mean and sd values were calculated for each of the spots and represented as relative value (ratio RA/control).
Table 1.
Identification of the differentially expressed spots by CID-MS/MS
| Spot no. | Identified protein | SwissProt ID | Sequenced peptides (position) | Match | E-value BLAST |
|---|---|---|---|---|---|
| 1 | Nucleophosmin | P06748 | DELHIVEAEAMNYEGSPIK (55–73) | 19/19 | 2e-11 |
| (NPM) | MSVQPTVSLGGFEITPPVVLR (81–101) | 21/21 | 1e-13 | ||
| 2 | Histone H1.5 | P16401 | ATGPPVSELITK (38–49) | 12/12 | 2e-04 |
| ALAAGGYDVEK (68–78) | 11/11 | 2e-03 | |||
| 3, 4 | HMG box 1 (HMGB1) | P09429 | RPPSAFFLFCSEYRPK (97–112) | 16/16 | 7e-10 |
| 5, 6 | hnRNP-C1/C2 | P07910 | GFAFVQYVNER (51–61) | 11/11 | 1e-04 |
| MIAGQVLDINLAAEPK (74–89) | 16/16 | 8e-09 | |||
| 7 | hnRNP-K | P61978 | ILSISADIETIGEILKK (87–103) | 17/17 | 2e-09 |
| IILDLISESPIK (208–219) | 12/12 | 5e-05 | |||
| NLPLPPPPPPR (306–316) | 11/11 | 1e-04 | |||
| 8 | Polyadenylate-binding protein-2 (PABP2) | Q86U42 | GFAYIEFSDKESVR (214–227) | 14/14 | 4e-07 |
| TSLALDESLFR (228–238) | 11/11 | 1e-03 |
ID, Identification.
Table 2.
Identification of the differentially expressed spots by MALDI-TOF/TOF-based peptide fingerprinting
| Spot no. | Identified protein | SwissProt ID | No. of different matched peptides | Coverage (%) | Score MASCOT |
|---|---|---|---|---|---|
| 2 | Histone H1.5 | P16401 | 2 | 12 | 70 |
| 3 | HMG box 1 (HMGB1) | P09429 | 13 | 67 | 368 |
| 5, 6 | hnRNP-C1/C2 | P07910 | 12 | 31 | 423 |
| 8 | Polyadenylate-binding protein-2 (PABP2) | Q86U42 | 7 | 32 | 71 |
| 9 | Histone H1.5 | P16401 | 11 | 41 | 316 |
ID, Identification.
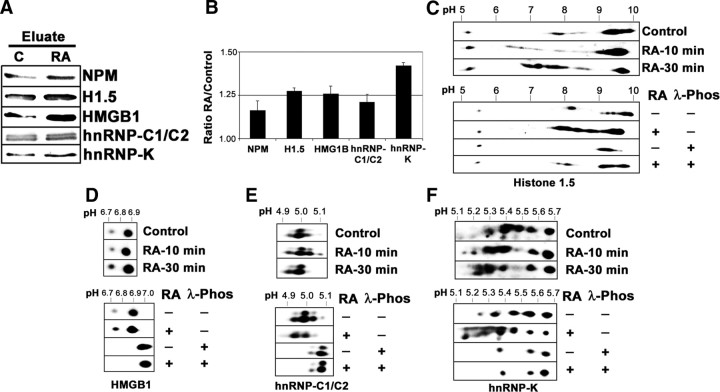
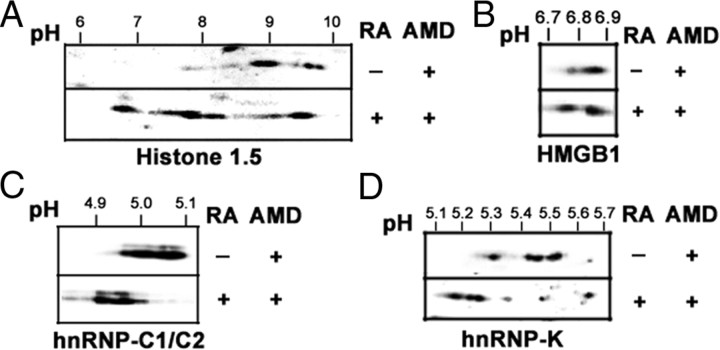
A first rapid validation experiment was performed by checking the levels of five of the identified proteins in the starting phosphoprotein-enriched extracts, through Western blot immunodetection. As shown in Fig. 4, A and B, all five proteins appeared increased to different extents in the nuclear phosphoprotein extract from RA-treated proteins. A second validation experiment was made using 2DE-Western blot immunodetection in unfractionated nuclear extracts. RA-induced phosphorylation was validated when the treatment induced a spot displacement toward acidic pH in 2DE-gels, which was abrogated by incubating the extract with an unspecific protein phosphatase such as λ-protein phosphatase. As shown in Fig. 4, C–F, four of the five proteins assayed [histone H1.5, high mobility group (HMG)B1, heterogenous nuclear ribonucleoprotein (hnRNP), C1/C2, and hnRNP K] fulfilled these criteria. In the case of histone H1.5, displacement was not completely reverted by the treatment with λ-phosphatase, suggesting that other posttranslational modifications could contribute to the strong pH displacement. The fifth protein assayed, nucleophosmin, could not be validated because the antibody used gave inconsistent patterns in 2DE-Western blot (data not shown). As part of the validation experiments, we wanted to test whether the RA-induced phosphorylation is a truly transcription-independent event. We have analyzed the effect of inhibiting transcription by actinomycin D (AMD) treatment on RA-induced protein phosphorylation on 2DE-Western blots. As shown in Fig. 5, AMD treatment did not affect RA-induced nuclear protein phosphorylation, and RA-induced spot displacement toward acidic pH was detected in all four proteins assayed, similarly to what was shown in the absence of the inhibitor in Fig. 4.
Fig. 4.
Validation of the obtained results. A, Representation of the identified proteins in the phosphoprotein eluates. Each lane contained 30 μg protein from the eluates from control and RA-treated (1 μm, 30 min) nuclear phosphoprotein fractions. Western blot membrane was incubated sequentially with specific antibodies against nucleophosmin (NPM), Histone H1.5, HMGB1, hnRNP-C1/C2, and hnRNP-K. B, Quantitative analysis of the experiment in panel A. The relative value (mean ± sd from three independent experiments) for the intensity of each band was shown. C, Validation of Histone H1.5 by 2DE-Western blot and phosphatase treatment. Nuclear proteins (25 μg) from control cells and cells treated with RA for 10 and 30 min, were run in 2DE-gels and transferred to nitrocellulose. Similarly, nuclear protein samples from control and RA-treated (1 μm, 30 min) cells, obtained in the absence of protein phosphatase inhibitors were submitted to digestion with 400 U λ-protein phosphatase (1 h, 30 C) or left untreated and were electrophoresed and transferred as above. The filters were incubated with specific antibodies for Histone H1.5. pH gradient in isoelectrofocusing was 3–10. D, Validation of HMGB1 by 2DE-Western blot and phosphatase treatment. Nuclear proteins (25 μg) from control cells and cells treated with RA for 10 and 30 min were run in 2DE-gels and transferred to nitrocellulose. Similarly, nuclear protein samples from control and RA-treated (1 μm, 30 min) cells, obtained in the absence of protein phosphatase inhibitors, were submitted to digestion with 400 U λ-protein phosphatase (1 h, 30 C) or left untreated and were electrophoresed and transferred as above. The filters were incubated with specific antibodies for HMGB1. pH gradient in isoelectrofocusing was 4–7. E, Validation of hnRNP-C1/C2 by 2DE-Western blot and phosphatase treatment. Nuclear proteins (25 μg) from control cells and cells treated with RA for 10 and 30 min, were run in 2DE-gels and transferred to nitrocellulose. Similarly, nuclear protein samples from control and RA-treated (1 μm, 30 min) cells, obtained in the absence of protein phosphatase inhibitors, were submitted to digestion with 400 U λ-protein phosphatase (1 h, 30 C) or left untreated and were electrophoresed and transferred as above. The filters were incubated with specific antibodies for hnRNP-C1/C2. pH gradient in isoelectrofocusing was 4–7. F, Validation of hnRNP-K by 2DE-Western blot and phosphatase treatment. Nuclear proteins (25 μg) from control cells and cells treated with RA for 10 and 30 min were run in 2DE-gels and transferred to nitrocellulose. Similarly, nuclear protein samples from control and RA-treated (1 μm, 30 min) cells, obtained in the absence of protein phosphatase inhibitors, were submitted to digestion with 400 U λ-protein phosphatase (1 h, 30 C) or left untreated and were electrophoresed and transferred as above. The filters were incubated with specific antibodies for HMGB1. pH gradient in isoelectrofocusing was 4–7. Phos, Phosphatase.
Fig. 5.
RA-induced changes in nuclear protein phosphorylation are transcription independent. SH-SY5Y neuroblastoma cells were pretreated with 1 μg/ml AMD for 30 min before treatment with 1 μm RA for 30 min in the presence of the inhibitor. As control, cells were treated with AMD alone. Nuclear proteins (25 μg) from AMD-treated cells and cells treated with RA for 30 min in the presence of the inhibitor were run in 2DE-gels and transferred to nitrocellulose. The filters were incubated with specific antibodies for Histone H1.5 (panel A), HMGB1 (panel B), hnRNP-C1/C2 (panel C) and hnRNP-K (panel D). pH gradient in isoelectrofocusing was 3–10 for histone H1.5 and 4–7 for HMGB1, hnRNP-C1/C2, and hnRNP-K.
The experiments show that the strategy of phosphoproteome comparison employed was useful to identify nuclear proteins the phosphorylation state of which was modified by RA treatment. Among the identified proteins we could find proteins related to chromatin, such as histone H1.5 or HMGB1. In addition, RNA-binding proteins, such as nucleophosmin, hnRNP C1/C2, hnRNP K, and PABP2 were also identified. However, the number of identified protein species is low, probably because the protocol relies on 2DE-gels stained with CBB, resulting in low-sensitivity protein detection. In addition, known 2DE drawbacks, such as the difficulty in resolving large proteins and too acidic or too basic proteins (16), could show more influence in our experiment, because of the nature of nuclear proteins.
Identification of RA-induced changes in phosphorylation of nuclear proteins by iTRAQ assay
One possibility for extending and improving our study would be to use a 2DE-independent method, having higher throughput, which would allow for comparison of nuclear phosphoproteomes and render quantitative results, such as the iTRAQ system (isolated tags for relative and absolute quantification (17); for recent reviews see Refs. 18, 19, 20). For this purpose we have used a strategy in which the enriched phosphoprotein fractions of neuroblastoma cells treated with RA for 15 and 30 min or left untreated were digested with trypsin and labeled independently with three of the iTRAQ reporter reagents. The three samples were combined and submitted to multidimensional nanoliquid chromatography (LC). The 12 peptide-containing fractions obtained from the first-dimension LC were submitted to the second-dimension LC, and the selected fractions were analyzed by tandem MS. The spectra obtained were used to identify and quantify proteins, using the Protein Pilot version 2.0 software.
We performed three independent iTRAQ assays, with phosphoproteins obtained from independent experiments. Approximately 150 different protein species could be identified from the MS spectra (with confidence >95%). For each experiment the identified proteins reported as having a statistically significant fold change value with respect to the untreated control (with P value < 0.05) were considered, and only those showing the same change trend in at least two of the three experiments were selected, with a total number of 63 different protein species. From these 63 different proteins, the phosphorylation of 36 of them was increased as the effect of RA treatments and the other 26 were dephosphorylated by the treatment. A protein belongs to the two groups because it was detected as decreased at 15 min treatment and increased at 30 min treatment. A complete relation of these proteins and their relative fold change values is shown in Tables 3 and 4.
Table 3.
Proteins identified in the iTRAQ assay as increased phosphorylation as effect of RA treatment (15 and 30 min)
| Name | Entry name | Accession | Ratio RA/Control | ||
|---|---|---|---|---|---|
| 15 min | 30 min | ||||
| Chromobox protein homolog 1 (HP1 β) | CBX1_HUMAN | P83916 | 1.2995 | 1.5161 | |
| Chromobox protein homolog 3 (HP1 γ) | CBX3_HUMAN | Q13185 | 1.0964 | 1.1999 | |
| Chromobox protein homolog 5 (HP1 α) | CBX5_HUMAN | P45973 | 1.3438 | ||
| hnRNP A/B | ROAA_HUMAN | Q99729 | 1.1215 | 1.3776 | |
| hnRNP A1 | ROA1_HUMAN | P09651 | 1.2672 | ||
| hnRNP A3 | ROA3_HUMAN | P51991 | 1.1674 | ||
| hnRNP D0 | HNRPD_HUMAN | Q14103 | 1.0895 | 1.3848 | |
| hnRNP G | HNRPG_HUMAN | P38159 | 1.3330 | ||
| hnRNP K | HNRPK_HUMAN | P61978 | 1.3181 | ||
| hnRNP Q | HNRPQ_HUMAN | O60506 | 1.7531 | ||
| hnRNP A2 /B11 | ROA2_HUMAN | P22626 | 0.8863 | 1.1729 | |
| HMG protein B1 (HMGB1) | HMGB1_HUMAN | P09429 | 1.6989 | 2.1734 | |
| HMG protein B2 (HMGB2) | HMGB2_HUMAN | P26583 | 1.5223 | 2.0522 | |
| HMG protein HMG-I/HMG-Y (HMGA1) | HMGA1_HUMAN | P17096 | 1.2226 | 1.2954 | |
| SF, arginine/serine-rich 1, SF2/ASF (SFRS1) | SFRS1_HUMAN | Q07955 | 1.2281 | ||
| SF, arginine/serine-rich 2, SC35 (SFSR2) | SFRS2_HUMAN | Q01130 | 1.2591 | ||
| SF, arginine/serine-rich 3, SRp20 (SFRS3) | SFRS3_HUMAN | P84103 | 1.3765 | ||
| SF, arginine/serine-rich 7, 9G8 (SFRS7) | SFRS7_HUMAN | Q16629 | 1.2293 | ||
| SF, arginine/serine-rich 9, SRp30C (SFRS9) | SFRS9_HUMAN | Q13242 | 1.1152 | 1.2155 | |
| Arsenite-resistance protein 2 (ARS2) | ARS2_HUMAN | Q9BXP5 | 1.1642 | ||
| Barrier-to-autointegration factor (BAF) | BAF_HUMAN | O75531 | 1.4720 | ||
| Complement component 1 Q | C1QBP_HUMAN | Q07021 | 1.2706 | 1.1944 | |
| Drebrin | DREB_HUMAN | Q16643 | 1.5005 | ||
| Nuclear protein Hcc-1 | HCC1_HUMAN | P82979 | 1.3270 | ||
| Nucleolin (C23) | NUCL_HUMAN | P19338 | 1.2772 | 1.3237 | |
| Nucleophosmin | NPM_HUMAN | P06748 | 1.1112 | 1.3664 | |
| HRP-3-Hepatoma-derived growth factor-related protein 3 | HDGR3_HUMAN | Q9Y3E1 | 1.1239 | 1.4624 | |
| PC4 and SFRS1-interacting protein | PSIP1_HUMAN | O75475 | 1.1608 | 1.4093 | |
| Protein DEK | DEK_HUMAN | P35659 | 1.1637 | 1.3236 | |
| Protein RED | RED_HUMAN | Q13123 | 1.2171 | 1.2655 | |
| Protein SET | SET_HUMAN | Q01105 | 1.3899 | 1.5030 | |
| RNA-binding protein 8A | RBM8A_HUMAN | Q9Y5S9 | 1.2135 | 1.3512 | |
| Scaffold attachment factor B1 (SAFB1) | SAFB1_HUMAN | Q15424 | 1.1379 | ||
| Splicing factor U2AF2 | U2AF2_HUMAN | P26368 | 1.2017 | ||
| Tropomyosin (1, 2, 3, or 4) | TPM3_HUMAN | P06753 | 1.1642 | ||
| U1 snRNP 70 kDa | RU17_HUMAN | P08621 | 1.4720 | ||
| U4/U6.U5 tri-snRNP-associated protein 1 (SART1) | SNUT1_HUMAN | O43290 | 1.2706 | 1.1944 | |
The name, the SWISS-PROT entry name and accession and the average values obtained for ratio RA/Control after 15 and 30 min RA treatment are shown. Only statistically significant values are shown.
hnRNP A2 /B1 is shown because it appears as decreased after 15 min and increased after 30 min RA treatment.
Table 4.
Proteins identified in the iTRAQ assay as decreased phosphorylation as effect of RA treatment (15 and 30 min)
| Name | Entry name | Accession | Ratio RA/control | ||
|---|---|---|---|---|---|
| 15 min | 30 min | ||||
| ATP-dependent DNA helicase 2 subunit 1 (70 kDa subunit) (Ku70) | KU70_HUMAN | P12956 | 0.7945 | ||
| ATP-dependent DNA helicase 2 subunit 2 (80 kDa subunit) (Ku80) | KU86_HUMAN | P13010 | 0.7280 | ||
| ATP-dependent RNA helicase A (DHX9) | DHX9_HUMAN | Q08211 | 0.8278 | 0.7545 | |
| ATP-dependent RNA helicase DDX39 | DDX39_HUMAN | O00148 | 0.8219 | ||
| Probable ATP-dependent RNA helicase DDX5 | DDX5_HUMAN | P17844 | 0.7272 | ||
| Spliceosome RNA helicase BAT1 | UAP56_HUMAN | Q13838 | 0.8312 | ||
| Nucleolar RNA helicase 2 | DDX21_HUMAN | Q9NR30 | 0.8583 | 0.7812 | |
| hnRNP U | HNRPU_HUMAN | Q00839 | 0.9095 | ||
| hnRNP U-like protein 1 | HNRL1_HUMAN | Q9BUJ2 | 0.7133 | ||
| hnRNP A2 /B11 | ROA2_HUMAN | P22626 | 0.8863 | 1.1729 | |
| 60S ribosomal protein L22 | RL22_HUMAN | P35268 | 0.7105 | ||
| Bromodomain adjacent to zinc finger domain protein 1B | BAZ1B_HUMAN | Q9UIG0 | 0.7057 | ||
| General transcription factor II-I (GTFII-I, TFII-I) | GTF2I_HUMAN | P78347 | 0.8136 | ||
| Histone H3 (1, 2, or 3) | H32_HUMAN | Q71DI3 | 0.7324 | ||
| Histone H4 | H4_HUMAN | P62805 | 0.6955 | ||
| Histone-binding protein RBBP4 | RBBP4_HUMAN | Q09028 | 0.8323 | ||
| Lamin-B1 | LMNB1_HUMAN | P20700 | 0.8128 | ||
| Non-POU domain-containing octamer-binding protein | NONO_HUMAN | Q15233 | 0.7489 | ||
| Nucleolar protein 5 (NOP5) | NOL5_HUMAN | Q9Y2 × 3 | 0.7909 | ||
| Nuclear mitotic apparatus protein 1 (NUMA1) | NUMA1_HUMAN | Q14980 | 0.7394 | ||
| SF, proline- and glutamine-rich (SFPQ) | SFPQ_HUMAN | P23246 | 0.8739 | ||
| Protein RRP5 homolog | RRP5_HUMAN | Q14690 | 0.6680 | ||
| RNA-binding protein Raly | RALY_HUMAN | Q9UKM9 | 0.8642 | ||
| SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 5 (SMARCA5) | SMCA5_HUMAN | O60264 | 0.6833 | ||
| Tubulin α−chain (A, B, or C) | TBA1A_HUMAN | Q71U36 | 0.8116 | ||
| U5 snRNP 200 kDa helicase (U5-200KD) | U520_HUMAN | O75643 | 0.8612 | ||
| Ubiquitin | UBIQ_HUMAN | P62988 | 0.8675 | 0.8993 | |
The name, the SWISS-PROT entry name and accession, and the average values obtained for ratio RA/Control after 15 and 30 min RA treatment are shown. Only statistically significant values are shown.
hnRNP A2 /B1 is shown because it appears as decreased after 15 min and increased after 30 min RA treatment.
In addition, the analysis detected 32 phosphorylated peptides, eight of them corresponding to new phosphorylation sites previously not reported in the databases (Table 5). However, only two phosphopeptides could be identified as induced by the RA treatment with enough statistical significance from the analysis of individual phosphopeptides, corresponding to phosphorylated Ser residues in hnRNP-A1 (Ser6) and nucleophosmin (Ser125). (Table 6).
Table 5.
Phosphorylation sites detected not present in the PhosphoSitePlus database (http://www.phosphosite.org) or Uniprot Protein KB (www.uniprot.org)
| Accession | Protein | Phospho-residue | Peptide sequence |
|---|---|---|---|
| Q9UIG0 | Bromodomain adjacent to zinc finger domain protein 1B (BAZ1B) | Thr661 | VLVILLQ(Pho)TLLQDEIAEDYGELGMK |
| Q14839 | Chromodomain helicase-DNA-binding protein 4 (CHD-4) | Thr242/Thr244 | GSSGASVAAAAAAAVAVVESMV(Pho)TA(Pho)TEVAPPPPPVEVP |
| P61978 | hnRNP K | Ser127 | IIPTLEEGLQLPSPTATSQLPLE(Pho)SDAVECLNYQHYK |
| Q6FI13 | Histone H2A type 2-A (H2A2A) | Thr60 | VGAGAPVYMAAVLEYL(Pho)TAEILELAGNAAR |
| GAPVYMAAVLEYL(Pho)TAEILELAGNAAR | |||
| Q8IUE6 | Histone H2A type 2-B (H2A2B) | Thr60 | VGAGAPVYLAAVLEYL(Pho)TAEILELAGNAAR |
| Q15424 | Scaffold attachment factor B1 (SAFB1) | Thr340 | TDCEPVGLEPAVEQSSAASELAEASSEELAEAP(Pho)TEAPSPEAR |
| Q14151 | Scaffold attachment factor B2 (SAFB2) | Thr339 | TDCEPVGLEPAVEQSSAASELAEASSEELAEAP(Pho)TEAPSPEAR |
Table 6.
Peptides detected as increased in phosphorylation as consequence of RA treatment with confidence >95%
| Protein | Accession | Peptide sequence | Residue | N | RA-15 min | RA-30 min | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | |||||||
| hnRNP-A1 | P09651 | SE(Pho)SPKEPEQLR | Ser6 | 12 | 1.37 | 0.15 | 1.53 | 0.23 | ||
| NPM | P06748 | CGSGPVHISGQHLVAVEEDAE(Pho)SEDEEEEDVK | Ser125 | 10 | 1.21 | 0.24 | 1.39 | 0.28 | ||
N represents the number of times that the peptide was identified in independent MS spectra.
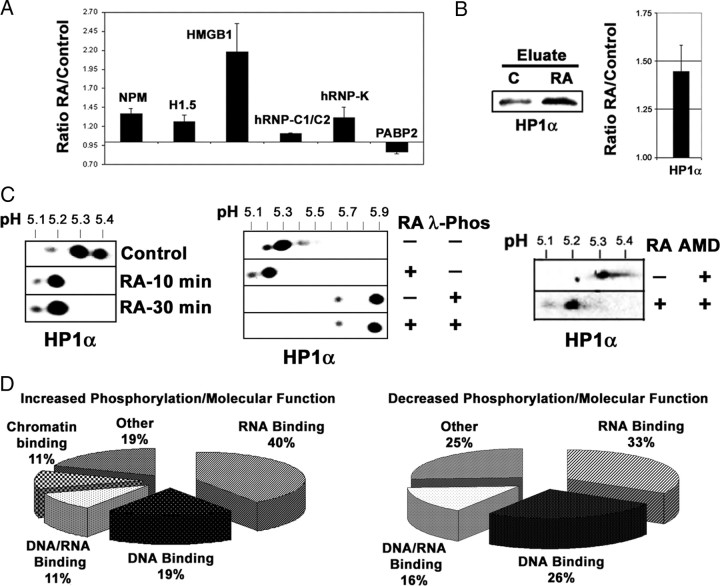
For comparison, the results obtained in the iTRAQ assay for the six proteins identified in the 2DE-gels are shown in Fig. 6A. All of them show the same change trend as in the 2DE-based experiment, although the quantitative values differ. In addition, a validation experiment made with the same criteria as shown for Figs. 4 and 5 is shown for HP1α, one of the prominent proteins the phosphorylation of which appeared increased by RA in the iTRAQ assay (Fig. 6, B and C).
Fig. 6.
Quantitative phosphoprotein comparison by iTRAQ analysis. A, Quantitative analysis by iTRAQ of the proteins identified by 2DE. The values obtained for change ratio (RA, 30 min/control) in mean ± sd from three independent iTRAQ assays was represented for the proteins identified with the 2DE-gel-based approach. In comparison, the change trend is the same for all proteins, but the fold change values obtained are lower for H1.5, hnRNP-C1/C2, and PABP2. For PABP2 the value taken was the one at 15 min RA treatment, because at 30 min RA treatment the value obtained was statistically not significant. B, Representation of HP1α protein in the phosphoprotein eluates. Each lane contained 30 μg protein from the eluates from control and RA-treated (1 μm, 30 min) nuclear phosphoprotein fractions. Western blot membrane was incubated with specific antibodies against HP1α. A quantitative analysis of the experiment is shown. The relative value (mean ± sd from three independent experiments) for the ratio of intensity of the band in the RA-treated eluate against that appearing in the control eluate is shown. C, Validation of HP1α by 2DE-Western blot, phosphatase treatment, and AMD treatment. Nuclear proteins (25 μg) from control cells and cells treated with RA for 10 and 30 min were run in 2DE-gels and transferred to nitrocellulose. Similarly, nuclear protein samples from control and RA-treated (1 μm, 30 min) cells, obtained in the absence of protein phosphatase inhibitors, were submitted to digestion with 400 U λ-protein phosphatase (1 h, 30 C) or left untreated and were electrophoresed and transferred as above. RA-induced phosphorylation of HP1α is transcription independent. Finally, nuclear proteins (25 μg) from AMD-treated cells and cells treated with RA for 30 min in the presence of the inhibitor were run in 2DE-gels and transferred to nitrocellulose. The filters were incubated with specific antibodies for HP1α. pH gradient in isoelectrofocusing was 4–7. D, Functional annotation of the differentially expressed phosphoproteins (increased in RA-treated, left; decreased in RA-treated, right) according to molecular function. Phos, Phosphatase.
A close inspection of the protein list shows again a majority of proteins related to chromatin dynamics and especially to RNA processing in a wide sense [RNA-binding proteins, hnRNPs, splicing factors (SFs) RNA helicases, etc.]. The presence of multiple RNA/DNA helicases among the dephosphorylated proteins appeared at least striking. To try to find the biological significance of our crude data, we have used the application FatiGO, included in the Babelomics suite (21) to group the proteins by biological process and molecular function. Among the proteins with increased phosphorylation by RA treatment, 40% belong to RNA binding (GO4), 19% to DNA binding (GO4), 11% to both DNA and RNA binding, and 11% to chromatin binding (GO3); 19% were assigned to other categories. For those the phosphorylation of which was reduced by RA treatment, 33% belong to RNA binding (GO4), 26% to DNA binding (GO4), 15% to RNA/DNA binding, and 26% assigned to other categories; 38% belong to the category ATPase activity (GO8) (Fig. 6D). With respect to biological process, the proteins the phosphorylation of which is increased as a consequence of RA treatment, 68% belong to RNA metabolism (GO5), 41% to RNA splicing (GO7), 27% to regulation of transcription (GO7), and 13% to RNA splicing, via transesterification reactions with bulged adenosine as nucleophile (GO9). Of those proteins with decreased phosphorylation as an effect of RA treatment, 63% belong to RNA metabolism (GO5), 38% to regulation of transcription (GO7), 31% to RNA splicing (GO7), and 15% to RNA splicing, via transesterification reactions with bulged adenosine as nucleophile (GO9).
An effect of rapid signaling actions on the transcriptional activity of nuclear receptors has been reported for steroid hormones, acting especially at the level of chromatin remodeling, suggesting a convergence between transcriptional and transcription-independent actions of the receptor (11, 12, 13). Recently, an effect of rapidly activated pathways in RARα phosphorylation and cofactor recruitment, resulting in increased transcriptional activation, has also been reported (14). In addition, our results underscore the involvement of nuclear proteins engaged in mRNA processing in a wide sense (splicing, transport, translational regulation, etc.) in the cellular response to RA. Therefore we propose as hypothesis that RA, through the rapid activation of signaling pathways, could regulate mRNA processing, as part of a cellular response orchestrated by the nuclear receptor RAR.
RA treatment influences the regulation of alternative splicing and mRNA translation via activation of signaling pathways
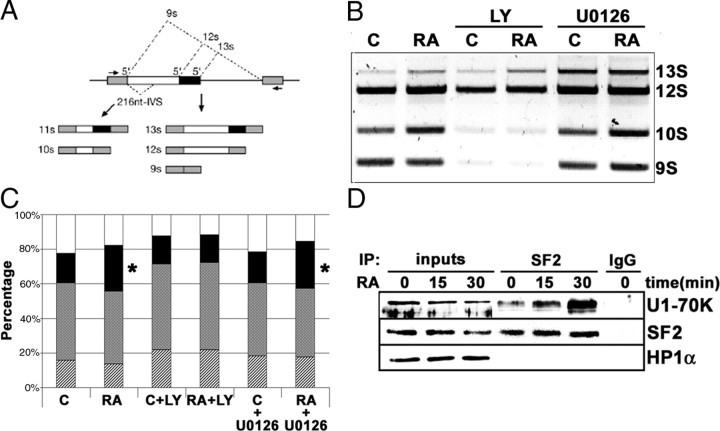
We first examined whether RA treatment of neuroblastoma cells had any effect on the regulation of mRNA splice site selection in vivo using the adenoviral E1A minigene splicing reporter (22). This reporter generates a splicing substrate containing competing splice sites allowing changes in splice site usage to be detected from the relative amounts of the different mRNA isoforms (Fig. 7A). After transfection of this reporter in neuroblastoma cells, the mRNA was analyzed by RT-PCR. The results showed that RA treatment altered the relative amounts of the different spliced mRNA forms (Fig. 7, B and C). A remarkable increase in the percentage of the 10S isoform occurs as consequence of RA treatment (from 16.94 ± 1.08% to 26.51 ± 0.87%). Treatment of the cells simultaneously with RA and the specific PI3K inhibitor LY294002 abolished this change (16.23 ± 2.36% and 15.93 ± 3.65%, for control and RA-treated cells in the presence of the inhibitor, respectively). However, RA-induced increase in the isoform 10S is not affected by inhibition of the ERK MAPK pathway by the specific MAP-ERK kinase (MEK) inhibitor U0126 (17.79 ± 1.51% and 27.01 ± 2.29% for control and RA-treated cells in the presence of the inhibitor) (Fig. 7, B and C). The amount of 10S mRNA isoform produced in RA-treated cells and RA+U0126-treated cells show statistically significant differences with the control, whereas all the other treatment showed no differences (P = 0.002 in ANOVA plus Bonferroni post hoc test). Therefore, we can conclude that RA treatment to neuroblastoma cells altered the regulation of splice site selection, through PI3K-dependent but ERK MAPK-independent mechanisms. Among the proteins the phosphorylation of which is affected by RA treatment, we could find the serine/arginine-rich (SR) protein family, which are important regulators of mRNA splicing (23). Splice site efficiency and specificity are determined by critical protein-protein interactions among pre-mRNA-binding proteins (24). Binding of U1 small nuclear ribonucleoprotein (snRNP)-70K protein to the 5′-splice site appears to be facilitated by serine/arginine-rich (SR) proteins bound to specific sequences of the pre-mRNA (23). Moreover, the interaction between SF2/ASF (alternate splicing factor) and U1-70K protein is enhanced by phosphorylation of the SR protein (25). To test whether RA treatment could influence the interactions between SF2/ASF and U1-70K protein, we performed an immunoprecipitation experiment in SH-SY5Y neuroblastoma cells. As shown in Fig. 7D, RA treatment rapidly increased binding of SF2/ASF to U1-70K protein.
Fig. 7.
RA treatment influences the regulation of alternative mRNA splicing via activation of signaling pathways. A, Schematic representation of the alternative splicing forms in the E1A minigene reporter and the RT-PCR fragments obtained from the different mRNA isoforms generated. [Adapted from Ref. 40 ]. B, Effect of RA treatment on the regulation of splice site selection. After transfection of the E1A minigene reporter, neuroblastoma cells were treated with 1 μm RA (24 h), plus LY294002 (10 μm) or U0126 (10 μm) as indicated. Total RNA was obtained, and the different splicing variants were detected by RT-PCR. After electrophoresis in an agarose gel, the bands were stained with ethidium bromide. C, Control cells; RA, RA-treated cells; LY, cells treated with the PI3K inhibitor LY294002 (10 μm); U0126, cells treated with the MEK inhibitor U0126 (10 μm). C, Quantitative analysis of the experiment shown in Fig. 6B. The relative percentages of the different mRNA isoforms generated by alternative splicing and the effect of the different treatments are shown. The graph includes the data from three independent experiments. Stippled bars, 13S; shadowed bars; 12 S; solid bars, 10S; open bars, 9S. Asterisks on the 10S isoform in RA and RA+U0126 samples indicate statistically significant differences with respect to control (P = 0.002). All the other samples were not statistically different from control (ANOVA plus Bonferroni post hoc test). D, RA treatment enhanced the interaction between SF2/ASF and the U1 snRNP-70K protein. Nuclear extracts were prepared from neuroblastoma cells treated with 1 μm RA for 0, 15, and 30 min. About 200 μg of nuclear proteins was incubated with 4 μg of SF2/ASF monoclonal antibody (lanes labeled “SF2”). As control, a parallel reaction was set with 4 μg of an unrelated antibody (lane labeled “IgG”). Nuclear extracts (25 μg) from cells treated with 1 μm RA for the times indicated in the figure were included for comparison (lanes labeled “inputs”). Immunocomplexes were precipitated using TrueBlot antimouse IgG beads (eBioscience), following the manufacturer’s instructions, and suspended in sample buffer containing freshly added 50 mm DTT. After Western blot, the filter was developed with anti-U1 snRNP-70K protein (U1-70K), using horseradish peroxidase-conjugated rabbit TrueBlot secondary antibody (eBioscience). The filter was reprobed additionally with anti-SF2/ASF monoclonal antibody (SF2), using horseradish peroxidase-conjugated rabbit TrueBlot secondary antibody (eBioscience). As specificity control, the filter was reprobed with an unrelated anti-HP1α antibody (HP1 α). IP, Immunoprecipitation; LY, LY294002.
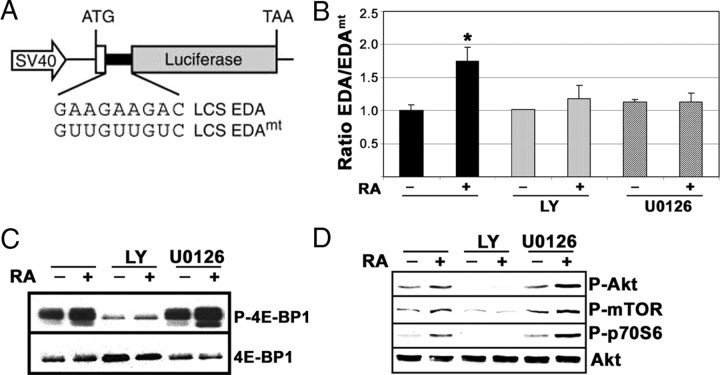
In addition to the actions of SR proteins in splicing, several postsplicing activities have been described for a subset of shuttling SR proteins, including regulation of mRNA export and translation (26, 27). To analyze a possible influence of the activated signaling pathways on SR protein-dependent translation, we used a translation reporter based on the fibronectin gene. The reporter contained a luciferase coding sequence (LCS) harboring the exonic splicing enhancer (ESE) as present in the fibronectin extradomain A (EDA’s ESE; pLCS-EDA) or a mutant version of this ESE (pLCS-EDAmt) that does not bind the SR protein SF2/ASF (Fig. 8A and Ref. 26). After transfection to neuroblastoma cells, the relative activity from pLCS-EDA over that obtained from pLCS-EDAmt in a parallel experiment was measured. The results obtained showed that RA treatment produced a modest but reproducible increase in the translation of the reporter (∼1.7-fold). This stimulation could be abrogated both by an inhibitor of the PI3K/Akt pathway as well as by an inhibitor of the ERK-MAPK pathway (Fig. 8B). The results suggest that RA treatment may affect the regulation of translation of specific mRNAs through specific phosphorylation of members of the SR protein family. It has been recently reported that the SR protein SF2/ASF promotes translation initiation by enhancing the phosphorylation of eIF4E-BP1, a competitive inhibitor of cap-dependent translation, and therefore suppressing its activity (28). Western blot immunodetection with a phospho-specific 4E-BP1 antibody showed a RA-induced increase in the phosphorylation of 4E-BP1, which could be abolished by simultaneous treatment with the PI3K inhibitor LY294002, but not by treatment with the inhibitor of the ERK MAPK pathway U0126 (Fig. 8C). The effect of these inhibitors on the activation of the PI3K/Akt signaling pathway by RA has been analyzed. As expected, LY294002 abolished the activation of Akt, mTOR, and p70S6 kinases, as demonstrated by Western blot with antibodies against the phosphorylated forms of the kinases. However, treatment with U0126 did not affect the activation of the three components of the PI3K/Akt pathway by RA (Fig. 8D). Therefore, despite the good correlation between mTOR activity and the phosphorylation of 4E-BP1, as reported (28), additional mechanisms depending on the activity of the ERK MAPK pathway appear to contribute to the establishment of a RA-dependent mRNA translation control.
Fig. 8.
RA treatment, through the activation of signaling pathways, influences the regulation of mRNA translation. A, Schematic representation of the reporter genes used for the analysis of mRNA translation regulation. [Adapted from Ref. 26 .]. B, The effect of the RA treatment on the translation of the LCS-EDA luciferase reporters. Neuroblastoma cells were transfected in parallel reactions with either the LCS-EDA or the LCS-EDAmt luciferase reporters, together with a promoterless Renilla luciferase reporter as internal control. After transfection, neuroblastoma cells were treated with 1 μm RA in the presence of LY294002 (10 μm) or U0126 (10 μm) as indicated. The ratio between the activities of the LCS-EDA and the LCS-EDAmt reporters, normalized for the efficiency of the transfection, is represented for control cells and cells treated with RA (1 μm, 24 h) alone (solid bars) or in the presence of the inhibitors LY294002 (10 μm, light gray bars) and U0126 (10 μm, dark gray bars) as indicated. Asterisk on RA-treated sample indicates a statistically significant difference with respect to control (P = 0.004). All the other samples were not statistically different from control. (ANOVA plus Bonferroni post hoc test). C, RA-induced changes in the phosphorylation of eIF4E-BP1. Cells were treated with RA (1 μm, 15 min) alone or in the presence of the inhibitors LY294002 (10 μm) and U0126 (10 μm) as indicated, and cell extracts were prepared. The phosphorylation state of 4E-BP1 was analyzed by Western blot with specific antibodies against the phosphorylated form of the protein (P-4E-BP1). The filter was reprobed finally with antibodies against total 4E-BP1 (4E-BP1). Each lane contains 30 μg of total protein. D, Effect of PI3K and MEK inhibitors in the activation of mTOR and p70S6 kinases by RA. Cells were treated with RA (1 μm, 15 min) alone or in the presence of the inhibitors LY294002 (10 μm) and U0126 (10 μm) as indicated, and whole-cell extracts were prepared. The phosphorylation state of Akt, mTOR, and p70S6 kinases was analyzed by Western blot with specific antibodies against the phosphorylated forms of the kinases (P-Akt, P-mTOR, and P-p70S6). The filter was reprobed finally with antibodies against total Akt (Akt). Each lane contains 30 μg of total protein. LY, LY294002.
Discussion
The members of the nuclear hormone receptor superfamily are involved in the transduction of the signals elicited by different hormones and signaling molecules that have profound effects on the physiology of the cells and contribute to crucial aspects of differentiation, development, and homeostasis in higher organisms. Nuclear hormone receptors are known to act through two different types of molecular actions, classified as transcriptional (genomic) and transcription independent (nongenomic). Genomic actions involve ligand-activated receptors bound to specific DNA elements in the promoter of their target genes, resulting in the recruitment of coactivators and other factors that promote chromatin remodeling and ultimately lead to transcriptional activation. Transcription-independent actions consist in the rapid and transient activation of kinase cascades through mechanisms involving protein-protein interactions between a subpopulation of nuclear receptors and components of the signal transduction pathway. In the case of RA we have previously shown that a subpopulation of the nuclear receptor RAR interacts with p85, the regulatory subunit of PI3K, and that this complex is located at the plasma membrane after ligand activation (10).
Although it appears logical that both sides of the response must integrate to achieve a coherent physiological cellular response to a certain signal, little information is available on the convergence of transcriptional and transcription-independent actions in gene regulation by nuclear hormone receptors. To address that point, we have identified proteins that become rapidly phosphorylated as a consequence of RA treatment in neuroblastoma cells. Because we wanted to confirm whether the rapid signaling response would play a role on the gene expression changes occurring during RA-induced differentiation, our study was restricted to nuclear proteins. By comparing the nuclear phosphoproteomes from untreated neuroblastoma cells and cells submitted to a short RA treatment, we could identify nuclear proteins that are target for the RA transcription-independent actions. The results obtained show that RA-induced phosphorylation changes occurred mainly in two families of nuclear proteins: those related to chromatin dynamics involved in transcriptional regulation and those related to mRNA processing and in particular mRNA splicing.
The involvement of rapid signaling response in chromatin remodeling and transcriptional regulation of steroid receptor target genes has been reported (11, 12, 13), and participation of transcription-independent actions in nuclear receptor phosphorylation and cofactor recruitment has been recently shown also for the RAR (14). In good agreement with this idea, chromatin-binding proteins involved in remodeling and transcriptional regulation constitute an important part of the nuclear proteins the phosphorylation of which is affected by RA. Special mention deserves the HP1 proteins, chromatin transcriptional repressors whose phosphorylation was increased by RA treatments. Transcriptional activation of the MMTV promoter by steroid receptors results in displacement of HP1γ, through a mechanism involving phosphorylation of serine 10 of histone H3, mediated by transcription-independent activation of members of the ERK MAPK pathway (11, 12). Phosphorylation of HP1 proteins impairs its repression activity (29), and therefore our results suggest that phosphorylation of HP1 proteins through rapid signaling actions of RAR could also contribute to the establishment of active chromatin and ultimately to gene activation.
The presence of a considerable number of RNA-binding proteins involved in mRNA processing and, in particular, mRNA splicing among those whose phosphorylation was modified by RA treatment uncovered the involvement of nuclear proteins engaged in mRNA processing in a wide sense (splicing, transport, translational regulation, etc.) in the cellular response to RA. Therefore we proposed as hypothesis that RA, through the rapid transcription-independent activation of signaling pathways, could regulate mRNA processing as part of a cellular response elicited by the nuclear receptor RAR. Consequently, we have found that RA treatments, through the activation of signaling pathways, regulate splice site selection in a simple assay as the E1A minigene splicing reporter. Splice site efficiency and specificity are determined by critical protein-protein interactions among pre-mRNA-binding proteins (24). Among the proteins the phosphorylation of which is affected by RA treatment, we could find members of the serine/arginine-rich (SR) protein family, which are important regulators of mRNA splicing (23). Binding of U1 snRNP-70K protein to the 5′-splice site appears to be facilitated by SR proteins bound to specific sequences of the pre-mRNA (23). We show that RA treatment promotes interactions among SFs such as SF2/ASF and U1-snRNP-70K protein, interactions that appear to rely on the phosphorylation of SF2/ASF (25). Much evidence supports the idea that signaling pathways could regulate alternative splicing, by phosphorylating RNA-binding proteins that are constituents of the spliceosome and/or function as splicing regulatory factors (30, 31, 32). This mechanism appears to overlap with the cotranscriptional coupling of RNA synthesis and splicing, previously reported for steroid receptors (33, 34, 35, 36). Nevertheless, cotranscriptional splicing regulation relies on transcriptional coactivators bound to nuclear hormone receptors, therefore acting only on receptor target genes (33, 34, 35, 36).
Translational control is an important strategy by which eukaryotic cells regulate gene expression. Translation is the last step in the genetic information flow, and regulation at this level allows a rapid response to different regulatory signals. We have also explored the possibility that RA-induced rapid signaling also affects specific mRNA translational efficiency. Several postsplicing activities have been described for a subset of shuttling SR proteins, including regulation of mRNA export and translation (26, 27). By using a translation reporter based on the fibronectin gene including binding sites for the SR factor SF2/ASF (26), we could show that RA treatment produced an increase in the translation of this reporter. This stimulation appeared to be dependant both on the activity of PI3K/Akt as well as the ERK-MAPK pathways. The results suggest that through specific phosphorylation of members of the SR protein family, RA treatment may affect the regulation of the translation of specific mRNAs. It has been recently reported that the SR protein SF2/ASF promotes translation initiation by enhancing the phosphorylation of eIF4E-BP1, a competitive inhibitor of cap-dependent translation, and therefore suppressing its activity (28). We have shown that RA treatment induced an increase in the phosphorylation of 4E-BP1 that could be abolished by inhibition of PI3K but not by inhibition of the ERK MAPK pathway. Therefore, despite the good correlation between mTOR activity and the phosphorylation of 4E-BP1, as reported elsewhere (28), additional mechanisms depending on the activity of the ERK MAPK pathway (37) appear to contribute to the establishment of a RA-dependent mRNA translation control. Translational control by RA is involved in important physiological aspects of the neuronal activity, as is synaptic plasticity. RA increases dendritic growth in hippocampal neurons by mechanisms related to that described here, in which a membrane-associated RARα regulates translation of specific mRNAs by activation of the ERK and mTOR pathways (38, 39).
The results shown here contribute to the idea that transcriptional and transcription-independent actions elicited by nuclear hormone receptors are integrated and converge at multiple levels in the regulation of gene expression. At the level of transcription, transcriptional actions elicited by promoter-bound receptors are potentiated by transcription-independent actions acting at the level of chromatin remodeling and cofactor recruitment, in which phosphorylation of the receptor appears to play an important role (11, 12, 13, 14). At the level of splicing, coactivator-mediated cotranscriptional regulation, which depends on DNA-bound receptors (33, 34, 35, 36), could converge with the signaling-dependent regulation of splicing described here, which relies on the phosphorylation of pre-mRNA-bound SFs. Finally, convergence at the gene expression level could involve the specific regulation of mRNA translation, depending on signaling events initiated by the receptor through its rapid transcription-independent actions.
Materials and Methods
Cell culture and treatments
SH-SY5Y human neuroblastoma cells (American Type Culture Collection no. CRL-2266; Manassas, VA) were cultured in DMEM with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell cultures were kept in a humidified incubator at 37 C with 5% CO2. The medium was replaced every 3 d, and the cells were split before they reached confluence. LY294002, U0126, AMD, and all-trans-retinoic acid were purchased from Sigma (St. Louis, MO). The different compounds were dissolved in dimethylsulfoxide and added to the culture medium at the indicated concentrations.
Nuclei preparation
Phosphate buffer was avoided through the procedure, because phosphate ions interfere with subsequent phosphoprotein affinity chromatography. Cells were washed and suspended with ice-cold TBS (10 mm Tris-HCl, pH 7.4; 150 mm NaCl). After centrifugation, cells were resuspended in 15 mm Tris-HCl (pH 7.4), 60 mm KCl, 0.15 mm spermine, 0.5 mm spermidine, 2 mm EDTA, 1 mm dithiothreitol (DTT), 0.5 m Sucrose, 0.1 mm phenylmethylsulfonyl fluoride, 1 mm NaF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mm sodium orthovanadate). Cell suspension was mixed with one volume of the same buffer containing 0.2% Nonidet P-40 and incubated for 10–15 min on ice. Nuclei were pelleted by centrifugation at 800 × g for 10 min at 4 C and washed with the same buffer without detergent. The nuclei were repelleted, and the nuclear pellets were stored at −80 C.
Protein extracts, SDS-PAGE, and Western blot
Whole-cell extracts were obtained by lysis of the cells in lysis buffer (50 mm Tris-HCl, pH 7.4; 150 mm NaCl) containing 1% Nonidet P-40, protease (1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) and phosphatase inhibitors (1 mm sodium orthovanadate, 1 mm NaF). After 30 min incubation on ice, the lysate was cleared by centrifugation (16,100 × g, 10 min, 4 C), and protein concentration was determined using detergent-compatible Bio-Rad DC Protein assay (Bio-Rad Laboratories, Inc., Hercules, CA).
Total nuclear extracts were obtained by lysis of the crude nuclear fraction in lysis buffer as above. SDS-PAGE and Western blot analysis of proteins were performed using standard methods as described elsewhere (9). 2DE-Western blot was performed similarly after 2DE (isoelectrofocusing/SDS-PAGE) (see below). Antibodies against Akt 1/2, hnRNP-C1/C2 hnRNP-K, Nucleophosmin SF2/ASF, U1 snRNP-70K, and HP1α were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against phospho-mTOR (Ser2448), phospho-p70S6 kinase (Thr389), phospho-4E-BP1 (Thr37/46), phospho-S/T Akt substrates, and 4E-BP1 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against phosphotyrosine (4G10) were from Upstate Biotechnology, Inc. (Lake Placid, NY). Antibodies against histone H1.5 and HMGB1 were obtained from Abcam PLC (Cambridge, UK), and those against phosphoserine were obtained from QIAGEN. Horseradish peroxidase-conjugated secondary antibodies were obtained from GE Healthcare (Piscataway, NJ) and Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Chemiluminescent signals were developed with ECL or ECL Plus (GE Healthcare) and images were captured with a LAS3000 Imager (Fuji Film Europe GmBH, Düsseldorf, Germany). Staining of SDS-PAGE gels with phosphoprotein-specific ProQ-Diamond dye, destaining, and subsequent restaining with Sypro Ruby dye was performed as recommended by the manufacturers (Molecular Probes, Inc., Eugene, OR; and Invitrogen, Carlsbad, CA). Detection of the fluorescent bands was made using a FLA5000 scanner with excitation lasers of 532 and 473 nm (Fuji Film).
Immunoprecipitation
Immunoprecipitation was carried out with the TrueBlot immunoprecipitation system (eBioscience, San Diego, CA), following the instructions of the manufacturer. For immunoprecipitation, approximately 200 μg protein from nuclear extracts prepared as above was incubated with 25 μl of mouse TrueBlot IgG beads for 30 min at 4 C in 500 μl of lysis buffer. After centrifugation, the precleared lysate was incubated with 4 μg anti-SF2/ASF monoclonal antibody during 1 h at 4 C. As specificity control, parallel reactions with an unrelated antibody were set. After addition of 50 μl of mouse TrueBlot IgG beads, the mixture was incubated under rotation during 16 h at 4 C. The immunocomplexes were recovered by centrifugation, washed three times with lysis buffer and resuspended in sample buffer containing freshly added 50 mm DTT. Western blot analysis of the immunocomplexes was carried out with primary antibodies against U1-snRNP-70K, SF2/ASF and HP1α, and horseradish peroxidase-conjugated TrueBlot secondary antibodies (eBioscience).
Phosphoprotein enrichment by affinity chromatography
Phosphoprotein-enriched fraction from nuclear proteins was obtained by affinity chromatography with the phosphoprotein purification kit (QIAGEN), following the instructions of the manufacturer. Benzonase was used at twice the recommended concentration because of the high amount of nucleic acids present in the cell nucleus. For each preparation 2.5 mg of nuclear proteins was used. After running the column, protein-containing fractions were desalted and concentrated with Nanosep 10K ultrafiltration spin columns (Pall Corp., Port Washington, NY).
2DE
For 2DE, 125 μg of nuclear phosphoprotein fractions was precipitated with 100% acetone and resuspended in 2DE-sample buffer (8 m urea, 4% 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate, 32 mm DTT, 0.002% bromophenol blue, and 2% of the corresponding ampholytes). 2DE was performed with isoelectrofocusing as first dimension and denaturing SDS-PAGE (12.5%) as second following standard protocols. Immobilized pH gradient strips (Immobiline DryStryp, 7 cm; GE Healthcare) for pH 3–10 or 4–7 were used following the recommendation of the manufacturer. 2DE gels were stained with CBB (SimplyBlue, Invitrogen), and scanned. Matched gel pairs from samples from RA-treated or untreated cells were compared with the PD-Quest package (Bio-Rad). For 2DE-Western blots 25–30 μg of total nuclear extract was electrophoresed as above and transferred to nitrocellulose membranes. For validation experiments, 25 μg of total nuclear protein extract lysed as above but omitting phosphatase inhibitors, was incubated with 400 U λ-protein phosphatase (New England Biolabs, Beverly, MA) in 50 mm Tris-HCl (pH 7.5), 100 mm NaCl, 2 mm MnCl2, 2 mm DTT, 0.1 mm EGTA, 0.01% Brij 35 during 60 min at 30 C. Finally proteins were precipitated with acetone and electrophoresed as indicated above.
Protein identification by MS
Relevant spots were manually extracted from the gel and reduced with DTT, alkylated with iodacetamide, and digested with trypsin (Roche Applied Science, Mannheim, Germany). The peptide mix was dried under vacuum and suspended in 50% acetonitrile, 0.1% trifluoroacetic acid (TFA). For peptide fingerprinting by MALDI-TOF, the sample was analyzed in a 4700 Proteomic analyzer MALDI-TOF/TOF (Applied Biosystems, Foster City, CA). Peptide fingerprint was identified using the MASCOT software (Matrix Science, Boston, MA). For peptide sequencing by collision-induced dissociation-MS/MS, peptide sample is ionized by nanoelectrospray, using a Q-Trap spectrometer (Applied Biosystems). Selected double- or triple-charged ions were fragmented, and the sequences of the peptides were obtained by manual interpretation of the fragmentation spectra.
iTRAQ assay
Phosphoprotein eluates obtained from nuclear extracts of untreated cells or cells treated with RA (1 μm) for 15 and 30 min were desalted by chloroform/methanol precipitation. Samples (100 μg of protein) were reduced with phosphine and cysteins blocked with methyl methanethiosulfonate. After trypsin digestion, the peptide mixtures were labeled with iTRAQ labeling reagents, following the instructions of the supplier. Reporter of mass 114, 116, and 117 were used for control, RA-15 min, and RA-30 min samples, respectively. Equal amounts of each sample were mixed and dried. Samples were resuspended in buffer A (20 mm triethanolamine in water) and loaded onto a Gemini 3 μ C18 110A 150- × 4-mm column (Phenomenex, Torrance, CA) equilibrated in the same buffer. Elution was performed with a 5–45% linear gradient of buffer B (20 mm triethanolamine in acetonitrile) of 60 min at a flow rate of 150 μl/min. Twelve fractions were collected, acidified with 10% TFA, and dried by centrifugation in vacuum. Each fraction was resuspended in 18 μl of 5% acetonitrile, 0.1% TFA, and 5 μl of the sample was loaded onto a trap column (PepMap C18, 300 μm × 5 mm; LC Packings, Amsterdam, The Netherlands) and desalted with 0.1% TFA at 40 μl/min during 5 min. The peptides were then loaded onto an analytical column (PepMap C18 3 μ 100A, 75 μm × 15 cm, LC Packings) equilibrated in 0.1% formic acid. Elution was carried out with a linear gradient 25–50% of solvent B (95% acetonitrile, 0.1% formic Acid) in 120 min at a flow rate of 300 nl/min. The eluted peptides were analyzed with a nano-ESI-Q-TOF mass spectrometer (QSTAR-XL; Applied Biosystems) in an information-dependent acquisition mode. MS/MS data were analyzed using ProteinPilot version 2.0 (Applied Biosystems), which uses the Paragon algorithm to perform database matching for protein identification and comparative quantification, and Progroup algorithm for protein grouping to remove redundant hits. The Swiss-Prot Homo sapiens protein database was used for all searches. All reported data were based on more than 95% confidence for protein identification as determined by ProteinPilot. For relative quantification, only those changes having a statistically significant change at P < 0.05 were considered.
Transfections, RNA extraction, RT-PCR, and luciferase assay
The E1A minigene splicing reporter plasmid (40) was transfected to SH-SY5Y neuroblastoma cells using Escort V transfection reagent (Sigma), following the recommendations of the supplier. Plasmid DNA (4 μg) was used for 60 mm plate. FBS (1%) was added to the cells 3–4 h after transfection. Cells were pretreated, 24 h after transfection, for 1 h with inhibitors or vehicle as indicated after which RA was added for 24 h in the presence of the inhibitors. Total RNA was isolated using QuickGene RNA cultured cell HC kit S (Fuji Film), and contaminating DNA was digested with RQ DNase I (Promega Corp., Madison, WI). cDNA was prepared by reverse transcription using oligo-deoxythymidine16 primer and Moloney murine leukemia virus reverse transcriptase (Promega) with 1 μg of RNA template in 25 μl reaction volume. An aliquot (2.5 μl) was then used for PCR (25 μl) with Taq DNA polymerase (Biotools, Madrid, Spain). The E1A splice forms were detected by PCR using E1A primers (E1A forward: 5′-GTTTTCTCCTCCGAGCCGCTCCGA; and E1A reverse: 5′-CTCAGGCTCAGGTTCAGACACAGG), with the following PCR steps: one cycle of 95 C for 5 min; 24 cycles of 95 C for 30 sec, 62 C for 20 sec, and 72 C for 40 sec; and one cycle of 72 C for 10 min. PCR products were separated on a 2% agarose gel, stained with ethidium bromide, documented, and quantified. The mean and sd values for each isoform were calculated from three independent experiments.
Reporter plasmids pLCS-EDAwt and pLCS-EDAmt (26) and promoterless Renilla luciferase (pGL4.70, Promega) were transfected to SH-SY5Y neuroblastoma using Lipofectamine 2000 reagent (Invitrogen), following the recommendations of the supplier. The corresponding firefly luciferase reporter (4 μg) was cotransfected with 1 μg of the Renilla reporter for each dish of a six-well microplate. FBS (1%) was added to the cells 3–4 h after transfection. cells were pretreated, 24 h after transfection, for 1 h with inhibitors or vehicle as indicated after which RA was added for 24 h in the presence of the inhibitors. Luciferase activities in cell lysates were measured using the Dual-Luciferase Reporter Assay System (Promega), following the instructions of the manufacturer. Firefly luciferase activity values were normalized for Renilla luciferase values, and the results are expressed as mean ± sd from three independent experiments performed in duplicate.
Acknowledgments
We thank Dr. G. Michlewski and Dr. J. F. Cáceres (Medical Research Council Human Genetics Unit, Edinburgh, Scotland, UK) for their generous help with the splicing and translation experiments and their comments on the manuscript; we also thank J. M. Escamilla [Instituto de Biomedicina de Valencia (IBV)] for technical assistance, A. Fernández (IBV) for the gift of Renilla luciferase plasmid, and Dr. J. Valcárcel and Dr. F. Gebauer (Centre de Regulació Genómica, Barcelona, Spain) for comments and suggestions.
NURSA Molecule Pages:
Ligands: all-trans-Retinoic acid.
Footnotes
This work was supported by grants of the Spanish former Ministry of Science and Technology and Ministry of Education and Science (SAF2003-00311, SAF2006-00647 and SAF2007-60780) and Generalitat Valenciana (ACOMP 06/212) (to D.B). E.J.L. was the recipient of a predoctoral fellowship/contract (FPI) from Consellería de Educación y Ciencia de la Generalitat Valenciana (Spain). The Proteomics Unit of the Centro de Investigación Príncipe Felipe is a member of the ProteoRed network (Spain).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 7, 2009
Abbreviations: AMD, Actinomycin D; CBB, Coomassie brilliant blue; 2DE: two-dimensional electrophoresis; DTT, dithiothreitol; ECL: enhanced chemiluminiscence; EDA: fibronectin extra domain A; ESE, exonic splicing enhancer; FBS, fetal bovine serum; HMG, high-mobility group; hnRNP, heterogenous nuclear ribonucleoprotein; iTRAQ, isolated tags for relative and absolute quantification; LC, liquid chromatography; LCS, luciferase coding sequence; MALDI, matrix-assisted laser desorption ionization; MEK, MAP-ERK kinase; MS, mass spectrometry; MS/MS, tandem MS; mTOR, mammalian target of rapamycin; PI3K, phosphatidyl-inositol-3 kinase; RA, retinoic acid; RAR, retinoic acid receptor; RXR, retinoid X receptor; SF, splicing factor; snRNP, small nuclear ribonucleoprotein; SR, serine/arginine-rich proteins; TFA, trifluoroacetic acid; TOF, time of flight.
References
- 1.Duester G2008. Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maden M2007. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8:755–765 [DOI] [PubMed] [Google Scholar]
- 3.Appel B, Eisen JS2003. Retinoids run rampant: multiple roles during spinal cord and motor neuron development. Neuron 40:461–464 [DOI] [PubMed] [Google Scholar]
- 4.Sidell N1982. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst 68:589–596 [PubMed] [Google Scholar]
- 5.Påhlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T1984. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbol ester-induced differentiation. Cell Differ 14:135–144 [DOI] [PubMed] [Google Scholar]
- 6.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP1999. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 341:1165–1173 [DOI] [PubMed] [Google Scholar]
- 7.Gronemeyer H, Gustafsson JA, Laudet V2004. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3:950–964 [DOI] [PubMed] [Google Scholar]
- 8.Lösel R, Wehling M2003. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4:46–56 [DOI] [PubMed] [Google Scholar]
- 9.López-Carballo G, Moreno L, Masiá S, Pérez P, Barettino D2002. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem 277:25297–25304 [DOI] [PubMed] [Google Scholar]
- 10.Masiá S, Alvarez S, de Lera AR, Barettino D2007. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol 21:2391–2402 [DOI] [PubMed] [Google Scholar]
- 11.Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M2006. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell 24:367–381 [DOI] [PubMed] [Google Scholar]
- 12.Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M2008. Convergence on chromatin of non-genomic and genomic pathways of hormone signaling. J Steroid Biochem Mol Biol 109:344–349 [DOI] [PubMed] [Google Scholar]
- 13.Quiles I, Millán-Ariño L, Subtil-Rodríguez A, Miñana B, Spinedi N, Ballaré C, Beato M, Jordan A2009. Mutational analysis of progesterone receptor functional domains in stable cell lines delineates sets of genes regulated by different mechanisms. Mol Endocrinol 23:809–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de Thé H, Rochette-Egly C2009. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARα to target promoters. EMBO J 28:34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin K, Steinberg TH, Goodman T, Schulenberg B, Kilgore JA, Gee KR, Beechem JM, Patton WF2003. Strategies and solid-phase formats for the analysis of protein and peptide phosphorylation employing a novel fluorescent phosphorylation sensor dye. Comb Chem High Throughput Screen 6:331–339 [DOI] [PubMed] [Google Scholar]
- 16.Rabilloud T2002. Two-dimensional gel electrophoresis in proteomics: old, old fashioned, but it still climbs up the mountains. Proteomics 2:3–10 [PubMed] [Google Scholar]
- 17.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ2004. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3:1154–1169 [DOI] [PubMed] [Google Scholar]
- 18.Wu WW, Wang G, Baek SJ, Shen RF2006. Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J Proteome Res 5:651–658 [DOI] [PubMed] [Google Scholar]
- 19.Schreiber TB, Mäusbacher N, Breitkopf SB, Grundner-Culemann K, Daub H2008. Quantitative phosphoproteomics—an emerging key technology in signal-transduction research. Proteomics 8:4416–4432 [DOI] [PubMed] [Google Scholar]
- 20.Paradela A, Albar JP2008. Advances in the analysis of protein phosphorylation. J Proteome Res 7:1809–1818 [DOI] [PubMed] [Google Scholar]
- 21.Al-Shahrour F, Carbonell J, Minguez P, Goetz S, Conesa A, Tárraga J, Medina I, Alloza E, Montaner D, Dopazo J2008. Babelomics: advanced functional profiling of transcriptomics, proteomics and genomics experiments. Nucleic Acids Res 36:W341–W346 [DOI] [PMC free article] [PubMed]
- 22.Cáceres JF, Stamm S, Helfman DM, Krainer AR1994. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science 265:1706–1709 [DOI] [PubMed] [Google Scholar]
- 23.Long JC, Caceres JF2009. The SR protein family of splicing factors: master regulators of gene expression. Biochem J 417:15–27 [DOI] [PubMed] [Google Scholar]
- 24.Smith CW, Valcárcel J2000. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem Sci 25:381–388 [DOI] [PubMed] [Google Scholar]
- 25.Xiao SH, Manley JL1997. Phosphorylation of the ASF/SF2 RS domain affects both protein-protein and protein-RNA interactions and is necessary for splicing. Genes Dev 11:334–344 [DOI] [PubMed] [Google Scholar]
- 26.Sanford JR, Gray NK, Beckmann K, Cáceres JF2004. A novel role for shuttling SR proteins in mRNA translation. Genes Dev 18:755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaustein M, Pelisch F, Tanos T, Muñoz MJ, Wengier D, Quadrana L, Sanford JR, Muschietti JP, Kornblihtt AR, Cáceres JF, Coso OA, Srebrow A2005. Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat Struct Mol Biol 12:1037–1044 [DOI] [PubMed] [Google Scholar]
- 28.Michlewski G, Sanford JR, Cáceres JF2008. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell 30:179–189 [DOI] [PubMed] [Google Scholar]
- 29.Lomberk G, Bensi D, Fernandez-Zapico ME, Urrutia R2006. Evidence for the existence of an HP1-mediated subcode within the histone code. Nat Cell Biol 8:407–415 [DOI] [PubMed] [Google Scholar]
- 30.Tarn WY2007. Cellular signals modulate alternative splicing. J Biomed Sci 14:517–522 [DOI] [PubMed] [Google Scholar]
- 31.Stamm S2008. Regulation of alternative splicing by reversible protein phosphorylation. J Biol Chem 283:1223–1227 [DOI] [PubMed] [Google Scholar]
- 32.Shin C, Manley JL2004. Cell signalling and the control of pre-mRNA splicing. Nat Rev Mol Cell Biol 5:727–738 [DOI] [PubMed] [Google Scholar]
- 33.Auboeuf D, Batsché E, Dutertre M, Muchardt C, O'Malley BW2007. Coregulators: transducing signal from transcription to alternative splicing. Trends Endocrinol Metab 18:122–129 [DOI] [PubMed] [Google Scholar]
- 34.Clark EL, Fuller-Pace FV, Elliott DJ, Robson CN2008. Coupling transcription to RNA processing via the p68 DEAD box RNA helicase androgen receptor co-activator in prostate cancer. Biochem Soc Trans 36:546–547 [DOI] [PubMed] [Google Scholar]
- 35.Auboeuf D, Dowhan DH, Dutertre M, Martin N, Berget SM, O'Malley BW2005. A subset of nuclear receptor coregulators act as coupling proteins during synthesis and maturation of RNA transcripts. Mol Cell Biol 25:5307–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monsalve M, Wu Z, Adelmant G, Puigserver P, Fan M, Spiegelman BM2000. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol Cell 6:307–316 [DOI] [PubMed] [Google Scholar]
- 37.Pyronnet S2000. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem Pharmacol 60:1237–1243 [DOI] [PubMed] [Google Scholar]
- 38.Chen N, Napoli JL2008. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARα. FASEB J 22:236–245 [DOI] [PubMed] [Google Scholar]
- 39.Maghsoodi B, Poon MM, Nam CI, Aoto J, Ting P, Chen L2008. Retinoic acid regulates RARα-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc Natl Acad Sci USA 105:16015–16020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cazalla D, Newton K, Cáceres JF2005. A novel SR-related protein is required for the second step of pre-mRNA splicing. Mol Cell Biol 25:2969–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]