Abstract
The potential roles of GnRH I and GnRH II have been assigned in promoting the invasive capacity of human trophoblasts by regulating matrix metalloproteinases-2 and -9, type I tissue inhibitor of matrix metalloproteinase, and urokinase plasminogen activator/plasminogen activator inhibitor protease systems during human placentation, and GnRH II has been shown to be more potent than GnRH I. However, the mechanisms for the differential effects of these two hormones remain unclear. In this study, we examined the invasion-promoting effects and the signaling pathways of GnRH I and GnRH II in human trophoblasts. The data revealed that both GnRH I and GnRH II were key autocrine and/or paracrine regulators in facilitating trophoblast invasion. The GnRH receptor antagonist (Antide) and specific small interfering RNA for GnRH receptor inhibited the regulatory effects of GnRH I, but not GnRH II, on trophoblast invasion. Both GnRH I and II activated protein kinase C, ERK1/2, and c-Jun N-terminal kinase to mediate their effects on trophoblast invasion, whereas only GnRH II elicited invasion-promoting action through transactivating the tyrosine kinase activity of epidermal growth factor receptor in trophoblasts. Our observations elucidate a ligand-dependent selective cross-communication between GnRH receptor and epidermal growth factor receptor signaling systems in human trophoblastic cell, and this would further our understanding on the differentially biological significance of these two forms of GnRH in extrapituitary tissues.
GnRH I and II facilitate human trophoblasts invasion via PKC and MAPK cascades, with the effects of GnRH II dependent upon activation of the EGF receptor.
Mammalian GnRH (GnRH I), a decapeptide that is typically produced by hypothalamic neurosecretory cells, plays key roles in the processes of reproduction (1, 2). It stimulates the biosynthesis of FSH and LH in the anterior pituitary gland, which in turn regulate gonadal steroidogenesis and gametogenesis in both sexes (2, 3). A second GnRH subtype (GnRH II) has been identified in humans, which differs from classical GnRH I at three amino acid residues (4, 5). In addition to their well-known endocrine function, it has become evident that GnRH I and GnRH II are important autocrine and/or paracrine regulators in some extrapituitary compartments such as the ovary, placenta, uterus, and immune system (1, 6).
GnRH I exists widely in various trophoblast subpopulations of human placenta throughout gestation (7, 8), whereas GnRH II expression is restricted in villous cytotrophoblasts and extravillous trophoblasts (EVTs) at the first trimester (8). The expression of GnRH receptor (GnRHR) is also present in human placenta (9, 10). Our previous work demonstrated that GnRH I and GnRH II regulated the expression of matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9), type I tissue inhibitor of MMP (TIMP-1), urokinase plasminogen activator (uPA), and plasminogen activator inhibitor (PAI) in the primary cultures of EVT cells (11, 12). All these molecules belong to the protease systems that contribute to extracellular matrix degradation and play crucial roles in the regulation of cell invasiveness (13, 14). These observations suggest that GnRH I and GnRH II are potential autocrine and/or paracrine regulators of trophoblasts behavior during the processes of implantation and placentation.
GnRH I transmits its signal via GnRHR, a specific G protein-coupled receptor (GPCR), which triggers MAPK cascades, leading to activation of ERK1/2, c-Jun N-terminal kinase (JNK), p38 MAPK, and big MAPK (15). Mitogenic signaling by GPCRs can also occur through protein kinase C (PKC), transactivation of tyrosine kinases of the Src family, focal adhesion kinases, and receptor tyrosine kinases (RTKs) (15, 16). The RTKs involved in GPCR-mediated activation of MAPKs include epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor, and IGF receptor (17). The initiation of MAPK cascades via transactivation of EGFR by GnRHR has been reported in neuron, fibroblast, and pituitary gonadotroph cells (18, 19, 20, 21, 22). Our previous study indicated the activation of MAPK signaling by GnRHR in human placental trophoblast cells (23). EGFR-triggered MAPK cascade was demonstrated in the regulation of trophoblast cell invasiveness and production of MMPs (24, 25, 26). However, the signaling cross talk between GnRHR and EGFR has yet to be identified in trophoblast cells. It remains to be elucidated whether the signaling pathways of the two types of GnRH are similar or not in regulating trophoblast cell invasion. In this study, we examined the invasion-promoting effects and the signaling pathways of GnRH I and GnRH II in primary cultures of EVTs propagated from first-trimester placental tissues.
Results
GnRH I and GnRH II enhance EVT cell invasion via MMP-2 and MMP-9 activities
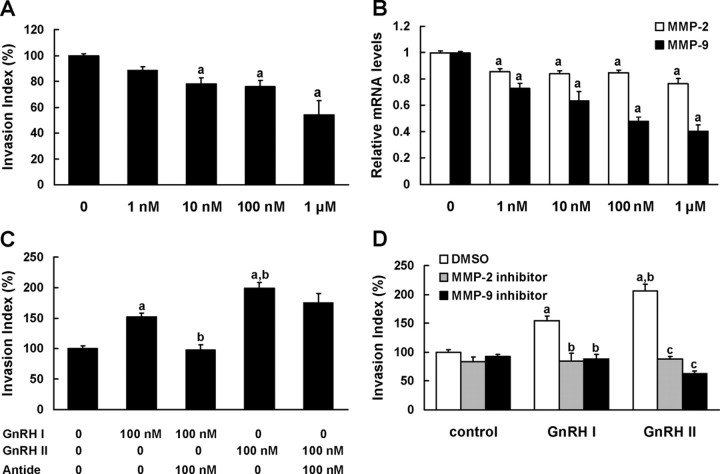
EVT cells, the highly invasive trophoblasts, are capable of expressing GnRH I, GnRH II, and the classical GnRH receptor (GnRHR) as evidenced by real-time PCR (data not shown). To determine the effect of endogenous GnRHs on EVT cell invasion, the cells were treated with Antide, a specific antagonist to GnRHR. As shown in Fig. 1A, 1 nm to 1 μm of Antide led to a dose-dependent decrease in cell invasiveness, with the nadir of invasive index being 54% of control at 1 μm of Antide. Meanwhile, the significant inhibition on the expression of MMP-2 and MMP-9 mRNA was observed, and the alteration of MMP-9 mRNA exhibited a dose-dependent manner (Fig. 1B).
Fig. 1.
GnRH I and GnRH II enhance EVT cell invasion via MMP-2 and MMP-9 activities. A, The EVTs were cultured in the presence or absence of increasing concentrations (1 nm to 1 μm as indicated) of Antide, a GnRHR antagonist, to block the actions of endogenous GnRH, and their invasive capacities were analyzed by invasion assay. B, Real-time PCR analysis revealing MMP-2 and MMP-9 mRNA levels in EVTs cultured in the presence or absence of increasing doses of Antide (1 nm to 1 μm) for 24 h. C, Invasion assay depicting the invasive capacities of EVTs treated with 100 nm GnRH I or GnRH II in the presence or absence of 100 nm Antide. D, Invasion assay of EVTs that were treated with GnRH I or GnRH II (100 nm) in the presence or absence of pretreatment with MMP-2 inhibitor (10 μm), MMP-9 inhibitor (10 μm), or vehicle (0.1% DMSO). The data derived from at least three independent sets of experiments were standardized to the corresponding control, and the statistical results are presented in the column graphs. a, P < 0.05 vs. control; b, P < 0.05 vs. treatment with GnRH I alone; c, P < 0.05 vs. treatment with GnRH II alone.
To reveal the respective effect of GnRH I and GnRH II, the EVT cells were treated with the native peptides of these two subtypes of GnRH. Both GnRH I and GnRH II treatment increased invasive capacity of EVTs, with the invasion index increasing to 1.5- and 2-fold of control cultures, respectively. Notably, GnRH II-stimulated invasiveness of EVTs was significantly higher than that of GnRH I, suggesting that GnRH II was more potent than GnRH I on trophoblastic cell invasion. Antide was able to fully block the invasion-promotion effect of GnRH I, but had no significant effect on that of GnRH II in EVTs (Fig. 1C).
To evaluate the contribution of MMP-2 and MMP-9 in GnRH-induced invasion of EVTs, the specific inhibitors for MMP-2 (10 μm) or MMP-9 (10 μm) were added in EVTs cultured in the presence of these two forms of GnRH native peptide. The results showed that inhibition of gelatinase activity of MMP-2 or MMP-9 significantly attenuated the stimulatory effect of GnRH I and GnRH II on EVT cell invasion (Fig. 1D).
GnRHR small interfering RNA (siRNA) inhibits GnRH I-induced trophoblastic cell invasion
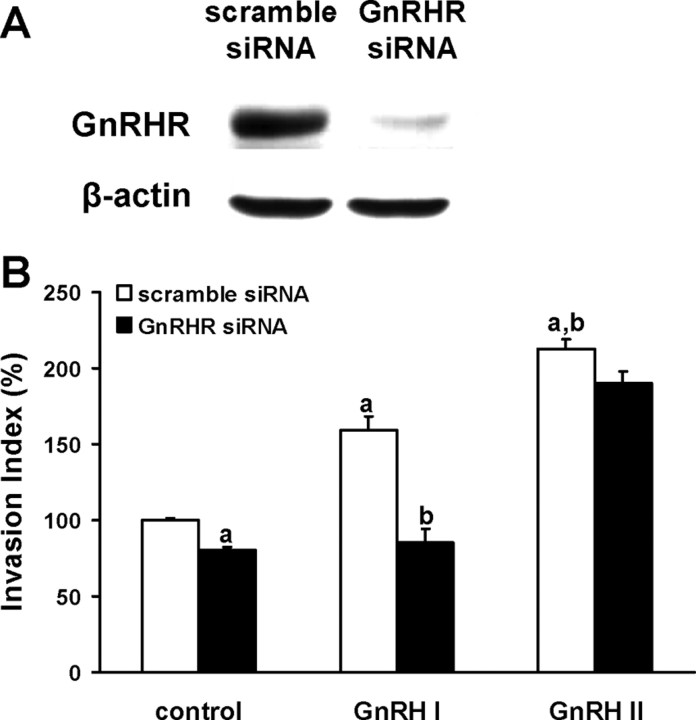
To further investigate the role of GnRHR in mediating effects of GnRH I and GnRH II on the invasiveness of trophoblastic cells, human choriocarcinoma JEG-3 cells, a widely used human trophoblastic cell line, were transfected with specific siRNA for GnRHR. The knockdown of GnRHR protein was confirmed by Western blot analysis (Fig. 2A). Invasion assay data showed that GnRHR siRNA effectively inhibited GnRH I-induced invasiveness of JEG-3 cells compared with that of GnRH I plus scramble siRNA-treated cells (Fig. 2B). However, the invasion-promotion effect of GnRH II on JEG-3 cells was not affected by GnRHR siRNA (Fig. 2B). It suggests a direct involvement of classic GnRHR in the acquisition of GnRH I- but not GnRH II-induced invasive phenotype in trophoblastic cells.
Fig. 2.
GnRHR siRNA inhibits GnRH I-induced trophoblastic cell invasion. A, JEG-3 cells were transiently transfected with scramble siRNA or GnRHR siRNA, and the expression of GnRHR was determined by Western blot with specific antibody. B, JEG-3 cells were transiently transfected with specific GnRHR siRNA or scramble siRNA. Transwell invasion assay was performed in the cells treated with GnRH I (100 nm) or GnRH II (100 nm). The data derived from at least three separate sets of experiments were standardized to the corresponding control, and the statistical results are presented in the column graphs. a, P < 0.05 vs. control cells transfected with scramble siRNA; b, P < 0.05 vs. GnRH I-treated cells transfected with scramble siRNA.
Activities of ERK1/2 and JNK are required for GnRH I- and GnRH II-induced EVT cell invasion
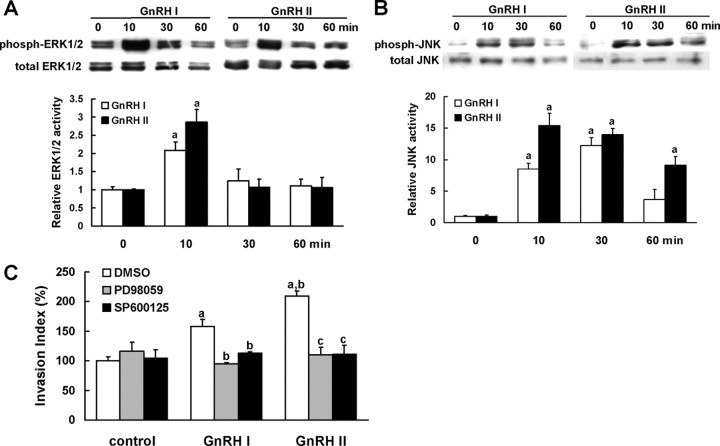
To address whether MAPK signaling is involved in the invasion-promotion effect of GnRHs in EVT cells, we first tested the phosphorylation of ERK1/2 and JNK in EVT cells treated with GnRH I or GnRH II. As shown in Fig. 3, A and B, addition of 100 nm of either GnRH I or GnRH II caused significant increase in the phosphorylation of ERK1/2 and JNK. The activation of ERK1/2 peaked at 10 min after treatment with GnRH I or GnRH II and decreased thereafter (Fig. 3A). However, activation of JNK was more sustainable and lasted until 60 min of treatment with GnRHs (Fig. 3B).
Fig. 3.
Activities of ERK1/2 and JNK are necessary for GnRH I- and GnRH II-induced EVT cell invasion. A and B, The EVTs were serum starved (>18 h) and then treated with 100 nm GnRH I or GnRH II for 10, 30, and 60 min, respectively. Phosphorylation of ERK1/2 (A) or JNK (B) was determined by Western blot with specific antibodies. The total amount of ERK1/2 or JNK was reprobed, and the relative density of phosphorylated ERK1/2 or JNK was normalized to total values of ERK1/2 or JNK, respectively. C, After 30-min pretreatment with vehicle (0.1% DMSO), PD98059 (10 μm, an ERK1/2 inhibitor), or SP600125 (10 μm, a JNK inhibitor), the EVTs were treated with GnRH I or GnRH II (100 nm), and the invasive capacity were analyzed by invasion assay. The data derived from at least three separate sets of experiments were standardized to the corresponding control, and the statistical results are presented in the column graphs. a, P < 0.05 vs. control; b, P < 0.05 vs. treatment with GnRH I alone; c, P < 0.05 vs. treatment with GnRH II alone.
To validate the functional significance of GnRH I- and GnRH II-activated ERK1/2 and JNK in EVT cell invasion, the cells were pretreated with specific inhibitors for ERK1/2 (PD98059, 10 μm) or JNK (SP600125, 10 μm), after which the invasive capacity of GnRHs-stimulated cells was examined. The data demonstrated that both PD98059 and SP600125 treatments resulted in significant reduction of cell invasiveness induced by GnRH I and GnRH II (Fig. 3C).
EGFR transactivation mediates the activation of ERK1/2 and JNK as well as cell invasion induced by GnRH II, but not by GnRH I, in EVTs
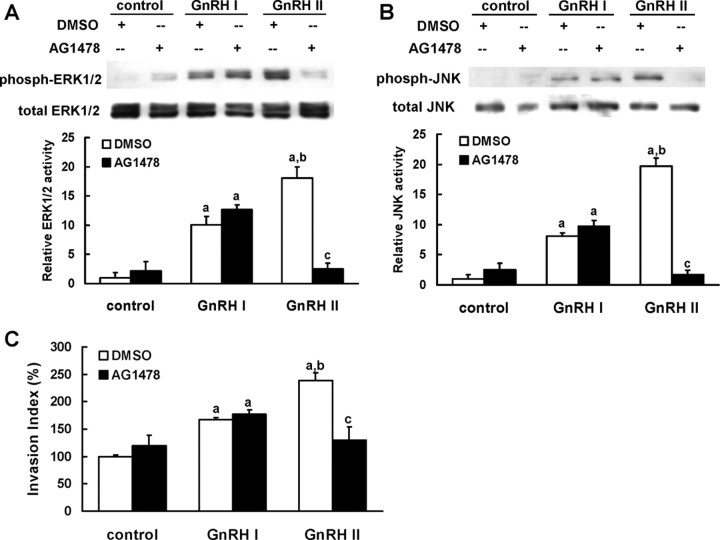
We further investigated the contribution of EGFR in GnRH-induced MAPK signaling and cell event in EVTs. Specific inhibition of EGFR tyrosine kinase activity by 5 μm of AG1478 completely abolished ERK1/2 and JNK activation as well as the promotion of cell invasion induced by GnRH II (Fig. 4, A–C). However, AG1478 failed to block GnRH I-induced effects on EVT cells (Fig. 4, A–C).
Fig. 4.
EGFR transactivation mediates the activation of ERK and JNK as well as cell invasion induced by GnRH II, but not by GnRH I, in EVTs. A and B, The EVTs that were serum starved as described were stimulated with GnRH I or GnRH II (100 nm, 10 min) in the presence or absence of 30-min pretreatment with vehicle (0.1% DMSO) or AG1478 (5 μm, an EGFR inhibitor). The phosphorylation of ERK1/2 (A) or JNK (B) was determined by Western blotting with specific antibodies. C, Invasive capacity of EVTs that were treated with GnRH I or GnRH II (100 nm), in the presence or absence of pretreatment with vehicle (0.1% DMSO) or AG1478 (5 μm), was measured by invasion assay. The data derived from at least three separate sets of experiments were standardized to the corresponding control, and the statistical results are presented in the column graphs. a, P < 0.05 vs. control; b, P < 0.05 vs. treatment with GnRH II alone; c, P < 0.05 vs. treatment with GnRH II alone.
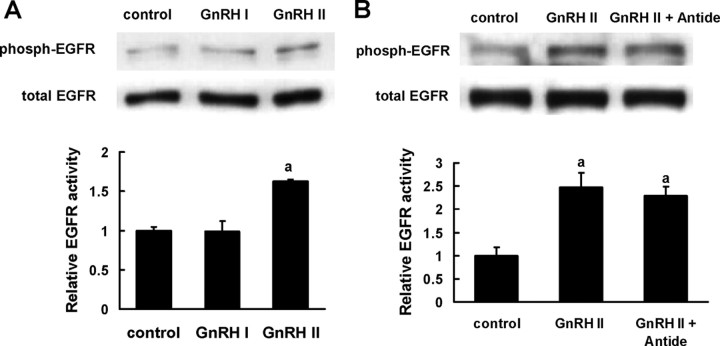
The phosphorylation status of EGFR was examined after GnRH treatment. As expected, treatment with GnRH II led to significantly elevated phosphorylation of EGFR in EVT cells, whereas GnRH I did not induce EGFR activation (Fig. 5A). Meanwhile, the transactivation of EGFR by GnRH II was not influenced by Antide, the GnRHR inhibitor (Fig. 5B), indicating that the classical receptor for GnRH might not be involved in GnRH II-induced EGFR activity in EVT cells.
Fig. 5.
Transactivation of EGFR by GnRH II. A, The EVTs that were serum starved as described were stimulated with GnRH I or GnRH II (100 nm, 10 min). B, After 30-min pretreatment with Antide (100 nm, a GnRHR antagonist), the EVTs were stimulated with GnRH II (100 nm, 10 min). The phosphorylation of EGFR was determined by Western blotting with specific antibody. The data derived from at least three separate sets of experiments were standardized to the corresponding control, and the statistical results are presented in the column graphs. a, P < 0.05 vs. control.
PKC is the upstream mediator of GnRH-induced MAPK signaling and EGFR transactivation in EVTs
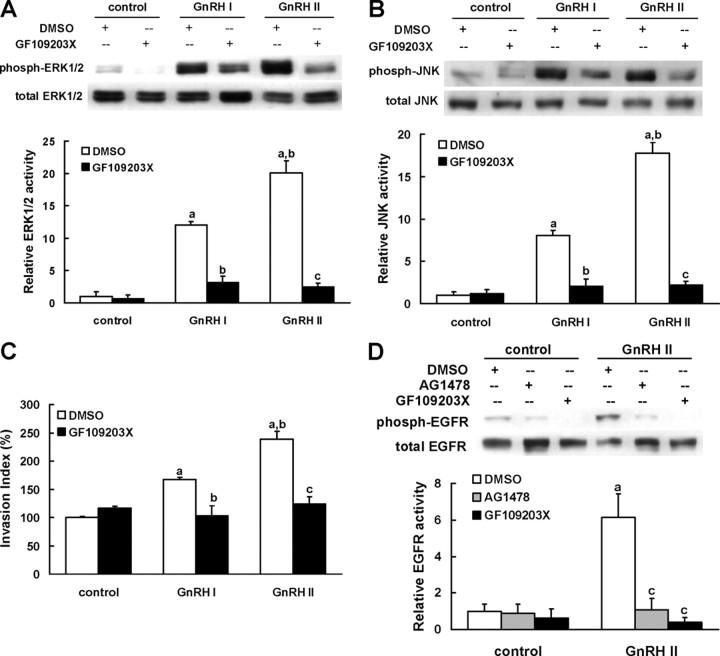
We further tested whether the up-regulation of EVT invasion was mediated by the GnRH I- and II-induced activation of PKC. The results showed that GF109203X, a specific inhibitor for PKC, attenuated GnRH I- and II-induced activation of ERK1/2 and JNK (Fig. 6, A and B) and consequently diminished GnRH-enhanced EVT invasion (Fig. 6C).
Fig. 6.
PKC is the upstream mediator of GnRH-induced MAPK signaling and EGFR transactivation in EVTs. A and B, The EVTs that were serum-starved as described were stimulated with GnRH I or GnRH II (100 nm, 10 min) in the presence or absence of 30-min pretreatment with vehicle (0.1% DMSO) or GF109203X (5 μm, a PKC inhibitor). The phosphorylation of ERK1/2 (A) or JNK (B) was detected by Western blotting with specific antibodies. C, Invasive capacity of EVTs that were treated with GnRH I or GnRH II (100 nm) in the presence or absence of pretreatment with vehicle (0.1% DMSO) or GF109203X (5 μm) were analyzed by invasion assay. D, The EVTs that were serum starved as described were stimulated with GnRH II (100 nm, 10min) in the presence or absence of pretreatment with vehicle (0.1% DMSO), AG1478 (5 μm), or GF109203X (5 μm). The phosphorylation of EGFR was determined by Western blotting with specific antibody. The data derived from at least three separate sets of experiments were standardized to the corresponding control, and the statistical results are presented in the column graphs. a, P < 0.05 vs. control; b, P < 0.05 vs. treatment with GnRH I alone; c, P < 0.05 vs. treatment with GnRH II alone.
To determine whether PKC acts upstream or downstream of EGFR, we examined the effect of PKC inhibition on GnRH II-stimulated EGFR activation. As shown in Fig. 6D, GF109203X completely abolished the phosphorylation of EGFR in GnRH II-treated EVT cells, indicating that PKC is the upstream activator of EGFR upon GnRH II stimulation.
Discussion
Although GnRH I and II have been identified in human placental tissues for decades (27, 28), the biological significance of placental GnRHs remains elusive. GnRH-like immunoreactivity has also been found in the placental extracts of other species, including species such as the rabbit and rat (29, 30), but no evidence shows the presence of GnRHs and their receptor in mouse placenta. Considering the placentation and trophoblast behaviors in rabbit and rodent differ from those in humans (31, 32), it is suggested that placental GnRHs may play different roles between human and these species. For instance, GnRH has been shown to play a crucial role in promoting secretion of human chorionic gonadotropin (CG) in placenta (28, 33), whereas rabbit placenta does not secrete CG (34), and the presence of rat CG is still controversial (35, 36). Therefore, there are some weaknesses of genetically manipulated animals to investigate the physiological roles of human placental GnRH in vivo. The primary cultures of human EVT propagated from explants of first-trimester chorionic villi are more representative of the invasive trophoblasts in vivo (37, 38). This valuable in vitro model has been well accepted to elucidate the physiological significance and molecular mechanisms of human placentation (11, 12). In present study, we used primary cultures of human EVT cells to characterize the two forms of GnRH as key regulators in facilitating trophoblastic cell invasion. We found that the actions of GnRHs were mediated by PKC- and MAPK-signaling cascades, and that the effects of GnRH II depended on EGFR tyrosine kinase activity in human trophoblastic cells.
In previous studies, we demonstrated that the expression of GnRHs in human placenta was relatively higher in the first trimester than at term, and the immunoreactivities were mainly localized in the invasive EVTs (8). It has been well-known that the EVT cells are highly invasive, penetrating maternal uterine wall as deeply as the upper third of myometrial layer in first trimester, which is the most important period in which to establish the uteroplacental circulation (39). The invasive potential of trophoblast cells decreased greatly at mid to late gestation (39). We found that the expression of GnRH I was very low, and that of GnRH II vanished in term placenta (8). The temporal changes of GnRH expression implicate the involvement of these peptides in the regulation of trophoblast cell invasiveness. This idea was proved by the present study, as evidenced by the fact that the exogenous native peptides of the two forms of GnRH significantly promoted cell invasion in cultured human EVT cells. Furthermore, the invasive potential of EVT cells was decreased when the endogenous action of GnRH was blocked by the inhibitor for GnRHR (Antide). We also found that the inhibition of MMP-2 and MMP-9 activities by specific inhibitors resulted in attenuation in GnRH-induced invasiveness of EVTs. These findings are in agreement with our previous data that GnRH I and GnRH II are able to up-regulate the expression of MMP-2, MMP-9, and uPA in human trophoblast cells (11, 12). Therefore, we conclude that both GnRH I and GnRH II regulate human trophoblast cell invasion in a paracrine and/or autocrine manner by facilitating the activities of various proteases including MMP-2 and MMP-9.
GnRH II was first identified in chicken hypothalamus (5), and White et al. (4) clarified its existence in humans. This second isoform of GnRH imitates the biological function of GnRH I in various extrapituitary tissues including placenta, ovary, and endometrium (6), but it appears more potential than GnRH I and/or its analogs. In human endometrial and ovarian cancer cells, GnRH II was shown to be a stronger inhibitor of cell proliferation than GnRH I (40). Our observations and those of others (11, 12, 41) in human placenta demonstrated that GnRH II was more effective than GnRH I in eliciting its regulation on the expression of MMPs, TIMP-1, uPA, and PAI as well as the secretion of leptin. In agreement with those findings, we showed in this study that GnRH II promoted EVT cell invasion more efficiently than GnRH I. Although the differential effects of these two forms of GnRH might be explained by distinct ligand-stabilized receptor conformations (42), different degradation pathways, or even different types of GnRHR (41), there has been little evidence to clarify the underlying molecular mechanisms. We were attempting to find the differences in the signaling pathways of these two hormones in this study. It is well established in gonadotroph cells that GnRH I interacts with its receptor and activates MAPK cascades including ERK1/2, JNK, p38 MAPK, and big MAPK, transmitting signals from the cell surface to the nucleus to initiate specific target gene expression (16). We previously showed that GnRH I also employed MAPKs as signaling mediators in ovarian and placental cells (23). In the present study, GnRH II mimicked GnRH I in terms of activating PKC and subsequent ERK1/2 and JNK to mediate their invasion-promoting actions in human trophoblasts. The activities of PKC, ERK1/2, and JNK were shown to be critical for their function, because the inhibition of these kinases led to a complete blocking of the effects of GnRH on cell invasiveness.
GPCRs activate MAPK-signaling cascades by a wide variety of processes, including the generation of second messengers (Ca2+, PKC, and cAMP), G protein subunit coupling to novel effectors, and activation of RTKs, such as EGFR, platelet-derived growth factor receptor, and IGF receptor (15, 16, 17). A recent major recognition in cancer cells revealed that the migration and invasion-promoting effects of many GPCRs are mediated through transactivation of EGFR, which elicits cellular responses mainly via its intrinsic tyrosine kinase activity (43, 44, 45, 46). GnRHR is a specific GPCR, and increasing experimental evidence supports the concept of the EGFR as a central integrator of diverse GnRHR signals in various cells (18, 19, 20, 21, 22). In this study, we investigated the possible involvement of EGFR in GnRH-induced cell invasion in human trophoblasts. Interestingly, only GnRH II was able to induce EGFR activation that resulted in ERK1/2 and JNK phosphorylation, whereas the responses of ERK1/2 and JNK to GnRH I were not coupled to EGFR activation. Consistently, EGFR tyrosine kinase activity was required in the up-regulation of EVT cell invasion induced by GnRH II, but not by GnRH I. It has been well documented that activation of EGFR mediated MAPK signaling cascades and played a crucial role in the invasive properties of trophoblasts (25, 26, 47, 48). EGFR homozygous mutant mouse died at midgestation due to placental defects (49). Therefore, we proposed that the more potent effect of GnRH II on human trophoblast cell invasion may be attributed, at least in part, to its effective enhancement on the activity of EGFR. On the other hand, it was shown in neuronal and gonadotropic cells that GnRH induced metalloprotease-dependent ectodomain shedding of heparin-binding EGF-like growth factor (HB-EGF) via sequential activation of PKC and Src/Pyk2, resulting in effective activation of EGFR upon binding to HB-EGF (19, 22). Our present study showed that inhibition of PKC activity by GF109203X was capable of abolishing the effects of GnRH II on EGFR phosphorylation as well as on EVT cell invasion. Moreover, evidence revealed that HB-EGF was a critical paracrine factor during the processes of placentation (50, 51), and one of its important effects was to induce the conversion of human trophoblast to invasive phenotype (50). Therefore, it will be of interest to further determine the ectodomain shedding of HB-EGF as well as its interaction with EGFR in response to PKC activation by GnRH II in human trophoblasts.
Another interesting observation in this study is the inability of Antide, an antagonist for classical GnRHR, to block the effects of GnRH II on EVT cell invasion and EGFR transactivation. Our previous studies have shown that Cetrorelix, another specific antagonist for GnRHR, was not capable of inhibiting the regulatory effects of GnRH II on the expression of MMPs, TIMP-1, uPA, and PAI in trophoblastic and endometrial cells (11, 12, 52). In our present study, knockdown of GnRHR expression by specific siRNA abolished the invasion-promoting effect of GnRH I, but failed to inhibit the action of GnRH II in JEG-3 cells. These data suggest the existence of a novel binding protein or receptor for GnRH II. As a matter of fact, reports in literature support that GnRH II may have a unique receptor to exert its action in extrapituitary tissues and cells. Grundker and associates [(53) and Emons and co-workers (54)] demonstrated that the antiproliferation activity of GnRH II on human endometrial and ovarian cancer cells was not mediated by the classical GnRHR, and they also found GnRH II receptor-like antigenicity in human placenta and cancer organs. Maiti et al. (55) suggested the existence of a novel GnRH II binding protein, in addition to the conventional GnRHR, in prostate cancer cells. The genes proposed to encode type II GnRH receptor were found in human, but they were thought to be nonfunctional pseudogenes due to the introduction of premature stop codons (56, 57). Therefore, it remains unclear whether the biological actions of these two hormones are elicited by distinct receptors.
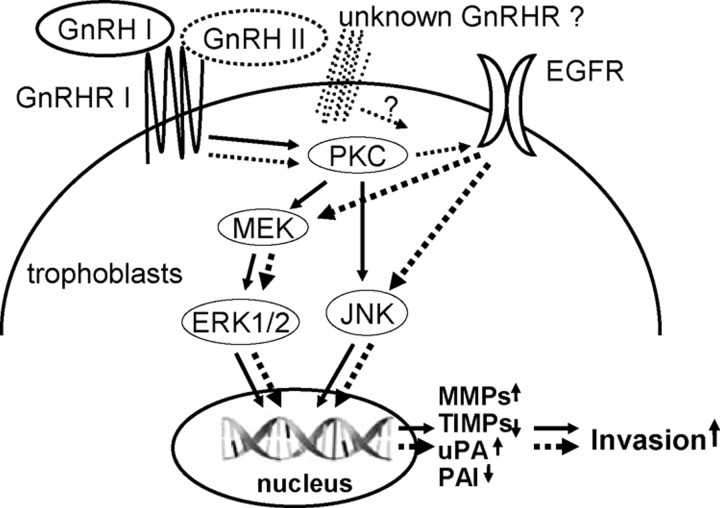
The signaling pathways mediating the invasion-promotion function of the two forms of GnRH in human trophoblasts is summarized in the diagram shown in Fig. 7. The ligand-dependent selective cross-communication between GnRHR and EGFR signaling in trophoblastic cell may further our understanding of the mechanisms underlying the differentially biological significance of GnRH I and GnRH II.
Fig. 7.
Schematic illustration of the distinct signaling mediating the effects of GnRH I and GnRH II on cell invasion in human trophoblasts. The solid lines indicate the pathways activated by GnRH I, and the broken lines indicate the pathways activated by GnRH II. MEK, MAPK kinase.
Materials and Methods
Materials
The native peptides of GnRH I and GnRH II were obtained from Peninsula Laboratories, Inc. (San Carlos, CA). GnRH antagonist (Antide), ERK1/2 inhibitor (PD98059), JNK inhibitor (SP600125), PKC inhibitor (GF109203X), and EGFR inhibitor (AG1478) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). MMP-2 inhibitor (OA-Hy) and MMP-9 inhibitor were products of CalBiochem/EMD Biosciences, Inc. (San Diego, CA). PD98059, SP600125, and AG1478, as well as the inhibitors of MMP-2 and MMP-9, were dissolved in dimethylsulfoxide (DMSO). The antibodies against phospho-ERK1/2 (Thr202/Tyr204), phospho-JNK/SAPK (Tyr185/Thr183), phospho-EGF receptor (Tyr992), pan-ERK1/2, pan-JNK/SAPK, and pan-EGFR were purchased from Cell Signaling Technology, Inc. (Beverly, MA). The antibody against GnRHR was purchased from NeoMarkers Inc. (Fremont, CA).
Tissue samples of the first-trimester placenta were obtained from women undergoing selective termination of pregnancy. The use of these tissues was approved by the Committee for Ethical Review of Research Involving Human Subjects, University of British Columbia, Canada. All patients provided informed written consents.
Isolation and culture of EVT cells
EVTs were propagated from the first-trimester placental tissue explants as previously described (11). Briefly, chorionic villi were washed thoroughly in DMEM (GIBCO-Invitrogen, San Diego, CA), and were minced finely and plated in 25-cm2 tissue culture flasks containing DMEM supplemented with antibiotics and 10% heated-inactivated fetal bovine serum (FBS; GIBCO-Invitrogen, San Diego, CA). These tissue explants were allowed to adhere for 2-3 d and were cultured for a further 10-14 d, with the culture medium being replaced every 2 d. EVTs were separated from the explants by trypsin digestion and plated in 60-mm2 culture dishes. The purity of the EVT cultures was determined by immunostaining with antibodies against cytokeratin 8 and 18 (BD Biosciences, Franklin, NJ) according to the methods described by MacCalman et al. (58). Only cell cultures that exhibited 100% immunostaining for cytokeratin were included in these studies. All experiments were carried out with EVTs at the second passage, and an 18-h serum starvation was performed before each hormone treatment for the cells. The starved cells were treated with Antide (100 nm), PD98059 (10 μm), SP600125 (10 μm), GF109203X (5 μm), AG1478 (5 μm), or vehicle (0.1% DMSO) for 30 min, and then stimulated with GnRH I or GnRH II. At different time points, cells were collected for RNA or protein extraction.
RNA preparation and reverse transcription to cDNA
Total RNA was extracted from EVT cells using TRIzol reagent (Invitrogen, Burlington, Ontario, Canada) according to the manufacturer’s instructions. The purity and concentration of total RNA were quantified by absorbance at 260 and 280 nm using a Du-64 UV-spectrophotometer (Beckman Coulter, Mississauga, Ontario, Canada). Aliquots (1 μg) of the total RNA extracts were subsequently reverse transcribed into cDNA using a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech, Oakville, Ontario, Canada).
Real-time PCR
The cDNA generated from the total RNA served as a template for real-time PCR using the ABI PRISM 7000 sequence detection system (PerkinElmer Applied Biosystems, Foster City, CA) equipped with a 96-well optical reaction plate. The primers used for SYBR Green Real-time quantitative PCR were designed using the Primer Express Software version 2.0 (PerkinElmer Applied Biosystems), and the sequences were as follows: MMP-2: 5′-TAC ACC AAG AAC TTC CGT CTG T-3′ (forward) and 5′-AAT GTC AGG AGA GGC CCC ATA-3′ (reverse); MMP-9: 5′-GGA CGA TGC CTG CAA CGT-3′ (forward) and 5′-CAA ATA CAG CTG GTT CCC AAT CT-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-ATG GAA ATC CCA TCA CCA TCT T-3′ (forward) and 5′-CGC CCC ACT TGA TTT TGG-3′ (reverse). The reactions were set up with 12.5 μl SYBR Green PCR master mix (PerkinElmer Applied Biosystems), 7.5 μl of primer mixture (300 nm), and 5 μl of cDNA template [diluted 1:7 (vol/vol)] under the following optimized conditions: 52 C for 2 min followed by 95 C for 10 min and 40 cycles of 95 C for 15 sec and 60 C for 1 min. All PCRs were performed in duplicate, with the mean values to determine the corresponding mRNA levels. A negative control with water instead of sample cDNA was included in each plate. The amplification efficiency was determined by plotting log cDNA dilution against ΔCT (ΔCT = CT.Target − CT.GAPDH), the slope of which was close to zero, indicating maximal and similar efficiency of the target and reference genes (data not shown). Relative mRNA levels were determined using the formula 2−ΔΔCT where ΔΔCT = (CT.Target − CT.GAPDH)X − (CT.Target − CT.GAPDH)0. In this formula, X represents any time point or experimental treatment, and control cultures are assigned a value of zero (59). Data were analyzed using SDS 2.0 software (PerkinElmer Applied Biosystems). This experimental approach was further validated by the observation that differences between the CT for the target gene and GAPDH remained relatively constant for each amount of cDNA examined.
Knockdown of GnRHR by siRNA in JEG-3 cells
The human choriocarcinoma cell line, JEG-3 cells, were purchased from American Type Culture Collection (ATCC, Manassas, VA). After thawing, the cells were maintained in DMEM supplemented with 10% FBS. The specific siRNA against GnRHR (5′-GCU CUC UGC GAC CUU UAA U-3′; Invitrogen) or scramble siRNA (5′-GCU UCC GAG CCU UUC UAA U-3′; Invitrogen) was transfected into JEG-3 cells using the Lipofectamine 2000 reagent according to the manufacturer’s instructions (Invitrogen). Transfection media were replaced 4 h later with fresh culture media and cells were further cultured for 24 h. The cells were collected and processed to Western blot analysis or invasion assay.
Transwell insert invasion assay
In vitro cell invasion was assayed by determining the ability of cells to invade a synthetic basement membrane. Briefly, 24-well fitted transwell inserts with membranes (8-μm pore size; BD Biosciences, Franklin, NJ) were coated with growth factor-reduced Matrigel (BD Biosciences, Franklin, NJ) at a concentration of 200 μg/ml and placed in 24-well plate. Cells (1 × 105) were seeded in each insert containing DMEM supplemented with 0.1% FBS. Lower chambers were loaded with culture medium containing 10% FBS. After pretreatment with various inhibitors or vehicle (0.1% DMSO) for 30 min, the cells were treated with 100 nm GnRH I or GnRH II for a further 48 h. The cells were then fixed and stained with crystal violet. Noninvaded cells on the upper surface of the membrane were removed using a cotton swab. The membranes were cut from inserts and mounted onto glass slides. The number of stained cells was counted in at least 15 randomly selected nonoverlapping fields of the membranes under light microscope. All experiments were done in triplicate, and the invasion index was expressed as the percentage of invasion compared with the corresponding control.
Western blot analysis
The cells were lysed in cell extraction buffer (Biosource International, Camarillo, CA) supplemented with 1.0 mm phenylmethylsulfonyl fluoride and protease-inhibitor cocktail. Protein extract (30 μg) was loaded on 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to the nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). The membrane was incubated with specific antibodies against GnRHR, phospho-ERK1/2, phospho-JNK, or phospho-EGFR at 4 C overnight and further incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. Final visualization was achieved using the enhanced chemiluminescence system (Amersham Pharmacia Biotech). The membranes were stripped and reprobed with β-actin, pan-ERK1/2, pan-JNK, and pan-EGFR antibodies, respectively. The relative intensity of phosphorylated forms of ERK1/2, JNK, and EGFR was separately normalized to densitometric value of total ERK1/2, JNK, and EGFR.
Statistical analysis
Data were shown as the mean ± sem according to at least three independent experiments, each with at least three dishes of cells per treatment. Statistical analysis was carried out using one-way ANOVA followed by Dunnett’s test, and differences were considered significant for P < 0.05.
Footnotes
This work was supported in part by the Chinese National Special Fund for Basic Research Project (Grant 2006CB944008) and the Knowledge Innovation Program in Chinese Academy of Sciences (Grant KSCX2-YW-R-53) in China (to Y.L.W.), and grant from the Canadian Institutes of Health Research (to P.C.K.L. and C.D.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 16, 2009
Abbreviations: CG, Chorionic gonadotropin; DMSO, dimethylsulfoxide; EGFR, Epidermal growth factor receptor; EVT, extravillous trophoblast; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GnRHR, GnRH receptor; GPCR, G protein-coupled receptor; HB-EGF, heparin-binding EGF-like growth factor; JNK, c-Jun N-terminal kinase; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; PKC, protein kinase C; RTK, receptor tyrosine kinase; siRNA, small interfering RNA; TIMP-1, type I tissue inhibitor of MMP; uPA, urokinase plasminogen activator.
References
- 1.Ortmann O, Diedrich K1999. Pituitary and extrapituitary actions of gonadotrophin-releasing hormone and its analogues. Hum Reprod 14(Suppl 1):194–206 [DOI] [PubMed] [Google Scholar]
- 2.Yen SS1975. Gonadotropin-releasing hormone. Annu Rev Med 26:403–417 [DOI] [PubMed] [Google Scholar]
- 3.Fink G1979. Neuroendocrine control of gonadotrophin secretion. Br Med Bull 35:155–160 [DOI] [PubMed] [Google Scholar]
- 4.White RB, Eisen JA, Kasten TL, Fernald RD1998. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci USA 95:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H1984. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci USA 81:3874–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CK, Leung PC2005. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev 26:283–306 [DOI] [PubMed] [Google Scholar]
- 7.Khodr GS, Siler-Khodr TM1980. Placental luteinizing hormone-releasing factor and its synthesis. Science 207:315–317 [DOI] [PubMed] [Google Scholar]
- 8.Chou CS, Beristain AG, MacCalman CD, Leung PC2004. Cellular localization of gonadotropin-releasing hormone (GnRH) I and GnRH II in first-trimester human placenta and decidua. J Clin Endocrinol Metab 89:1459–1466 [DOI] [PubMed] [Google Scholar]
- 9.Cheng KW, Nathwani PS, Leung PC2000. Regulation of human gonadotropin-releasing hormone receptor gene expression in placental cells. Endocrinology 141:2340–2349 [DOI] [PubMed] [Google Scholar]
- 10.Lin LS, Roberts VJ, Yen SS1995. Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion. J Clin Endocrinol Metab 80:580–585 [DOI] [PubMed] [Google Scholar]
- 11.Chou CS, Zhu H, Shalev E, MacCalman CD, Leung PC2002. The effects of gonadotropin-releasing hormone (GnRH) I and GnRH II on the urokinase-type plasminogen activator/plasminogen activator inhibitor system in human extravillous cytotrophoblasts in vitro. J Clin Endocrinol Metab 87:5594–5603 [DOI] [PubMed] [Google Scholar]
- 12.Chou CS, Zhu H, MacCalman CD, Leung PC2003. Regulatory effects of gonadotropin-releasing hormone (GnRH) I and GnRH II on the levels of matrix metalloproteinase (MMP)-2, MMP-9, and tissue inhibitor of metalloproteinases-1 in primary cultures of human extravillous cytotrophoblasts. J Clin Endocrinol Metab 88:4781–4790 [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty C, Gleeson LM, McKinnon T, Lala PK2002. Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol 80:116–124 [DOI] [PubMed] [Google Scholar]
- 14.Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R2000. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci 57:77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruf F, Fink MY, Sealfon SC2003. Structure of the GnRH receptor-stimulated signaling network: insights from genomics. Front Neuroendocrinol 24:181–199 [DOI] [PubMed] [Google Scholar]
- 16.Naor Z, Benard O, Seger R2000. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab 11:91–99 [DOI] [PubMed] [Google Scholar]
- 17.Luttrell LM, Daaka Y, Lefkowitz RJ1999. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11:177–183 [DOI] [PubMed] [Google Scholar]
- 18.Grosse R, Roelle S, Herrlich A, Höhn J, Gudermann T2000. Epidermal growth factor receptor tyrosine kinase mediates Ras activation by gonadotropin-releasing hormone. J Biol Chem 275:12251–12260 [DOI] [PubMed] [Google Scholar]
- 19.Shah BH, Farshori MP, Jambusaria A, Catt KJ2003. Roles of Src and epidermal growth factor receptor transactivation in transient and sustained ERK1/2 responses to gonadotropin-releasing hormone receptor activation. J Biol Chem 278:19118–19126 [DOI] [PubMed] [Google Scholar]
- 20.Shah BH, Soh JW, Catt KJ2003. Dependence of gonadotropin-releasing hormone-induced neuronal MAPK signaling on epidermal growth factor receptor transactivation. J Biol Chem 278:2866–2875 [DOI] [PubMed] [Google Scholar]
- 21.Kraus S, Benard O, Naor Z, Seger R2003. c-Src is activated by the epidermal growth factor receptor in a pathway that mediates JNK and ERK activation by gonadotropin-releasing hormone in COS7 cells. J Biol Chem 278:32618–32630 [DOI] [PubMed] [Google Scholar]
- 22.Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T2003. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem 278:47307–47318 [DOI] [PubMed] [Google Scholar]
- 23.Kang SK, Tai CJ, Cheng KW, Leung PC2000. Gonadotropin-releasing hormone activates mitogen-activated protein kinase in human ovarian and placental cells. Mol Cell Endocrinol 170:143–151 [DOI] [PubMed] [Google Scholar]
- 24.Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S2004. Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod 10:229–235 [DOI] [PubMed] [Google Scholar]
- 25.Qiu Q, Yang M, Tsang BK, Gruslin A2004. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 128:355–363 [DOI] [PubMed] [Google Scholar]
- 26.Qiu Q, Yang M, Tsang BK, Gruslin A2004. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod 10:677–684 [DOI] [PubMed] [Google Scholar]
- 27.Siler-Khodr TM, Khodr GS1978. Content of luteinizing hormone-releasing factor in the human placenta. Am J Obstet Gynecol 130:216–219 [DOI] [PubMed] [Google Scholar]
- 28.Siler-Khodr TM, Grayson M2001. Action of chicken II GnRH on the human placenta. J Clin Endocrinol Metab 86:804–810 [DOI] [PubMed] [Google Scholar]
- 29.Nowak RA, Wiseman BS, Bahr JM1984. Identification of a gonadotropin-releasing hormone-like factor in the rabbit fetal placenta. Biol Reprod 31:67–75 [DOI] [PubMed] [Google Scholar]
- 30.Sarkar DK1986. Gonadotropin-releasing hormone-like immunoreactivity in rat placenta. Neuroendocrinology 44:397–400 [DOI] [PubMed] [Google Scholar]
- 31.Pijnenborg R, Robertson WB, Brosens I, Dixon G1981. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 2:71–91 [DOI] [PubMed] [Google Scholar]
- 32.Carter AM2007. Animal models of human placentation—a review. Placenta 28(Suppl 1):S41–S47 [DOI] [PubMed]
- 33.Khodr G, Siler-Khodr TM1978. The effect of luteinizing hormone-releasing factor on human chorionic gonadotropin secretion. Fertil Steril 30:301–314 [DOI] [PubMed] [Google Scholar]
- 34.Kim HH, Mui KL, Nikrodhanond AA, Tamayo NC2007. Regulation of gonadotropin-releasing hormone in nonhypothalamic tissues. Semin Reprod Med 25:326–336 [DOI] [PubMed] [Google Scholar]
- 35.Tabarelli M, Kofler R, Schwarz S, Wick G1982. Rat placental hormones: attempts for identification of rat chorionic gonadotrophin and rat placental lactogen by in vivo experiments. Acta Endocrinol (Copenh) 99:288–294 [DOI] [PubMed] [Google Scholar]
- 36.Carr FE, Chin WW1985. Absence of detectable chorionic gonadotropin subunit messenger ribonucleic acids in the rat placenta throughout gestation. Endocrinology 116:1151–1157 [DOI] [PubMed] [Google Scholar]
- 37.Genbacev O, Schubach SA, Miller RK1992. Villous culture of first trimester human placenta—model to study extravillous trophoblast (EVT) differentiation. Placenta 13:439–461 [DOI] [PubMed] [Google Scholar]
- 38.Bischof P1997In vitro models used to study implantation, trophoblast invasion and placentation: a review. Placenta 18(Suppl 2):67–82 [Google Scholar]
- 39.Aplin JD1991. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci 99:681–692 [DOI] [PubMed] [Google Scholar]
- 40.Gründker C, Günthert AR, Millar RP, Emons G2002. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J Clin Endocrinol Metab 87:1427–1430 [DOI] [PubMed] [Google Scholar]
- 41.Islami D, Bischof P, Chardonnens D2003. Possible interactions between leptin, gonadotrophin-releasing hormone (GnRH-I and II) and human chorionic gonadotrophin (hCG). Eur J Obstet Gynecol Reprod Biol 110:169–175 [DOI] [PubMed] [Google Scholar]
- 42.Pfleger KD, Pawson AJ, Millar RP2008. Changes to gonadotropin-releasing hormone (GnRH) receptor extracellular loops differentially affect GnRH analog binding and activation: evidence for distinct ligand-stabilized receptor conformations. Endocrinology 149:3118–3129 [DOI] [PubMed] [Google Scholar]
- 43.Thomas SM, Bhola NE, Zhang Q, Contrucci SC, Wentzel AL, Freilino ML, Gooding WE, Siegfried JM, Chan DC, Grandis JR2006. Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer Res 66:11831–11839 [DOI] [PubMed] [Google Scholar]
- 44.Schäfer B, Gschwind A, Ullrich A2004. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene 23:991–999 [DOI] [PubMed] [Google Scholar]
- 45.Han C, Michalopoulos GK, Wu T2006. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol 207:261–270 [DOI] [PubMed] [Google Scholar]
- 46.Arora P, Cuevas BD, Russo A, Johnson GL, Trejo J2008. Persistent transactivation of EGFR and ErbB2/HER2 by protease-activated receptor-1 promotes breast carcinoma cell invasion. Oncogene 27:4434–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ1994. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol 164:550–561 [DOI] [PubMed] [Google Scholar]
- 48.LaMarca HL, Dash PR, Vishnuthevan K, Harvey E, Sullivan DE, Morris CA, Whitley GS2008. Epidermal growth factor-stimulated extravillous cytotrophoblast motility is mediated by the activation of PI3-K, Akt and both p38 and p42/44 mitogen-activated protein kinases. Hum Reprod 23:1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC1995. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269:230–234 [DOI] [PubMed] [Google Scholar]
- 50.Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR2004. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol 266:223–237 [DOI] [PubMed] [Google Scholar]
- 51.Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE2006. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 133:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou CS, MacCalman CD, Leung PC2003. Differential effects of gonadotropin-releasing hormone I and II on the urokinase-type plasminogen activator/plasminogen activator inhibitor system in human decidual stromal cells in vitro J Clin Endocrinol Metab 88:3806–3815 [DOI] [PubMed] [Google Scholar]
- 53.Eicke N, Günthert AR, Viereck V, Siebold D, Béhé M, Becker T, Emons G, Gründker C2005. GnRH-II receptor-like antigenicity in human placenta and in cancers of the human reproductive organs. Eur J Endocrinol 153:605–612 [DOI] [PubMed] [Google Scholar]
- 54.Gründker C, Schlotawa L, Viereck V, Eicke N, Horst A, Kairies B, Emons G2004. Antiproliferative effects of the GnRH antagonist cetrorelix and of GnRH-II on human endometrial and ovarian cancer cells are not mediated through the GnRH type I receptor. Eur J Endocrinol 151:141–149 [DOI] [PubMed] [Google Scholar]
- 55.Maiti K, Oh DY, Oh DY, Moon JS, Acharjee S, Li JH, Bai DG, Park HS, Lee K, Lee YC, Jung NC, Kim K, Vaudry H, Kwon HB, Seong JY2005. Differential effects of gonadotropin-releasing hormone (GnRH)-I and GnRH-II on prostate cancer cell signaling and death. J Clin Endocrinol Metab 90:4287–4298 [DOI] [PubMed] [Google Scholar]
- 56.Faurholm B, Millar RP, Katz AA2001. The genes encoding the type II gonadotropin-releasing hormone receptor and the ribonucleoprotein RBM8A in humans overlap in two genomic loci. Genomics 78:15–18 [DOI] [PubMed] [Google Scholar]
- 57.Neill JD2002. GnRH and GnRH receptor genes in the human genome. Endocrinology 143:737–743 [DOI] [PubMed] [Google Scholar]
- 58.MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss 3rd JF1996. Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn 206:201–211 [DOI] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]