Abstract
Oxytocin plays an important role in the progression, timing, and modulation of uterine contraction during labor and is widely used as an uterotonic agent. We investigated the mechanisms regulating oxytocin receptor (OTR) signaling in human primary myometrial smooth muscle cells and the ULTR cell-line. Oxytocin produced concentration-dependent increases in both total [3H]inositol phosphate accumulation and intracellular Ca2+ concentration ([Ca2+]i); however, responses were greater and more reproducible in the ULTR cell line. Assessment of phospholipase C activity in single cells revealed that the OTR desensitizes rapidly (within 5 min) in the presence of oxytocin (100 nm). To characterize OTR desensitization further, cells were stimulated with a maximally effective concentration of oxytocin (100 nm, 30 sec) followed by a variable washout period and a second identical application of oxytocin. This brief exposure to oxytocin caused a marked decrease (>70%) in OTR responsiveness to rechallenge and was fully reversed by increasing the time period between agonist challenges. To assess involvement of G protein-coupled receptor kinases (GRKs) in OTR desensitization, cells were transfected with small interfering RNAs to cause specific ≥75% knockdown of GRKs 2, 3, 5, or 6. In both primary myometrial and ULTR cells, knockdown of GRK6 largely prevented oxytocin-induced OTR desensitization; in contrast, selective depletion of GRKs 2, 3, or 5 was without effect. These data indicate that GRK6 recruitment is a cardinal effector of OTR responsiveness and provide mechanistic insight into the likely in vivo regulation of OTR signaling in uterine smooth muscle.
G protein-coupled receptor kinase type 6 (GRK6) selectively regulates oxytocin receptor responsiveness to agonist stimulation in a primary human myometrial cell background.
The peptide hormone oxytocin has many functions in the human body including facilitating milk expulsion during lactation (1) and mediating maternal behavior (2). However, oxytocin is best known as a powerful uterotonic agent stimulating uterine contractions during labor and is also frequently used to augment labor and to prevent postpartum hemorrhage (3). The physiological effects of oxytocin are primarily mediated through a specific G protein-coupled receptor (GPCR), the oxytocin receptor (OTR). The OTR preferentially couples to Gαq/11 proteins to activate phospholipase C (PLC), which hydrolyzes the membrane phospholipid phosphatidylinositol 4,5-bisphosphate to form inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. These second messengers can initiate (IP3) and sustain (diacylglycerol) smooth muscle contraction through activation of Ca2+ mobilization and influx pathways (4, 5, 6).
Throughout pregnancy the myometrium is maintained in a quiescent state and is subjected to an increasing degree of mechanical stretch. Gαq/11-coupled GPCRs are tightly controlled during pregnancy to prevent myometrial contraction and the onset of preterm labor. The molecular processes involved in maintaining myometrial quiescence are not fully understood but are likely to involve desensitization and/or down-regulation of receptors as well as hormonal regulation of expression levels (7, 8, 9, 10). Indeed, OTRs are known to desensitize rapidly, and the mRNA encoding the receptor is also regulated (11, 12), particularly when synthetic oxytocin agonists are used to initiate or augment labor. The initial stages of the GPCR desensitization process usually require G protein-coupled receptor kinase (GRK)-mediated phosphorylation of the agonist-occupied receptor (13, 14) and subsequent binding of arrestin proteins (15), which both sterically inhibit receptor-G protein interactions and promote receptor internalization (16, 17). Interestingly, myometrial GRK expression alters during pregnancy, with GRK2 and GRK6 expression levels increasing as pregnancy proceeds (18, 19). These data suggest that specific GRKs may well play an important role in regulating receptors that stimulate myometrial contractility. Evidence from heterologous expression systems suggest that OTRs are phosphorylated by GRK2, and receptor desensitization is inhibited by overexpression of a catalytically inactive, dominant-negative GRK2 mutant (20).
Accumulating evidence suggests that regulation of recombinantly expressed GPCRs can often differ substantially from that of the same GPCR endogenously expressed in its native cell environment (21, 22, 23, 24, 25, 26). Previous studies highlight the fact that OTRs can rapidly desensitize in myometrial cells (6, 11, 27); however, little is known regarding the potential roles of the endogenous GRKs in mediating this process in myometrium. Therefore, in the present study, we have addressed this question in human primary myometrial cells and the ULTR cell line (28, 29, 30, 31, 32, 33) using small interfering RNAs (siRNAs) to selectively deplete endogenous GRKs and assess their role in regulating OTR responsiveness.
Results
Expression of smooth muscle α-actin and calponin in ULTR and human primary myometrial cells
Our group and others have previously used the immortalized human myometrial cell line ULTR to study myometrial physiology (29, 30, 33, 34). The GPCR profile of this cell line is similar to that seen in human primary myometrial cells (32, 33). mRNA analyses have indicated that ULTR cells are likely to express estrogen receptors α and β, progesterone receptors A and B, connexin 43, cyclooxygenase 2, relaxin receptors, α-actin, and smoothelin (Taylor, A. H., Department of Cancer Studies and Molecular Medicine, University of Leicester, personal communication). Here we used immunocytochemistry to compare contractile protein expression in ULTR and human primary myometrial cells. Both ULTR and primary myometrial cells express α-actin and calponin proteins (Fig. S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). The maintenance of a contractile phenotype in ULTR cells is further supported by visual evidence of oxytocin-induced ULTR cell contraction, combined with previously observed ULTR cell contractions after exposure to agonists such as histamine, which stimulates PLC activity via endogenous H1 histamine receptors (33).
Characterization of OTR signaling in ULTR and human primary myometrial cells
Preliminary experiments established that oxytocin (100 nm) stimulates near-linear [3H]inositol phosphate ([3H]IPx) accumulations in the presence of Li+ (as an index of PLC activity) over a 20-min time course in both ULTR and myometrial cells (data not shown). In myometrial (Fig. 1A) and ULTR (Fig. 1C) cells oxytocin caused concentration-dependent [3H]IPx responses [pEC50 (M) values: 8.06 ± 0.14 and 8.46 ± 0.06, respectively]. However, in primary myometrial cells, oxytocin-stimulated [3H]IPx responses were quite variable between patient donors (all traces are shown in Fig. 1A for clarity), and essentially no responses were observed in cells beyond passages 2–3. Histamine stimulated more robust and more reproducible [3H]IPx responses in primary myometrial cells (Fig. 1B), whereas maximal responses to histamine and oxytocin were comparable in ULTR cells (Fig. 1C). Oxytocin also caused concentration-dependent increases in [Ca2+]i in both primary myometrial and ULTR cells (Fig. 1D). Again, oxytocin-stimulated responses were smaller and more variable in the primary myometrial cells and were only observed at low passage. These data indicate that whereas OTR expression in the immortalized ULTR cell line is likely to be stable, passage-dependent decreases in OTR expression are likely to be occurring in the primary myometrial cells.
Fig. 1.
Characterization of oxytocin-stimulated PLC-signaling in ULTR and primary human uterine smooth muscle cells. Concentration-response relationships for oxytocin- and histamine-stimulated total [3H]inositol phosphate accumulations in primary (A and B) and ULTR (C) cells. [3H]Inositol phosphate accumulation was stimulated by addition of increasing oxytocin (0.1–1000 nm) or histamine (0.1–1000 μm) concentrations for 20 min under lithium blockade (see Materials and Methods). Individual curves are shown from each patient donor cell preparation indicated PT1–4 (A and B), or as mean data for ULTR cell responses (C). To assess oxytocin-stimulated Ca2+ responses (D), cells were Fluo4-AM-loaded (see Materials and Methods) and stimulated with increasing concentrations of oxytocin (0.01–1000 nm). [Ca2+]i changes were measured as increased cytosolic fluorescence and expressed as fold-over-basal. Data are shown as means ± sem for n = 4 for ULTR cells and three patient donors for the primary preparations.
OTR desensitization and resensitization
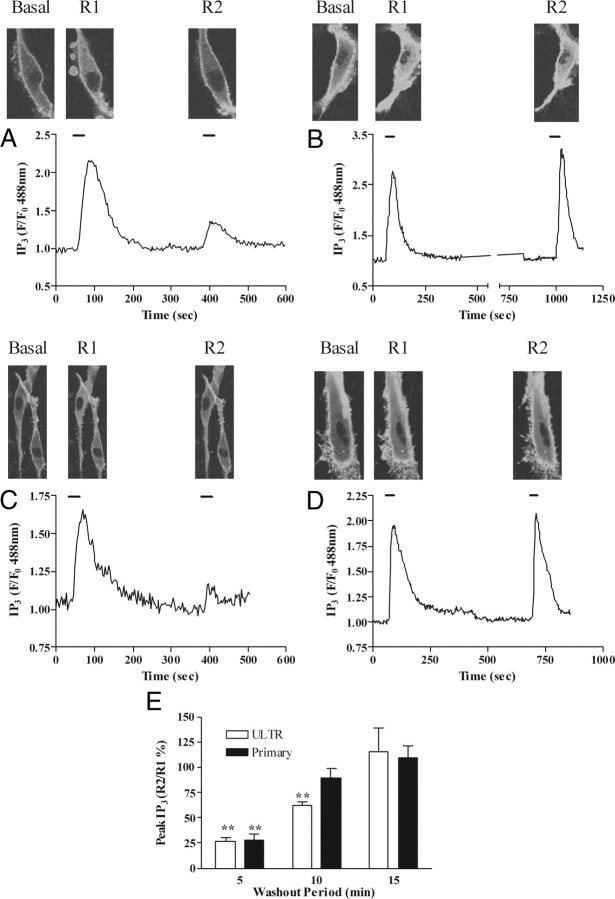
To observe the time course of OTR desensitization and resensitization, we used the enhanced green fluorescent protein (eGFP)-tagged pleckstrin homology (PH) domain of PLCδ1 (eGFP-PH) as an indicator of changes in IP3 (32, 33, 35, 36) (and see supplemental video), and the Ca2+-sensitive dye Fluo4. ULTR or primary myometrial cells were transfected with a plasmid encoding eGFP-PH, or were loaded with Fluo4-AM (3 μm, 30 min). To assess receptor desensitization, cells were stimulated with a maximal oxytocin concentration (100 nm) for 30 sec (termed R1), followed by a wash period (5, 10, or 15 min) and then a second oxytocin challenge (100 nm, 30 sec, termed R2). In both ULTR (Fig. 2, A and B) and primary myometrial (Fig. 2, C and D) cells oxytocin challenge (R1) increased eGFP-PH translocation from the plasma membrane to the cytoplasm, and this translocation reversed over a 5-min time course on washout of oxytocin (Fig. 2A). A second oxytocin challenge (R2) applied 5 min after R1 produced substantially less cytoplasmic translocation of eGFP-PH (Fig. 2A). The reduction in the R2:R1 ratio is interpreted as an indication of OTR desensitization (32, 33, 35, 36, 37). Similar results were obtained for both ULTR and primary myometrial cells, with this protocol causing approximately 75% reductions in the R2 relative to R1 response in both cell backgrounds, indicating equivalent extents of OTR desensitization (Fig. 2, A and C). Increasing the wash period between R1 and R2 revealed a time-dependent recovery of the OTR-mediated eGFP-PH translocation response (Fig. 2, B and D), with essentially complete recovery (i.e. R1 ≈ R2) occurring when a 15-min washout period was used (Fig. 2E).
Fig. 2.
Desensitization and resensitization of OTR-stimulated PLC signaling in ULTR and primary myometrial cells. Cells expressing eGFP-PH were challenged with oxytocin (100 nm, 30 sec) before (R1) and after (R2) a variable wash period. Representative images and traces showing the extent of OTR desensitization 5 min (A and C), 15 min (B), and 10 min (D) after initial oxytocin challenge in ULTR (A and B) and primary myometrial (C and D) cells. F/F0, Fluorescence divided by fluorescence at time zero. OTR desensitization was determined as the relative change in R2 response compared with R1. Cumulative data (E) are expressed as means ± sem for the % change in R2 relative to R1; n = 8–14 cells for each time-point, from at least eight separate experiments. Primary cells experiments were from at least three separate patient donors; **, P < 0.01; R2:R1 ratios for 5 min and 10 min vs. 15 min delay after R1.
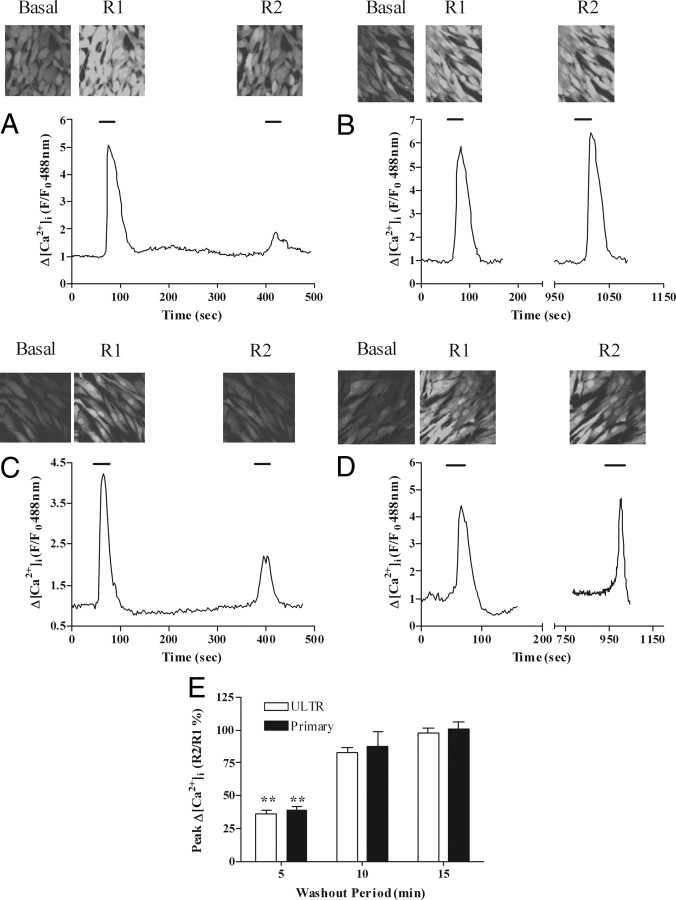
These data could be confirmed by measuring agonist-stimulated changes in [Ca2+]i while applying the R1:R2 protocol (Fig. 3). In this case the maximal decrease in R1:R2 was approximately 60% in both ULTR (Fig. 3, A and B) and primary myometrial (Fig. 3, C and D) cells, and within 10 min of washout a full recovery of the R2 signal could be observed (Fig. 3E). Attempts to examine R1:R2 ratios using shorter washout periods were frustrated by a lack of full recovery of the IP3/[Ca2+]i signals to baseline; therefore, all subsequent desensitization experiments, using either eGFP-PH or Fluo4 as reporters of cellular responses to agonist challenge, were undertaken with a 5-min washout period between R2 and R1.
Fig. 3.
Desensitization and resensitization of OTR-stimulated Ca2+ signaling in ULTR and primary myometrial cells. Cells loaded with the Ca2+-sensitive dye Fluo4 were subjected to the OTR desensitization protocol, where cells were stimulated with oxytocin (R1, 100 nm, 30 sec) followed by variable washout periods and rechallenge with oxytocin (R2, 100 nm, 30 sec). Representative images and traces indicate the extent of OTR desensitization 5 min (A and C) and 15 min (B and D) after initial oxytocin challenge in ULTR (A and B) and primary myometrial (C and D) cells. OTR desensitization was determined as the relative change in R2 response compared with R1. Cumulative data (E) are expressed as means ± sem for the % change in R2 relative to R1; n = 54–59 cells for each time point, from at least six separate experiments for each condition for ULTR cells and from at least three patient donors for primary cells; **, P < 0.01; R2:R1 ratios for 5 min vs. 10 and 15 min delay after R1.
Identification and selective depletion of endogenous GRK isoenzymes
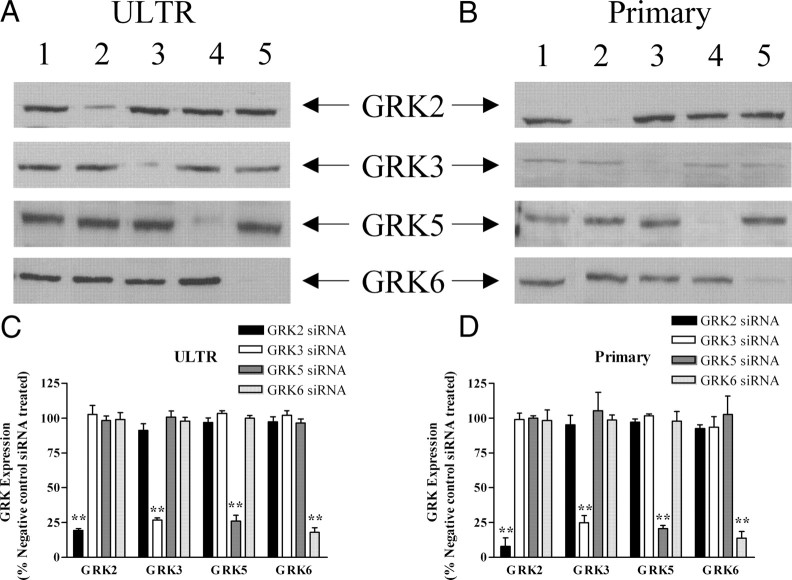
To examine endogenous OTR regulation by GRKs, we adopted a previously successful siRNA strategy to specifically and systematically deplete the expression of individual GRKs (33). In agreement with our previous findings, transfection of ULTR or primary myometrial cells produced maximal knockdown of GRKs 48 h after transfection using specific anti-GRK siRNA oligonucleotides. siRNAs for GRKs 2, 3, 5, or 6 were identified that caused at least 75% knockdown of the targeted kinase (33), whereas a negative control siRNA was without effect on endogenous GRK expression (Fig. 4). Importantly, knockdown of each targeted GRK was specific because no changes were seen in the expression of other endogenous GRKs (Fig. 4).
Fig. 4.
siRNA-mediated depletion of endogenous GRKs in ULTR and primary myometrial cells. Cells were transfected with siRNA constructs targeting endogenous GRK2, GRK3, GRK5, and GRK6 as described in Materials and Methods. After 48 h cells were lysed and 40 μg of protein loaded per lane for SDS-PAGE separation and immunoblotting. Representative immunoblots showing effects of anti-GRK siRNA treatment on endogenous GRK expression in ULTR (A) and primary myometrial (B) cells: lane 1, negative control; lane 2, anti-GRK2; lane 3, anti-GRK3; lane 4, anti-GRK5; lane 5, anti-GRK6. Densitometric analysis was undertaken on all blots and data are shown to highlight changes in GRK2, GRK3, GRK5, and GRK6 expression in (C) ULTR or (D) primary myometrial cells after anti-GRK2, anti-GRK3, anti-GRK5, or anti-GRK6 siRNA treatments. Data are shown as means ± sem for three to six separate experiments in which GRK isoenzyme expression was compared with cells transfected with a negative control siRNA (=100% for each GRK isoenzyme); **, P < 0.01 comparing anti-GRK2/anti-GRK3/anti-GRK5/anti-GRK6 siRNA treatments with respective negative control siRNA treatments.
It should be noted that expression levels for GRKs 2, 5, and 6 were similar in ULTR and primary myometrial cells, but GRK3 expression was (∼4-fold) greater in ULTR cells (supplemental Fig. S2).
Effects of selective depletion of GRK isoenzymic expression on OTR desensitization
To identify which endogenous GRKs modulate OTR responsiveness, ULTR cells were cotransfected with eGFP-PH (0.5 μg) and one of the siRNAs (concentrations used: negative control, 100 nm; anti-GRK2, 10 nm; anti-GRK3, 100 nm; anti-GRK5, 100 nm; anti-GRK6, 10 nm). None of the siRNAs affected the R1 response to oxytocin. In ULTR cells transfected with negative control, anti-GRK2, anti-GRK3, or anti-GRK5 siRNAs (Fig. 5, A–D and F) the R2 response was consistently decreased by 75–80% compared with R1, indicating a reduction in OTR responsiveness similar to that observed in untransfected cells. In contrast, transfection of ULTR cells with anti-GRK6 siRNA resulted in R2 responses that were only decreased by 15–30% relative to R1, indicating a highly significant attenuation of oxytocin-induced receptor desensitization caused by the cellular loss of GRK6 activity (Fig. 5, E and F).
Fig. 5.
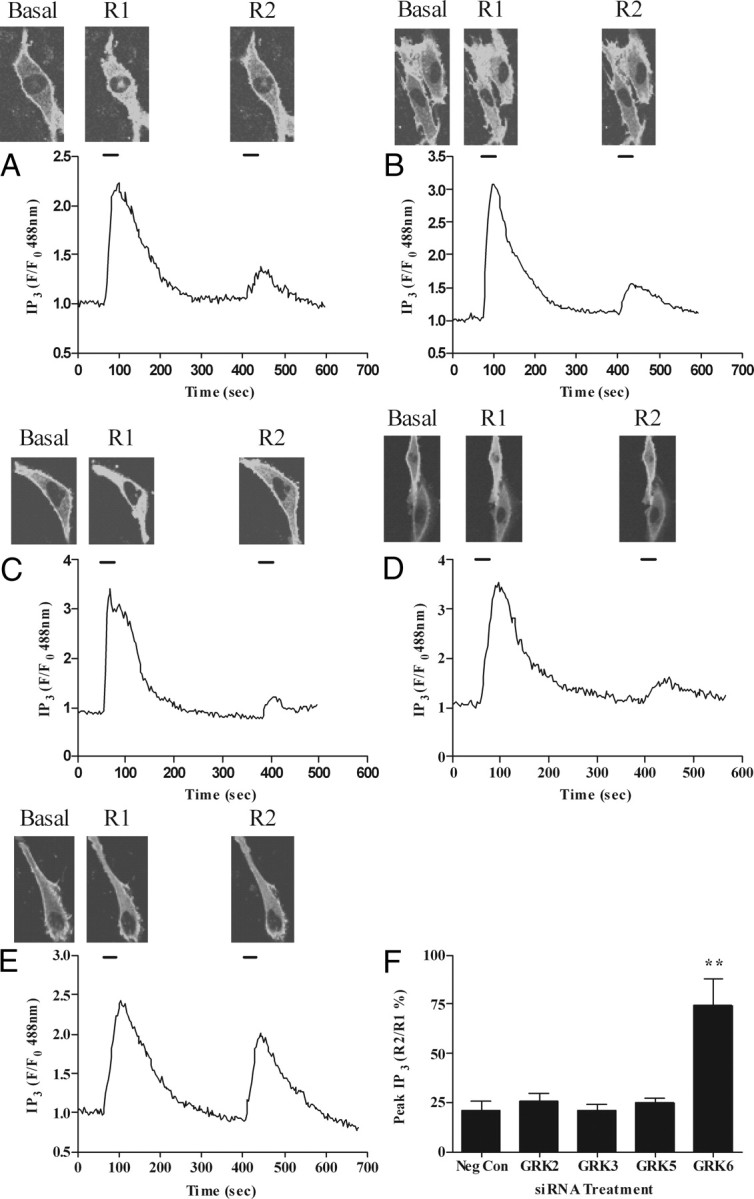
Effects of depleting GRK2, GRK3, GRK5, or GRK6 on OTR desensitization in ULTR cells. Cells were cotransfected with eGFP-PH and either anti-GRK2 (10 nm), anti-GRK3 (100 nm), anti-GRK5 (100 nm), anti-GRK6 (10 nm), or negative control siRNA (100 nm). After 48 h cells were subjected to the standard (R1, R2 = 30 sec, with 5-min washout between oxytocin additions) OTR desensitization protocol. Representative images and traces showing effects of negative control (A), anti-GRK2 (B), anti-GRK3 (C), anti-GRK5 (D), and anti-GRK6 (E) on OTR desensitization. Cumulative data (F) show that depletion of endogenous GRK6 significantly (P < 0.01) prevents OTR desensitization. Data are shown as means ± sem for the % change in R2:R1 ratio for between eight and 17 cells from at least seven separate experiments for each treatment group. **, P < 0.01 comparing anti-GRK6 siRNA treatment with respective negative-control siRNA, anti-GRK2, anti-GRK3, and anti-GRK5 treatments.
To determine whether OTRs are similarly regulated in human primary myometrial cells, we used Amaxa-nucleofection to increase transfection efficiency and achieve better knockdown of endogenous GRK expression (see Materials and Methods and Ref. 33). Preliminary experiments showed that R1:R2 responses to oxytocin challenge in cells 48 h after treatment were similar those observed in cells not subjected to the Amaxa nucleofection protocol (data not shown). Primary myometrial cells were cotransfected with eGFP-PH (0.5 μg) and either negative control or anti-GRK siRNAs (concentrations used identical to those given above for ULTR cells). Like ULTR cells, primary cells transfected with negative control, anti-GRK2, anti-GRK3, or anti-GRK5 siRNAs responded similarly to untransfected cells with respect to their R2:R1 ratio responses to oxytocin (Fig. 6, A–D and F). In contrast, in anti-GRK6 siRNA-transfected cells the response to oxytocin rechallenge (R2) was only 20–30% reduced relative to R1 (Fig. 6, E and F).
Fig. 6.
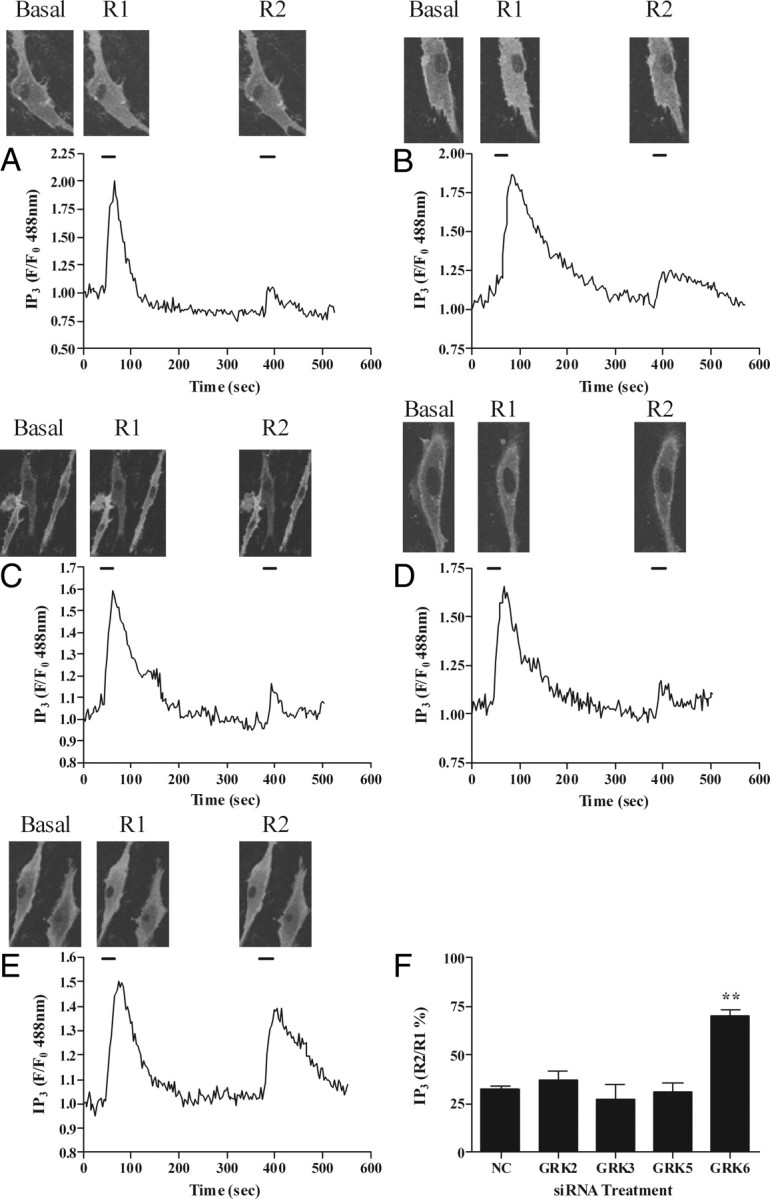
Effects of GRK2, GRK3, GRK5, or GRK6 depletion on OTR desensitization in primary myometrial cells. Cells were cotransfected with eGFP-PH and either anti-GRK2 (10 nm), anti-GRK3 (100 nm), anti-GRK5 (100 nm), anti-GRK6 (10 nm), or negative control siRNA (100 nm). After 48 h cells were subjected to the standard (R1, R2 = 30 sec, with 5-min washout between oxytocin additions) OTR desensitization protocol. Representative images and traces showing effects of negative control (A), anti-GRK2 (B), anti-GRK3 (C), anti-GRK5 (D), and anti-GRK6 (E) on OTR desensitization. Cumulative data (F) show that depletion of endogenous GRK6 significantly (P < 0.01) prevents OTR desensitization. Data are shown as means ± sem for the % change in R2:R1 ratio for between eight and 15 cells from at least eight separate experiments for each treatment group. Data were obtained from cells prepared from at least three different patient donors. **, P < 0.01 comparing anti-GRK6 siRNA treatment with respective negative-control siRNA, anti-GRK2, anti-GRK3, and anti-GRK5 treatments. NC, Negative control.
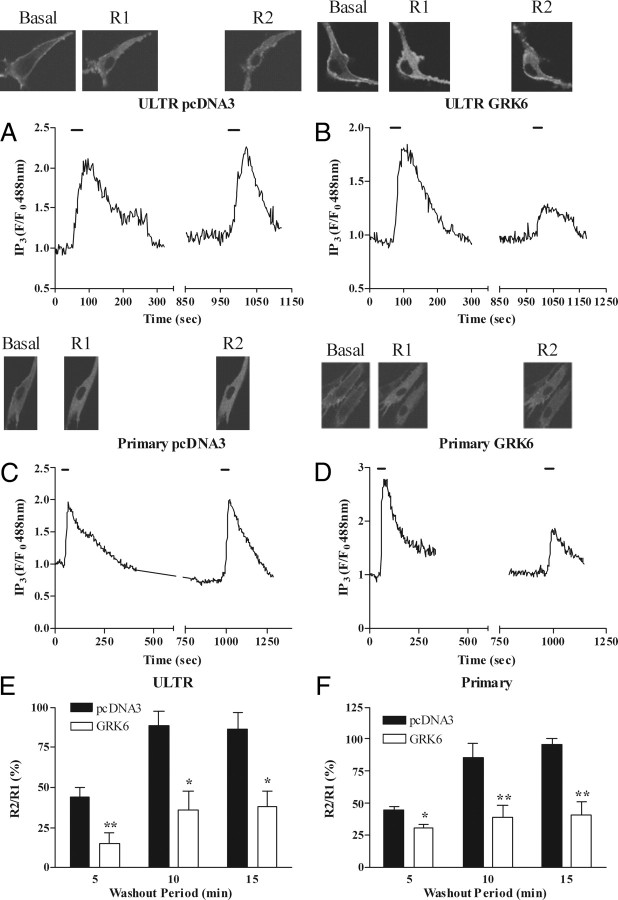
Recombinantly overexpressing GRK6 in either ULTR or primary myometrial cells increased the extent of oxytocin-induced OTR desensitization and dramatically delayed resensitization (Fig. 7). Collectively, the siRNA and overexpression data indicate that GRK6 is the key isoenzyme regulating OTR responsiveness in ULTR and primary myometrial smooth muscle cells.
Fig. 7.
Overexpression of GRK6 enhances OTR desensitization and delays resensitization. ULTR or primary myometrial cells were cotransfected with eGFP-PH and either pcDNA3 (vector control) or human GRK6. After 48 h cells were subjected to the standard desensitization protocol (R1, R2 = 30 sec, with 5-, 10-, or 15-min washout periods between oxytocin additions). Representative images and traces showing effects of pcDNA3 (A and C) or GRK6 (B and D) overexpression on OTR desensitization with 15-min wash periods between oxytocin challenges, in ULTR (A and B) or primary myometrial (C and D) cells. E and F, Cumulative data show that GRK6 overexpression increased the extent of OTR desensitization and prevented resensitization even after a 15-min washout period. Data are shown as means ± sem for the % change in R2:R1 ratio for between four and eight cells from at least four separate experiments for each treatment group. Data were obtained from cell preparations from at least three different patient donors. *, P < 0.05; **, P < 0.01 comparing GRK6 overexpression with pcDNA3 expression for the indicated recovery time periods.
Finally, we assessed whether changes in GRK expression might contribute to the decline in oxytocin-stimulated IP3/Ca2+ responses observed in myometrial smooth muscle cells with increasing passage. GRK6 expression does not appear to change with passage (supplemental Fig. S3), whereas OTR transcript levels have been found to decrease after myometrial cell isolation and primary culture for 7 d or more (Tribe, R., Division of Reproduction and Endocrinology, Kings College, UK personal communication).
Discussion
OTR-mediated contractions play an important role in the progression, if not in the initiation, of labor (38). Considering its physiological importance, it is surprising how little is known regarding the regulation of this receptor within native tissues. In the present study we characterized OTR signaling in the immortalized human myometrial smooth muscle cell line, ULTR (28), and primary human myometrial cells. OTR stimulation by oxytocin resulted in similar patterns of phosphoinositide turnover and Ca2+ signaling in both primary and ULTR cells. However, responses were more robust and maintained during passage in ULTR cells, unlike primary cells in which responses to oxytocin had waned toward zero after two passages. These data suggest that ULTR cells are likely to express a higher and more stable level of OTRs than nonpregnant myometrial cells, making them a favorable model in which to study endogenous OTR regulation. Nevertheless, here we have used both ULTR and primary cultures to assess endogenous OTR regulation.
Continued or repeated agonist stimulation desensitizes many GPCRs, providing a potential protection against the generation of prolonged and/or inappropriate cellular responses to external stimuli (39, 40). In agreement with previous studies, our data indicate that OTRs desensitize rapidly in nonpregnant tissues (6, 32, 37), even in response to modest agonist exposures. This contrasts with a study of the time course of OTR desensitization in isolated myometrial cells from pregnant term patients (27) where a half time for decreasing OTR responsiveness was reported as 4.2 h, suggesting that OTR regulation may vary greatly depending on physiological status. It should be stressed that OTR expression is increased (41), along with other contraction-associated proteins such as cyclooxygenase 2 (42) and connexin 43 (43, 44), before labor, and this may play some role in the apparent resistance of OTR to desensitization (especially when assessed at the level of an amplified, distal response such as [Ca2+]i); however, other mechanisms are also likely to be involved. It is also conceivable that that OTR recycling and/or resensitization rates are more rapid during labor.
In general, GPCR desensitization is initiated through the recruitment of GRK proteins that phosphorylate agonist-occupied receptors (13, 14) to initiate homologous desensitization. Receptor phosphorylation usually leads to β-arrestin binding, which acutely occludes further interaction with G proteins and promotes receptor internalization (15, 17). A number of studies have provided convincing evidence for GRK involvement in OTR regulation, at least in model cell systems (20, 37). Thus, OTRs are phosphorylated in an agonist-dependent manner when recombinantly expressed in human embryonic kidney (HEK)293 or COS-7 cells (20, 45), with two C-terminal Ser-Ser-Ser (368-370/377-379) clusters being implicated as targets for GRK phosphorylation and β-arrestin binding (45). Hasbi et al. (20) found that overexpression of GRK2 enhanced OTR desensitization when expressed in HEK293 or COS7 cells, whereas expression of a kinase-dead, dominant-negative GRK2 mutant (K220MGRK2) prevented OTR phosphorylation and internalization. Further evidence from Smith et al. (37) confirmed that overexpression of a dominant-negative GRK2 attenuated desensitization of OTR in a HEK293 cell background and showed that OTR internalization proceeds via a β-arrestin- and dynamin-dependent route. With these prior findings in mind, we examined whether GRKs were able to regulate endogenously expressed OTRs using ULTR and primary human myometrial cells.
Although in previous studies we have successfully used dominant-negative GRKs to disrupt GPCR interactions with endogenous GRKs, this method requires overexpression of the dominant-negative constructs and raises concerns about the potential for off-target effects. Therefore, here we have employed validated siRNAs to knock down the expression of individual endogenous GRK isoenymes (33). Despite decreasing endogenous GRK2 expression by ≥80% using siRNAs, which almost completely abolished H1 histamine receptor desensitization in these cells (33), OTR desensitization was unaffected. Furthermore, depletion of GRK3 and GRK5 also had no effect on oxytocin-mediated OTR desensitization. In contrast, siRNA knockdown of GRK6 markedly attenuated OTR desensitization in both ULTR and primary myometrial cells. Similarly, GRK6 overexpression was shown to increase the extent of, and delay recovery from, OTR desensitization.
Collectively, these data suggest that GRK6 is the key GRK isoenzyme regulating the initial phase of OTR desensitization in cells endogenously expressing this receptor subtype. Why this role switches to GRK2 when OTRs are recombinantly expressed in HEK or COS-7 cells is unclear, as is the issue of whether the Ser-Ser-Ser (368-370/377-379) clusters identified by Oakley et al. (45) in the C-terminal of OTR are also the sites of GRK6-mediated phosphorylation. That our data do not match previous findings is perhaps not surprising given the accruing evidence that GPCRs are often regulated very differently in their native environment compared with when they are overexpressed in model cell systems (20, 21, 22, 23, 24, 25, 26, 37).
We have observed that GRK6 expression is similar in ULTR and primary myometrial smooth muscle cells, and the level of this isoenzyme does not change with passage in the latter cells. Interestingly, myometrial GRK6 expression has been reported to alter during pregnancy and to be significantly increased at term (18, 19). It is therefore tempting to speculate that increased expression of GRK6 may contribute to clinically observed resistance to oxytocin induction therapy (46). Our observations also suggest that GRK6 may play a role in regulating oxytocin-mediated uterine contractions during labor and might be a pharmacological target worthy of investigation in the context of labor management. For example, it is possible that reducing GRK6 expression or activity during labor may be beneficial during oxytocin-augmented labor because it would retard desensitization and maintain a functional OTR population.
The OTR has been reported to couple to Gαi proteins to inhibit adenylyl cyclase and decrease cAMP-dependent protein kinase (protein kinase A) activity in rat myometrial cells (47). Because protein kinase A can negatively regulate phosphoinositide signaling, this mechanism might facilitate oxytocin-induced myometrial contractions; however, we could find no evidence for OTR-Gαi coupling in human myometrial cells (data not shown).
The process of parturition follows well-defined stages. Uterine contractions are highly regulated with contractions of finite length interspersed with relaxation, gradually shortening the myometrial layer and opening the cervix. Interestingly, there is a positive correlation between contraction rate and the pulse frequency of oxytocin release during labor (48). Cessation of contraction, despite the presence of circulating procontractile ligands, suggests a coordinated inhibition or desensitization of the contraction process. Inhibition of myometrial contraction is a complex process controlled at multiple levels balancing the activity of excitatory [e.g. Ca2+ channel activity (49), Na+ channel activity, PLC-coupled receptor activity (50)], and inhibitory [e.g. plasma membrane and sarcoplasmic reticulum Ca2+ ATPase, and Na+/Ca2+ exchangers (50), cAMP concentrations (51), and K+ channel activity (52)] pathways. However the input from Gαq/11-coupled receptors is one important excitatory component because stimulation of PLC activity leads to increased [Ca2+]i and muscle contraction (50). Therefore, the relative activity of PLC-coupled GPCRs will, in part, determine myometrial tone. Because oxytocin helps maintain and enhance contraction, desensitization of OTR responses will reduce or potentially halt contraction. Our new data suggest that GRK6 may play an important contributory role in the coordinated contractions seen during labor.
In summary, the present study has identified GRK6 as the predominant GRK-terminating OTR signaling in ULTR and primary myometrial uterine smooth muscle cells. These data identify an important role for GRK6 in acute OTR desensitization and highlight a potential new therapeutic approach in the management of labor. However, future studies are now required to examine OTR signaling and desensitization pathways in pregnant primary uterine smooth muscle from term patients to further test our working hypothesis.
Materials and Methods
Cell culture
ULTRs, an immortalized human myometrial cell line, were grown in DMEM, supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (2.5 μg/ml). Cells were maintained under humidified conditions at 37 C, in air/5% CO2.
Tissue collection and primary cell culture
All protocols for human tissue collection were approved by the University Hospitals of Leicester R&D, and the Leicestershire, Northamptonshire, and Rutland Research Ethics Committees (Reference No. 6816). Uterine samples were obtained at hysterectomy from women undergoing surgery for nonneoplastic indications, e.g. dysfunctional uterine bleeding. All patients gave a signed informed consent. Primary myometrial cells were isolated and cultured as for the ULTR cells and as described previously (53).
Immunocytochemical detection of α-smooth muscle actin and calponin
Cells were plated onto 13-mm glass coverslips and grown for 2 d. Cells were washed three times in PBS (pH 7.4) before fixation and permeabilization with ice-cold methanol (10 min). Cells were then washed three times with PBS before incubation overnight at 4 C with primary antibodies against α-actin (1:1000), or calponin (1:10,000) (Sigma, Poole, Dorset, UK) in PBS containing 10% goat serum. After an additional three washes with PBS, coverslips were incubated with a goat antimouse fluorescein isothiocyanate-tagged secondary antibody (1:200 dilution,) for 2 h at room temperature in the dark. Cells were then washed an additional three times in PBS before addition of the nuclear stain propidium iodide diluted 1:3000 in PBS for 5 min. After extensive washing, cells were mounted and fixed. Calponin and α-actin staining was visualized using an Olympus FV500 scanning laser confocal microscope (Olympus Corp., Lake Success, NY) with 488-nm laser excitation. Images were captured using a × 60 objective.
Assessment of OTR-stimulated Ca2+ signaling in ULTR cells
Cells were plated into 96-well multiplates and when 90% confluent monolayers were loaded with the Ca2+-sensitive dye fluo4-AM (3 μm) at room temperature for 1 h. Oxytocin-stimulated changes in intracellular Ca2+ levels were determined as the relative change in fluorescence measured using a NovoStar imaging system (BMG Labtech, Aylesbury, UK).
Single-cell Ca2+ imaging
Cells were loaded with the Ca2+-sensitive dye fluo4-AM (3 μm, 60 min) at room temperature. To assess changes in intracellular Ca2+, cells were excited at 488 nm, using an Olympus FV500 scanning laser confocal microscope as described previously (36). Cells were maintained at 37 C and perfused with Krebs-Henseleit buffer (NaCl, 134 mm; KCl, 6 mm; MgCl2, 1 mm; glucose, 10 mm; HEPES, 10 mm; and CaCl2, 1.3 mm, pH 7.4) at 5 ml/min. Single-cell images were captured using an oil immersion × 60 objective and intracellular Ca2+ changes determined as the relative change in fluorescence in a particular area of interest as described previously (33, 36). Drugs were added via the perfusion system for the times stated in Results.
[3H]inositol phosphate accumulation assay
Cells were seeded into 24-well plates at approximately 50% confluency. After 24 h cells were incubated with [3H]inositol (1 μCi/ml) in standard medium for a further 48 h. Near-confluent cell monolayers were then washed twice with Krebs-Henseleit buffer and incubated for 15 min at 37 C. LiCl (final concentration 10 mm) was added to each well for 30 min before addition of oxytocin. Agonist stimulation was terminated by addition of trichloroacetic acid (final concentration 0.5 m) and samples were left on ice for 30 min. Sample neutralization and recovery of the total [3H]inositol phosphate ([3H]-IPx) fraction was achieved as described previously (54).
Determination of OTR-stimulated IP3 accumulation in single cells
OTR-stimulated PLC activity was assessed in real time using the fluorescent IP3 biosensor eGFP-PH. Cells were plated onto 25-mm glass coverslips and transfected with eGFP-PH (0.5 μg) using Lipofectamine2000 as per the manufacturer’s instructions. After 48 h oxytocin-stimulated eGFP-PH membrane-cytoplasmic translocations were assessed using an Olympus FV500 scanning laser confocal microscope as described previously (33, 36). Cells were incubated at 37 C using a temperature controller and microincubator (PDMI-2 and TC202A; Burleigh, Digitimer, Cambridge, UK) and perfused at 5 ml/min in Krebs-Henseleit buffer (composition as described in the Ca2+ imaging section). Images were captured using an oil immersion ×60 objective, and IP3 levels were determined as the relative change in cytosolic fluorescence in a defined area of interest exactly as described previously.
Western blotting and assessment of endogenous GRK expression
Endogenous GRK expression levels were determined by Western blotting following transfection with siRNAs as described previously (33). Briefly, ULTR cells were plated at a density of 150,000 per well of a six-well plate and 24 h later transfected with either negative control, anti-GRK2 siRNA (5′-GGCAGCCAGUGACCAAAAAtt-3′), anti-GRK3 siRNA (5′-GGAACUUCUUUCCUGUUCAtt-3′), anti-GRK5 (5′-CGUCUACCGAGAUCUGAAAtt-3′), anti-GRK5 (5′-CGUCUACCGAGAUCUGAAAtt-3′), or anti-GRK6 siRNA (5′-GGACACAAAAGGAAUCAAGtt-3′) (Ambion, Applied Biosystems, Warrington, UK) using the Interferin transfection reagent (Autogen Bioclear, Calne, Wiltshire, UK) according to the manufacturer’s instructions. Primary myometrial cells were transfected with anti-GRK siRNAs using the Nucleofection technique (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer’s optimized protocol. Briefly, 1 × 106 cells per reaction were transfected with anti-GRK2 (10 nm), anti-GRK3 (100 nm), anti-GRK5 (100 nm), anti-GRK6 (10 nm), or negative control siRNAs, before seeding onto six-well plates.
After 48 h, cells were lyzed and subjected to SDS-PAGE separation and transfer to nitrocellulose as described previously (55). GRK expression was determined using polyclonal antibodies against GRKs 2, 3, 5, and 6 and visualized using ECL reagent and Hyperfilm (GE Healthcare, Little Chalfont, UK). The relative expression of individual GRK proteins was determined using the GeneGnome image analysis system and software (Syngene, Cambridge, UK).
Data analysis
Concentration-response curves were generated and EC50 values were determined using nonlinear regression analysis software (GraphPad Prism version 3.0; GraphPad Software Inc., San Diego, CA). Data were analyzed using one-way ANOVA, followed by Bonferroni’s or Dunnett’s post hoc test (Excel 5.0; Microsoft, Redmond, WA). Significance was accepted when P < 0.05.
Acknowledgments
We thank Tobias Meyer (Stanford University, Stanford, CA) for generously making the eGFP-PHPLCδ biosensor probe available to us and James McDougall (Fred Hutchinson Cancer Research Center, Seattle, WA) for the generous gift of the ULTR cell line.
Footnotes
Disclosure Summary: The authors have nothing to declare.
First Published Online May 7, 2009
Abbreviations: [Ca2+]i, Intracellular Ca2+ concentration; eGFP, enhanced green fluorescent protein; eGFP-PH, PH domain of PLCδ1 tagged to eGFP; GPCR, G protein-coupled receptor; GRK, G protein-coupled receptor kinase; IP3, inositol 1,4,5-trisphosphate; OTR, oxytocin receptor; PH, pleckstrin homology; PLC, phospholipase C; siRNA, small interfering RNA.
References
- 1.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM1996. Oxytocin is required for nursing, but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA 93:11699–11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young LJ, Wang Z, Insel TR1998. Neuroendocrine bases of monogamy. Trends Neurosci 21:71–75 [DOI] [PubMed] [Google Scholar]
- 3.Wise A, Clark V2008. Strategies to manage major obstetric haemorrhage. Curr Opin Anaesthesiol 21:281–287 [DOI] [PubMed] [Google Scholar]
- 4.Ku CY, Qian A, Wen Y, Anwer K, Sanborn BM1995. Oxytocin stimulates myometrial guanosine triphosphatase and phospholipase-C activities via coupling to Gαq/11 Endocrinology 136:1509–1515 [DOI] [PubMed] [Google Scholar]
- 5.Sanborn BM2001. Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. Exp Physiol 86:223–237 [DOI] [PubMed] [Google Scholar]
- 6.Holda JR, Oberti C, Perez-Reyes E, Blatter LA1996. Characterization of an oxytocin-induced rise in [Ca2+]i in single human myometrium smooth muscle cells. Cell Calcium 20:43–51 [DOI] [PubMed] [Google Scholar]
- 7.Helmer H, Hackl T, Schneeberger C, Knöfler M, Behrens O, Kaider A, Husslein P1998. Oxytocin and vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol 179:1572–1578 [DOI] [PubMed] [Google Scholar]
- 8.Brodt-Eppley J, Myatt L1999. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet Gynecol 93:89–93 [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Sagawa N, Yoshida M, Mori T, Tanaka I, Mukoyama M, Kotani M, Nakao K1997. The prostaglandin E2 and F2α receptor genes are expressed in human myometrium and are down-regulated during pregnancy. Biochem Biophys Res Commun 238:838–841 [DOI] [PubMed] [Google Scholar]
- 10.Fuchs AR, Fuchs F, Husslein P, Soloff MS1984. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 150:734–741 [DOI] [PubMed] [Google Scholar]
- 11.Phaneuf S, Asbóth G, Carrasco MP, Liñares BR, Kimura T, Harris A, Bernal AL1998. Desensitization of oxytocin receptors in human myometrium. Hum Reprod Update 4:625–633 [DOI] [PubMed] [Google Scholar]
- 12.Phaneuf S, Rodríguez Liñares B, TambyRaja RL, MacKenzie IZ, López Bernal A2000. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J Reprod Fertil 120:91–97 [DOI] [PubMed] [Google Scholar]
- 13.Willets JM, Challiss RA, Nahorski SR2003. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci 24:626–633 [DOI] [PubMed] [Google Scholar]
- 14.Kelly E, Bailey CP, Henderson G2008. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153(Suppl 1):S379–S388 [DOI] [PMC free article] [PubMed]
- 15.Lefkowitz RJ, Shenoy SK2005. Transduction of receptor signals by β-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- 16.Luttrell LM, Lefkowitz RJ2002. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115:455–465 [DOI] [PubMed] [Google Scholar]
- 17.Gesty-Palmer D, Luttrell LM2008. Heptahelical terpsichory. Who calls the tune? J Recept Signal Transduct Res 28:39–58 [DOI] [PubMed] [Google Scholar]
- 18.Brenninkmeijer CB, Price SA, López Bernal A, Phaneuf S1999. Expression of G-protein-coupled receptor kinases in pregnant term and non-pregnant human myometrium. J Endocrinol 162:401–408 [DOI] [PubMed] [Google Scholar]
- 19.Simon V, Mhaouty-Kodja S, Legrand C, Cohen-Tannoudji J2001. Concomitant increase of G protein-coupled receptor kinase activity and uncoupling of β-adrenergic receptors in rat myometrium at parturition. Endocrinology 142:1899–1905 [DOI] [PubMed] [Google Scholar]
- 20.Hasbi A, Devost D, Laporte SA, Zingg HH2004. Real-time detection of interactions between the human oxytocin receptor and G protein-coupled receptor kinase-2. Mol Endocrinol 18:1277–1286 [DOI] [PubMed] [Google Scholar]
- 21.Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ1996. Phosphorylation of the type 1A angiotensin II receptor by G protein-coupled receptor kinases and protein kinase C. J Biol Chem 271:13266–13272 [DOI] [PubMed] [Google Scholar]
- 22.Simon V, Robin MT, Legrand C, Cohen-Tannoudji J2003. Endogenous G protein-coupled receptor kinase 6 triggers homologous β-adrenergic receptor desensitization in primary uterine smooth muscle cells. Endocrinology 144:3058–3066 [DOI] [PubMed] [Google Scholar]
- 23.Kong G, Penn R, Benovic JL1994. A β-adrenergic receptor kinase dominant negative mutant attenuates desensitization of the β2-adrenergic receptor. J Biol Chem 269:13084–13087 [PubMed] [Google Scholar]
- 24.Willets JM, Challiss RA, Kelly E, Nahorski SR2001. G protein-coupled receptor kinases 3 and 6 use different pathways to desensitize the endogenous M3 muscarinic acetylcholine receptor in human SH-SY5Y cells. Mol Pharmacol 60:321–330 [DOI] [PubMed] [Google Scholar]
- 25.Willets JM, Challiss RA, Nahorski SR2002. Endogenous G protein-coupled receptor kinase 6 Regulates M3 muscarinic acetylcholine receptor phosphorylation and desensitization in human SH-SY5Y neuroblastoma cells. J Biol Chem 277:15523–15529 [DOI] [PubMed] [Google Scholar]
- 26.Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT2003. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38:291–303 [DOI] [PubMed] [Google Scholar]
- 27.Robinson C, Schumann R, Zhang P, Young RC2003. Oxytocin-induced desensitization of the oxytocin receptor. Am J Obstet Gynecol 188:497–502 [DOI] [PubMed] [Google Scholar]
- 28.Perez-Reyes N, Halbert CL, Smith PP, Benditt EP, McDougall JK1992. Immortalization of primary human smooth muscle cells. Proc Natl Acad Sci USA 89:1224–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ball A, Wang JW, Wong S, Zielnik B, Mitchell J, Wang N, Stemerman MB, Mitchell BF2006. Phorbol ester treatment of human myometrial cells suppresses expression of oxytocin receptor through a mechanism that does not involve activator protein-1. Am J Physiol Endocrinol Metab 291:E922–E928 [DOI] [PubMed]
- 30.Olson DM, Zaragoza DB, Shallow MC, Cook JL, Mitchell BF, Grigsby P, Hirst J2003. Myometrial activation and preterm labour: evidence supporting a role for the prostaglandin F receptor—a review. Placenta 24(Suppl A):S47–S54 [DOI] [PubMed]
- 31.Zaragoza DB, Wilson RR, Mitchell BF, Olson DM2006. The interleukin 1β-induced expression of human prostaglandin F2α receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFκB. Biol Reprod 75:697–704 [DOI] [PubMed] [Google Scholar]
- 32.Willets JM, Shaw H, Challiss RAJ, Konje JC2008. G protein-coupled receptor kinase (GRK) 6 selectively regulates oxytocin receptor responsiveness in ULTR human myometrial smooth muscle cells. Reprod Sci 15:64a–64a (Abstract) [Google Scholar]
- 33.Willets JM, Taylor AH, Shaw H, Konje JC, Challiss RA2008. Selective regulation of H1 histamine receptor signaling by G protein-coupled receptor kinase 2 in uterine smooth muscle cells. Mol Endocrinol 22:1893–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brighton PJ, Willets JM, Konje JC2008. Anandamide activates extracellular regulated kinase signaling in ULTR human myometrial cells. Reprod Sci 15:112a–112a (Abstract) [Google Scholar]
- 35.Willets JM, Mistry R, Nahorski SR, Challiss RA2003. Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell M3 muscarinic acetylcholine receptor signaling. Mol Pharmacol 64:1059–1068 [DOI] [PubMed] [Google Scholar]
- 36.Willets JM, Nahorski SR, Challiss RA2005. Roles of phosphorylation-dependent and -independent mechanisms in the regulation of M1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 in hippocampal neurons. J Biol Chem 280:18950–18958 [DOI] [PubMed] [Google Scholar]
- 37.Smith MP, Ayad VJ, Mundell SJ, McArdle CA, Kelly E, López Bernal A2006. Internalization and desensitization of the oxytocin receptor is inhibited by dynamin and clathrin mutants in human embryonic kidney 293 cells. Mol Endocrinol 20:379–388 [DOI] [PubMed] [Google Scholar]
- 38.Blanks AM, Thornton S2003. The role of oxytocin in parturition. BJOG 110(Suppl 20):46–51 [DOI] [PubMed] [Google Scholar]
- 39.Dromey JR, Pfleger KD2008. G protein-coupled receptors as drug targets: the role of β-arrestins. Endocr Metab Immune Disord Drug Targets 8:51–61 [DOI] [PubMed] [Google Scholar]
- 40.Premont RT, Gainetdinov RR2007. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 69:511–534 [DOI] [PubMed] [Google Scholar]
- 41.Soloff MS, Alexandrova M, Fernstrom MJ1979. Oxytocin receptors: triggers for parturition and lactation? Science 204:1313–1315 [DOI] [PubMed] [Google Scholar]
- 42.Slater DM, Dennes WJ, Campa JS, Poston L, Bennett PR1999. Expression of cyclo-oxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod 5:880–884 [DOI] [PubMed] [Google Scholar]
- 43.Balducci J, Risek B, Gilula NB, Hand A, Egan JF, Vintzileos AM1993. Gap junction formation in human myometrium: a key to preterm labor? Am J Obstet Gynecol 168:1609–1615 [DOI] [PubMed] [Google Scholar]
- 44.Garfield RE, Sims S, Daniel EE1977. Gap junctions: their presence and necessity in myometrium during parturition. Science 198:958–960 [DOI] [PubMed] [Google Scholar]
- 45.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG2001. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J Biol Chem 276:19452–19460 [DOI] [PubMed] [Google Scholar]
- 46.Rezapour M, Bäckström T, Ulmsten U1996. Myometrial steroid concentration and oxytocin receptor density in parturient women at term. Steroids 61:338–344 [DOI] [PubMed] [Google Scholar]
- 47.Zhou XB, Lutz S, Steffens F, Korth M, Wieland T2007. Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and non-pregnant rat uterine myocytes: implications for myometrial contractility. Mol Endocrinol 21:740–752 [DOI] [PubMed] [Google Scholar]
- 48.Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E1991. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol 165:1515–1523 [DOI] [PubMed] [Google Scholar]
- 49.Sanborn BM, Ku CY, Shlykov S, Babich L2005. Molecular signaling through G-protein-coupled receptors and the control of intracellular calcium in myometrium. J Soc Gynecol Invest 12:479–487 [DOI] [PubMed] [Google Scholar]
- 50.Sanborn BM2007. Hormonal signaling and signal pathway crosstalk in the control of myometrial calcium dynamics. Semin Cell Dev Biol 18:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan W, Lopez Bernal A2007. Cyclic AMP signalling pathways in the regulation of uterine relaxation. BMC Pregnancy Childbirth 7(Suppl 1):S10 [DOI] [PMC free article] [PubMed]
- 52.Sanborn BM2000. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Invest 7:4–11 [DOI] [PubMed] [Google Scholar]
- 53.Fu X, Favini R, Kindahl K, Ulmsten U2000. Prostaglandin F2α-induced Ca2+ oscillations in human myometrial cells and the role of RU 486. Am J Obstet Gynecol 182:582–588 [DOI] [PubMed] [Google Scholar]
- 54.Jenkinson S, Patel N, Nahorski SR, Challiss RA1993. Comparative effects of lithium on the phosphoinositide cycle in rat cerebral cortex, hippocampus, and striatum. J Neurochem 61:1082–1090 [DOI] [PubMed] [Google Scholar]
- 55.Willets J, Kelly E2001. Desensitization of endogenously expressed δ-opioid receptors: no evidence for involvement of G protein-coupled receptor kinase 2. Eur J Pharmacol 431:133–141 [DOI] [PubMed] [Google Scholar]