Abstract
Endometriosis is a benign chronic gynecological disease of reproductive-age women characterized by the presence of functional endometrial tissues outside the uterine cavity. It is an estrogen-dependent disease. Current treatment modalities to inhibit biosynthesis and actions of estrogen compromise menstruation, pregnancy, and the reproductive health of women and fail to prevent reoccurrence of disease. There is a critical need to identify new specific signaling modules for non-estrogen-targeted therapies for endometriosis. In our previous study, we reported that selective inhibition of cyclooxygenase-2 prevented survival, migration, and invasion of human endometriotic epithelial and stromal cells, which was due to decreased prostaglandin E2 (PGE2) production. In this study, we determined mechanisms through which PGE2 promoted survival of human endometriotic cells. Results of the present study indicate that 1) PGE2 promotes survival of human endometriotic cells through EP2 and EP4 receptors by activating ERK1/2, AKT, nuclear factor-κB, and β-catenin signaling pathways; 2) selective inhibition of EP2 and EP4 suppresses these cell survival pathways and augments interactions between proapoptotic proteins (Bax and Bad) and antiapoptotic proteins (Bcl-2/Bcl-XL), facilitates the release of cytochrome c, and thus activates caspase-3/poly (ADP-ribose) polymerase-mediated intrinsic apoptotic pathways; and 3) these PGE2 signaling components are more abundantly expressed in ectopic endometriosis tissues compared with eutopic endometrial tissues during the menstrual cycle in women. These novel findings may provide an important molecular framework for further evaluation of selective inhibition of EP2 and EP4 as potential therapy, including nonestrogen target, to expand the spectrum of currently available treatment options for endometriosis in women.
Selective blockade EP2 and EP4 receptors suppresses cell survival and activates intrinsic apoptotic pathways thereby inhibiting survival of human endometriotic epithelial and stromal cells.
Endometriosis is a common benign chronic gynecological disease of reproductive-age women characterized by the presence of functional endometrial tissues outside the uterine cavity. More commonly, endometriosis lesions are found in the pelvic cavity/peritoneal organs where these tissues respond to the menstrual hormonal changes and menses (1). The prevalence of this disease is approximately 10–20%, depending on the population of women studied and diagnostic methods used, and increases to 20–30% in women with subfertility and 40–60% in women with dysmenorrhea or severe menstrual pain (2). Two major symptoms of endometriosis are intolerable pelvic pain and infertility, which profoundly affect the quality life in women of reproductive age (1, 2). Despite its high prevalence, pathogenesis of endometriosis is largely unknown. The most widely accepted theory is that the viable endometrial tissue fragments are refluxed through the oviducts into the pelvic cavity during retrograde menstruation (3). Endometriosis has been traditionally viewed as an estrogen-responsive disease (1, 4, 5); however, a recent report suggests that endometriosis is also a progesterone-unresponsive disease (6).
Current treatment strategies are surgical intervention, medical therapy, or a combination of both. After surgical removal of endometriosis lesions, the disease reestablishes within 3–5 yr in approximately 30–50% of women. Surprisingly, the disease reoccurs in approximately 10% of women who had uterus and both ovaries removed (7). Hormonal therapy to induce a hypoestrogenic state through the use of oral contraceptives, progestagens, and GnRH analogs and androgenic agents can be prescribed only for a short time due to unacceptable side effects, pseudomenopause, and bone density loss in reproductive-age women (1, 2, 7). Nevertheless, the recurrence rate is approximately 50–60% after cessation of therapy within a year (7). Furthermore, two apparently expensive unsuccessful clinical trials on the use of fulvestrant, an estrogen receptor antagonist, and raloxifene, a selective estrogen receptor modulator, to inhibit estrogen actions for the treatment of endometriosis in women were discontinued due to unfavorable outcomes (7). Together, existing treatment modalities fail to prevent reoccurrence of disease and affect pregnancy and reproductive health of women. This suggests a crucial need to identify potential cell signaling pathways for targeted therapies, including nonestrogen targets, for endometriosis.
Lack of information on molecular endocrinology of human endometriotic cells remains one of the major limitations to identify potential targeted therapies for this disease (7, 8). A growing body of evidence indicates that prostaglandins (PGs) contribute to the pathophysiology/pathogenesis of endometriosis (9, 10, 11, 12, 13, 14). Concentrations of PGE2 in peritoneal fluid are higher in women suffering from endometriosis compared with disease-free women (15), and this increased PGE2 is considered to be involved in endometriosis-associated pain (9). Data from our laboratory and others have shown that cyclooxygenase-2 (COX-2) is more abundantly expressed in ectopic endometriotic tissues compared with eutopic endometrial tissues during the menstrual cycle in women (11, 13, 14). A placebo-controlled double-blinded study reported that selective COX-2 inhibitor rofecoxib at 25 mg/d for 6 months effectively suppressed the pelvic pain symptoms in endometriosis patients in Europe (16). However, no clinical trial has been approved to test the use of COX-2 inhibitors for the treatment of endometriosis in women in the United States. In an animal model for endometriosis, selective COX-2 inhibitor celecoxib decreased establishment of endometriosis and number and size of endometriotic implants in rat model (17), and selective COX-2 inhibitor NS-398 induced regression of endometriotic implant through caspase-3-dependent apoptosis in a hamster model (10). However, nonselective or partially selective COX-2 inhibitor nimesulide failed to decrease number and size of endometriotic lesions in a nude mouse model (18), and the lack of effect could be due to low dose and duration of treatment and ability of nimesulide to inhibit COX-2 activity in endometriotic implants compared with celecoxib or NS-398 (19). In vitro studies indicated that PGE2 regulates genes involved in steroid biosynthesis and estradiol production, and in turn, estradiol increases COX-2-derived PGE2 production by establishing a positive loop between PGE2 and estradiol in primary cultured endometriotic stromal cells and endometriosis lesions per se in humans (20, 21, 22). Celecoxib prevents growth and survival of primary cultured eutopic endometrial epithelial cells from endometriosis patients (23). We have recently found that selective inhibition of COX-2 using NS-398 prevents survival, migration, and invasion of human immortalized endometriotic epithelial cells 12Z and stromal cells 22B, which is associated with decreased PGE2 production (11). Collectively, these studies strongly indicate a prerequisite for COX-2/PGE2 in pathogenesis of endometriosis and inhibition of COX-2/PGE2 could emerge as a potential therapy for this disease in human.
Ablation of the COX-2 gene resulted in multiple reproductive failures in mice (24), and therapeutic use of COX-2 inhibitors resulted in undesirable cardiovascular side effects (25). There is a need to discover alternative targets downstream of COX-2 to inhibit actions of PGE2 selectively at its receptor level. Multifaceted actions of PGE2 are primarily mediated through G protein-coupled membrane receptors designated EP that include EP1, EP2, EP3, and EP4 (26). EP2 and EP4 receptors are coupled to G protein α-subunit s type (Gs) proteins that activate adenylate cyclase and generate cAMP, which in turn activates the protein kinase A pathway (26). One of the fascinating aspects of EP2 and EP4 signaling is its prone cross talk with the epidermal growth factor receptor (EGFR) and β-catenin pathways through protein kinase A (PKA)-dependent or -independent intracellular pathways (25, 27, 28, 29, 30, 31). The EP1 receptor is coupled to Gq protein and activates phospholipase C, which results in generation of two second messengers, inositol triphosphate, which liberates intracellular calcium (Ca2+), and diacylglycerol, which activates protein kinase C (26). There are four EP3 isoforms, EP3A, EP3B, EP3C, and EP3D, coupled to Gq, Gs, and Gi proteins. Activation of EP3 receptors produces a wide range of complex and even opposite actions from inhibition or induction of cAMP production to increases in Ca2+ and inositol triphosphate (26).
PGE2 is an important antiapoptotic mediator and prevents cells from undergoing programmed cell death or apoptosis by activating several cell survival and antiapoptotic pathways (25, 32, 33, 34). Human endometriotic cells are resistant to apoptosis, and this plays an intrinsic role in establishment and growth of endometriosis lesions in women (35, 36, 37). It has been proposed that medical strategies to intervene antiapoptotic or proapoptotic pathways may lead to identify effective treatment modalities for the treatment of endometriosis in women (35, 36, 37). Hence, understanding of functional association between PGE2 signaling and endometriotic cell apoptosis and survival will not only advance our current understanding of pathophysiology of endometriosis but also lead us to identify new specific signaling modules to inhibit growth and survival of endometriosis lesions in women.
The objectives of the present study were to 1) determine the PGE2 signaling network supporting survival of human endometriotic cells, 2) examine whether selective inhibition of PGE2 receptors can be used as potential molecular targets to induce apoptosis of human endometriotic cells, and 3) determine the spatial expression of PGE2 signaling components in ectopic endometriotic and eutopic endometrial tissues from women during the menstrual cycle. Our findings indicate that PGE2 promotes survival of human endometriotic cells through EP2 and EP4 receptors by transactivating EGFR, AKT (protein kinase B), ERK1/2, nuclear factor-κB (NFκB), and β-catenin pathways, and selective combined inhibition of EP2 and EP4 induces apoptosis of human endometriotic cells by compromising these survival pathways and activating intrinsic apoptosis pathways.
Results
Expression of PGE2 receptors in human endometriosis tissues
In deciphering PGE2 signaling in pathogenesis of endometriosis, first we determined relative expression of EP receptors in endometriosis and compared with endometrial tissues in women. Results (Fig. 1) indicated that EP2 and EP4 proteins were abundantly expressed (P < 0.05), EP1 protein was expressed at very low levels (P < 0.05), and EP3 protein was undetectable in endometriosis tissues (ectopic endometrium) and endometrial tissues (eutopic endometrium) from women with or without endometriosis during the proliferative phase of the menstrual cycle. Interestingly, relative spatial expression of EP2 and EP4 proteins in glandular epithelium and stroma were higher (P < 0.05) in ectopic compared with eutopic endometria; however, this increase was not significant between eutopic endometria from women with and without endometriosis. These results suggest that EP2 and EP4 might be the major PGE2 receptors that mediate PGE2 signaling in the pathogenesis of endometriosis in women.
Fig. 1.

Immunohistochemical localization of PGE2 receptors EP1, EP2, EP3, and EP4 proteins in ectopic and eutopic endometria in women during the proliferative phase of the menstrual cycle. 1–4, Ectopic endometria; 5–8. eutopic endometria from women with endometriosis; 9–12, eutopic endometria from women without endometriosis; 13–16, negative controls (control serum or IgG); 17, densitometry of relative spatial expression of EP1, EP2, EP3, and EP4 receptors in glandular epithelium (GLE) and stroma (STR), quantified using Image-Pro Plus. *, P < 0.05, ectopic vs. eutopic endometria; n = 12. Relative spatial expression of EP2 and EP4 proteins in glandular epithelium and stroma were higher in ectopic compared with eutopic endometria. Immunohistochemistry was performed using Vectastain Elite ABC kit and representative photomicrographs at ×40 magnification are shown.
Selective inhibition of EP2 and EP4 induces apoptosis of human endometriotic cells
We used the well-characterized human immortalized endometriotic epithelial cells 12Z and stromal cells 22B as a model system (see Materials and Methods for more details about these cells) to understand PGE2 signaling in the pathogenesis of endometriosis in women. These endometriotic cells produce large amounts of PGE2 and inhibition of PGE2 biosynthesis by a selective COX-2 inhibitor induces apoptosis of these cells (11, 38, 39). PGE2 receptors EP2 and EP4 are abundantly expressed, EP1 is expressed at a very low level, and EP3 is undetectable (11, 38, 39) in these endometriotic cells. Therefore, we inhibited signaling of EP1, EP2, or EP4 using pharmacological and genomic approaches. The inhibitors used for EP1 (SC19220), EP2 (AH6809), and EP4 (AH23848) competitively bind with the respective EP receptors and inhibit their activation (40, 41, 42).
Results (Fig. 2, A–P) of the terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) assay using flow cytometry indicated that pharmacological inhibition (Fig. 2, A, C, and E–H) of either EP2 or EP4 signaling induced (P < 0.05) apoptosis of 12Z and 22B cells approximately 55 and 45%, respectively. Combined inhibition of EP2 and EP4 produced synergistic effects and induced apoptosis of 12Z and 22B cells approximately 85 and 75%, respectively. Interestingly, inhibitory effects of EP2 and EP4 on apoptosis were about 10% higher (P < 0.05) in 12Z cells compared with 22B cells. Inhibition of EP1 signaling did not induce apoptosis of 12Z and 22B cells. Consistent with these results, TUNEL assay based on fluorescence microscopy clearly showed that inhibition of EP2 and EP4 signaling induced DNA fragmentation in both 12Z and 22B cells (Fig. 2, I–L).
Fig. 2.
Selective inhibition of EP2 and EP4 induces apoptosis of human endometriotic epithelial cells 12Z (panel 1) and stromal cells 22B (panel 2): A–D, TUNEL assay based on flow cytometry: A and C, pharmacological inhibition of EP1, EP2, and EP4; B and D, EP2 and EP4 siRNA. E–H, Representative DNA histogram. Cells under M1 showed apoptotic cell population. I–L, TUNEL assay based on fluorescence microscopy. Representative photomicrographs at ×40 magnification are shown. Arrows show DNA fragments in TUNEL-labeled cells. The cells were treated with EP-I for EP1 (SC19220, 100 μm), EP2 (AH6809, 75 μm), and EP4 (AH23848, 50 μm) for 24 h. *, P < 0.05, control vs. EP-I or siRNA; n = 3. Inhibition of EP2, EP4, or EP2/EP4 increases apoptosis in 12Z and 22B cells. M–P, Effects of EP2 or EP4 siRNA on expression of EP2 and EP4 proteins at 96 h after siRNA. **, P < 0.05, control (Cont) vs. EP2 siRNA; ***, P < 0.05, control vs. EP4 siRNA; n =3 (see Materials and Methods for siRNA details).
Then, we used a small interfering RNA (siRNA) approach to knock down EP2 and EP4 genes to confirm their roles in the survival of the human endometriotic cells. Gene silencing using SMARTpool siRNA approach resulted in efficient knockdown of EP2 and EP4 genes and resulted in decreased expression of EP2 and EP4 proteins up to 70–80% in both 12Z and 22B cells (Fig. 2, M–P). Effects of silencing of EP2 or EP4 genes on induction of apoptosis of human 12Z and 22B cells were similar to those involving pharmacological inhibition of EP2 and EP4 signaling. Double knockdown of EP2 and EP4 genes resulted in synergistic effects and induced (P < 0.05) apoptosis approximately 75% in 12Z cells and 65% in 22B cells (Fig. 2, B and D).
Together, these results indicate that PGE2 promotes survival of human endometriotic cells 12Z and 22B primarily through EP2 and EP4 receptors, and selective inhibition of EP2 and EP4 receptors induced apoptosis of 12Z and 22B cells. Furthermore, these results suggest the existence of a compensatory mechanism between EP2 and EP4 receptors in mediating PGE2 signaling in human endometriotic cells, and combined inhibition of both EP2 and EP4 is obligatory to understand PGE2 signaling in the pathogenesis of endometriosis in humans. Based on these data, pharmacological inhibition of both EP2 and EP4 signaling was further evaluated considering therapeutic use of EP2 and EP4 inhibitors for the treatment of endometriosis.
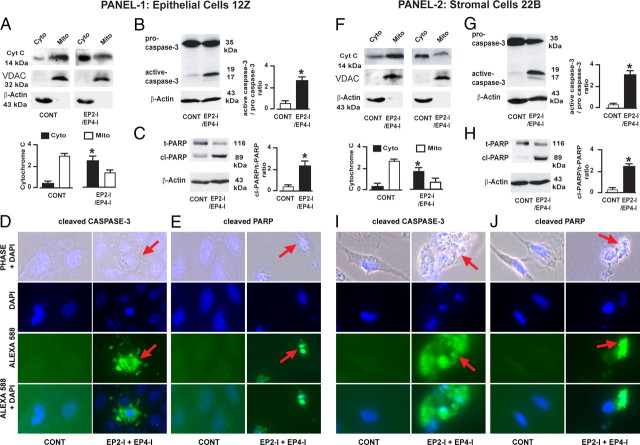
Selective inhibition of EP2 and EP4 releases cytochrome c and activates caspase-3 and poly (ADP-ribose) polymerase (PARP) in human endometriotic cells
To understand the molecular mechanisms by which inhibition of EP2- and EP4-mediated PGE2 signaling resulted in apoptosis of human endometriotic cells, we determined interactions between PGE2 signaling and apoptotic machinery. Release of cytochrome c from mitochondria into the cytosol and activation of caspase-3 and nuclear PARP enzymes are important terminal events that promote apoptosis of cells (43). Results (Fig. 3, A–J) indicated that combined inhibition of EP2 and EP4 facilitated release (P < 0.05) of cytochrome c from mitochondria into cytosol (Fig. 3, A and F) and activated/cleaved (P < 0.05) caspase-3 (B and G) and PARP (C and H) proteins in 12Z and 22B cells. Consistent with these results, fluorescence microscopy analyses clearly showed that inhibition of EP2 and EP4 induced activation of caspase-3 (Fig. 3, D and I) and PARP (E and J) proteins in 12Z and 22B cells. These results together suggest that selective inhibition of EP2 and EP4 induces apoptosis of human endometriotic cells through cytochrome c/caspase-3/PARP pathways.
Fig. 3.
Selective inhibition of EP2 and EP4 activates the cytochrome c/caspase-3/PARP pathway in the human endometriotic epithelial cells 12Z (panel 1) and stromal cells 22B (panel 2). A and F, Western blot of cytochrome c; B and G, Western blot of caspase-3; C and H, Western blot of PARP; D, E, I, and J, immunofluorescence analysis; D and I, cleaved caspase-3; E and J, cleaved PARP. Arrows show cytosolic and nuclear localization of caspase-3 and PARP proteins, respectively, in cells undergoing apoptosis. Mitochondrial-specific voltage-dependent anion channel (VDAC) and cytosolic-specific β-actin were measured as internal control proteins. *, P < 0.05, control (CONT) vs. EP2-I/EP4-I; n = 3. The cells were treated with EP-I for EP2 (AH6809, 75 μm) and EP4 (AH23848, 50 μm) for 24 h. Inhibition of EP2 and EP4 facilitated release of cytochrome c (Cyt-c) from mitochondria (Mito) into cytosol (Cyto) and activated/cleaved caspase-3 and PARP proteins in 12Z and 22B cells.
Selective inhibition of EP2 and EP4 augments interactions between antiapoptotic and proapoptotic proteins in human endometriotic cells
The balance between antiapoptotic proteins [B-cell lymphoma 2 (Bcl-2) and basal cell lymphoma-extra large (Bcl-XL)] and proapoptotic proteins [Bcl-2-associated death promoter (Bad) and Bcl-2-associated X protein (Bax)] determines whether cells live or die (44). In the absence of apoptotic stimuli, Bax and Bad protein phosphorylated at serine 112 and 136 bind with 14-3-3 proteins and are sequestered in the cytosol (45). In response to apoptotic stimuli, Bad and/or Bax proteins are dissociated from 14-3-3 proteins, translocate from cytosol to the mitochondrial outer membrane, and dimerize with Bcl-XL and/or Bcl-2 proteins and thereby facilitate the release of cytochrome c (43, 44, 45). Therefore, we determined whether inhibition of EP2- and EP4-mediated PGE2 signaling augments interactions between Bcl-2/Bcl-XL and Bad/Bax proteins in human endometriotic cells. Results (Fig. 4, A–H) demonstrated that inhibition of EP2 and EP4 decreased (P < 0.05) expression of Bcl-2 and Bcl-XL proteins (A and E), increased (P < 0.05) expression of Bax protein, and dephosphorylated (P < 0.05) Bad protein at serine 112 and 136 sites (B and F) in 12Z and 22B cells. Furthermore, immunoprecipitation results (Fig. 4, C, D, G, and H) indicated that inhibition of EP2 and EP4 increased (P < 0.05) interactions between Bax and Bcl-2, Bax and Bcl-XL, Bad and Bcl-2, and Bad and Bcl-XL proteins in 12Z and 22B cells. These results together suggest that selective inhibition of EP2 and EP4 augments interactions between antiapoptotic and proapoptotic proteins and thereby activates intrinsic apoptotic pathways in human endometriotic cells.
Fig. 4.
Selective inhibition of EP2 and EP4 augments interactions between antiapoptotic and proapoptotic proteins in the human endometriotic epithelial cells 12Z (panel 1) and stromal cells 22B (panel 2). A and E, Western blot of Bcl-2 and Bcl-XL; B and F, Western blot of Bax and Bad; C, D, G, and H, immunoprecipitation/Western blot; C and G, interaction between Bax and Bcl2 or Bcl-XL; D and H, interaction between Bad and Bcl2 or Bcl-XL. β-Actin was measured as an internal control. *, P < 0.05, control (CONT) vs. EP2-I/EP4-I; n = 3. The cells were treated with EP-I for EP2 (AH6809, 75 μm) and EP4 (AH23848, 50 μm) for 24 h. Inhibition of EP2 and EP4 decreased expression of Bcl-2 and Bcl-XL, increased expression of Bax, dephosphorylated Bad at serine 112 and 136 sites, and increased interactions between Bax and Bcl-2, Bax and Bcl-XL, Bad and Bcl-2, and Bad and Bcl-XL proteins in 12Z and 22B cells.
PGE2 signaling network
PGE2 transactivates EGFR signaling through a tyrosine kinase enzyme (c-Src)/β-arrestin 1 complex that in turn activates ERK1/2 and phosphoinositide-3 kinase-AKT pathways (25, 27, 28, 31), activates Wnt/β-catenin signaling pathways through binding with the regulator of G protein signaling (RGS) domain of axin and AKT-mediated phosphorylation/inactivation of glycogen synthase kinase-3β (GSK3β) (29, 30), and interacts with NFκB pathways (25) in malignant tumor cells. EGFR signaling phosphorylates Bad protein through ERK1/2 and AKT pathways (46, 47). NFκB signaling increases expression of antiapoptotic proteins Bcl2 and Bcl-XL (48). The β-catenin pathway interacts with proapoptotic and antiapoptotic proteins directly or indirectly (49). Therefore, we sought to determine interactions between PGE2 signaling and EGFR, NFκB, and β-catenin pathways to understand how these survival pathways are compromised to facilitate apoptosis in human endometriotic cells.
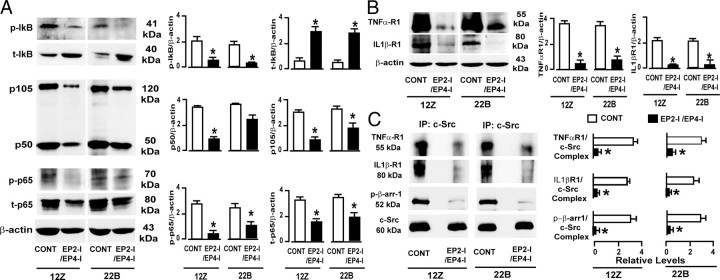
First, we examined whether selective inhibition of EP2/EP4 affects EGFR signaling pathways in human endometriotic cells. It is well known that activation/phosphorylation of EGFR in turn triggers Ras-Raf-ERK1/2 and phosphoinositide-3 kinase-AKT signaling modules, and activation of EGFR protein can be inhibited by dephosphorylation and/or by down-regulation (50). Results (Fig. 5A) indicated that inhibition of EP2 and EP4 dephosphorylated and down-regulated (P < 0.05) EGFR protein in 12Z and 22B cells. ERK1/2 protein was dephosphorylated (P < 0.05) in 12Z cells but not in 22B cells. Interestingly, AKT protein was dephosphorylated (P < 0.05) in both 12Z and 22B cells. To determine whether this cross talk between EP2/EP4 and EGFR is mediated through c-Src and β-arrestin 1, we studied their expression levels. Results (Fig. 5B) demonstrated that inhibition of EP2 and EP4 dephosphorylated (P < 0.05) c-Src and β-arrestin 1 proteins in 12Z and 22B cells. Next, we determined interactions among EP2, EP4, c-Src, β-arrestin 1, and EGFR to gain further mechanistic insights. Results (Fig. 5C) indicated that inhibition of EP2 and EP4 decreased (P < 0.05) interactions among EP2/EP4, c-Src, β-arrestin 1, and EGFR proteins in 12Z and 22B cells. Taken together, these results suggest that PGE2 transactivates EGFR through EP2 and EP4 receptors, and this signaling is transmitted intracellularly through a c-Src/β-arrestin 1 complex in human endometriotic cells.
Fig. 5.
PGE2 transactivates EGFR in the human endometriotic epithelial cells 12Z and stromal cells 22B. A, Western blot of EGFR, ERK1/2, and AKT; B, Western blot of c-Src and β-arrestin 1; C, immunoprecipitation (IP)/Western blot showed the interactions among EP2, EP4, EGFR, c-Src, and β-arrestin (β-arr 1) 1. β-Actin was measured as an internal control. *, P < 0.05, control (CONT) vs. EP2-I/EP4-I; n = 3. The cells were treated with EP-I for EP2 (AH6809, 75 μm) and EP4 (AH23848, 50 μm) for 24 h. Inhibition of EP2 and EP4 affected phosphorylation of EGFR, ERK1/2, AKT, c-Src, and β-arrestin 1 in a cell-specific manner and decreased interactions among EP2/ EP4, c-Src, β-arrestin 1, and EGFR proteins in 12Z and 22B cells.
Second, we examined whether selective inhibition of EP2/EP4-mediated PGE2 signaling affects NFκB signaling cascades in human endometriotic cells. Heterodimer complex of p50/p65 is the most common active form of NFκB in the majority of cells. In the absence of NFκB stimuli, p50 and p65 proteins are sequestered in the cytoplasm with their inhibitory protein κBα (IκBα) and form an inactive protein complex, p50/p65/IκB. In response to cytokines TNFα and IL-1β or other stimuli, IκBα protein is phosphorylated and targeted for degradation and allows formation of active p50/p65 heterodimer and translocation into the nucleus (48). Results (Fig. 6A) indicated that inhibition of EP2 and EP4 1) dephosphorylated (P < 0.05) IκBα, 2) decreased (P < 0.05) phosphorylation and abundance of p65 protein, and 3) decreased (P < 0.05) abundance of precursor p105 in 12Z and 22B cells, whereas it decreased (P < 0.05) abundance of active p50 proteins only in 12Z cells but not in 22B cells. Next, we determined whether this NFκB signaling complex is dependent or independent of TNFα receptor 1 (TNFαR1) and/or IL-1βR1 involvement. Results (Fig. 6B) demonstrated that inhibition of EP2 and EP4 decreased (P < 0.05) expression of TNFαR1 and IL-1βR1 in 12Z and 22B cells. This led us to explore interactions between EP2/EP4 and TNFαR1 or IL-1βR1 to derive further mechanistic insights. We hypothesized that c-Src/β-arrestin 1 protein complex may mediate cross talk between EP2/EP4 and TNFαR1 or IL-1βR1 in 12Z and 22B cells, because this protein complex is involved in mediating cross talk between EP2/EP4 and EGFR in these cells. In support of this notion, immunoprecipitation results (Fig. 6C) clearly demonstrated that inhibition of EP2 and EP4 decreased (P < 0.05) interaction between the c-Src/β-arrestin 1 complex and TNFαR1 or IL-1βR1 in 12Z and 22B cells. These results together suggest that PGE2 activates NFκB pathways through EP2 and EP4 receptors by transactivating TNFαR1 and IL-1βR1, and this signaling is routed intracellularly through a c-Src/β-arrestin 1 complex in human endometriotic cells. Furthermore, selective inhibition of EP2- and EP4-mediated PGE2 signaling disturbs NFκB signaling pathways at multiple levels, that is, at processing of active p50, dephosphorylation of p65, and dephosphorylation and stabilization of IκBα proteins in a cell-specific manner. It appears that stabilization of IκBα is the key step that could result in reestablishment of inactive complex p50/p65/IκB and affect the NFκB downstream signal in both 12Z and 22B cells.
Fig. 6.
PGE2 transactivates NFκB pathways in the human endometriotic epithelial cells 12Z and stromal cells 22B. A, Western blot of IκB, p105/p50, and p65; B, Western blot of TNFαR1 and IL-1βR1; C, immunoprecipitation/Western blot showed the interactions among TNFαR1, IL-1βR1, c-Src, and β-arrestin 1 (β-arr 1). β-Actin was measured as an internal control. *, P < 0.05, control vs. EP2-I/EP4-I; n = 3. The cells were treated with EP-I for EP2 (AH6809, 75 μm) and EP4 (AH23848, 50 μm) for 24 h. Inhibition of EP2 and EP4 dephosphorylated IκBα and p65; decreased expression of p105, p50, TNFαR1, and IL-1βR1; and decreased interaction between c-Src/β-arrestin 1 complex and TNFαR1 or IL-1βR1 proteins in 12Z and 22B cells.
Third, we examined whether selective inhibition of EP2/EP4-mediated PGE2 signaling affects β-catenin pathways in human endometriotic cells. In the absence of Wnt/β-catenin or other stimuli, β-catenin is sequestered in the cytosol by a destruction complex consisting of GSK3β, axin, and adenomatous polyposis coli (APC) and targeted for degradation. In response to Wnt/β-catenin stimuli, β-catenin is released from this destruction complex, translocates into the nucleus, and forms a transcriptionally active complex with the Tcf/Lef family of transcription factors (49). Results (Fig. 7A) indicated that inhibition of EP2 and EP4 resulted in degradation (P < 0.05) of active β-catenin in 12Z and 22B cells, decreased (P < 0.05) expression of β-catenin binding transcriptional factors T-cell factor (TCF)-1 and TCF-4 in 12Z and 22B cells and did not decrease LEF-1 in both cells. Next, we examined whether this degradation of β-catenin is due to changes in destruction complex proteins GSK3β, axin, and Gs. Results (Fig. 7B) indicated that inhibition of EP2 and EP4 dephosphorylated (P < 0.05) GSK3β in 12Z cells and but not in 22B cells and did not affect relative expression of either Gs or axin proteins in 12Z and 22B cells. Then, we examined the mechanisms by which inhibition of EP2- and EP4-mediated PGE2 signaling led to destruction of β-catenin. First, we examined the Gs/axin/β-catenin axis. Results (Fig. 7C) indicated that inhibition of EP2 and EP4 decreased (P < 0.05) interactions between axin and Gs and increased association between axin and β-catenin in 12Z and 22B cells. Second, we examined the GSK3β/β-catenin axis. Results (Fig. 7D) indicated that inhibition of EP2 and EP4 increased interactions between β-catenin and GSK3β in 12Z but not in 22B cells. Next, the basis for cell-specific differences of EP2- and EP4-mediated PGE2 signaling on regulation of GSK3β in 12Z and 22B cells was analyzed. We hypothesized that one of the constitutively active signaling pathways might contribute to this cell-specific regulation of GSK3β in 22B cells. Previous studies have reported that GSK3β is regulated by AKT and ERK1/2 pathways in cancer cells (30, 51). The results shown in Fig. 5 indicated that in response to inhibition of EP2 and EP4, AKT but not the ERK1/2 pathway was inhibited in 22B compared with 12Z cells; therefore, we examined interactions among GSK3β, ERK1/2, and AKT. In support of this notion, immunoprecipitation results (Fig. 7D) demonstrated that inhibition of EP2 and EP4 decreased (P < 0.05) interactions between GSK3β and AKT and/or ERK1/2 in 12Z cells. Although in 22B cells, inhibition EP2 and EP4 decreased (P < 0.05) interactions between GSK3β and AKT but failed to decrease interaction between GSK3β and ERK1/2. These results together suggest that selective inhibition of EP2 and EP4 degrades β-catenin in a cell-specific manner by impairing Gs/axin/β-catenin, ERK1/2/GSK3β/β-catenin, and AKT/GSK3β/β-catenin signaling modules in 12Z cells and Gs/axin/β-catenin and AKT/GSK3β/β-catenin signaling modules in 22B cells.
Fig. 7.
PGE2 transactivates β-catenin pathways in the human endometriotic epithelial cells 12Z and stromal cells 22B. A, Western blot of β-catenin (β-cate), TCF-1, TCF-4, and LEF-1; B, Western blot of p-GSKα/β, t-GSKα/β, axin, and Gs.β-Actin was measured as an internal control. C, Immunoprecipitation/Western blot showed the interactions among axin, Gs, and β-catenin; and D, interactions among GSK3β, p-ERK1/2, p-AKT, and β-catenin. *, P < 0.05, control (CONT) vs. EP2-I/EP4-I; n = 3. The cells were treated with EP-I for EP2 (AH6809, 75 μm) and EP4 (AH23848, 50 μm) for 24 h. Inhibition of EP2 and EP4 decreased active β-catenin, TCF-1, and TCF-4; dephosphorylated GSK3β; and affected interactions between axin and Gs, GSK3β and AKT, and GSK3β and ERK1/2 proteins in a cell-specific manner in 12Z and 22B cells.
Finally, we determined whether inhibition of EP2 and EP4 affects the important transcriptional factors associated with EGFR, AKT, ERK1/2, NFκB, and β-catenin signaling pathways in human endometriotic cells. Results (Fig. 8) indicated that inhibition of EP2 and EP4 decreased (P < 0.05) expression of c-fos, SP1, and early growth response factor-1 (EGR-1) and phosphorylation of c-myc and cAMP response element-binding proteins in 12Z and 22B cells. In contrast, decreased phosphorylation of c-Jun protein was observed in 12Z cells but not in 22B cells. These results suggest that EP2- and EP4-mediated PGE2 signaling regulate expression and activation of multiple transcriptional factors in human endometriotic cells.
Fig. 8.
PGE2 regulates expression of multiple transcriptional factors in human endometriotic epithelial cells 12Z and stromal cells 22B. Inhibition of EP2 and EP4 affects expression/activation of c-fos, c-Jun, c-myc, cAMP response element-binding (CREB), Sp1, and EGR-1 proteins in a cell-specific manner in 12Z and 22B cells. The cells were treated with EP-I for EP2 (AH6809, 75 μm) and EP4 (AH23848, 50 μm) for 24 h. β-Actin was measured as an internal control. *, P < 0.05, control (CONT) vs. EP2-I/EP4-I; n = 3.
Expression of PGE2 signaling components in endometriotic tissues in human
To conclude, we examined the relative expression of major PGE2 signaling components in endometriosis and endometrial tissues from women. Results (Fig. 9) indicated that Bcl2, Bcl-XL, p-Bad112, p-Bad136, p-EGFR, p-ERK1/2, p-AKT, p-IκB, and active β-catenin proteins were more abundantly expressed in endometriosis tissues during the proliferative phase of the menstrual cycle. Interestingly, the spatial relative expression of these proteins in glandular epithelium and stroma was higher (P < 0.05) in endometriosis (ectopic endometrium) compared with healthy endometrial tissues (eutopic endometrium). These results suggest that PGE2 signaling components associated with antiapoptosis/prosurvival pathways are abundantly expressed in endometriosis tissues in women.
Fig. 9.

Expression of PGE2 signaling components in ectopic and eutopic endometria during the proliferative phase of the menstrual cycle in women. 1 and 2, IgG or serum; 3 and 4, Bcl-2; 5 and 6, Bcl-XL; 7 and 8, p-Bad112; 9 and 10, p-Bad136; 11 and 12, p-EGFR; 13 and 14, p-ERK1/2; 15 and 16, p-AKT; 17 and 18, p-IκB; 19 and 20, active β-catenin; 21, densitometry of relative spatial expression of these proteins in glandular epithelium (GLE) and stroma (STR). *, P < 0.05, ectopic vs. eutopic endometria; n = 12. Relative spatial expression of PGE2 signaling proteins in glandular epithelium and stroma were higher in ectopic compared with eutopic endometria. Immunohistochemistry was performed using Vectastain Elite ABC kit, and representative photomicrographs at ×40 magnification are shown.
Discussion
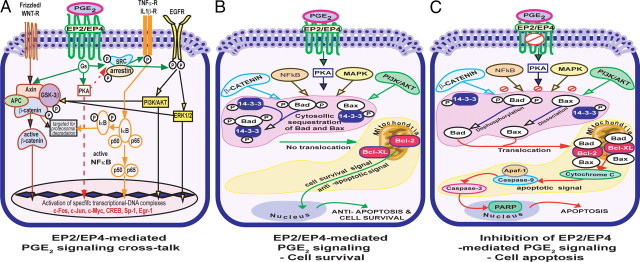
Involvement of prostaglandins in the pathogenesis of endometriosis has been reported for a long time; however, the underlying mechanisms are largely unknown. Analysis of PGE2-induced protection of human endometriotic cells from apoptosis has revealed a complex organization of signaling pathways. Our results strongly suggest that the ability of human endometriotic cells to circumvent apoptosis signals is associated with increased PGE2 signaling, abundant expression of Bcl2 and Bcl-XL proteins, low expression of Bax protein, phosphorylation/inactivation of Bad protein, and activation of multiple cell survival signaling pathways. Based on the results of the present study, we propose molecular mechanisms by which PGE2 promotes survival of human endometriotic cells as illustrated in Fig. 10. PGE2 regulates these complex molecular interactions and promotes survival of human endometriotic cells through EP2 and EP4 receptors by transactivating multiple complex signaling modules c-Src/β-arrestin 1/EGFR/ERK1/2 or AKT, c-Src/βarrestin 1/TNFαR1 and/or IL-1βR1/IκB/NFκB, Gsα/axin/β-catenin, ERK1/2/GSK3β/β-catenin, and AKT/GSK3β/β-catenin. Selective inhibition of EP2 and EP4 impairs these cell survival pathways and augments interactions between proapoptotic proteins (Bax/Bad) and antiapoptotic proteins (Bcl-2/Bcl-XL) facilitates the release of cytochrome c and thus activates caspase-3/PARP pathways.
Fig. 10.
A, PGE2-EP2/EP4 signaling network in the human endometriotic cells: PGE2 binds with EP2 and EP4 receptors. Upon activation, these receptors are coupled to Gs proteins and activate three major signaling pathways: 1) EP2/EP4 interacts with c-Src kinase and β-arrestin 1 protein complex and transactivates EGFR intracellularly, which in turn activates ERK1/2 and AKT pathways; 2) EP2/EP4 interacts with c-Src kinase and β-arrestin 1 and transactivates TNFαR1 and IL-1β1R intracellularly, which consecutively activates NFκB pathways (note that these interactions might be mediated through PKA-dependent or -independent mechanisms, which was not determined in this study); and 3) EP2/EP4 activates β-catenin pathways intracellularly by disestablishing Gs and axin complex directly and inhibiting GSK3β through AKT and ERK1/2 pathways. Transactivation of these three linear-cell signaling pathways activates specific transcriptional/DNA complexes. B, EP2/EP4-mediated PGE2 signaling leads to cell survival. PGE2 could transactivate ERK1/2, AKT, NFκB, and β-catenin pathways through EP2 and EP4 receptors. Activation of these cell survival pathways phosphorylates Bad protein at serine 112 and 136, sequestrates Bad and Bax proteins in the cytosol with 14-3-3 proteins, and prevents translocation of Bad and Bax from the cytosol to the mitochondria. This prevents interactions between Bax/Bad and Bcl-2/Bcl-XL and thereby promotes survival of human endometriotic cells. C, Inhibition of EP2/EP4-mediated PGE2 signaling leads to cell apoptosis. Selective inhibition of EP2 and EP4 impairs ERK1/2, AKT, NFκB, and β-catenin pathways. Inhibition of these cell survival pathways results in dephosphorylation of Bad protein at serine 112 and 136, dissociation of Bad and Bax from 14-3-3 proteins, permits translocation of Bad and Bax proteins from the cytosol to the mitochondria, and thereby augments interaction between Bax/Bad and Bcl-2/Bcl-XL proteins. These sequential interactions result in release of cytochrome c from the mitochondria into the cytosol and activation of caspase-3 and PARP enzymes and eventually culminate in apoptosis of human endometriotic cells.
The present study did not determine the sequential order of signal transduction through which selective inhibition of EP2 and EP4 receptors suppresses or activates these major cell signaling pathways in 12Z and 22B cells using spatial and temporal experiments, which is our future focus. Nevertheless, the data presented in this manuscript allowed us to speculate that activation of EP2/EP4 receptors by PGE2 in turn activates Src/β-arrestin 1 complex, which is the first step in EP2/EP4 signal transduction. The Src/β-arrestin 1 complex could then mediate transactivation between EP2/EP4 and EGFR or TNFα/ILβ receptors. At present, we do not know which of these receptors is activated first, however; we hypothesize that EGFR could be activated earlier than TNFα/ILβ receptors. Second, activation of EGFR and TNFα/ILβ receptors could result in activation/suppression of multiple downstream cell signaling pathways. Third, activated AKT and ERK could establish activation of β-catenin. Thus, PGE2 could integrate EGFR, NFκB, and β-catenin pathways intracellularly that in turn could activate or inactivate transcription of genes, translation of proteins, or protein-protein interactions in 12Z and 22B cells. Ongoing experiments are focused to dissect these signal transductions in 12Z and 22B cells.
The remarkable redundancy of signaling pathways that control interactions between proapoptotic and antiapoptotic proteins argues for the importance of inhibiting multiple pathways to prevent the survival of human endometriotic cells. Relative expressions of proteins involved in PGE2 signaling including EP2, EP4, p-Bad112, p-Bad136, Bcl2, Bcl-XL, p-ERK1/2, p-AKT, p-IκB, and β-catenin are significantly higher in ectopic endometriotic tissues compared with eutopic endometrial tissues in women in vivo, suggesting ERK1/2, AKT, NFκB, or β-catenin pathways are highly activated in endometriosis. Therefore, inhibition of each of these pathways is possible using selective inhibitors. However, the limitations of inhibiting a single pathway are 1) possible compensation by other linear survival pathways resulting in rescue of endometriotic cells from apoptosis and 2) complete inhibition of a single pathway by selective inhibitors may block/abolish the particular pathway required for normal reproductive, physiological, developmental, and homeostasis processes. The most exciting finding of the present study is that selective inhibition of EP2 and EP4 partially suppresses but does not abolish multiple signaling pathways including ERK1/2, AKT, NFκB, and β-catenin pathways. Furthermore, signal transduction pathways that promote cell survival are compromised, whereas pathways that promote cell apoptosis are augmented. Taken together, our data suggest that selective inhibition of EP2 and EP4 could potentially suppress the adverse effects of up-regulated ERK1/2, AKT, NFκB, and β-catenin pathways in the pathogenesis of endometriosis and could preserve the beneficial role of these important pathways in normal endometrial cell functions. Therefore, selective inhibition of EP2 and EP4 receptors has advantages over inhibition of ERK1/2, AKT, NFκB, or β-catenin pathways separately.
Interestingly, inhibition of EP2 and EP4 induces apoptosis to a greater degree in the endometriotic epithelial cells 12Z compared with stromal cells 22B. This cell-specific effect is similar to that of selective inhibition of COX-2 (11) in these cells. It appears that a difference in the level of suppression of ERK1/2, NFκB, or β-catenin pathways might be one of the reasons for increased survival of 22B compared with 12Z cells. In support of this notion, phosphorylation of ERK1/2, NFκB, phosphorylation of GSK3β, stability of β-catenin, and activation of c-jun are not completely suppressed by inhibition of EP2 and EP4 in 22B compared with 12Z cells. Microscopically, endometriosis lesions are characterized by the presence of endometriosis glands lined with cylindrical and flattened epithelial cells and surrounded by dense stromal cells. Variations in the growth patterns of endometriosis lesions are considered to be regulated by epithelial-stromal interactions (8, 52). One of the most interesting aspects of the present study is that selective inhibition of EP2 and EP4 inhibits survival of epithelial cells by 85% and stromal cells by 75%, suggesting the inhibition of epithelial-stromal interactions and formation of endometriosis glands. Molecular characterization of this complex organization of PGE2 signaling network is currently underway to determine cell-specific functional significance of individual ERK1/2, AKT, NFκB, or β-catenin pathway in survival of 12Z and 22B cells.
Expression of EP2 mRNA in endometrium was not modulated across the human menstrual cycle; however, EP4 mRNA expression was significantly higher in proliferative endometrium than in secretory endometrium (53). Because EP2/EP4 receptors are expressed in endometrium during the menstrual cycle in women, these receptors would be involved in mechanisms associated with menstruation, differentiation of endometrium after menstruation, and establishment of pregnancy. Whether pharmacological inhibition of EP2/EP4 receptors could affect these processes in women is not known. Interestingly, data from EP2 and EP4 knockout mice indicated that EP4 gene knockout mice were fertile (54), and EP2 gene knockout mice had normal ovulation and implantation but suffered from a fertilization defect that was overcome by in vitro fertilization (55). These data might not necessarily mirror the human condition because mice are nonmenstruating animals. The baboon could be the ideal preclinical model system to evaluate the effects of EP2/EP4 inhibitors on menstruation and pregnancy. Until a clinical trial has been conducted with the use of EP2/EP4 inhibitors for the treatment of endometriosis in pregnant or nonpregnant women, it would not be possible to predict whether this therapy would be safe or unsafe. Furthermore, we propose that if combined inhibition of EP2/EP4 affects menstruation and pregnancy in women, it could be possible to overcome or minimize this effect by blocking EP2 or EP4 independently to get 35–40% growth arrest of endometriotic cells. This would allow compensatory mechanisms between these two receptors to mediate the physiological functions and thus would allow menstruation and successful pregnancy in endometriosis women.
The advantages of selective inhibition of EP2 and EP4 might include but are not limited to 1) inhibition of growth and survival of endometriotic cells; 2) decreased PGE2-induced inflammation and pain; 3) a permissive action on menstruation, ovulation, and pregnancy; and 4) the absence of a hypoestrogenic state or temporary menopause in reproductive-age women resulting in improved reproductive health. These are the goals for endometriosis therapies envisioned for several decades. Based on our results, we speculate that inhibition of EP2/EP4 receptors could emerge as a potential therapy for treatment of endometriosis, preferably stages I and II (active phase of disease characterized by red peritoneal lesions). Current studies in our laboratory are targeted to analyze the therapeutic use of EP2 and EP4 inhibitors for the treatment of endometriosis using xenograft mice models.
In conclusion, results of the present study for the first time indicate that PGE2 promotes survival of human endometriotic cells through EP2 and EP4 by activating multiple cell survival signaling pathways. Selective and combined inhibition of EP2 and EP4 impairs these survival pathways and activates intrinsic apoptotic pathways and thereby induces apoptosis of human endometriotic cells. Inhibition of EP2- and EP4-mediated signaling could emerge as a potential non-estrogen-targeted therapy for the treatment of endometriosis in women. Results of the present study suggest potential opportunities for translational studies that could lead to preclinical and clinical-phase trials.
Materials and Methods
Materials
The following reagents used in this study were purchased from the following suppliers: prestained protein markers and Bio-Rad assay reagents and standards (Bio-Rad Laboratories, Hercules, CA); Protran BA83 nitrocellulose membrane (Whatman Inc., Sanford, ME); Pierce ECL and mitochondria isolation kit (Pierce Biotechnology, Rockford, IL); protease inhibitor cocktail tablets complete EDTA-free and PhosStop (Roche Applied Biosciences, Indianapolis, IN); antibiotic-antimycotic, and trypsin-EDTA, Alexa Fluor 488, APO-BrdU TUNEL assay kit, and ProLong Gold antifade reagent (Invitrogen Life Technologies Inc., Carlsbad, CA); Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, CA); Blue X-Ray film (Phenix Research Products, Hayward, CA); fetal bovine serum (FBS) (HyClone, Logan, UT); Lab-Tek II chamber slides (Nunc, Rochester, NY), and tissue culture dishes and plates (Corning Inc., Corning, NY). EP2 and EP4 siRNA, siGLORISC-free siRNA and DharmaFect-1 were obtained from Dharmacon Inc., Lafayette, CO. Antagonists/inhibitors for EP1 (SC19220), EP2 (AH6809), EP4 (AH23848), and EP1, EP2, EP3, and EP4 antibodies were purchased from Cayman Chemicals (Ann Arbor, MI). All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA) or Santa Cruz Biotechnology (Santa Cruz, CA) except β-actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO), goat antirabbit or antimouse IgG conjugated with horseradish peroxidase (Kirkegaard & Perry Laboratories, Gaithersburg, MD). The chemicals used were molecular biological grade from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich.
Human endometriotic cells and culture
Clinically, endometriosis lesions are classified into red, white, and bluish-black lesions. The red lesions are highly vascularized and extremely proliferative, adhesive, and invasive and represent the active/progressive phase of the disease (52, 56, 57). Immortalized human endometriotic epithelial cells 12Z and stromal cells 22B used in this study were derived from these active red lesions of peritoneal endometriosis from women, and these share several phenotypic and molecular characteristics of primary cultured endometriotic cells (58). Accumulating information from our and other laboratories indicates that these endometriotic cells mimic the active/progressive phase of the diseases and are a potential tool to develop targeted therapy (7, 8, 11, 39, 58, 59, 60, 61). We reported that molecular and cellular behavior of these 12Z and 22B cells differ from those of human eutopic endometrial epithelial cells and stromal cells from endometriosis-free woman in various aspects. Endometriotic cells 12Z and 22B produced large amounts of PGE2, genes associated with cytokine and growth factor were more abundantly expressed, and 12Z and 22B cells were highly migratory and invasive compared with human eutopic endometrial epithelial cells and stromal cells (11, 39). We have further compared molecular and cellular behavior of 12Z and 22B cells with available information from primary cultured endometriotic cells and eutopic endometrial cells from endometriosis women, and endometriosis lesions in women and animal models for endometriosis, indicating that 12Z and 22B cell possess several similarities with their counterparts (39, 58). Interestingly, xenograft of a mixed population of these 12Z and 22B cells into the peritoneal cavity of nude mice is able to proliferate, attach, invade, reorganize, and establish peritoneal endometriosis-like lesions, and histomorphology of these lesions is similar to that of spontaneous peritoneal endometriosis lesions in women (8). These 12Z and 22B cells expressed COX-2, EP2, and EP4 proteins abundantly in vitro (11, 39) and in vivo in induced endometriosis-like lesions in nude mice (8). These results together suggest that human endometriotic cells 12Z and 22B is an ideal model system to study PGE2 signaling in the pathogenesis of endometriosis in women.
These well-characterized 12Z and 22B cells were cultured in DMEM/F12 without special steroid treatment containing 10% FBS and penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin-B 2.5 μg/ml in humidified 5% CO2 and 95% air at 37 C as we described previously (11, 39). At 70–80% confluency, the cells were cultured in DMEM/F12 with 2% dextran-charcoal-treated FBS and treated with EP inhibitors (EP-I) for EP1 (SC19220, 100 μm), EP2 (AH6809, 75 μm), and/or EP4 (AH23848, 50 μm) for 24 h.
Protein extraction and Western blot
Total protein was isolated from endometriotic cells and immunoblotting/Western blotting was performed as we described previously (11, 62). Briefly, the cells were harvested using 1% Trypsin-EDTA and pelleted. The cell lysates were sonicated in sonication buffer that consisted of 20 mm Tris-HCl, 0.5 mm EDTA, 100 μm diethyldithiocarbonate, 1% Tween 20, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablets: complete EDTA-free (1 tablet/50 ml) and PhosStop (1 tablet /10 ml). Sonication was performed using a Microson ultrasonic cell disruptor (Microsonix Inc., Farmingdale, NY). Protein concentration was determined using the Bradford method (63) and a Bio-Rad protein assay kit. Protein samples (75 μg) were resolved using 7.5, 10, or 12.5% SDS-PAGE. Chemiluminescent substrate was applied according to the manufacturer’s instructions (Pierce). The blots were exposed to Blue X-Ray film, and densitometry of autoradiograms was performed using an Alpha Imager (Alpha Innotech Corp., San Leandro, CA).
Mitochondria/cytosol isolation
The 12Z and 22B cells were cultured, treated, and harvested as described above. The mitochondrial and cytosolic fractions were isolated using a mitochondria isolation kit for cultured cells according to the manufacturer’s instructions (Pierce). Cytosolic fractions were concentrated by Amicon Ultra 3K column (Millipore, Bedford, MA). Both protein fractions were briefly sonicated. Protein concentration was determined using the Bradford method (63) and a Bio-Rad protein assay kit.
Immunoprecipitation
The 12Z and 22B cells were cultured, treated, and harvested, and then total cell lysates were prepared as described above. Total cell lysate (1 mg) was precleared by incubating with appropriate preclearing matrix (Santa Cruz) for 30 min at 4 C. The precleared cell lysate was incubated with primary antibody overnight at 4 C at the recommended concentrations given by manufacturers (Cell Signaling and/or Santa Cruz) and then further incubated with immunoprecipitation matrix-ExactraCruz (Santa Cruz) overnight at 4 C. Protein-antibody complexes were precipitated using protocols provided by Santa Cruz Biotechnology and/or Cell Signaling Technology.
Immunofluorescence
The 12Z and 22B cells were seeded at 50,000 cells per well on Lab-Tek II chambered slides and cultured as described above. At 70–80% confluency, the cells were treated with EP2 and EP4 inhibitors as described above. The procedure given by Cell Signaling Technology was followed with minor modifications. Cells were rinsed in PBS, fixed in 1% paraformaldehyde for 15 min at room temperature, and permeabilized for 10 min in 100% methanol at −20 C. Cells were blocked for 1 h in 10% normal serum from the same species in which secondary antibody was developed and then incubated overnight with primary antibodies at the concentrations recommended by manufacturer. For the negative control, serum or IgG from respective species with reference to the primary antibody at the respective dilution was used. After washing in 0.2 m PBS/0.3% Tween 20, cells were incubated with Alexa Fluor 488-conjugated secondary antibodies for 1 h. Cells were washed and mounted with ProLong Gold antifade reagent. Images were visualized by using digital imaging and an image analysis workstation consisting of a Zeiss Axioplan 2 research microscope interfaced with a Zeiss Axiocam HR high-resolution color CCD camera with Zeiss Axiovision (Carl Zeiss, Thornwood, NY).
TUNEL assay
Nonadherent and adherent cells were harvested, mixed together, and resuspended at the concentrations of 1 × 106 cells/ml. Nicks in the DNA were determined by terminal deoxynucleotidyl transferase and 5-bromo-2′-deoxyuridine (BrdU) 5′-triphosphate labeling using APO-BrdU TUNEL assay kit. Detection of BrdU incorporation at DNA break sites was achieved through Alexa Fluor 488 dye-labeled anti-BrdU antibody. The staining procedures were performed as recommended by manufacturers. Numbers of apoptotic cells were analyzed by a flow cytometer (FACSCaliber; Becton Dickinson, San Jose, CA) using Cell Quest software. In addition, the presence of TUNEL-labeled DNA fragments was determined by fluorescence microscopy. Digital images were captured using a Zeiss Axioplan 2 research microscope with an Axiocam HR digital camera.
EP2/EP4 siRNA
The 12Z and 22B cells (3.0 × 105 per well) were cultured in antibiotic-free DMEM/F12 with 10% FBS in six-well tissue culture plates. At 70–80% confluency, cells were used for EP2, EP4, or EP2/EP4 knockdown experiments using SMART pool siRNA duplex delivered by DharmaFect-1 as we described previously (11) and per manufacturer’s instructions (Dharmacon). As an internal control, mock siRNA was used. According to the manufacturer’s instructions, SMARTpool siRNA consisted of at least four individual siRNA duplexes targeted against a specific gene and designed using a bioinformatics technology known as SMARTselection. This resulted in the generation of siRNAs more than 97% of the time, and the targeted message level was reduced by more than 70% within 24 h after transfection. We preferred SMARTpool siRNA to use more than one siRNA duplex from different regions of the gene of interest to avoid nonspecific indirect effects of a single siRNA duplex to a single region of the target gene. Briefly, siRNA duplexes (100 nm/well) and DharmaFect-1 (3 μl/well) were diluted in 50 μl antibiotic- and serum-free DMEM/F12 medium separately and mixed gently and incubated for 5 min at room temperature. Afterward, EP2, EP4, or EP2/EP4 siRNA and DharmaFect-1 were mixed (total volume 100 μl) and incubated at room temperature for 20 min. Then, 100 μl siRNA-DharmaFect-1 complex was diluted with 2 ml antibiotic-free medium with 10% FBS and added to the well. After 24 h, the medium was replaced with fresh DMEM/F12 with 10% FBS and incubated for 24 h. Fluorescence-labeled siGLO RISC-free siRNA was transfected separately, and transfection efficiency was estimated using a fluorescence microscope. Transfection efficiency of more than 80% was considered as optimal condition for further experiments. Efficiency of siRNA on silencing of EP2 and EP4 genes and proteins was assessed by RT-PCR and Western blot, respectively, 72 h after transfection. Knockdown efficiency was 70–80% in both 12Z and 22B cells.
Endometriosis and endometrial tissues
The following tissues were collected from women presented at the Obstetrics and Gynecology Unit and processed at Anatomic Pathology Laboratory for diagnostic purposes, Scott and White Memorial Hospital, Texas A&M University System Health Science Center. Ectopic endometria from peritoneal lesions (endometriotic tissue, n = 12) were collected from women with endometriosis. Eutopic endometria (n = 12) were collected from women with endometriosis. Normal eutopic endometria (n = 12) were collected from endometriosis-free women undergoing hysterectomy for benign gynecological indications. Each of the women reported regular menstrual cycles (25–40 d cycle length) and no hormonal medication in the last 3 months. All the endometriotic and endometrial tissues were collected from proliferative phase of the menstrual cycle that was confirmed by patient’s last menstrual period, progesterone profile, and histology of endometriotic and endometrial tissues. Ectopic endometria were classified stages I–IV based on criteria established by the American Society for Reproductive Medicine (57). Stages I and II were included in this investigation. For the present study, additional sections were cut from these archived paraffin-embedded tissues that were not needed for patient care. These studies were approved by the Institutional Review Board of Scott and White Memorial Hospital, Texas A&M University System Health Science Center.
Immunohistochemistry
Ectopic and eutopic endometrial tissue sections were fixed in 4% buffered paraformaldehyde in saline for 4 h at 4 C and processed using standard procedures (11, 62). Paraffin sections (5 μm) were used for immunohistochemical localization of proteins involved in PGE2 signaling using a Vectastain Elite ABC kit as we previously described (11, 62) and according to the manufacturer’s protocols. The tissue sections were incubated with specific antibodies at the concentrations recommended by manufacturers overnight at 4 C. Then, tissue sections were further incubated with the secondary antibody (biotinylated IgG) for 45 min at room temperature. For the negative control, serum or IgG from respective species with reference to the primary antibody at the respective dilution was used. Digital images were captured using a Zeiss Axioplan 2 research microscope with an Axiocam HR digital camera. The intensity of staining for each protein was quantified using Image-pro Plus as we described previously (62) according to the manufacturer’s instructions (Media Cybernetics, Inc., Bethesda, MD). We preferred immunohistochemistry followed by densitometry compared with Western blot for the following reasons: 1) quantity of endometrial and endometriotic tissues obtained from each patient is not enough to extract adequate proteins and to analyze various proteins using Western blot, and 2) immunohistochemistry is being used as a primary technique to confirm endometriosis (presence of endometriosis glands lined by epithelial cells and surrounded by stromal cells) in women clinically; therefore, immunohistochemistry will provide details on spatial expression of a specific protein in glandular epithelial and stromal cells of endometriosis, whereas Western blot provide information only on total steady-state expression levels of a particular protein in the given tissue.
Statistical analyses
Statistical analyses were performed using general linear models of Statistical Analysis System (SAS, Cary, NC). Relative spatial expression of different proteins in glandular epithelium and stroma in ectopic and eutopic endometria in vivo and the effects of inhibition of EP receptors on cell apoptosis and expression levels of different proteins in 12Z and 22B cells in vitro were analyzed by one-way ANOVA followed by Tukey-Kramer honestly significant difference test. The numerical data are expressed as mean ± sem. Statistical significance was considered at P < 0.05.
Acknowledgments
Services provided by Dr. Robert C. Burghardt and the Image Analysis Laboratory at College of Veterinary Medicine and Biomedical Sciences Texas A&M University and Flowcytometry Core Facility at the College of Medicine, Texas A&M Health Science Center, are gratefully acknowledged.
Footnotes
This work was supported by program development award (to J.A.A.) from the Department of Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 30, 2009
S.K.B. and J.L. equally contributed to this work.
Abbreviations: Bad, Bcl-2-associated death promoter; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Bcl-XL, basal cell lymphoma-extra large; BrdU, 5-bromo-2′-deoxyuridine; COX-2, cyclooxygenase-2; c-Src, tyrosine kinase enzyme; EGFR, epidermal growth factor receptor; EGR-1, early growth response factor-1; EP-I, EP inhibitor; FBS, fetal bovine serum; Gs, G protein α-subunit s type; GSK3β, glycogen synthase kinase-3β; IκBα, inhibitory κBα protein; NFκB, nuclear factor-κB; PAR, poly (ADP-ribose) polymerase; PG, prostaglandin; siRNA, small interfering RNA; TCF, T-cell factor; TNFαR1, TNFα receptor 1; TUNEL, terminal deoxynucleotide transferase dUTP nick end labeling.
References
- 1.Giudice LC, Kao LC2004. Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- 2.Olive DL2008. Gonadotropin-releasing hormone agonists for endometriosis. N Engl J Med 359:1136–1142 [DOI] [PubMed] [Google Scholar]
- 3.Sampson J1927. Peritoneal endometritis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:442–469 [Google Scholar]
- 4.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S2002. Estrogen production and metabolism in endometriosis. Ann NY Acad Sci 955:75–85; discussion 86–88, 396–406 [DOI] [PubMed] [Google Scholar]
- 5.Hastings JM, Fazleabas AT2003. Future directions in endometriosis research. Semin Reprod Med 21:255–262 [DOI] [PubMed] [Google Scholar]
- 6.Osteen KG, Bruner-Tran KL, Eisenberg E2005. Endometrial biology and the etiology of endometriosis. Fertil Steril 84:33–34; discussion 38–39 [DOI] [PubMed] [Google Scholar]
- 7.Guo SW, Olive DL2007. Two unsuccessful clinical trials on endometriosis and a few lessons learned. Gynecol Obstet Invest 64:24–35 [DOI] [PubMed] [Google Scholar]
- 8.Banu SK, Starzinski-Powitz A, Speights VO, Burghardt RC, Arosh JA2009. Induction of peritoneal endometriosis in nude mice with use of human immortalized endometriosis epithelial and stromal cells: a potential experimental tool to study molecular pathogenesis of endometriosis in human. Fertil Steril 91:2199–2209 [DOI] [PubMed] [Google Scholar]
- 9.Wu MH, Shoji Y, Chuang PC, Tsai SJ2007. Endometriosis: disease pathophysiology and the role of prostaglandins. Exp Rev Mol Med 9:1–20 [DOI] [PubMed] [Google Scholar]
- 10.Laschke MW, Elitzsch A, Scheuer C, Vollmar B, Menger MD2007. Selective cyclo-oxygenase-2 inhibition induces regression of autologous endometrial grafts by down-regulation of vascular endothelial growth factor-mediated angiogenesis and stimulation of caspase-3-dependent apoptosis. Fertil Steril 87:163–171 [DOI] [PubMed] [Google Scholar]
- 11.Banu SK, Lee J, Speights Jr VO, Starzinski-Powitz A, Arosh JA2008. Cyclooxygenase-2 regulates survival, migration and invasion of human endometriotic cells through multiple mechanisms. Endocrinology 149:1180–1189 [DOI] [PubMed] [Google Scholar]
- 12.Ozawa Y, Murakami T, Tamura M, Terada Y, Yaegashi N, Okamura K2006. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis xenografts via antiangiogenic activity in severe combined immunodeficiency mice. Fertil Steril 86(Suppl 4):1146–1151 [DOI] [PubMed] [Google Scholar]
- 13.Chishima F, Hayakawa S, Sugita K, Kinukawa N, Aleemuzzaman S, Nemoto N, Yamamoto T, Honda M2002. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am J Reprod Immunol 48:50–56 [DOI] [PubMed] [Google Scholar]
- 14.Ota H, Igarashi S, Sasaki M, Tanaka T2001. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod 16:561–566 [DOI] [PubMed] [Google Scholar]
- 15.De Leon FD, Vijayakumar R, Brown M, Rao CV, Yussman MA, Schultz G1986. Peritoneal fluid volume, estrogen, progesterone, prostaglandin, and epidermal growth factor concentrations in patients with and without endometriosis. Obstet Gynecol 68:189–194 [PubMed] [Google Scholar]
- 16.Cobellis L, Razzi S, De Simone S, Sartini A, Fava A, Danero S, Gioffrè W, Mazzini M, Petraglia F2004. The treatment with a COX-2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol 116:100–102 [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki S, Canis M, Darcha C, Dallel R, Okamura K, Mage G2004. Cyclooxygenase-2 selective inhibitor prevents implantation of eutopic endometrium to ectopic sites in rats. Fertil Steril 82:1609–1615 [DOI] [PubMed] [Google Scholar]
- 18.Hull ML, Prentice A, Wang DY, Butt RP, Phillips SC, Smith SK, Charnock-Jones DS2005. Nimesulide, a COX-2 inhibitor, does not reduce lesion size or number in a nude mouse model of endometriosis. Hum Reprod 20:350–358 [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki S, Canis M2005. Is the dose to inhibit the COX-2 enzyme in nude mice also adequate in ‘human’ endometrial tissues? Hum Reprod 20:2665; author reply 2665–2666 [DOI] [PubMed] [Google Scholar]
- 20.Attar E, Bulun SE2006. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update 12:49–56 [DOI] [PubMed] [Google Scholar]
- 21.Ebert AD, Bartley J, David M2005. Aromatase inhibitors and cyclooxygenase-2 (COX-2) inhibitors in endometriosis: new questions–old answers? Eur J Obstet Gynecol Reprod Biol 122:144–150 [DOI] [PubMed] [Google Scholar]
- 22.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, Bulun SE2009. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab 94:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares C, Bilotas M, Buquet R, Borghi M, Sueldo C, Tesone M, Meresman G2008. Effects of a selective cyclooxygenase-2 inhibitor on endometrial epithelial cells from patients with endometriosis. Hum Reprod 23:2701–2708 [DOI] [PubMed] [Google Scholar]
- 24.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK1997. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- 25.Cha YI, DuBois RN2007. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med 58:239–252 [DOI] [PubMed] [Google Scholar]
- 26.Narumiya S, Sugimoto Y, Ushikubi F1999. Prostanoid receptors: structures, properties, and functions. Physiol Rev 79:1193–1226 [DOI] [PubMed] [Google Scholar]
- 27.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS2002. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8:289–293 [DOI] [PubMed] [Google Scholar]
- 28.Regan JW2003. EP2 and EP4 prostanoid receptor signaling. Life Sci 74:143–153 [DOI] [PubMed] [Google Scholar]
- 29.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN2006. Role of β-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA 103:1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS2005. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 310:1504–1510 [DOI] [PubMed] [Google Scholar]
- 31.Jabbour HN, Sales KJ2004. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol Metab 15:398–404 [DOI] [PubMed] [Google Scholar]
- 32.Wang D, Dubois RN2006. Prostaglandins and cancer. Gut 55:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisinger AL, Prescott SM, Jones DA, Stafforini DM2007. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat 82:147–154 [DOI] [PubMed] [Google Scholar]
- 34.Clevers H2006. Colon cancer: understanding how NSAIDs work. N Engl J Med 354:761–763 [DOI] [PubMed] [Google Scholar]
- 35.Harada T, Taniguchi F, Izawa M, Ohama Y, Takenaka Y, Tagashira Y, Ikeda A, Watanabe A, Iwabe T, Terakawa N2007. Apoptosis and endometriosis. Front Biosci 12:3140–3151 [DOI] [PubMed] [Google Scholar]
- 36.Izawa M, Harada T, Deura I, Taniguchi F, Iwabe T, Terakawa N2006. Drug-induced apoptosis was markedly attenuated in endometriotic stromal cells. Hum Reprod 21:600–604 [DOI] [PubMed] [Google Scholar]
- 37.Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E, Terakawa N2004. Apoptosis in human endometrium and endometriosis. Hum Reprod Update 10:29–38 [DOI] [PubMed] [Google Scholar]
- 38.Arosh JA, Lee J, Rodriguez R, Starzinski-Powitz A, Banu SK2007. Prostaglandin E2 signaling in pathophysiology of endometriosis in human. Biol Reprod 77:157-b (Abstract) [Google Scholar]
- 39.Banu SK, Lee J, Starzinski-Powitz A, Arosh JA2008. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril 90:972–987 [DOI] [PubMed] [Google Scholar]
- 40.Coleman RA, Smith WL, Narumiya S1994. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46:205–229 [PubMed] [Google Scholar]
- 41.Woodward DF, Pepperl DJ, Burkey TH, Regan JW1995. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem Pharmacol 50:1731–1733 [DOI] [PubMed] [Google Scholar]
- 42.Crider JY, Griffin BW, Sharif NA2000. Endogenous EP4 prostaglandin receptors coupled positively to adenylyl cyclase in Chinese hamster ovary cells: pharmacological characterization. Prostaglandins Leukot Essent Fatty Acids 62:21–26 [DOI] [PubMed] [Google Scholar]
- 43.Jiang X, Wang X2004. Cytochrome C-mediated apoptosis. Annu Rev Biochem 73:87–106 [DOI] [PubMed] [Google Scholar]
- 44.Adams JM, Cory S1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326 [DOI] [PubMed] [Google Scholar]
- 45.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619–628 [DOI] [PubMed] [Google Scholar]
- 46.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–1362 [DOI] [PubMed] [Google Scholar]
- 47.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241 [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Takada Y, Boriek AM, Aggarwal BB2004. Nuclear factor-κB: its role in health and disease. J Mol Med 82:434–448 [DOI] [PubMed] [Google Scholar]
- 49.Grigoryan T, Wend P, Klaus A, Birchmeier W2008. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of β-catenin in mice. Genes Dev 22:2308–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS2007. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal 19:2013–2023 [DOI] [PubMed] [Google Scholar]
- 51.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC2005. Erk associates with and primes GSK-3β for its inactivation resulting in up-regulation of β-catenin. Mol Cell 19:159–170 [DOI] [PubMed] [Google Scholar]
- 52.Nisolle M, Casanas-Roux F, Donnez J1997. Immunohistochemical analysis of proliferative activity and steroid receptor expression in peritoneal and ovarian endometriosis. Fertil Steril 68:912–919 [DOI] [PubMed] [Google Scholar]
- 53.Milne SA, Perchick GB, Boddy SC, Jabbour HN2001. Expression, localization, and signaling of PGE2 and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab 86:4453–4459 [DOI] [PubMed] [Google Scholar]
- 54.Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH1997. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature 390:78–81 [DOI] [PubMed] [Google Scholar]
- 55.Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH1999. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest 103:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J1993. Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril 59:681–684 [PubMed] [Google Scholar]
- 57.American Society for Reproductive Medicine1997. Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil Steril 67:817–821 [DOI] [PubMed] [Google Scholar]
- 58.Zeitvogel A, Baumann R, Starzinski-Powitz A2001. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol 159:1839–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS2008. Tumor necrosis factor-α regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor-κB in human endometriotic epithelial cells. Mol Pharmacol 73:1394–1404 [DOI] [PubMed] [Google Scholar]
- 60.Wu Y, Starzinski-Powitz A, Guo SW2008. Prolonged stimulation with tumor necrosis factor-α induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil Steril 70:234–237 [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, Starzinski-Powitz A, Guo SW2007. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod Sci 14:374–382 [DOI] [PubMed] [Google Scholar]
- 62.Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, MacLaren LA, Fortier MA2003. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology 144:3076–3091 [DOI] [PubMed] [Google Scholar]
- 63.Bradford MM1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]