Abstract
Hypercortisolemia and glucocorticoid treatment cause elevated level of circulating free fatty acids (FFAs). The basis of this phenomenon has long been linked to the effect of glucocorticoids permitting and enhancing the adipose lipolysis response to various hormones. In this study, we demonstrate that glucocorticoids directly stimulate lipolysis in rat primary adipocytes in a dose- and time-responsive manner; this lipolytic action was attenuated by treatment with the glucocorticoid antagonist RU486. Dexamethasone down-regulates mRNA and protein levels of cyclic-nucleotide phosphodiesterase 3B, thereby elevating cellular cAMP production and activating protein kinase A (PKA). On inhibition of PKA but not other kinases, the lipolysis response ceases. Furthermore, dexamethasone induces phosphorylation and down-regulation of perilipin, a lipid droplet-associating protein that modulates lipolysis; this effect is restored by RU486 or PKA inhibitor H89. Dexamethasone up-regulates mRNA and protein levels of hormone-sensitive lipase (HSL) and adipose triglyceride lipase; these effects, parallel to increased lipolysis, are attenuated by RU486 or actinomycin D. Phosphorylation at Ser-563 and Ser-660 residues of HSL and activity of cellular lipases are elevated on dexamethasone stimulation but abrogated by the coaddition of H89. However, dexamethasone does not induce HSL translocation to the lipid droplet surface in differentiated adipocytes. We show that elevated FFA concentration in plasma is associated with increased lipase activity and lipolysis in vivo in adipose tissues of dexamethasone-treated rats. Therefore, the lipolytic action of glucocorticoids liberates FFA efflux from adipocytes to the bloodstream, which could be a cellular basis of systemic FFA elevation in response to glucocorticoid challenge.
The direct lipolysis action of glucocorticoids is mediated through PKA signaling in adipocytes and contributes to elevation of circulating free fatty acids in response to glucocorticoid challenge.
Elevated levels of plasma free fatty acids (FFAs) restrict glucose utilization and induce insulin resistance (1). Regulation of circulating FFA concentrations depends primarily on the lipolysis of adipocytes in response to various hormones, which governs the breakdown of triglycerides and the release of fatty acids to the bloodstream. Catecholamines are the major hormones that stimulate lipolysis through elevating cAMP production and activating cAMP-dependent protein kinase [protein kinase A (PKA)] (2). Cytokines such as TNF-α induce lipolysis via activating ERK1/2 (3, 4). To date, the activation of PKA and ERK1/2 signaling were thought to be the major events during lipolysis (2, 3), but PKC may also modulate lipolysis in a separate pathway (5). In the downstream lipolytic cascade, the phosphorylation (6, 7, 8) and down-regulation of perilipin (4, 9) and activation of hormone-sensitive lipase (HSL) (7, 10) and adipose triglyceride lipase (ATGL) (11, 12, 13) cooperatively regulate the full lipolytic reaction in adipocytes.
Glucocorticoids are the counterregulatory hormones in the body and are also widely used drugs, with broad effects on antiinflammation and carbohydrate, lipid, and protein metabolism. The mechanisms of the biological effects of glucocorticoids are diverse (e.g. involving transcription regulation and nongenomic action) (14, 15). Hypercortisolemia or glucocorticoid treatment is associated with increased levels of circulating FFAs and insulin resistance in humans (16, 17, 18) and rodents (19, 20). However, the cellular basis for glucocorticoid-associated elevation of serum FFAs is not completely understood. In general, glucocorticoids may not or only slightly increase adipose lipolysis on short-term (<6 h) stimulation. By contrast, glucocorticoids ensure that adipocytes produce normal (21, 22) and enhanced (23, 24) lipolysis responses to various hormones, an action previously termed the permissive effect (22, 24, 25) (for review, see Ref. 16). For example, glucocorticoids enhance the lipolysis of adipocytes in response to catecholamines, thyronines, and GH (23, 24). However, an early study has provided a clue showing that prolonged stimulation with dexamethasone for 24 h increased glycerol release from adipocytes (26). This observation implied that glucocorticoids could have a direct role in lipolysis stimulation, although the characteristics and mechanism of this action are unknown.
In this study, we examined the characteristics of the direct lipolysis response to glucocorticoids and investigated the multiple mechanisms by which dexamethasone stimulates lipolysis in isolated rat primary adipocytes. We also examined the in vivo lipolytic action of dexamethasone and its association with systemic FFA elevation in rats.
Results
Dexamethasone directly stimulated lipolysis in primary adipocytes in a dose- and time-dependent manner
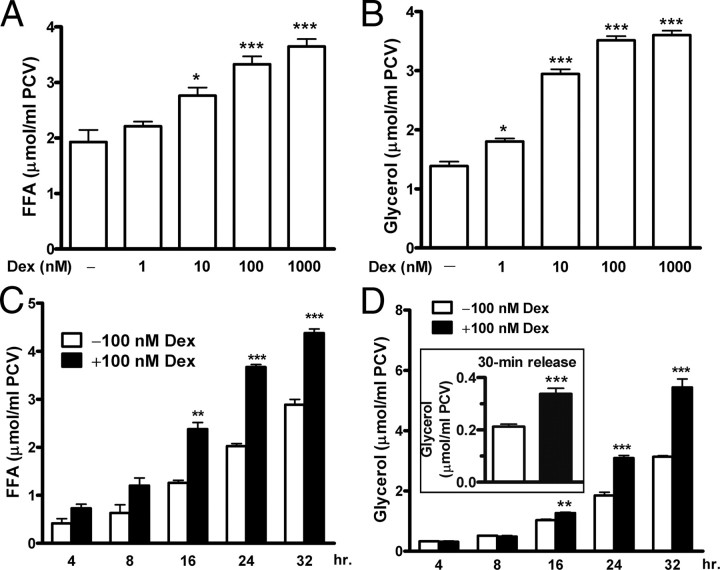
Primary adipocytes were isolated from epididymal adipose tissues of normal rats and incubated with dexamethasone. FFA and glycerol release in the incubation medium was assayed as an index of lipolysis. After 24 h incubation, dexamethasone gradually stimulated release of FFAs (Fig. 1A) and glycerol (Fig. 1B) in a dose-dependent manner. Dexamethasone slightly increased glycerol release at 1 nm and induced robust lipolysis at 10–100 nm; lipolysis peaked at 1000 nm (Fig. 1, A and B). Furthermore, 100 nm dexamethasone stimulated release of FFAs (Fig. 1C) and glycerol (Fig. 1D) in a time-dependent manner. FFA release was detectable at 4–8 h, when glycerol release remained quiescent. The inconsistent rates of fatty acid and glycerol breakdown and different partial oxidation or reesterification of these metabolites within adipocytes may account for the inconsistent rates of FFA and glycerol release from adipocytes. However, both FFA and glycerol release became apparent at 16 h and continually increased to 32 h (Fig. 1, C and D). In addition, we examined a 30-min rate of glycerol release from adipocytes, which reflects a dynamic lipolysis after prolonged incubation. The adipocytes were stimulated with dexamethasone for 24 h and then washed and incubated for another 30 min in fresh medium. The 30-min rate of glycerol release from dexamethasone-stimulated adipocytes increased by 1.6-fold that from unstimulated adipocytes (Fig. 1D, inset).
Fig. 1.
Dexamethasone stimulates lipolysis in primary adipocytes in a dose- and time-dependent manner. Primary adipocytes were isolated from epididymal adipose tissues of normal rats and incubated for indicated periods in the presence or absence of dexamethasone (Dex). The levels of FFA and glycerol release in the medium were assayed and served as lipolytic indexes. After incubation for 24 h, dexamethasone stimulated FFA (A) and glycerol (B) release in a concentration-dependent manner. Next, incubation with 100 nm dexamethasone increased FFA (C) and glycerol (D) release in a time-dependent manner. In addition, after adipocytes were stimulated with dexamethasone for 24 h, the cells were washed and incubated for another 30 min in the fresh medium. The 30-min rate of glycerol release was assayed and reflected dynamic lipolysis of adipocytes after prolonged incubation (inset). The data are mean ± sem of three (A–D) or six (inset) measurements from three separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. untreated adipocytes.
Glucocorticoid antagonist RU486 specifically blocked dexamethasone-stimulated lipolysis
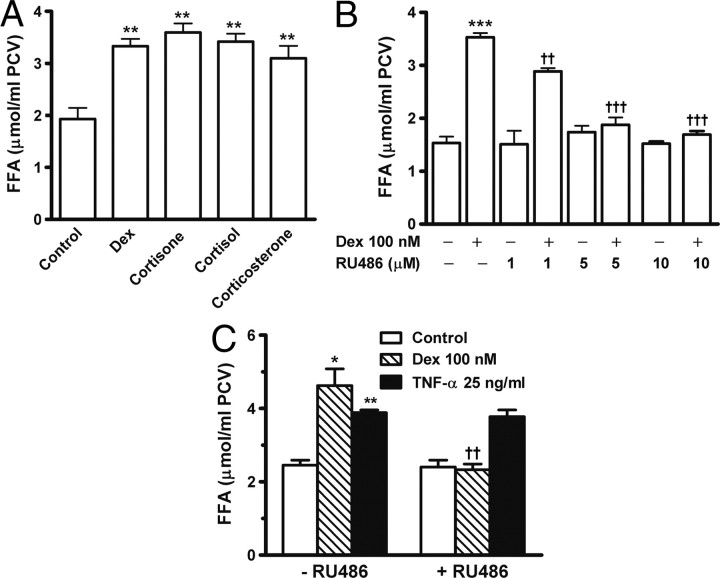
To examine whether other glucocorticoids also stimulate lipolysis, adipocytes were stimulated for 24 h with cortisone, cortisol, corticosterone, or dexamethasone. The four steroids increased FFA release to a similar extent (Fig. 2A). RU486 is an antagonist for glucocorticoid receptors (27). Adipocytes were incubated in the presence or absence of RU486, dexamethasone, or both. The addition of RU486 did not affect FFA release in unstimulated adipocytes. The lipolytic action of dexamethasone was already inhibited by 1 μm RU486 and completely blocked by RU486 at 5–10 μm (Fig. 2B, P < 0.001). Similar to dexamethasone, TNF-α also induces lipolysis on prolonged treatment (3, 4). After 24 h incubation, RU486 did not block TNF-α-stimulated FFA release, which suggests that inhibition by RU486 on dexamethasone-induced lipolysis was specific (Fig. 2C).
Fig. 2.
Dexamethasone-stimulated lipolysis is blocked by glucocorticoid antagonist RU486. Primary adipocytes were incubated for 24 h in the presence or absence of indicated agents, and then FFA release in the medium was assayed. A, 100 nm dexamethasone (Dex), 1 μm cortisone, 1 μm cortisol, and 1 μm corticosterone stimulated lipolysis and increased FFA release. B, Preaddition of glucocorticoid receptor antagonist RU486 attenuated dexamethasone-induced FFA release. C, RU486 specifically blocked dexamethasone- but not TNF-α-induced lipolysis. The data are mean ± sem of three separate experiments performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. untreated control. ††, P < 0.01; †††, P < 0.001 vs. dexamethasone (Dex) alone.
Dexamethasone elevates intracellular cAMP level and activates PKA
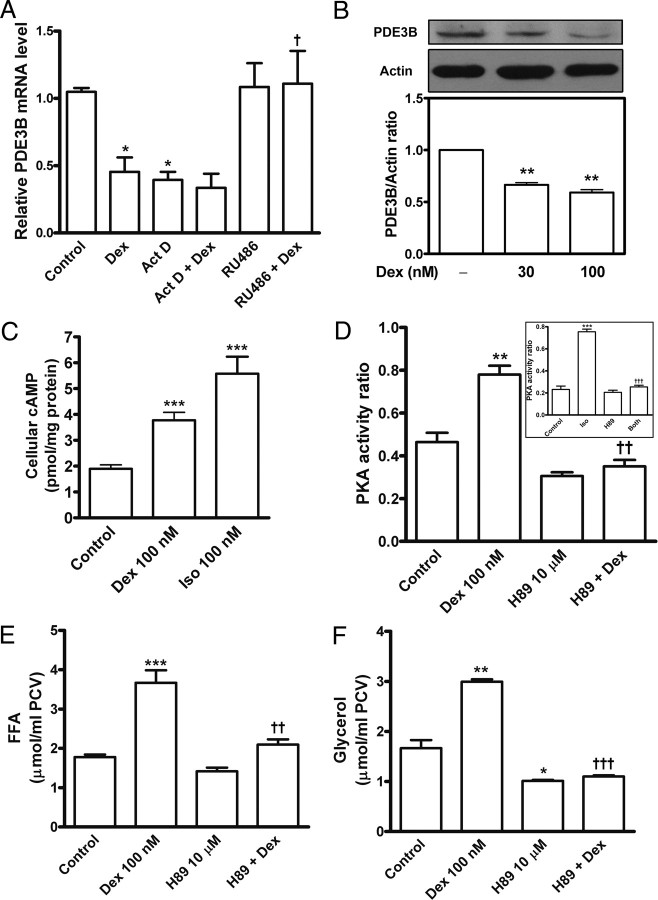
Elevation of cellular cAMP level and activation of PKA induce lipolysis. Cyclic-nucleotide phosphodiesterase 3B (PDE3B) is the major enzyme responsible for cAMP hydrolysis in adipocytes (2). To investigate the cellular basis of glucocorticoid inducing lipolysis, we isolated total RNA from primary adipocytes. Real-time PCR data revealed that expression of PDE3B mRNA was sensitive to transcription inhibition with actinomycin D. Dexamethasone down-regulated PDE3B transcription; this effect was attenuated by RU486 (Fig. 3A). Immunoblot analysis revealed that PDE3B protein level was lower in adipocytes treated with 30 or 100 nm dexamethasone than in unstimulated adipocytes (Fig. 3B). Then, cellular cAMP contents were determined by 125I-RIA. Dexamethasone treatment for 24 h resulted in an approximately 2-fold increase (P < 0.001) in cAMP content, compared with an approximately 3-fold elevation of cAMP production in adipocytes stimulated with isoproterenol (Fig. 3C). On [γ-32P]ATP PKA activity assay, dexamethasone increased PKA activity ratio by 1.7-fold, and this promotion was abolished by 10 μm H89, a potent PKA inhibitor (Fig. 3D, P < 0.01). To examine the relative effectiveness of H89 in PKA activation, stimulation with isoproterenol (as a positive control) caused rapid and strong elevation of PKA activity, by approximately 3.3-fold, which was sufficiently inhibited by H89 (Fig. 3D, inset, P < 0.001). These results suggested that cAMP/PKA might be major signals in the lipolysis cascade with dexamethasone treatment. To further test this speculation, adipocytes were preincubated for 1 h with or without H89 and then stimulated with dexamethasone for 24 h. PKA inhibition with 10 μm H89 significantly attenuated dexamethasone-stimulated release of FFAs (Fig. 3E, P < 0.01) and glycerol (Fig. 3F, P < 0.001). H89 at lower doses also partially suppressed PKA activity and lipolysis increased by dexamethasone; H89 at 10 μm did not affect the cell viability of adipocytes (data not shown).
Fig. 3.
Dexamethasone elevates intracellular cAMP and activates PKA. Primary adipocytes were preincubated with actinomycin D (Act D), RU486, or H89 and incubated for 24 h with or without dexamethasone (Dex) and then underwent the following examinations. A, Total RNA was extracted and underwent reverse transcription and quantitative real-time PCR to detect relative mRNA levels of PDE3B and β-actin as described in Materials and Methods. B, Adipocyte lysates underwent immunoblotting with primary antibody against PDE3B. The blots were then stripped and reprobed with anti-actin antibodies. After densitometric quantitation, the ratio of PDE3B to actin of the band density was calculated. C, cAMP RIA. The cytosolic fractions of adipocytes were prepared and used for cAMP 125I-RIA. Stimulation with 100 nm isoproterenol (Iso) for 30 min served as a positive control. The data were normalized to picomoles cAMP per milligram cytosolic proteins. D, PKA activity ratio was assayed in the cytosolic extracts of the adipocytes stimulated with dexamethasone, H89, or both. Inset in D showed PKA activity ratio assay of adipocytes incubated for 30 min with 100 nm isoproterenol, 10 μm H89, or both. The release of FFAs (E) and glycerol (F) in the medium was measured. The values are mean ± sem of at least three separate experiments in triplicate or sextuplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. untreated control. †, P < 0.05; ††, P < 0.01; †††, P < 0.001 vs. dexamethasone (Dex) alone or isoproterenol (Iso) alone.
Dexamethasone-induced lipolysis was not attenuated by inhibition of ERK1/2, c-Jun-NH2-terminal kinase (JNK), p38 MAPK, and PKC
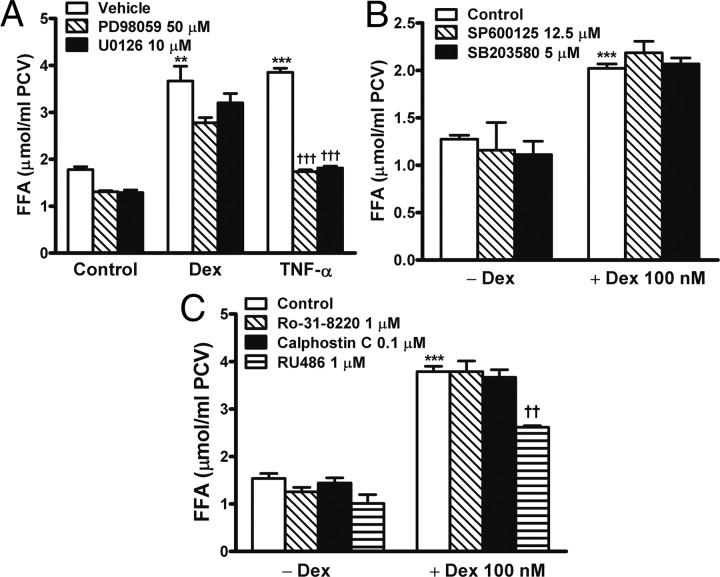
ERK1/2 and PKC are involved in lipolysis regulation (3, 5). We investigated whether these kinases mediate lipolysis induced by dexamethasone. Adipocytes were incubated for 1, 2, or 6 h in the presence or absence of 100 nm dexamethasone. On immunoblotting, dexamethasone did not alter the levels of native and phosphorylated ERK1/2 proteins (data not shown). Next, adipocytes were preincubated for 1 h with different kinase inhibitors and then stimulated for 24 h with 100 nm dexamethasone. Dexamethasone-induced FFA release was only slightly attenuated by two ERK1/2 inhibitors, PD98059 at 50 μm (P = 0.06) and U0126 at 10 μm (P = 0.28) (Fig. 4A). By contrast, these inhibitors effectively inhibited TNF-α-stimulated lipolysis (Fig. 4A, P < 0.001), an action known to be mediated typically via ERK1/2 signaling (3, 4). SB203580 at 5 μm and SP600125 at 12.5 μm, specific inhibitors for p38 MAPK and JNK, respectively, did not affect dexamethasone-induced lipolysis (Fig. 4B). Also, dexamethasone-stimulated FFA release was not inhibited by the PKC inhibitors calphostin C and Ro-31-8220 but was efficiently attenuated by the glucocorticoid antagonist RU486 (Fig. 4C).
Fig. 4.
Dexamethasone-induced lipolysis not attenuated by inhibition of ERK1/2, JNK, p38 MAPK, and PKC. Adipocytes were preincubated for 1 h with indicated agents and then stimulated for 24 h with 100 nm dexamethasone. FFA release in the medium was measured. A, ERK1/2 inhibition with PD98059 (P = 0.06) and U0126 (P = 0.28) only slightly attenuated FFA release in response to dexamethasone but significantly (P < 0.001) prevented the lipolysis of TNF-α. B, Inhibition of JNK and p38 MAPK with SP600125 and SB203580, respectively, did not affect dexamethasone-induced lipolysis. C, PKC inhibitors Ro-31–8220 or calphostin C did not alter FFA release induced by dexamethasone; RU486 was added as an inhibitory control. The data are mean ± sem of three separate experiments performed in triplicate. **, P < 0.01; ***, P < 0.001 vs. untreated control. ††, P < 0.01; †††, P < 0.001 vs. dexamethasone (Dex) alone or TNF-α alone.
Dexamethasone caused phosphorylation and down-regulation of perilipins
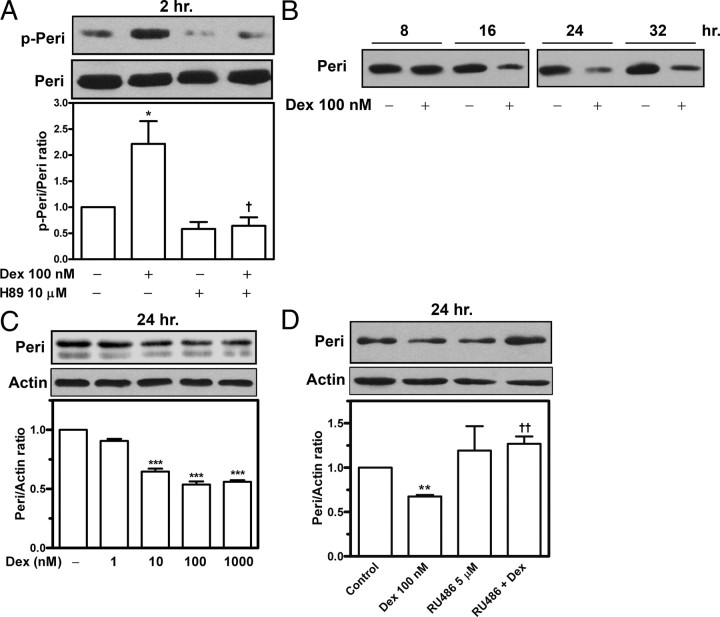
Perilipin coats the surface of intracellular lipid droplets. Phosphorylation and down-regulation of perilipins facilitate lipolysis (4, 6, 8). The fat cake extracts of adipocytes underwent immunoblotting with use of an antibody against a specific motif (RRXS/T) of PKA phosphosubstrate, which recognizes phosphorylated perilipins in adipocytes (4). Incubation with dexamethasone induced perilipin phosphorylation at 2 h (Fig. 5A); at 6 h, this phosphorylation was higher than that in unstimulated adipocytes (data not shown). Inhibition of PKA with H89 suppressed dexamethasone-induced perilipin phosphorylation, which indicates that PKA activation may account for this effect (Fig. 5A). Next, we examined changes in protein level of perilipins in adipocytes stimulated with dexamethasone. Incubation with 100 nm dexamethasone effectively reduced perilipin protein level at 16 h (Fig. 5B). After 24 h incubation, dexamethasone decreased perilipin protein level by 37% at 10 nm and by approximately 50% at 100 nm; this reduction was not further enhanced with 1000 nm dexamethasone (Fig. 5C). The addition of RU486 restored perilipin down-regulation caused by dexamethasone (P < 0.01, Fig. 5D), so this down-regulation could be mediated via glucocorticoid receptors.
Fig. 5.
Dexamethasone causes down-regulation and phosphorylation of perilipins. Primary adipocytes were incubated in the presence or absence of dexamethasone, H89, or RU486. Fat cake fractions were then prepared, and the extracts underwent immunoblotting. A, Phosphorylated perilipins (p-Peri) were detected with use of an antibody against a specific motif (RRXS/T) of PKA phosphosubstrate, and then the blots were stripped and reprobed with antiperilipin antibodies to detect native perilipins (Peri). B–D, Dexamethasone down-regulated perilipins in a time-dependent (B) and dose-dependent (C) manner; this down-regulation was restored by treatment with the anti-glucocorticoid RU486 (D). The density of the protein bands was quantitated, and the results are presented as mean ± sem of three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. untreated control. †, P < 0.05; ††, P < 0.01 vs. dexamethasone (Dex) alone.
Dexamethasone regulation of adipose lipases
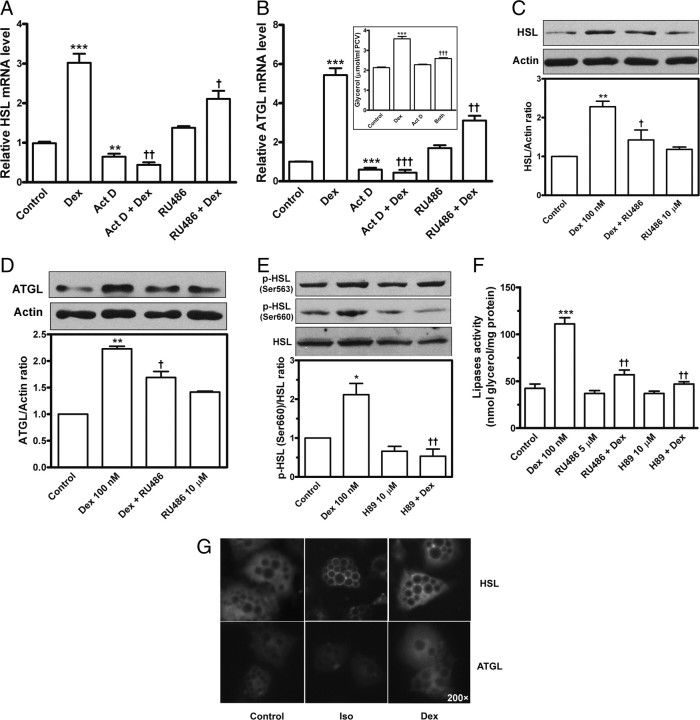
HSL and ATGL are two major lipases in adipocytes (12, 13). Real-time PCR data indicated that dexamethasone greatly up-regulated the transcription of HSL and ATGL in adipocytes, which was attenuated by treatment with the anti-glucocorticoid RU486 or by inhibiting transcription with actinomycin D (Fig. 6, A and B); in parallel to the transcription changes, lipolysis induced by dexamethasone was suppressed by actinomycin D (Fig. 6B, inset). Immunoblot results showed that dexamethasone elevated protein levels of HSL and ATGL, by 128 and 123%, respectively; these effects were suppressed by RU486 (Fig. 6, C and D). Moreover, stimulation with dexamethasone promoted HSL phosphorylation slightly at the Ser-563 residue but obviously at the Ser-660 residue, which was abrogated on PKA inhibition with 10 μm H89 (Fig. 6E). Lipase activity assay revealed that dexamethasone promoted lipase activity in adipocytes by 162% (P < 0.001), but this increase was significantly inhibited by RU486 and H89 (Fig. 6F, P < 0.01). The changes in lipase activity were consistent with changes in extent of lipolysis reaction (data not shown). HSL is translocated from the cytosol to the lipid droplets during lipolysis of PKA stimulation (7). We differentiated rat preadipocytes to adipocytes and performed immunofluorescent staining. Dexamethasone stimulation slightly enhanced immunofluorescence of HSL and ATGL but did not induce HSL translocation from the cytosol to the surface of lipid droplets at 24 h (Fig. 6G); in contrast, PKA stimulation with isoproterenol (as a positive control) efficiently induced the HSL translocation to the lipid droplets in adipocytes (Fig. 6G).
Fig. 6.
Modulation of dexamethasone on adipose lipases. Primary adipocytes were preincubated with actinomycin D (Act D), RU486, or H89 and stimulated for 24 h in the presence or absence of 100 nm dexamethasone (Dex). A and B, Total RNA was extracted and underwent reverse transcription and quantitative real-time PCR to detect relative mRNA levels of HSL (A) and ATGL (B). An inset in B shows that actinomycin attenuated the lipolysis induced by dexamethasone. C and D, Adipocyte extracts underwent immunoblotting with primary antibodies against HSL (C) or ATGL (D). The blots were then stripped and reprobed with anti-actin antibody (C and D). E, Phosphorylated HSL was detected with the use of two antibodies against phospho-Ser-563 and -660 motifs of HSL. The densitometric measurement is shown below the blot images. F, The lipase activity was assayed by measuring a 60-min glycerol release rate of triolein hydrolysis in the reaction containing the cytosol extracts of adipocytes. G, On immunofluorescent detection, differentiated rat adipocytes were treated for 24 h with 100 nm dexamethasone or stimulated for 30 min with 1 μm isoproterenol (Iso, positive control). The fixed cells were immunostained with primary antibodies against HSL or ATGL and with fluorescein-conjugated secondary antibodies. The dark circles inside adipocytes indicate intracellular lipid droplets. HSL was translocated from the cytosol to the lipid droplets surface on stimulation with isoproterenol but not dexamethasone (G). The values are mean ± sem of three separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. untreated control. †, P < 0.05; ††, P < 0.01; †††, P < 0.001vs. dexamethasone (Dex) alone.
Administration of dexamethasone increased lipolysis in adipose tissues and elevated plasma FFA level in rats
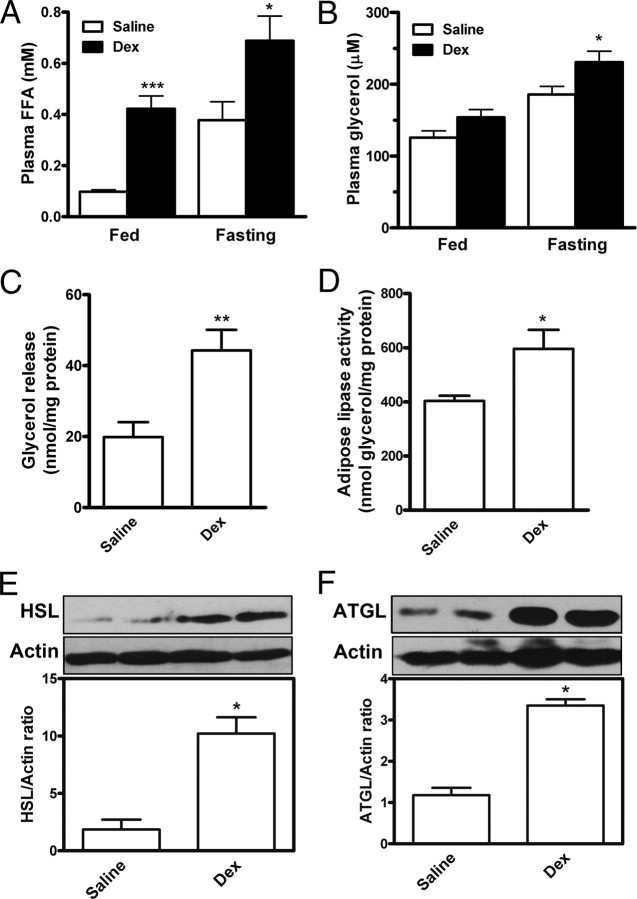
Regulation of circulating FFA concentrations depends mainly on lipolysis in adipose tissues, especially in the fasting stage. We examined whether systemic FFA elevation is associated with the in vivo lipolysis response to glucocorticoid challenge in rats. Male rats were sc injected with 0.9% saline (n = 6) or low-dose dexamethasone sodium phosphate (0.1 mg/kg body weight/d, n = 6). In comparison with saline control, dexamethasone greatly elevated plasma FFA concentrations by 4.3-fold (P < 0.001) in ad libitum-fed rats at wk 6 and by 1.8-fold (P < 0.05) in fasting rats at wk 7 (Fig. 7A); plasma glycerol was increased by 1.46-fold in fasting rats (Fig. 7B, P < 0.05). We then examined the in vivo lipolysis in epididymal adipose tissues isolated from the treated rats. As compared with saline control, dexamethasone injection resulted in 223% (Fig. 7C, P < 0.01) increase in glycerol efflux from adipose tissues; lipase activity was significantly increased (Fig. 7D, P < 0.05), and the protein levels of HSL and ATGL were greatly up-regulated (Fig. 7, E and F, P < 0.05) in adipose tissues of rats treated with dexamethasone. These observations suggest that dexamethasone increased the in vivo lipase activity and lipolysis in adipose tissues, thereby increasing FFA efflux from adipocytes to the bloodstream.
Fig. 7.
Elevation of FFA levels in plasma and lipolytic activity in adipose tissues of rats treated with dexamethasone. Rats were sc injected with dexamethasone sodium phosphate (0.1 mg/kg body weight · d) or saline. At the end of wk 7, rats were fasted for 12 h before being killed. A and B, Plasma FFA (A) and glycerol (B) levels. C, The epididymal adipose tissues isolated from fasted rats were minced and incubated for 60 min without addition. Glycerol release in the medium was measured and served as an index of lipolysis. D–F, In parallel, lipase activity was assayed (D), and protein levels of HSL (E) and ATGL (F) were detected in isolated epididymal adipose tissues. Data are mean ± sem of six animals (n = 6), each measured in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. saline group. Fed indicates ad libitum-fed rats at wk 6, and fasting indicates 12-h fasted rats at end of wk 7. Dex, Dexamethasone.
Discussion
Glucocorticoids permit adipocytes to produce normal and enhanced lipolysis responses to various hormones (16, 22, 24, 25). This permissive effect has long been thought to be the major basis by which glucocorticoids modulate FFA mobilization. In this study, we demonstrated that dexamethasone directly stimulates lipolysis in isolated primary adipocytes through multiple mechanisms. This agent also promotes lipase activity and lipolysis in vivo in epididymal adipose tissues, thus elevating plasma FFA concentrations in rats. The lipolytic action of glucocorticoids liberates FFA efflux from adipocytes to the bloodstream, which could be a cellular basis for systemic FFA elevation and hence insulin resistance in hypercortisolemia or with glucocorticoid treatment.
In primary adipocytes, dexamethasone and other glucocorticoids directly stimulated lipolysis in a dose- and time-dependent manner. The lipolytic action of dexamethasone occurred at a low concentration, 1 nm, and became robust at 10–100 nm, a level commonly used in therapy (10–600 nm) (28). When FFA efflux induced by dexamethasone slightly increased at 4–8 h, glycerol release remained undetectable. Both FFA and glycerol release were apparent 16–24 h after incubation. Thus, the lipolysis action of glucocorticoids appears to be a chronic process on prolonged stimulation, which is different from the acute lipolysis response to catecholamines (2) but similar to the chronic lipolysis with TNF-α (4). This chronic feature could be relevant to the gene regulatory features of glucocorticoids and may explain why glucocorticoids may not increase or only slightly increase lipolysis during short-term (<6 h) stimulation, as noted earlier (21, 23, 24). An early study showed that prolonged incubation with dexamethasone for 24 h but not 4 h increased lipolysis in adipocytes (26), which is consistent with our findings.
Adipose cells have many glucocorticoid receptors (29). We observed that RU486, a glucocorticoid receptor antagonist (14), blocked the lipolytic action of dexamethasone but not that of TNF-α, which suggests that steroids could stimulate lipolysis via glucocorticoid receptors. Subsequently, we observed that dexamethasone down-regulated expression levels of PDE3B mRNA and proteins perhaps through a postreceptor process; these effects could be a basis for decreased 3′,5′-AMP phosphodiesterase (PDE) activity in differentiated 3T3-L1 adipocytes (30) and in primary adipocytes isolated from dexamethasone-injected rats (31, 32). PDE3B is responsible for cAMP hydrolysis in adipocytes, and its down-regulation accounts for cAMP elevation during TNF-α-induced lipolysis (4). Our RIA results confirm that, parallel to PDE3B down-regulation, cAMP production is increased by approximately 2-fold during dexamethasone-stimulated lipolysis. However, dexamethasone may also directly increase cAMP production by promoting basal and forskolin-stimulated activity of adenylate cyclase in adipocytes (33). Therefore, dexamethasone may have a two-step strategy to promote cAMP accumulation by inhibiting cAMP degradation and stimulating adenylate cyclase. The stimulation of dexamethasone promoted PKA activity ratio by approximately 1.7-fold, compared with an approximately 3.3-fold elevation of PKA activity on isoproterenol stimulation. Importantly, when promoted PKA activity was inhibited by H89, dexamethasone-induced release of FFAs and glycerol ceased. Although ERK1/2 participates in the regulation of lipolysis response to catecholamines or TNF-α (3, 4), the lipolytic action of dexamethasone was not significantly attenuated by inhibiting ERK1/2, JNK, p38 MAPK, or PKC. Therefore, cAMP elevation and PKA activation are crucial for dexamethasone-stimulated lipolysis.
Perilipins coat lipid droplets and function as a barrier to restrict lipase access to the triglyceride core within the droplet (6). Perilipin phosphorylation (7, 8) and/or down-regulation (4, 9) impair the barrier function, thereby facilitating lipolysis. We observed a two-stage perilipin regulation by dexamethasone via different pathways: an induction of perilipin phosphorylation that could be suppressed on PKA inhibition with H89 and a down-regulation of perilipin proteins that was prevented by treatment with the anti-glucocorticoid RU486. In parallel, inhibition of phosphorylation or down-regulation of perilipins attenuated the lipolysis stimulated by dexamethasone. Perilipin is regulated mainly via posttranscriptional machinery (34). When perilipin cannot be posttranslationally stabilized by intracellular stored neutral lipids (34), it is degraded by proteasome (35); whether perilipin down-regulation by dexamethasone is mediated by proteasome requires further study.
HSL and ATGL are two major lipases consisting of approximately 95% triglyceride hydrolase activity in adipocytes (12, 13). Early studies suggested that transcriptional inhibition with actinomycin D may attenuate the glucocorticoid-enhanced lipolysis response to other hormones (21, 24), which suggests that increases in mRNA synthesis, perhaps of cellular lipases, could be involved in the lipolytic regulation by glucocorticoids. Our study indicated that ATGL and HSL mRNA were greatly up-regulated by dexamethasone in primary adipocytes, which is consistent with previous observations that dexamethasone increased ATGL (36) and HSL (26) mRNA in differentiating and primary adipocytes. When dexamethasone-up-regulated HSL and ATGL transcription was inhibited by actinomycin D or RU486, the lipolytic action was attenuated. Protein levels of ATGL and HSL were also up-regulated by dexamethasone, and this effect was suppressed by RU486. Thus, these observations suggest a postreceptor and transcriptional regulation of dexamethasone on the lipases and hence lipolysis. ATGL is not phosphorylated by PKA, and its phosphorylation on enzymatic function is unclear (11). HSL activation depends on its phosphorylation. HSL is phosphorylated by PKA at Ser-563, -659, and -660 residues, of which Ser-659 and Ser-660, rather than Ser-563, are the major sites for controlling HSL activity (37). No antibody against the phospho-Ser-659 motif is currently available; however, we observed that dexamethasone stimulation slightly increased Ser-563 phosphorylation but obviously promoted Ser-660 phosphorylation of HSL, and this action was likely mediated via PKA signaling because it could be abolished on PKA inhibition with H89.
Dexamethasone promoted lipase activity in primary adipocytes, and this effect was inhibited by H89 and RU486, which suggests an involvement of a glucocorticoid receptor and PKA activation in the modulation of lipase activity. The studies in the last decade demonstrate that lipase activity seems to not parallel simply lipase protein levels but instead could be modulated by trafficking and interaction with other scaffold-like proteins such as perilipin and CGI-58 (12). Moreover, HSL translocation from the cytosol to the lipid droplets in adipocytes confers full lipolysis with typical PKA stimulation of catecholamines or forskolin, which requires simultaneous phosphorylation of both HSL and perilipin (7, 8, 10). Although dexamethasone caused moderate PKA activation, up-regulated HSL proteins, and promoted HSL phosphorylation, it failed to induce HSL translocation to the lipid droplets in our study. This finding is perhaps due to dexamethasone stimulation being weaker or different from typical PKA stimulation (phosphorylation) of catecholamine and thus not inducing a strong perilipin phosphorylation or not sufficiently inducing simultaneous phosphorylation of both HSL and perilipin to ensure an induction of HSL translocation. In fact, parts of ATGL (11) and HSL (38) are already associated with the lipid droplets in unstimulated adipocytes. ATGL is predominantly responsible for hydrolysis of triglycerides (11, 13), whereas the hydrolysis activity of HSL for diglycerides is 10 times that for triglycerides (39). Therefore, in dexamethasone-stimulated adipocytes, HSL might efficiently manipulate the hydrolysis of ATGL-generated diglycerides in the cytoplasm rather than (translocation) at the lipid droplet surface. Likely, the regulation of glucocorticoids on the lipases and, thereby lipolysis, is complex, which requires further investigation.
In conclusion, the present findings give evidence of a novel pathway of dexamethasone directly stimulating lipolysis and increasing FFA mobilization from adipocytes to the bloodstream. This lipolytic action could be a cellular basis of elevated level of circulating FFAs in hypercortisolemia and with glucocorticoid treatment.
Materials and Methods
Materials
Dexamethasone, dexamethasone 21-phosphate disodium salt, cortisone, cortisol, corticosterone acetate, PD98059, U0126, and phenol red-free DMEM were from Sigma (St. Louis, MO). Enzyme materials used for enzymatic assays were from Totobo Co. (Tokyo, Japan). Rabbit polyclonal antibodies against rat perilipin and HSL were generous gifts from C. Londos at the National Institutes of Health (Bethesda, MD). Antibodies recognizing phosphorylated HSL were from Cell Signaling (Boston, MA). Modified Lowry protein assay kit, nitrocellulose membrane, and enhanced chemiluminescence detection kit were from Applygen Technologies (Beijing, China).
Isolation and culture of primary rat adipocytes
Adipocytes were isolated from epididymal fat pads of normal male Sprague-Dawley rats (160–180 g) according to our laboratory method (4, 8, 40, 41). Adenosine (200 nm) was included in all subsequent incubations. Rat fat pads were excised, minced, and digested in Krebs-Ringer solution containing 0.75 mg/ml type I collagenase, 200 nm adenosine, 25 mm HEPES (pH 7.4), and 1% defatted BSA. After incubation for 40 min at 37 C in a water bath shaken at 100 cycles/min, cells were filtered through a nylon mesh and washed three times in warmed phenol-free DMEM. Adipocytes floating in the tube were collected and packed by centrifugation at 200 × g for 3 min. The packed cell volume of adipocytes was determined (8). Adipocytes were preincubated in an atmosphere of 5% CO2 at 37 C for 1 h before treatments.
RNA extraction and real-time PCR
Total RNA was extracted from primary rat adipocytes by use of RNAtrip reagent (Applygen, Beijing, China). Two micrograms of total RNA per sample were reverse transcribed, and real-time PCR involved use of the Mx3000 Multiplex quantitative PCR system (Stratagene, La Jolla, CA) and SYBR Green I dye. The sets of forward and reverse primers were PDE3B, ggaaggattctcagtcaggt and tggaatacagcaactcttcg; HSL, gaatatcacggagatcgagg and ccgaagggacacggtgatgc; ATGL, cactttagctccaaggatga and tggttcagtaggccattcct; and β-actin, cgtgggccgccctaggcacca and ctctttgatgtcacgcacgatttc. Amplification reactions in duplicate were carried out for 40 cycles at 94 C for 30 sec, 58 C for 30 sec, and 72 C for 30 sec. The correct PCR products obtained were confirmed by sequencing amplicons. The relative target mRNA levels were analyzed with use of Stratagene Mx3000 software and normalized to that of internal control β-actin.
Fatty acid assay
The concentration of FFAs in the culture medium was determined by colorimetric assay by our laboratory method (4, 40). Briefly, 50 μl culture medium was mixed with 120 μl isooctane and 80 μl cupric acetate-pyridine. The mixture was vortexed and centrifuged for 10 min at 12,000 × g at room temperature. The upper organic phase (80 μl) was mixed with 180 μl of the color development reagent consisting of diphenylcarbazone and diphenylcarbazide in methanol. After a vortex, the reaction was developed immediately for color. The absorbance at 540 nm was spectrophotometrically measured.
Glycerol assay
The incubation medium was heated at 70 C for 10 min to inactivate residue lipase activity in the samples. Glycerol content was determined by a colorimetric assay (GPO Trinder reaction) from the absorption of 490 nm and served as an index of lipolysis. Lipolysis data are expressed as micromoles of glycerol per milliliter packed cell volume of adipocytes (4, 8, 40).
Immunoblotting
Adipocytes were packed and lysed in ice-cold fractionation buffer [50 mm Tris-HCl (pH 7.4), 255 mm sucrose, 1 mm EDTA, 0.1 mm sodium orthovanadate, and 50 mm sodium fluoride]. The lysate was incubated on ice for 15 min and then centrifuged at 15,000 × g for 30 min at 4 C. The solidified fat cake of intracellular lipid droplets floating on top of the tube was used for perilipin immunoblotting (8, 41). The cytosolic fraction was localized below the layer of the fat cake. For immunoblotting detection of other proteins, whole adipocyte lysates or the cytosol fractions were used. The protein content was determined by modified Lowry protein assay. Equal amounts of proteins were separated by SDS-PAGE and underwent immunoblotting analysis. The blots were developed with enhanced chemiluminescence detection reagents. If required, the blots were stripped and then reprobed. Densitometric analysis of protein bands involved use of NIH Image software.
cAMP RIA
According to our previous method (4, 40), 20 μl adipocytes was mixed with 100 μl ice-cold lysis buffer [20 mm Tris-HCl (pH 7.4) and 1 mm EDTA]. The lysate was incubated on ice for 20 min and centrifuged at 15,000 × g for 20 min at 4 C. Then, 90 μl cytosol fraction was mixed with 30 μl 40% trichloroacetic acid and incubated on ice for 5 min. The tubes were vortexed and centrifuged at 12,000 × g for 5 min at 4 C. The supernatant was collected and used for cAMP assay according to the protocol of a commercial 125I-RIA kit (Isotope Laboratory of Shanghai University of Chinese Medicine, Shanghai, China). Cellular cAMP concentration was expressed as picomoles cAMP per milligram protein.
Assay of PKA activity ratio
A corrected PKA activity ratio, which more faithfully reflects the true activity of cAMP-dependent protein kinase in adipocytes, was determined as described (42) with our minor modifications (43). Briefly, 20 μl of the cytosolic extract was added in the 30-μl assay medium. PKA activity in each cytosol extract was assayed six times under three separate conditions: a) a quiescent reaction consisting of 50 mm Tris-HCl (pH 7.4), 10 mm MgCl2, 1 mm 3-isobutyl-1-methylxanthine, 20 μCi/ml [γ-32P]ATP, 0.3 mm ATP, and 100 μm Kemptide (a PKA substrate); b) a stimulatory reaction consisting of reaction a plus 2 μm cAMP to activate PKA; and c) an inhibitory reaction consisting of reaction a plus 10 μm PKI inhibitor to suppress PKA. The reactions were incubated for 10 min at 30 C and terminated by cooling the tubes in a 0 C ice bath. Then, 40 μl reaction mixture was passed through a P81 phosphocellulose filter. The filter was washed four times for 5 min each with ice-cold 75 mm phosphoric acid and then for twice for 5 min each with 100% ethanol. The filter was air dried and placed into 3 ml liquid scintillation fluid for counting the 32P radioactivity. Results are expressed as a ratio [(a − c)/(b − c)] of PKA activity (42, 43).
Assay of lipase activity
According to our previous method (4, 40, 43), 50 μl packed adipocytes was washed twice with PBS and mixed with 120 μl lysis buffer containing 50 mm Tris-HCl (pH 7.4), 1 mm MgCl2, and 1 mm EDTA. The lysate was vortexed and centrifuged at 15,000 × g for 10 min at 4 C. The infranatant phase below the fat cake was carefully transferred to a new tube, centrifuged again at 15,000 × g for 10 min at 4 C, and collected without disrupting the floating triglyceride residues. Each cytosol fraction was added to an emulsified triolein substrate solution (reaction A) and, in parallel, to a control solution without triolein substrates (reaction B). The reactions were incubated for 60 min at 37 C, when the cytosolic lipases hydrolyze emulsified triolein to produce novel glycerol in the reaction A. The reaction B contained only endogenous glycerol derived from the cytosol preparation. Next, glycerol contents of the reactions A and B were determined. A value of (A − B) was calculated, which indicated a 60-min rate of glycerol release by lipase-catalyzed triolein hydrolysis and represented activity of adipose lipases.
Differentiation of rat preadipocytes and immunostaining
Adipose precursor cells isolated from rat epididymal fat pads were differentiated into adipocytes for 3 d in serum-free DMEM/F12 (1:1) medium supplemented with 5 μg/ml insulin, 33 μm biotin, and 200 pm T3, as we previously described (8, 41). At d 4, the differentiating adipocytes were transferred to serum-free DMEM/F12 (1:1) medium and incubated for 24 h. At d 5, the differentiated adipocytes were stimulated with dexamethasone or isoproterenol and then were fixed with 4% paraformaldehyde and blocked with 5% donkey serum for 60 min. The cells were incubated with rabbit antisera against HSL at 1:400 or ATGL at 1:500 overnight at 4 C and then with fluorescein isothiocyanate-conjugated donkey antirabbit IgG at 1:300 for 2 h in the dark at room temperature (41, 44). Immunofluorescent signals of HSL or ATGL were observed with use of a Nikon Eclipse TE2000-U microscope.
Animal studies
The animal study was approved by the Animal Care and Use Committee of Peking University Health Science Center. Sprague-Dawley male rats (150 ± 5 g) were randomly divided into two groups (n = 6 each) and sc injected with 0.9% saline or dexamethasone sodium phosphate (0.1 mg/kg body weight · d). The rats were fed ad libitum, and tail venous blood was collected weekly. At the end of 7 wk, the rats underwent 12 h fasting from midnight and then were killed. The blood samples were centrifuged at 1000 × g for 15 min at 4 C, and the plasma was collected. The plasma FFA and glycerol were determined with use of the commercial assay kits (Applygen). Subsequently, epididymal fat tissues were isolated and used for assaying glycerol release, lipase activity, and immunoblotting.
Statistical analysis
Data are expressed as mean ± sem. Student’s t test or one-way ANOVA Tukey’s test was used for statistical analysis. A value of P < 0.05 was considered statistically significant.
NURSA Molecule Pages:
Ligands: Dexamethasone | RU486.
Footnotes
This work was supported by the National Natural Science Foundation of China (30890042, 30770803, and 30670779) and by the National Basic Research Program of China (2009CB941603 and 2006CB503910).
Current address of J.H.: Center for Pharmacogenetics and Department of Pharmaceutical Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Current address of H.J.: Department of Medicine, Columbia University Medical Center. New York, New York 10032.
Disclosure Summary: Authors have no conflict of interest to disclosure.
First Published Online May 14, 2009
Abbreviations: ATGL, Adipose triglyceride lipase; FFA, free fatty acid; HSL, hormone-sensitive lipase; JNK, c-Jun-NH2-terminal kinase; PDE3B, cyclic-nucleotide phosphodiesterase 3B; PKA, protein kinase A.
References
- 1.Arner P2002. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev 18(Suppl 2):S5–S9 [DOI] [PubMed]
- 2.Londos C, Brasaemle DL, Schultz CJ, Adler-Wailes DC, Levin DM, Kimmel AR, Rondinone CM1999. On the control of lipolysis in adipocytes. Ann NY Acad Sci 892:155–168 [DOI] [PubMed] [Google Scholar]
- 3.Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L, Lonnqvist F, Arner P2002. Mapping of early signaling events in tumor necrosis factor-α-mediated lipolysis in human fat cells. J Biol Chem 277:1085–1091 [DOI] [PubMed] [Google Scholar]
- 4.Zu L, Jiang H, He J, Xu C, Pu S, Liu M, Xu G2008. Salicylate blocks lipolytic actions of tumor necrosis factor-α in primary rat adipocytes. Mol Pharmacol 73:215–223 [DOI] [PubMed] [Google Scholar]
- 5.Fricke K, Heitland A, Maronde E2004. Cooperative activation of lipolysis by protein kinase A and protein kinase C pathways in 3T3-L1 adipocytes. Endocrinology 145:4940–4947 [DOI] [PubMed] [Google Scholar]
- 6.Londos C, Sztalryd C, Tansey JT, Kimmel AR2005. Role of PAT proteins in lipid metabolism. Biochimie 87:45–49 [DOI] [PubMed] [Google Scholar]
- 7.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C2003. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Jiang H, Tansey JT, Tang C, Pu S, Xu G2006. Calyculin and okadaic acid promote perilipin phosphorylation and increase lipolysis in primary rat adipocytes. Biochim Biophys Acta 1761:247–255 [DOI] [PubMed] [Google Scholar]
- 9.Ren T, He J, Jiang H, Zu L, Pu S, Guo X, Xu G2006. Metformin reduces lipolysis in primary rat adipocytes stimulated by tumor necrosis factor-α or isoproterenol. J Mol Endocrinol 37:175–183 [DOI] [PubMed] [Google Scholar]
- 10.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C2000. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta 1483:251–262 [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386 [DOI] [PubMed] [Google Scholar]
- 12.Watt MJ, Steinberg GR2008. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem J 414:313–325 [DOI] [PubMed] [Google Scholar]
- 13.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241 [DOI] [PubMed] [Google Scholar]
- 14.Bamberger CM, Schulte HM, Chrousos GP1996. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 17:245–261 [DOI] [PubMed] [Google Scholar]
- 15.Cato AC, Nestl A, Mink S2002. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE 2002:RE9 [DOI] [PubMed]
- 16.Macfarlane DP, Forbes S, Walker BR2008. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol 197:189–204 [DOI] [PubMed] [Google Scholar]
- 17.Djurhuus CB, Gravholt CH, Nielsen S, Mengel A, Christiansen JS, Schmitz OE, Møller N2002. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab 283:E172–E177 [DOI] [PubMed]
- 18.Divertie GD, Jensen MD, Miles JM1991. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes 40:1228–1232 [DOI] [PubMed] [Google Scholar]
- 19.Severino C, Brizzi P, Solinas A, Secchi G, Maioli M, Tonolo G2002. Low-dose dexamethasone in the rat: a model to study insulin resistance. Am J Physiol Endocrinol Metab 283:E367–E373 [DOI] [PubMed]
- 20.Novelli M, Pocai A, Chiellini C, Maffei M, Masiello P2008. Free fatty acids as mediators of adaptive compensatory responses to insulin resistance in dexamethasone-treated rats. Diabetes Metab Res Rev 24:155–164 [DOI] [PubMed] [Google Scholar]
- 21.Goodman HM1970. Permissive effects of hormones on lypolysis. Endocrinology 86:1064–1074 [DOI] [PubMed] [Google Scholar]
- 22.Exton JH, Friedmann N, Wong EH, Brineaux JP, Corbin JD, Park CR1972. Interaction of glucocorticoids with glucagon and epinephrine in the control of gluconeogenesis and glycogenolysis in liver and of lipolysis in adipose tissue. J Biol Chem 247:3579–3588 [PubMed] [Google Scholar]
- 23.Fain JN, Scow RO, Chernick SS1963. Effects of glucocorticoids on metabolism of adipose tissue in vitro. J Biol Chem 238:54–58 [Google Scholar]
- 24.Fain JN, Kovacev VP, Scow RO1965. Effect of growth hormone and dexamethasone on lipolysis and metabolism in isolated fat cells of the rat. J Biol Chem 240:3522–3529 [PubMed] [Google Scholar]
- 25.Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Møller N, Schmitz O2004. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab 286:E488–E494 [DOI] [PubMed]
- 26.Slavin BG, Ong JM, Kern PA1994. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res 35:1535–1541 [PubMed] [Google Scholar]
- 27.Spitz IM, Bardin CW1993. Mifepristone (RU 486): a modulator of progestin and glucocorticoid action. N Engl J Med 329:404–412 [DOI] [PubMed] [Google Scholar]
- 28.Czock D, Keller F, Rasche FM, Häussler U2005. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44:61–98 [DOI] [PubMed] [Google Scholar]
- 29.Rebuffé-Scrive M, Lundholm K, Björntorp P1985. Glucocorticoid hormone binding to human adipose tissue. Eur J Clin Invest 15:267–271 [DOI] [PubMed] [Google Scholar]
- 30.Elks ML, Manganiello VC, Vaughan M1984. Effect of dexamethasone on adenosine 3′,5′-monophosphate content and phosphodiesterase activities in 3T3-L1 adipocytes. Endocrinology 115:1350–1356 [DOI] [PubMed] [Google Scholar]
- 31.Manganiello V, Vaughan M1973. An effect of insulin on cyclic adenosine 3′:5′-monophosphate phosphodiesterase activity in fat cells. J Biol Chem 248:7164–7170 [PubMed] [Google Scholar]
- 32.Osegawa M, Makino H, Kanatsuka A, Suzuki T, Yoshida S1984. Effects of changes in serum insulin in response to dexamethasone and adrenalectomy on insulin-sensitive phosphodiesterase in rat fat cells. Metabolism 33:754–759 [DOI] [PubMed] [Google Scholar]
- 33.Lacasa D, Agli B, Giudicelli Y1988. Permissive action of glucocorticoids on catecholamine-induced lipolysis: direct “in vitro” effects on the fat cell β-adrenoreceptor-coupled-adenylate cyclase system. Biochem Biophys Res Commun 153:489–497 [DOI] [PubMed] [Google Scholar]
- 34.Brasaemle DL, Barber T, Kimmel AR, Londos C1997. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem 272:9378–9387 [DOI] [PubMed] [Google Scholar]
- 35.Xu G, Sztalryd C, Londos C2006. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta 1761:83–90 [DOI] [PubMed] [Google Scholar]
- 36.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS2004. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 279:47066–47075 [DOI] [PubMed] [Google Scholar]
- 37.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C1998. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273:215–221 [DOI] [PubMed] [Google Scholar]
- 38.Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ2000. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem 275:5011–5015 [DOI] [PubMed] [Google Scholar]
- 39.Fredrikson G, Strålfors P, Nilsson NO, Belfrage P1981. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem 256:6311–6320 [PubMed] [Google Scholar]
- 40.Zu L, He J, Jiang H, Xu C, Pu S, Xu G2009. Bacterial endotoxin stimulates adipose lipolysis via toll-like receptor 4 and extracellular signal-regulated kinase pathway. J Biol Chem 284:5915–5926 [DOI] [PubMed] [Google Scholar]
- 41.Jiang H, He J, Pu S, Tang C, Xu G2007. Heat shock protein 70 is translocated to lipid droplets in rat adipocytes upon heat stimulation. Biochim Biophys Acta 1771:66–74 [DOI] [PubMed] [Google Scholar]
- 42.Honnor RC, Dhillon GS, Londos C1985. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem 260:15122–15129 [PubMed] [Google Scholar]
- 43.Zhang T, He J, Xu C, Zu L, Jiang H, Pu S, Guo X, Xu G2009. Mechanisms of metformin inhibiting lipolytic response to isoproterenol in primary rat adipocytes. J Mol Endocrinol 42:57–66 [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, Dorward H, Kimmel AR, Londos C2005. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem 280:42841–42847 [DOI] [PubMed] [Google Scholar]