Abstract
We describe a review of human adenovirus (HAdV) infections occurring among adults in a tertiary hospital in Singapore from February to May 2013. A similar increase in cases was observed among children and military personnel during the same time period. The majority of isolates were identified as HAdV-7, likely an emerging pathogen in Asia.
Keywords: adenovirus, outbreak, severe infection, surveillance
Emerging and reemerging viruses have caused outbreaks of varying scales in recent years. These often result not only in public health emergencies, but they also have significant impact on individual health outcomes.
Human adenoviruses (HAdVs) are nonenveloped deoxyribonucleic acid viruses that cause upper respiratory tract infections and, infrequently, severe pneumonia [1]. There are more than 52 serotypes of HAdVs with serotypes B, C, and E responsible for most of these infections documented in preschool children and military recruits, the latter due to crowding, increased physical and psychological stress [2], among others. More recently, multiple HAdV outbreaks have been reported in Asia [3–5], suggesting that HAdV might be reemerging in the region. Indeed, an outbreak of severe HAdV-7 disease among children occurred in Singapore from 2012 to 2013 [6]. Available epidemiological information initially directed surveillance attention to pediatric patients and military personnel [6]. In this study, we describe a retrospective review of adult HAdV infections in one tertiary hospital in Singapore during the same period, focusing in particular on the epidemiological, clinical, and molecular characteristics of patients with severe infections.
METHODS
Singapore General Hospital, an adult public tertiary care hospital with an acute care medical facility, routinely screens respiratory specimens for viruses using multiplex polymerase chain reaction (PCR) assays. Indications for multiplex PCR sampling are patients presenting with symptoms suggestive of a respiratory tract infection, including but not limited to fever, cough, sore throat, and rhinorrhea. In February 2013, an astute microbiologist observed an unusually high HAdV PCR positivity rate that initiated further investigations. A case was defined as a patient whose respiratory sample was positive for HAdV monoinfection by multiplex PCR. As the prospective investigations commenced well after the first cases were highlighted to the hospital infection control unit, a retrospective analysis of all the HAdV cases that were managed in the hospital before, during, and after the “heightened” period was thus conducted (Figure 1A).
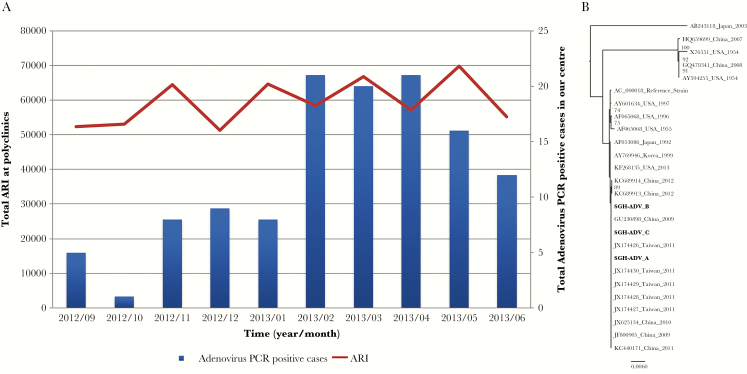
Figure 1.
(A) Total monthly number of patients seeking treatment at primary care clinics for acute respiratory infections (ARIs) and adenovirus polymerase chain reaction (PCR)-positive cases in our center from September 2012 to June 2013. (B) Hexon gene sequences of the adenovirus type 7 viruses from this study in relation to representative sequences from around the world. Branch lengths are indicative of the number of substitutions per site. Sequences from the current study are depicted in bold.
We reviewed all PCR results of respiratory samples from the Department of Pathology, from February 2012 to June 2013. Patient records of HAdV cases were retrieved, and their clinical, laboratory, and radiological characteristics were reviewed. Where possible, respiratory samples were retrieved and HAdV was typed by sequencing a ~750-base pair segment containing the hexon gene hypervariable regions 1–6. Severe HAdV infection was defined as infection resulting in admission to the intensive care unit. Nosocomial infection was defined as a case with a positive HAdV PCR, with no other copathogen detected that could account for the patient’s clinical presentation, and that had a date of specimen collection >48 hours after date of hospitalization.
RESULTS
The epidemiologic curve shows a peak in HAdV cases between February 1 and May 31, 2013, relative to the other months in the study period (Figure 1A). It is notable that the peak occurred during approximately the same time period that a sudden increase in severe HAdV-7 disease among pediatric inpatients and military personnel in Singapore was reported [6]. A total of 35 of 555 (6.3%) respiratory specimens collected from inpatients were positive for HAdV PCR during the outbreak period compared with the PCR-positive rate of 1.4% during the same period in the previous year (P < .001). Two of these HAdV-positive cases had dual infections, which were removed from further analysis because the disease could not be confidently attributed to HAdV infection. Of the 33 HAdV monoinfections, we attempted typing of 9 respiratory viral isolates from 9 different patients: 6 (66.6%) were identified as HAdV-7, 1 as HAdV-4, and 2 were untyped due to insufficient specimen volume. Only 9 patients (27.2%) were classified as having nosocomial infection, with the remaining 24 patients being diagnosed with HAdV within 48 hours of admission.
Four HAdV-infected patients had severe infection. All 4 patients were males with a mean age of 51 years (95% confidence interval, 31.8–62.2 years). Only 1 patient had underlying diabetes mellitus, whereas the remaining 3 had no other existing comorbidities. All 4 patients had abnormal pulmonary infiltrates on chest radiograph on presentation. Among patients with severe infection, 2 (50%) had HAdV-7 infection and 1 (25%) had HAdV-4. The fourth patient had an untyped HAdV: this patient died, giving an overall mortality of 3%. All 4 patients received intravenous cidofovir.
Because HAdV-7 was the predominant strain, whole-genome sequencing of 3 HAdV-7 isolates with sufficient ribonucleic acid samples were performed. All 3 isolates were identical, with the exception of a small indel in the 3’-untranslated region of the L2 gene in 2 of the isolates. It is notable that the sequenced isolates were >99% identical to KC440171 (Figure 1B), which has been found to cause a number of adenovirus outbreaks in southern China [7].
DISCUSSION
This description of HAdV infections, with a predominance of HAdV-4 and HAdV-7, among adult civilians is the first of its kind in Singapore. Although the strain is not novel, it seems to be new in the local population, resulting in a significant number of severe cases with increased mortality and morbidity.
Although there is no systemic surveillance of HAdV in Singapore, published data thus far indicate that HAdV infection is sporadic. Doraisingham and Ling [8] noted outbreaks caused by influenza and parainfluenza, but not HAdV in children from January 1977 to December 1979. Furthermore, an epidemiological study of respiratory viral pathogens among Singapore military servicemen between 2009 and 2012 identified HAdV monoinfection in only 3.7% of cases with febrile respiratory illness [9]. Other isolated outbreaks have been reported in Singaporean military servicemen [10] and in the pediatric population [6].
In Asia, several HAdV outbreaks have been reported recently [4, 5, 7], including those attributed to HAdV7. A 2012–2013 study of HAdV-7 associated with severe pediatric infections in Singapore showed 100% nucleotide similarity with the sequences reported from outbreaks in Taiwan and China [6], suggesting transboundary transmission of virus in the region. This trend in HAdV-7 emergence may be a relatively recent trend because a serosurvey reported in 2001 showed low immunity to HAdV-7 in Singapore [11]. Furthermore, infection with HAdV-7 in Singapore also appears to be associated with more severe disease [12]. However, the possibility that HAdV-7 has evolved to acquire greater virulence is unlikely because sequence data from our isolates are >99% identical to KC440171, which has been found to cause a number of outbreaks in China [7], Korea [13], Taiwan [14]. Our findings thus call for more detailed prospective studies into the correlates of outcome in healthy adults with HAdV-7 infection.
It is also interesting that acute respiratory infection (ARI) surveillance data in Singapore, which was based on syndromic reporting from selected primary healthcare clinics, did not show any significant change in trends within the same period in time as our study (Figure 1A). However, respiratory samples from a subset of these patients are routinely tested only for influenza but not for other viruses. It is thus possible that there existed a community-based outbreak of HAdV-7 in Singapore that was compensated by reduction in the prevalence of the other respiratory viruses, hence resulting in a lack of change in the number of ARI cases. Alternatively, the overall prevalence of HAdV infection in Singapore had remained constant during that time, but the proportion of cases that developed severe disease markedly increased. However, this explanation would be more compelling had we observed significant changes in the nucleotide sequence of the HAdV-7 genome compared with those reported from elsewhere. An etiological surveillance in patients presenting with ARI would have been useful to delineate the underlying reason for the sudden surge in severe HAdV-7 cases.
Our findings also suggest that a reliance on a standard case definition for ARI reporting may also limit the utility of such surveillance methods. Amongst our HAdV cases, 27% presented with atypical symptoms such as chest pain and/or abdominal pain. These presentations do not meet the standard definition for ARI and would otherwise have been missed by the current ARI surveillance system that relies solely on classic patterns of presentation. Indeed, in dengue fever where patients tend to present with atypical clinical manifestations, this often results in underdiagnosis and failure of passive surveillance to detect outbreaks [15]. The diagnosis of ARI that is used for community surveillance is based on clinical symptoms. In contrast, by incorporating rapid molecular diagnostics into ARI surveillance, both sensitivity and speed of outbreak identification can be significantly improved for early implementation of disease control measures.
A limitation of this study is its retrospective design. Furthermore, the true extent of the outbreak could have been much larger than what was estimated because patients with atypical ARI presentations at our hospital may not have been systematically sampled for multiplex PCR.
CONCLUSIONS
The emergence of HAdV, especially HAdV-4 and HAdV-7, as causes of severe respiratory infections warrant urgent prospective studies and etiological surveillance to identify correlates of outcome to guide prompt public health interventions.
Acknowledgments
We assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was granted ethics approval by the SingHealth Centralised Institutional Review Board (Approval ID: 2013/233/C).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Louie JK, Kajon AE, Holodniy M, et al. Severe pneumonia due to adenovirus serotype 14: a new respiratory threat? Clin Infect Dis 2008; 46:421–5. [DOI] [PubMed] [Google Scholar]
- 2. Russell KL, Hawksworth AW, Ryan MA, et al. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999–2004. Vaccine 2006; 24:2835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hwang SM, Park DE, Yang YI, et al. Outbreak of febrile respiratory illness caused by adenovirus at a South Korean military training facility: clinical and radiological characteristics of adenovirus pneumonia. Jpn J Infect Dis 2013; 66:359–65. [DOI] [PubMed] [Google Scholar]
- 4. Huang G, Yu D, Zhu Z, et al. Outbreak of febrile respiratory illness associated with human adenovirus type 14p1 in Gansu Province, China. Influenza Other Respir Viruses 2013; 7:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin KH, Lin YC, Chen HL, et al. A two decade survey of respiratory adenovirus in Taiwan: the reemergence of adenovirus types 7 and 4. J Med Virol 2004; 73:274–9. [DOI] [PubMed] [Google Scholar]
- 6. Ng OT, Thoon KC, Chua HY, et al. Severe pediatric adenovirus 7 disease in Singapore linked to recent outbreaks across Asia. Emerg Infect Dis 2015; 21:1192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao S, Wan C, Ke C, et al. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in Southern China after a twenty-one year absence. Sci Rep 2014; 4:7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doraisingham S, Ling AE. Acute non-bacterial infections of the respiratory tract in Singapore children: an analysis of three years’ laboratory findings. Ann Acad Med Singapore 1981; 10:69–78. [PubMed] [Google Scholar]
- 9. Tan XQ, Zhao X, Lee VJ, et al. Respiratory viral pathogens among Singapore military servicemen 2009–2012: epidemiology and clinical characteristics. BMC Infect Dis 2014; 14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kajon AE, Dickson LM, Metzgar D, et al. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol 2010; 48:1438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nishio O, Matsui K, Goh KT, et al. Prevalence of adenovirus types 3 and 7 antibodies in Singapore. Jpn J Infect Dis 2001; 54:128–9. [PubMed] [Google Scholar]
- 12. Rajkumar V, Chiang CS, Low JM, et al. Risk factors for severe adenovirus infection in children during an outbreak in Singapore. Ann Acad Med Singapore 2015; 44:50–9. [PubMed] [Google Scholar]
- 13. Choi EH, Lee HJ, Kim SJ, et al. Ten-year analysis of adenovirus type 7 molecular epidemiology in Korea, 1995–2004: implication of fiber diversity. J Clin Virol 2006; 35:388–93. [DOI] [PubMed] [Google Scholar]
- 14. Tsou TP, Tan BF, Chang HY, et al. Community outbreak of adenovirus, Taiwan, 2011. Emerg Infect Dis 2012; 18:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ooi EE, Gubler DJ. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cad Saude Publica 2009; 25(Suppl 1):S115–24. [DOI] [PubMed] [Google Scholar]