Abstract
Background
There is lack of reliable predictors of disease severity and mortality in dengue. The present study was carried out to identify these predictors during the 2015 outbreak in India.
Methods
This prospective observational study included confirmed adult dengue patients hospitalized between August and November 2015 in a tertiary care centre in New Delhi, India. Appropriate statistical tests were used to compare clinicolaboratory characteristics, derive predictors of severe disease and mortality, and compute a predictive score for mortality. Serotyping was done.
Results
Data of 369 patients were analyzed (mean age, 30.9 years; 67% males). Of these, 198 (54%) patients had dengue fever, 125 (34%) had dengue hemorrhagic fever (grade 1 or 2), and 46 (12%) developed dengue shock syndrome (DSS). Twenty-two (6%) patients died. Late presentation to the hospital (≥5 days after onset) and dyspnea at rest were identified as independent predictors of severe disease. Age ≥24 years, dyspnea at rest and altered sensorium were identified as independent predictors of mortality. A clinical risk score was developed (12*age + 14*sensorium + 10*dyspnea), which, if ≥22, predicted mortality with a high sensitivity (81.8%) and specificity (79.2%). The predominant serotypes in Delhi (2015) were dengue virus DENV2 and DENV4.
Conclusion
Age ≥24 years, dyspnea at rest, and altered sensorium were identified as independent predictors of mortality. Platelet counts did not determine outcome in dengue patients. Timely referral/access to healthcare is important. The clinical risk score for mortality prediction that was developed in this study can be used in all healthcare settings, after validation in larger cohorts.
Keywords: dengue shock, mortality, outbreak, predictors, severity
Dengue is a self-limiting, vector-borne disease caused by the dengue virus (DENV). Recent decades have witnessed an emergence and re-emergence of dengue, with more frequent and severe outbreaks, in previously unaffected areas. It is now endemic in more than 100 countries with half the world’s population at risk [1]. Although the World Health Organization (WHO) estimates an annual incidence of 50–100 million infections globally, the actual numbers may be as high as 390 million due to inadequate surveillance, notification, and misclassification [2]. There are 4 distinct but closely related serotypes of dengue (DENV1 to DENV4). A fifth serotype was first isolated and reported in October 2013 from forests of Malaysia, but it has not yet been reported from India [3]. Infection by one serotype produces lifelong immunity against reinfection by that serotype (homotypic immunity), but there is an increased risk of severe disease in case of reinfection with a different serotype, due to the phenomenon of antibody-dependent immune enhancement [4]. There has been a significant debate over the utility of the original 1997 and the revised 2009 classification criteria for dengue proposed by the WHO [5, 6]. Although the 2009 classification is easier to apply from a public health point of view and has higher sensitivity and specificity, it is relatively broad in its context and has limited usefulness in a research setting where more stringent definitions of severity are required [7, 8]. Hence, the 1997 classification criteria have been used in the present study. Considering the limitations of each, a revised classification scheme incorporating elements from both classification systems may be forthcoming [8].
India has long been endemic to dengue, although its epidemiology has witnessed a major change over time. The frequency of outbreaks has risen, and certain states/union territories such as Delhi have become hyperendemic [9]. In 2015, India experienced one of its largest outbreaks (99913 notified cases; 220 deaths) with Delhi being most severely affected (15867 notified cases; 60 deaths) [10]. Identification of reliable and validated predictors of disease severity and mortality is central to reduction of morbidity and mortality associated with dengue. Studies to the above end have been limited and have yielded varied combinations of clinical and biochemical predictors of disease severity, due to problems related to study design and/or limited sample size leading to underpowered results. In addition, identification of predictors of dengue mortality is a largely unexplored domain. In an attempt to overcome these limitations, this prospective observational study was undertaken, wherein we describe the clinical and laboratory features of adult dengue patients hospitalized during the outbreak and correlate these factors with disease outcome. Predictors of disease severity and mortality have been identified, and a predictive score for mortality has been calculated. The circulating dengue serotypes in Delhi during the outbreak have also been identified.
MATERIALS AND METHODS
This prospective hospital-based observational study was carried out at the All India Institute of Medical Sciences, an apex tertiary care centre in New Delhi, which is a major dengue hotspot in the country. The study was approved by the Institutional Ethics Committee. The study included all microbiologically confirmed dengue cases admitted under the Department of Internal Medicine between August and November 2015. Written informed consent was taken from the study participants. Refusal to give written informed consent, age <14 years, patients without a serologically confirmed diagnosis, and patients managed on outpatient basis were excluded from the study (Supplementary Figure). Standard WHO definitions were used for classifying suspected dengue infection [5]. Microbiological confirmation was done using (1) NS-1 antigen (Panbio Dengue Early ELISA, Standard Diagnostics Inc., Republic of Korea) if the patient presented within 5 days of disease onset and (2) dengue serum immunoglobulin M (NIV DEN Immunoglobulin [IgM] Capture ELISA, National Institute of Virology, Pune, India) if the patient presented after 5 days of onset. Patients were classified into dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) according to the WHO 1997 criteria [5]. Demographic and clinical details were recorded at admission in a predesigned pro forma, whereas laboratory findings were recorded daily until discharge or death. Point-of-care malaria card test (which could distinguish between Plasmodium falciparum, Plasmodium vivax, or a mixed infection) and chikungunya serology (NIV Chikungunya IgM ELISA, National Institute of Virology, Pune, India) were done for all patients. Widal test (in-house), leptospira serology (Panbio Leptospira IgM ELISA, Standard Diagnostics Inc., Republic of Korea), and scrub typhus serology (InBios International Scrub typhus IgM Indirect ELISA, Seattle, WA) were done in patients with acute febrile illness with rash and/or hepatorenal dysfunction. Japanese encephalitis serology (Panbio Japanese Encephalitis/Dengue IgM Combo ELISA, Australia) was done for all patients with altered sensorium. Seventy-five (25.8%) NS-1-positive but IgM-negative serum samples collected within 5 days of disease onset were sent to National Institute of Virology (Pune) for serotyping by reverse-transcriptase polymerase chain reaction (RT-PCR). Acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI) were defined using the Berlin criteria and the Kidney Disease: Improving Global Outcomes criteria, respectively [11, 12]. A diagnosis of myocarditis was made in the setting of sinus tachycardia with/without ST-T changes on electrocardiogram, elevated troponin I, and/or N-terminal probrain natriuretic peptide (NT-proBNP) or decreased left ventricular ejection fraction on echocardiography.
Statistical Analysis
Data were recorded in a predesigned pro forma and managed on an excel spreadsheet. Categorical variables were summarized as frequency (percentage) and analyzed using χ2 or Fisher’s exact test. Continuous variables were summarized as mean and standard deviation (SD) or median and range (when SD was >50% of mean) and analyzed using parametric (one-way analysis of variance) or nonparametric tests (Kruskal-Wallis test), as appropriate. Clinical and laboratory correlates of mortality and severe disease with P value <.2 in univariable logistic regression analysis were included in multivariable analysis. A predictive score for mortality was derived using coefficients of variables from the regression model. Receiver operating characteristics (ROC) curve analysis was used to develop cutoff value of predictive score for mortality. Characteristics and performance of score cutoff was reported using sensitivity, specificity, and likelihood ratio. Statistical analysis was performed using Stata 12 software (StataCorp [2011], College Station, TX). A P value <.05 was considered statistically significant.
RESULTS
A total of 369 patients were analyzed. The mean age of participants was 30.9 ± 13.9 years (SD), and the majority (67.5%) were males (Table 1). The mean duration of illness at presentation was 4.9 ± 2.4 days (SD), which was higher (6 ± 3 days) in DSS patients (P = .001). A majority [198 (54%)] had DF, 125 (34%) patients had DHF (grade 1 or 2), and the remaining 46 (12%) developed DSS. Two hundred ninety-one (78.9%) patients were dengue positive by NS-1 antigen, and 78 (21.1%) were positive by IgM enzyme-linked immunosorbent assay. Comorbidities such as diabetes mellitus, hypertension, coronary artery disease, and chronic obstructive airway disease/bronchial asthma were seen in 47 (~13%) patients (more common in DSS [P = .001]). Nine (~2.5%) had coinfections (malaria [4], hepatitis B [1], filariasis [1], active pulmonary tuberculosis [1], lower respiratory tract infection [2]).
Table 1.
Comparison of Demographic and Clinical Features in Patients With DF, DHF, and DSSa
| Parameter | DF (n = 198) | DHFb (n = 125) | DSS (n = 46) | P Value | Overall (n = 369) |
|---|---|---|---|---|---|
| Demographic and Epidemiological Parameters | |||||
| Age (years) | 31.8 ± 14.2 | 29.1 ± 12.2 | 32 ± 16.8 | .2 | 30.9 ± 13.9 |
| Male gender | 140 (70.7%) | 83 (66.4%) | 26 (56.5%) | .17 | 249 (67.5%) |
| Duration of illness at presentation (days) | 4.6 ± 2.2 | 5.1 ± 2.4 | 6.0 ± 3.0 | .001 | 4.9 ± 2.4 |
| Comorbiditiesc | 22 (11.1%) | 11 (8.8%) | 14 (30.4%) | .001 | 47 (12.7%) |
| Coinfectionsd | 4 (2%) | 2 (1.6%) | 3 (6.5%) | .18 | 9 (2.4%) |
| Clinical Parameters | |||||
| Fever | 195 (98.5%) | 125 (100%) | 46 (100%) | .52 | 366 (99.1%) |
| Headache | 164 (82.8%) | 107 (85.6%) | 29 (63.0%) | .003 | 300 (81.3%) |
| Retro-orbital pain | 49 (24.8%) | 44 (35.2%) | 6 (13%) | .009 | 99 (26.8%) |
| Conjunctival congestion | 5 (2.5%) | 6 (4.8%) | 7 (15.2%) | .002 | 18 (4.9%) |
| Sore throat | 12 (6.1%) | 4 (3.2%) | 2 (4.4%) | .51 | 18 (4.9%) |
| Rash | 37 (18.7%) | 23 (18.4%) | 5 (10.9%) | .44 | 65 (17.6%) |
| Abdominal pain | 64 (32.3%) | 54 (43.2%) | 21 (45.6%) | .07 | 139 (37.7%) |
| Vomiting | 93 (47%) | 68 (54.4%) | 30 (65.2%) | .06 | 191 (51.8%) |
| Diarrhoea | 25 (12.6%) | 9 (7.2%) | 4 (8.7%) | .29 | 38 (10.3%) |
| Abdominal distension | 5 (2.5%) | 4 (3.2%) | 7 (15.2%) | .004 | 16 (4.3%) |
| Dyspnea at rest | 4 (2%) | 4 (3.2%) | 21 (45.6%) | <.001 | 29 (7.9%) |
| Chest pain | 3 (1.5%) | 2 (1.6%) | 0 | 1 | 5 (1.4%) |
| Altered sensorium | 0 | 0 | 13 (28.3%) | <.001 | 13 (3.5%) |
| Crepitations on chest auscultation | 5 (2.5%) | 5 (4%) | 11 (23.9%) | <.001 | 21 (5.7%) |
| Abdominal tenderness | 18 (9.1%) | 9 (7.2%) | 14 (30.4%) | <.001 | 41 (11.1%) |
| Hepatomegaly | 18 (9.1%) | 12 (9.6%) | 5 (10.9%) | .93 | 35 (9.5%) |
| Splenomegaly | 7 (3.5%) | 6 (4.8%) | 2 (4.4%) | .75 | 15 (4.1%) |
| Assisted ventilation | 1 (0.5%) | 1 (0.8%) | 25 (54.4%) | <.001 | 27 (7.3%) |
| Hemodialysis | 0 | 0 | 6 (13%) | <.001 | 6 (1.6%) |
| Encephalitis | 0 | 0 | 7 (15.2%) | <.001 | 7 (1.9%) |
| Myocarditis | 1 (0.5%) | 1 (0.8%) | 3 (6.5%) | .02 | 5 (1.4%) |
| Platelet transfusion (%) | 80 (40.6%) | 81 (65.3%) | 30 (65.2%) | <.001 | 191 (52%) |
Abbreviations: DF, dengue fever; DHF, dengue hemorrhagic fever; DSS, dengue shock syndrome; SD standard deviation.
aCategorical variables are summarized as n (%). Continuous variables are represented as mean ± SD or median (range) if SD >50% of mean.
bGrade 1 and 2 DHF.
cComorbidities included diabetes mellitus, hypertension, coronary artery disease, and chronic obstructive airway disease/asthma.
dCoinfections included malaria, hepatitis B, filariasis, pulmonary tuberculosis, and lower respiratory tract infection.
Serotyping Data
Seventy-five of 291 (25.8%) NS-1-positive samples were sent for serotyping. Seventeen of these were positive by RT-PCR: 13 were DENV2 and 4 were DENV4, suggesting a mixed outbreak.
Clinical Presentation
Typical manifestations (headache, rash, retro-orbital pain) were more common in DF patients, whereas atypical manifestations (dyspnea, altered sensorium) were more frequently observed among DSS patients: the difference was statistically significant (Table 1). Bleeding manifestations were seen in 149 patients (125 DHF and 24 DSS). Most common sites of bleeding were as follows: gastrointestinal ([GI] 59%), gums (19.5%), and epistaxis (15.4%). Hematuria, hemoptysis, and subconjunctival hemorrhage were less frequent. Two patients each had spontaneous extradural hemorrhage and intramuscular hematoma formation.
The WHO “expanded dengue syndrome” manifestations [13] included the following: encephalitis (7 cases [~2%]) and myocarditis (5 cases [~1.5%]). Twenty-seven (~7%) patients required assisted ventilation, 14 (~4%) of them developed ARDS. Thirty-five (10%) cases developed AKI with 6 (~1.5%) of them requiring hemodialysis. Six patients with pregnancy had uncomplicated DF with a favorable outcome.
Twenty-two patients died (all-cause mortality) during the study period; all had DSS. Nineteen deaths were attributable directly to dengue, whereas 3 died due to unrelated causes (aortic aneurysm rupture [1], ventilator-associated pneumonia complicating a prolonged intensive care unit [ICU] stay [2]). It is noteworthy that almost 1 in 2 patients (47.8%) with DSS succumbed to the disease. Ten patients were referred from other hospitals; all had DSS and 8 of them (80%) died. Mean age of patients who died was 41.1 ± 3.5 years.
Laboratory and Radiological Investigations
Statistically significant differences were noted among the 3 groups in the baseline hemoglobin, total leucocyte count, and renal and liver functions (Table 2). Fifteen patients (10%) had bleeding manifestations without thrombocytopenia (defined as platelet counts <100000/mm3). In contrast, 15 patients had no bleeding even with platelet counts below 10000/mm3 (median = 5000/mm3). Leucocytosis was more prevalent in DSS cases, whereas patients with milder form of disease had leucopenia. Radiological findings have been summarized in Table 2.
Table 2.
Comparison of Laboratory Parameters and Radiological Findings in Patients With DF, DHF, and DSSa
| Parameter | DF (n = 198) | DHFb (n = 125) | DSS (n = 46) | P Value | Overall (n = 369) |
|---|---|---|---|---|---|
| Laboratory Parameters | |||||
| Hemoglobinc (g/dL) | 13.3 ± 2.3 | 13.5 ± 2.3 | 11.9 ± 3.7 | .0007 | 13.2 ± 2.5 |
| Hematocritc (%) | 40.8 ± 6.7 | 40.9 ± 6.7 | 36.0 ± 12.1 | .0004 | 40.2 ± 7.7 |
| Platelet count | |||||
| Meanc (×103/mm3) | 39 (2–213) | 32 (5–295) | 31.5 (5–274) | .05 | 36 (2–295) |
| Thrombocytopeniac,d (%) | 162 (81.8%) | 110 (88%) | 40 (90.9%) | .16 | 312 (85%) |
| Lowest platelet count (×103/mm3) | 28 (2–170) | 22 (5–147) | 21.5 (5–72) | .007 | 25 (2–170) |
| TLC | |||||
| Meanc (/mm3) | 4400 (700–22400) | 4600 (1300–27600) | 8050 (2500–39900) | .0001 | 4700 (700–39900) |
| Leucopeniac,e (%) | 74 (38%) | 44 (36.1%) | 6 (14.3%) | .01 | 124 (34.5%) |
| Leucocytosisc,f (%) | 9 (4.6%) | 9 (7.4%) | 16 (38.1%) | <.001 | 34 (9.5%) |
| Ureac (mg/dL) | 16 (2–113) | 16 (5–93) | 25.5 (8–337) | .0001 | 16 (2–337) |
| Creatininec (mg/dL) | 0.7 (0.2–2.8) | 0.7 (0.2–1.4) | 0.8 (0.2–21.9) | .02 | 0.7 (0.2–21.9) |
| Bilirubinc (mg/dL) | 0.5 (0.1–8.2) | 0.6 (0.1–4.9) | 0.7 (0.2–41) | .001 | 0.6 (0.1–41) |
| AST | |||||
| Meanc (U/L) | 72 (18–613) | 80.5 (9–1319) | 210.5 (7–11110) | .03 | 81 (7–11110) |
| Elevated ASTg (%) | 41 (63.1%) | 29 (72.5%) | 16 (72.7%) | .52 | 86 (67.7%) |
| Peak AST (U/L) | 111.5 (20–1294) | 138.5 (27–2345) | 226 (7–18700) | .0001 | 129 (7–18700) |
| ALT | |||||
| Meanc (U/L) | 46 (13–405) | 57.5 (10–538) | 136.5 (3–2600) | .006 | 53 (3–2600) |
| Elevated ALTh (%) | 31 (44.9%) | 21 (52.5%) | 17 (70.8%) | .09 | 69 (51.9%) |
| Peak ALT (U/L) | 71 (10–665) | 88 (16–823) | 170 (17–2600) | .0004 | 83 (10–2600) |
| PTc (seconds) | 12.6 ± 1.5 | 12.4 ± 1.5 | 13.4 ± 3.1 | .13 | 12.6 ± 1.6 |
| ARDSi | 0 | 0 | 14 (30.4%) | <.001 | 14 (3.8%) |
| DIC | 0 | 0 | 7 (15.2%) | <.001 | 7 (1.9%) |
| Metabolic acidosis | 0 | 2 (1.6%) | 15 (32.6%) | <.001 | 17 (4.6%) |
| Radiological Findings | |||||
| Serositisj on ultrasound abdomen | 113 (57.4%) | 89 (71.2%) | 32 (78%) | .006 | 234 (64.4%) |
| Pleural effusion on chest x-ray | 91 (46.2%) | 68 (54.4%) | 21 (50%) | .36 | 180 (49.4%) |
| Bilateral | 64 (70.3%) | 48 (70.6%) | 14 (66.7%) | 126 (70%) | |
| Right sided | 24 (26.4%) | 16 (23.5%) | 7 (33.3%) | 47 (26.1%) | |
| Left sided | 3 (3.3%) | 4 (5.9%) | 0 | 7 (3.9%) | |
Abbreviations: ALT, alanine transaminase; ARDS, acute respiratory distress syndrome; AST, aspartate transaminase; DF, dengue fever; DHF, dengue hemorrhagic fever; DIC, disseminated intravascular coagulation; DSS, dengue shock syndrome; PT, prothrombin time; SD standard deviation; TLC, total leucocyte count.
aCategorical variables are summarized as n (%). Continuous variables are represented as mean ± SD or median (range) if SD >50% of mean.
bGrade 1 and 2 DHF.
cAt presentation (baseline).
dDefined as platelet count <100000/mm3.
eDefined as TLC <4000/mm3.
fDefined as TLC >11000/mm3.
gDefined as AST >50 U/L.
hDefined as ALT >50 U/L.
iIn accordance with the 2012 Berlin definition.
jGall bladder wall edema and/or ascites.
Treatment Received
A significant proportion of DSS patients received antibiotics (58.7%) and antimalarials (32.6%) on an empirical basis, because severe dengue is often difficult to distinguish from other febrile illnesses with hepatorenal dysfunction. Two hundred seventy-four patients (75%) required infusion of intravenous fluids (crystalloids). One hundred ninety-one (52%) patients received random donor platelets transfusion; 32 of these also received single-donor platelets transfusion. Fourteen patients (~4%) required packed red cells transfusion, and 12 of these had DSS. Twenty-six (56.5%) of 46 DSS patients responded to fluid resuscitation, whereas the remaining 20 (43.5%) required vasopressor support; 18 (90%) of these ultimately died. Eighty-five patients (23%) received vitamin C and folate therapy.
Stepwise Logistic Regression
Predictors of Severe Disease
Considering DHF and DSS as “severe” disease, late presentation to the hospital (≥5 days after disease onset), abdominal pain, vomiting, dyspnea at rest, conjunctival congestion, leucocytosis, serum bilirubin >1 mg/dL and serum creatinine >1 mg/dL at presentation, peak transaminases >250 U/L during the course of illness, and sonographic evidence of gall bladder wall edema were identified as predictors of severe disease on univariable analysis (Table 3). Amongst these, late presentation to the hospital (P = .03) and dyspnea at rest (P = .005) were identified as independent predictors of severe disease (Table 3). Abdominal pain (P = .09) and leucocytosis at presentation (P = .078) showed a trend towards statistical significance as predictors of disease severity.
Table 3.
Predictors of Severe Denguea: Univariable and Multivariable Logistic Regression Analysis
| Variables | Severe Denguea | Unadjusted OR (95% CI); P Value | Adjusted OR (95% CI); P Value | |
|---|---|---|---|---|
| Yes (171) n (%) | No (198) n (%) | |||
| Age ≥24 yearsb | 97 (56.7) | 117 (59.1) | 0.9 (0.6–1.4); .65 | — |
| Duration of illness at presentation ≥5 days | 104 (60.8) | 93 (47) | 1.8 (1.2–2.6); .008 | 1.1 (1.0–1.2); .03 |
| Conjunctival congestion | 13 (7.6) | 5 (2.5) | 3.2 (1.1–9.1); .031 | — |
| Dyspnea at rest | 25 (14.6) | 4 (2) | 8.3 (2.8–24.4); <.001 | 5.1 (1.6–15.7); .005 |
| Abdominal pain | 75 (43.9) | 64 (32.3) | 1.6 (1.1–2.5); .023 | 1.5 (0.9–2.3); .09 |
| Vomiting | 98 (57.3) | 93 (47) | 1.5 (1.0–2.3); .048 | — |
| Lowest platelet count ≤20000/mm3 | 77 (46.7) | 71 (37.4) | 1.5 (1.0–2.2); .077 | — |
| Leucocytosis at presentation | 25 (15.2) | 9 (4.6) | 3.7 (1.7–8.2); .001 | 2.2 (0.9–5.1); .078 |
| Creatinine at presentation >1mg/dL | 25 (17) | 12 (6.7) | 2.8 (1.4–5.9); .005 | — |
| Bilirubin at presentation >1 mg/dL | 36 (25.7) | 16 (9.7) | 3.2 (1.7–6.1); <.001 | — |
| Peak AST >250U/L | 41 (26.3) | 29 (16.5) | 1.8 (1.1–3.1); .03 | — |
| Peak ALT >250U/L | 30 (19.4) | 16 (9.1) | 2.4 (1.2–4.6); .008 | — |
| Sonographic evidence of gall bladder wall edema | 112 (73.2) | 98 (58) | 2.0 (1.2–3.2); .004 | — |
Abbreviations: ALT, alanine transaminase; ARDS, acute respiratory distress syndrome; AST, aspartate transaminase; CI, confidence interval; DIC, disseminated intravascular coagulation; DHF, dengue hemorrhagic fever; DSS, dengue shock syndrome; OR, odds ratio.
aSevere dengue includes DHF and DSS.
bIn accordance with the United Nations definition for youth: 15–24 years [14].
(Note: Variables such as presence of shock, ARDS, encephalitis, myocarditis, DIC, metabolic acidosis, etc, which are, by definition, severe dengue have been excluded from this list of “predictors”.)
Predictors of Mortality Among Dengue Shock Syndrome Patients
Because all the patients who died had DSS, predictors of mortality were identified in this subgroup: dyspnea at rest, altered sensorium, hepatic or renal dysfunction or ARDS, disseminated intravascular coagulation, metabolic acidosis, and vasopressor requirement were identified as predictors of mortality on univariable analysis (Table 4). Leucocytosis and age ≥24 years also correlated with an adverse outcome. Of these, age ≥24 years (P = .007), dyspnea at rest (P = .04), and altered sensorium (P = .03) were identified as independent predictors of mortality (Table 4).
Table 4.
Predictors of In-Hospital Mortality Among DSS Patients: Univariable and Multivariable Logistic Regression Analysis
| Variables | DSS (46) | Unadjusted OR (95% CI); P Value | Adjusted OR (95% CI); P Value | |
|---|---|---|---|---|
| Dead (22) n (%) | Alive (24) n (%) | |||
| Age ≥24 yrsa | 19 (86.4) | 7 (29.2) | 15.4 (3.4–69.1); <.001 | 15.8 (2.1–119.0); .007 |
| Leucocytosis at presentation | 11 (50) | 5 (20.8) | 5.0 (1.3–19.0); .02 | — |
| Dyspnea at rest | 14 (63.6) | 7 (29.2) | 4.2 (1.2–14.6); .02 | 7.5 (1.1–52.1); .04 |
| ARDS | 11(50) | 3 (12.5) | 7 (1.6–30.4); .01 | — |
| Creatinine at presentation >1 mg/dL | 11 (50) | 6 (25) | 3.7 (1.0–13.1); .04 | — |
| AKIb | 13 (59.1) | 5 (20.8) | 5.5 (1.5–20.2); .008 | — |
| Metabolic acidosis | 14 (63.6) | 1 (4.2) | 40.2 (4.5–356.9); <.001 | — |
| Altered sensorium | 10 (45.4) | 3 (12.5) | 5.8 (1.3–25.4); .02 | 11.1 (1.2–102.6); .03 |
| Encephalitis | 6 (27.3) | 1 (4.2) | 8.6 (0.9–78.7); .04 | — |
| Peak AST >500 U/L | 8 (36.4) | 5 (20.8) | 5.1 (1.2–21.5); .03 | — |
| Peak ALT >500 U/L | 8 (36.4) | 4 (16.7) | 6.7 (1.5–30.1); .01 | — |
| DIC | 7 (100) | 0 | One sided; .003 | — |
| Vasopressor support | 18 (81.8) | 2 (8.3) | 49.5 (8.1–301.9); <.001 | — |
Abbreviations: AKI, acute kidney injury; ALT, alanine transaminase; ARDS, acute respiratory distress syndrome; AST, aspartate transaminase; CI, confidence interval; DIC, disseminated intravascular coagulation; DSS, dengue shock syndrome; KDIGO, Kidney Disease: Improving Global Outcomes; OR, odds ratio.
aIn accordance with the United Nations definition for youth: 15–24 years [14].
bIn accordance with the 2012 KDIGO criteria.
Predictive Score for Mortality Among Dengue Shock Syndrome Patients
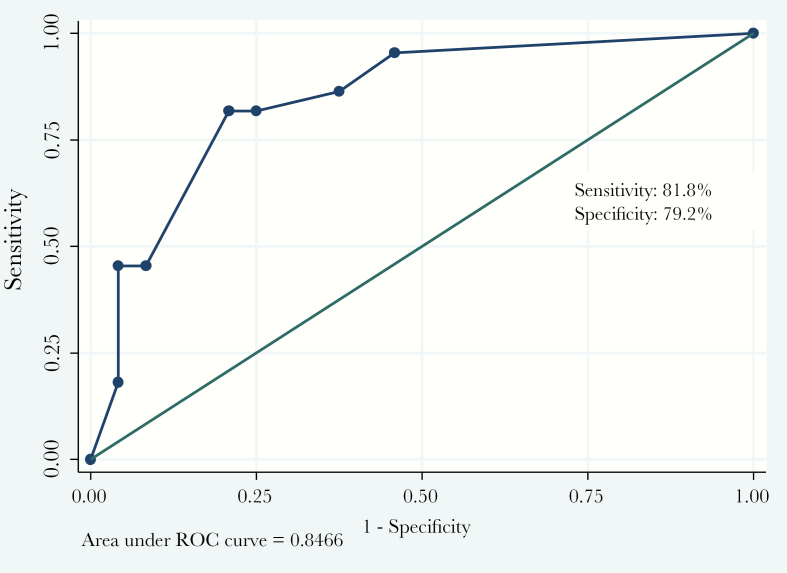
Three variables found significant in multivariable regression model were used to calculate a predictive score: 12*age + 14*sensorium + 10*dyspnea (estimated regression coefficient of variable = logistic regression coefficient of variable/logistic regression coefficient of dyspnea*10). Dyspnea was assigned a value of “1” if present and “0” if absent. Sensorium was assigned a value of “1” if altered and “0” if normal. Age was assigned a value of “0” if <24 years and “1” if ≥24 years. Based on ROC curve analysis, optimal score cutoff for prediction of mortality among DSS patients was found to be ≥22 with a sensitivity, specificity, and positive likelihood ratio of 81.8%, 79.2%, and 3.9, respectively. The area under the ROC curve was 0.85 (95% confidence interval, 0.73–0.96) (Figure 1).
Figure 1.
Receiver operating characteristics (ROC) curve for the derived predictive score for mortality in dengue shock syndrome patients. Area under the curve = 84.7%.
DISCUSSION
Dengue has become a major global public health concern in recent years, posing a large financial burden on society, especially in developing countries. With 99913 cases and 220 deaths, the 2015 dengue outbreak was the largest India has witnessed in recent years [10]. Despite media awareness, people with even trivial illness flocked to hospitals in large numbers. In this setting, it is imperative to triage cases using predictors of disease severity for optimal utilization of limited healthcare resources.
In our analysis, late presentation to the hospital (≥5 days after disease onset) was identified as an independent predictor of severity, highlighting the importance of timely access to healthcare. However, duration of illness at presentation did not correlate with mortality, reflecting the unpredictability in disease course and progression. On the other hand, presence of dyspnea at rest was identified both as a predictor of disease severity as well as a predictor of mortality among patients with severe dengue. Amongst the existing warning signs specified by the WHO in its 2009 classification, none came out to be an independent predictor of disease severity in the present study. Thus, we concur with the findings of a recent systematic review that WHO warning signs need validation studies [7]. Although biochemical parameters such as leucocytosis, elevated serum bilirubin and creatinine >1 mg/dL at presentation, and sonographic evidence of gall bladder wall edema had statistically significant P values on univariable analysis, none of these reached significance as independent predictors on multivariable analysis. These need to be evaluated in future studies with large sample size to comment on their significance. A lower transaminase cutoff of 250 U/L needs to be examined in subsequent studies for use as “predictor” of severe disease, in tandem with the WHO cutoff of 1000 U/L for “defining” severe disease [6].
Although DF, DHF, and DSS form a continuum in terms of severity, progression along this spectrum is unpredictable. Thus, predictors of mortality among patients with severe dengue need to be identified to cut down the mortality associated with the disease. The present study identified older age, presence of dyspnea at rest, and altered sensorium as independent predictors of mortality among patients with dengue shock syndrome. Neutrophilic leucocytosis (instead of leucopenia) and onset of multiorgan dysfunction (MOD) were identified as predictors of mortality on univariable analysis and need future studies with larger sample size to determine their significance. It is an established fact that a cytokine “storm” underlies the pathogenesis of DHF and DSS [5, 15]. Through this study, we hypothesize that dengue shock due to vasculopathy and capillary leakage ultimately leads to a state of dengue viral “sepsis” with accompanying leucocytosis and MOD; this is a harbinger of mortality. In accordance with the WHO SEARO 2011 guidelines [13], MOD is included under the “expanded dengue syndrome” and is related to prolonged periods of shock, thus lending credibility to our hypothesis. Eighty percent of patients referred to our center succumbed to the disease, highlighting the importance of early and timely referral to higher centers in severe disease. Almost 1 in 2 (47.8%) DSS patients and 90% of patients requiring vasopressor support ultimately died. No correlation between thrombocytopenia and mortality or disease severity was found. It is thus concluded from the study that platelet count does not influence disease outcome in dengue patients.
Platelet count was also found to not correlate with rash or bleeding manifestations. Evidence of bleeding manifestations even with normal platelet counts suggests the presence of concomitant platelet functional defects [16]. In the present study, 10% of patients had bleeding manifestations in the absence thrombocytopenia (median = 130000/mm3). In contrast, 15 patients had no bleeding even with platelet counts below 10000/mm3 (median = 5000/mm3). Although not advocated by the WHO [6], national guidelines (National Vector Borne Diseases Control Program) still recommend transfusion of platelets to asymptomatic patients with platelet counts below 10000/mm3, an arbitrary cutoff not supported by scientific evidence [17]. Thus, we concur with the recommendation against prophylactic platelet transfusion [18]. Recent efforts to chase the platelet count to more than 50000/mm3 in patients without bleeding manifestations (“dengue panic syndrome”) [19] should also be discouraged because it leads to a shortage of blood products and poses undue risks of transfusion reactions and transmission of infections. Presence of comorbidities, coinfections, or pregnancy did not affect patient outcome in the present study, although the numbers were small. Alternative medications such as ascorbic acid, folate, and steroids and traditional medicines such as goat milk, papaya, and giloy extracts were used during the outbreak; good-quality, randomized, controlled trials are needed to provide scientific evidence of their benefit.
The case-fatality rate (CFR) of 0.22% in the 2015 outbreak was significantly lower compared with the previous outbreaks (3.3% in 1996, 0.4% in 2010) [10]. In 2015, India received significant premonsoon rainfall from March to May (138% of long-predicted average [LPA]), which probably contributed to earlier detection of dengue cases and was a major factor in this outbreak being India’s largest despite total monsoon rainfall being on the lower side (86% of LPA) [20]. In addition, the optimum ambient temperature during the premonsoon period may have favored hastening of life cycle of the vector resulting in production of smaller mosquitoes, which take more frequent blood meals and thus favor rapid spread of the disease [21]. The CFR (0.38%) in Delhi (15 870 cases, 60 deaths) was almost twice the national average of 0.22% [10]. This can be attributed to several reasons. First, DENV2 and DENV4 were isolated from Delhi in the 2015 outbreak. Secondary infection with DENV2 and -4 is known to cause more severe disease [22]. The dominant serotypes isolated in other states were as follows: DENV1, -2, and -3 in Kerala and Karnataka; DENV2, -3, and -4 in Maharashtra; DENV1 and -4 in Arunachal Pradesh; and all 4 serotypes in Tamil Nadu (unpublished data). Second, serotypes isolated previously from Delhi were as follows: DENV2 in 1967; DENV1 and -2 in 1982; DENV2 in 1988 and 1996; DENV1, -2, -3, and -4 in 2003; DENV1 and -2 in 2011; DENV1, -2, and -3 in 2012; and DENV1 and -2 in 2014 (unpublished data). As is evident, DENV2 has been in circulation in Delhi for the last few years, but isolation of DENV4 is new. In addition, mutations in precirculating dengue serotypes might also have played a role in increased severity. Third, Delhi has the highest population density amongst all states and union territories, which creates more biting opportunities for the Aedes mosquito [23]. The observed all-cause mortality in this study was 6%, because the study site, being an apex tertiary care referral centre, catered to sicker patients.
This study is the first to have computed a risk score for prediction of mortality in dengue with a good sensitivity (81.8%) and specificity (79.2%) (area under the curve 84.7%). Because the predictive score developed in this study is entirely clinical, it is easy to apply and can be used even in a primary-level healthcare setting where facilities for laboratory testing may or may not be available. A retrospective study done from Taiwan identified age, leucocytosis at presentation, platelet count >100 000/mm3, and minor GI bleed as predictors of severe dengue, but risk factors for mortality were not identified [24]. A prospective study by Chen et al [25] among severe dengue patients admitted to ICU identified low Glasgow Coma Scale score, low platelet counts, and MOD as predictors of mortality. We believe that identification of MOD as a predictor of mortality in previous studies may be clinically less relevant because the pathogenetic process is usually irreversible by the time MOD has set in and prognosis is extremely poor. On the other hand, the predictors of mortality identified through our study have the potential to pick up cases at risk of death at an early stage, thus increasing the chances of patient salvageability. In a study from south India, altered sensorium and presence of hypertension were identified as risk factors of mortality; however, only 10 dengue deaths were analyzed, and the study design was retrospective [26]. Presence of comorbidities, thrombocytopenia, and severe myalgias were not found to be predictors of mortality in this study, as opposed to a few other underpowered studies [26–28]. The other strengths of this study include its prospective design and inclusion of large number of DHF-DSS cases. It is the first study to present a serotype analysis of the 2015 Delhi outbreak. Limitations of the study include that it is a single-center, hospital-based study and that preferentially sick patients requiring hospitalization were included.
CONCLUSIONS
Identification of reliable and validated predictors of disease severity and death is essential to cut down the morbidity and mortality associated with dengue. The predictive score for mortality derived in this study is entirely clinical and can be applied bedside (even out-of-hospital), obviating the need for laboratory investigations. This will facilitate early recognition of dengue patients likely to have an adverse outcome, with subsequent reduction in dengue-related mortality.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Author contributions. S. J., A. M., S. K. S., M. S., A. B., and R. S. J. conceived and designed the study; S. J. and A. M. collected and organized the data; A. D. U. and R. M. P. performed statistical analysis; C. D. and M. B. K. performed the serotyping analysis; S. J., A. M., S. K. S., and M. S. wrote the manuscript; A. B., R. S. J., and S. K. S. edited the manuscript; S. J., A. M., S. K. S., A. D. U., R. M. P., M. S., A. B., R. S. J., C. D., and M. B. K. approved the final manuscript.
Acknowledgments. We thank the following: Dr. A. C. Dhariwal (Director, National Vector Borne Diseases Control Program) for providing data and valuable technical guidance; Dr. D. T. Mourya (Director, National Institute of Virology, Pune, India) for facilitating serotyping; residents and faculty of the Departments of Internal Medicine and Microbiology; nursing and other paramedical staff; and the All India Institute of Medical Sciences administration for providing infrastructure and other logistic support for smooth conduct of the study.
Financial support. S. K. S. was supported by JC Bose Fellowship of Ministry of Science and Technology, Government of India.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Dengue and severe dengue. Fact Sheet No. 117. Updated April 2016 Available at: http://www.who.int/mediacentre/factsheets/fs117/en/ Accessed 18 July 2016.
- 2. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore C. UTMB Galveston researchers discover first new dengue feverserotype in 50-years. BIONews Texas 2013. Available at: https://bionews-tx.com/news/2013/10/25/utmb-galveston-researchers-discover-first-new-dengue-fever-serotype-in-50-years/. Accessed 14 February 2017 https://bionews-tx.com/news/2013/10/25/utmb-galveston-researchers-discover-first-new-dengue-fever-serotype-in-50-years/ .
- 4. Flipse J, Diosa-Toro MA, Hoornweg TE, et al. Antibody-dependent enhancement of dengue virus infection in primary human macrophages; balancing higher fusion against antiviral responses. Sci Rep 2016; 6:29201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed Geneva: World Health Organization, 1997. [Google Scholar]
- 6. World Health Organization/TDR. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition2009. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 7. Horstick O, Jaenisch T, Martinez E, et al. Comparing the usefulness of the 1997 and 2009 WHO dengue case classification: a systematic literature review. Am J Trop Med Hyg 2014; 91:621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hadinegoro SR. The revised WHO dengue case classification: does the system need to be modified? Paediatr Int Child Health 2012; 32(Suppl 1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakravarti A, Matlani M, Kashyap B, Kumar A. Awareness of changing trends in epidemiology of dengue fever is essential for epidemiological surveillance. Indian J Med Microbiol 2012; 30:222–6. [DOI] [PubMed] [Google Scholar]
- 10. National Vector Borne Diseases Control Programme, Government of India. Dengue cases and deaths in the country since 2010 Available at: http://www.nvbdcp.gov.in/den-cd.html Accessed 22 July 2016.
- 11. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 12. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney InjuryWork Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138. [Google Scholar]
- 13. World Health Organization. Regional Office for South-East Asia. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Hemorrhagic Fever Revised and Expanded Edition 2011: [Google Scholar]
- 14. The United Nations. Definition of Youth Available at: http://www.un.org/esa/socdev/documents/youth/fact-sheets/youth-definition.pdf Accessed 18 June 2016.
- 15. Espada-Murao LA, Morita K. Dengue and soluble mediators of the innate immune system. Trop Med Health 2011; 39:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michels M, Alisjahbana B, De Groot PG, et al. Platelet function alterations in dengue are associated with plasma leakage. Thromb Haemost 2014; 112:352–62. [DOI] [PubMed] [Google Scholar]
- 17. National Vector Borne Diseases Control Programme. Government of India. Guidelines for Clinical Management of Dengue Fever 2014 Available at: http://www.nvbdcp.gov.in/Doc/Dengue-National-Guidelines-2014.pdf Accessed 18 June 2016.
- 18. Sharma S, Sharma SK, Mohan A, et al. Clinical profile of dengue hemorrhagic fever in adults during 1996-outbreak in Delhi, India. Dengue Bull 1998; 22:20–27. [Google Scholar]
- 19. Ahluwalia G, Sharma SK. Dengue: current trends and challenges–an Indian perspective. J Assoc Physicians India 2004; 52:561–3. [PubMed] [Google Scholar]
- 20. India Meteorological Department. Annual Climate Summary 2015 Available at: http://www.imdpune.gov.in/Links/annual%20summary%202015.pdf Accessed 20 August 2016.
- 21. Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg 2008; 79:688–95. [PMC free article] [PubMed] [Google Scholar]
- 22. Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol 1984; 120:653–69. [DOI] [PubMed] [Google Scholar]
- 23. Census of India. Provisional population totals, paper 1 of 2011 India, Series-1, Office of the Registrar General & Census Commissioner, New Delhi. 2011 Available at: http://censusindia.gov.in/2011-prov-results/data_files/india/Final_PPT_2011chapter7.pdf Accessed 26 August 2016http://www.census2011.co.in/density.php .
- 24. Lee IK, Liu JW, Chen YH, et al. Development of a simple clinical risk score for early prediction of severe dengue in adult patients. PLoS One 2016; 11:e0154772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen CM, Chan KS, Yu WL, et al. The outcomes of patients with severe dengue admitted to intensive care units. Medicine 2016; 95:e4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karunakaran A, Ilyas WM, Sheen SF, et al. Risk factors of mortality among dengue patients admitted to a tertiary care setting in Kerala, India. J Infect Public Health 2014; 7:114–20. [DOI] [PubMed] [Google Scholar]
- 27. Pinto RC, de Castro DB, de Albuquerque BC, et al. Mortality predictors in patients with severe dengue in the State of Amazonas, Brazil. PLoS One 2016; 11:e0161884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thein TL, Leo YS, Fisher DA, et al. Risk factors for fatality among confirmed adult dengue inpatients in Singapore: a matched case-control study. PLoS One 2013; 8:e81060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.