Abstract
Background and Objectives
Migration flux is an increasing phenomenon in Italy, and it raises several public health issues and concerns in pediatric infectious diseases. This study investigated the clinical characteristics and outcomes of a pediatric population at high-risk for tuberculosis (TB) and the potential role of immigration as a risk factor.
Design
We performed an observational retrospective study of children referred to the only Pediatric Infectious Diseases Unit for Northern Sardinia over a 6-year-period (2009–2014). Main variables assessed included TB skin test (TST), confirmed by quantiFERON Gold in Tube test, thorax X-ray (TX), microbiological culture, direct microscopy for acid-fast bacilli and molecular assays.
Results
Of the 246 children (mean age = 5.8 ± 3.9 years) identified, 222 (90.2%) were native to Sardinia and 24 (9.8%) were immigrants. The majority of children (n=205; 83%) were TB-exposed but not infected based on a negative TST and TX. Among the TST positive group (n= 39; 16%), 19 (49%) had latent TB (TX negative), while 20 (51%) had active TB (TX positive). The percent of TST positive children was significantly higher in the immigrant than the native group (42.5% versus 14%, p<0.001). Clinical presentations included pulmonary involvement with hilar lymphadenopathy (72%), pleurisy (13,5%), lateral-cervical lymphadenopathy (9%), pneumonia with calcifications (4.5%) and disseminated TB (4.5%). One child had multidrug-resistant tuberculosis.
Conclusions
Pediatric TB represents a relevant and potentially worsening public health problem in Northern Sardinia. A strict surveillance system and appropriate treatment can prevent the most severe forms and reduce TB transmission.
Keywords: tuberculosis household contacts, latent tuberculosis, active tuberculosis, multi-drug resistant tuberculosis
Introduction
Over the past two decades, migration and the phenomenon of “boat migration” to Italy and the island of Sardinia1 have progressively increased, causing significant demographical changes. From 2009 to 2014, the Italian National Centre for Statistics (ISTAT) recorded the arrival of about 20.000 new immigrants to the island, of whom roughly one-third reside in the Northern provinces. Upon arrival, migrants undergo medical assistance and evaluation, especially for infectious diseases.1
According to the Italian government guidelines, migrants coming from high tuberculosis (TB) endemic countries receive special care for potential Mycobacterium tuberculosis (MT) infection.1–2 About one-third of the population worldwide encounters MT during their lifespan, remaining asymptomatic, a condition termed latent tuberculosis infection (LTBI).3,4 However, 5 to 10% of the infected subjects are at risk of developing clinically evident TB, in part depending on their immune system status. In fact, children aged less than 5 years who are co-infected with HIV or treated with immunosuppressants for other diseases are considered at higher risk for TB.3–5 Among children less than 5 years, those less than 2 years are at even greater risk of severe disseminated TB, including pulmonary miliary TB and meningitis. The diagnosis of LTBI is made when the tuberculosis skin test (TST) is positive in subjects who are not vaccinated and in the absence of any other clinical and/or radiological evidence for active TB. Subjects with LTBI harbor an inactive form of MT. Adequate prophylaxis with isoniazid in LTBI subjects is able to reduce if not eliminate the risk of active disease.3,4 However, the distinction between latent and active TB may be at times particularly challenging, leading to the concept of a “pediatric TB spectrum” ranging from asymptomatic to lethal TB.5–7 Children with positive TST but negative chest X-ray (TX) have been recently found to have active TB by microbiological analysis that was confirmed by chest CT scan.6 As a consequence of this complexity, the “Paediatric Tuberculosis Network European Trials group” has recently proposed to replace LTBI with the new definition of “TB with a positive immunological test result [TST and/or interferon-gamma release assay (IGRA)] in the absence of active disease”, in which the word “latent” is omitted.5
Data recently published by the TB surveillance and monitoring in 29 European States for the year 2012, have shown that 2,845 (4%) out of the total 68,423 cases of TB were found in children.7 These data show a decrease of 2% compared to the year 2011.8
According to the World Health Organization, Italy is one of the countries with a low incidence of TB.8 However, the progressive reduction of surveillance measures along with the increasing number of immigrants coming from countries at high TB incidence are reasons for the recent alarming reports of a reemerging TB in developed countries.8
In Italy, inter-regional differences exist. A recent study on the prevalence of LTBI among 733 healthcare students in Genoa reported a prevalence as low as 1.4%, with migrants coming from a geographical area at high incidence of TB being those most commonly affected.9 Differently, in Tuscany, the study of a large series of children with TB (n=484), almost half of whom were immigrants, has found no difference between native and immigrant youth. 10 This study has also reported an increase in pediatric TB incidence in Tuscany, where children less than 5 years were at high risk of severe TB.10
The proportion of TB affected children in Sardinia, and the impact of the migration flux from TB endemic countries is unknown. Our study is the first report on the clinical characteristics and outcomes of pediatric TB in Northern Sardinia, assessed by TST, IGRA, TX, microbiological culture, direct microscopy for acid-fast bacilli and molecular assays.
Study Population and Methods
We performed an observational retrospective study relative to the 6-year-period 2009–2014 by reviewing clinical records of a TB high-risk pediatric population at the Pediatric Infectious Diseases Unit of the Pediatric Clinic, University of Sassari, Italy. This is the only referral Center for pediatric TB in the Northern Sardinia and is responsible for the evaluation of children referred from the Public Health Service because of household or occasional contacts of TB index cases. Less commonly, symptomatic children come to the Center for a general clinical evaluation; in these cases, it is not always possible to identify the index case. Both de novo active TB children and children identified because of close or occasional contacts with TB index cases were included in the present study. We classified as native children those born from both native parents, while as immigrant children those with at least one foreign-born parent.
For the purpose of this study, any child referred because of household/occasional contact or affected by active TB underwent a personal and family history collection, complete physical examination, TB skin test (TST), interferon-gamma release assay (IGRA) and a thorax X-ray (TX).10–11 The TST (Biocine Test PPD; Chiron, Siena, Italy) was considered positive when producing a diameter ≥ 5 mm at 48 to 72 hours.
Interferon-gamma release assay (IGRA)
The IGRA assay was the quantiFERON Gold in Tube test (QFT). One mL of blood sample was added into three QFT tubes containing either TB antigens ESAT-6, CFP-10 and TB7.7, a positive control (mitogen) or a negative control (Nil). After 16–24 hours incubation at 37°C, plasma IFN-γ concentration was measured by ELISA. QFT results were scored as indicated by the manufacturer (cut-off value for a positive test was ≥0.35 IU/ml).11–12
Microbiological diagnosis of tuberculosis
To confirm active TB, standard microbiological culture and molecular genetic assays were performed on patients’ biological samples as previously described.11–12 Gastric aspirates were collected from children who were TST and/or IGRA positive. Staining and microscopy, nested polymerase chain reaction (PCR) and culture tests were performed as previously described.11–12 All clinical samples were stained with Zielh-Neelsen and inoculated into liquid and solid medium (MGIT 960 and Lowenstein-Jensen respectively). Molecular tests were carried out using the Dx MTB Assay (Bio-Rad), a Real-time PCR that targets the IS6110 element and the RD9 specific region. DNA was extracted using a chelating ion-exchange resin (InstaGene matrix; Bio-Rad, Hercules, CA) and amplified according to the manufacturer’s instructions.
Patient management
Depending on the absence/presence of clinical symptoms, TST/IGRA and TX results, patients were classified into three standard categories of (1) exposed but not infected (asymptomatic, negative to both TST and TX), (2) LTBI (asymptomatic, TST positive but TX negative) and (3) active TB (symptomatic, TX positive or negative, TST positive or negative).
The exposed but not infected children did not receive any treatment but were re-tested for TST and IGRA three months later (Table 1). In accordance with WHO global guidelines,4 children in this category who were less than 5 years or at risk for developing active TB (e.g,, children in close contact with an index case of TB) received isoniazid at the dosage of 10 mg/kg/day for 3 months before being re-tested (Table 1). Isoniazid was discontinued if both tests were still negative at the second evaluation.
Table 1.
Anti-TB medications and protocols.8
| EXPOSED* | LTBI | ACTIVE TB | ||
|---|---|---|---|---|
| Pulmonary TB or Cervical TB adenopathy | Complex pulmonary TB | |||
| Starting Therapy and Duration | Isoniazid | Isoniazid | Isoniazid Rifampin Pyrazinamide | Isoniazid Rifampin Pyrazinamide Ethambutol |
| 3 months | 6–9 months | 2 months | 2 months | |
| Continued Therapy and Duration | Isoniazid Rifampin | Isoniazid Rifampin | ||
| 4 months | 4 months | |||
only children at high risk or less than 5 years of age
All children who were LTBI received isoniazid prophylaxis for 9 months (Table 1), while all children with active TB underwent a further investigation to confirm the diagnosis. In children with active TB, a DNA fingerprinting assay was performed according to standard protocols.11–12 Children affected by active TB received the conventional anti-tubercular therapy13 (Table 1). Treatment for TB lateral-cervical adenopathy was both medical and surgical when indicated. The surgical excision of the affected nodes was used for both diagnostic (microbiological analysis) and therapeutic purposes.
Statistical analysis
Statistical comparisons between groups were performed with the χ2 test or the Fisher’s exact test when indicated. A p-value < 0.05 was considered to be statistically significant.
Results
Over the 6 years period of study, a total of 246 children (0–14 years; mean age = 5.8 ± 3.9 years; M: F=1:1) were observed at our unit, among whom 222 (90.2%) were native Sardinians, and 24 (9.8%) were immigrants.
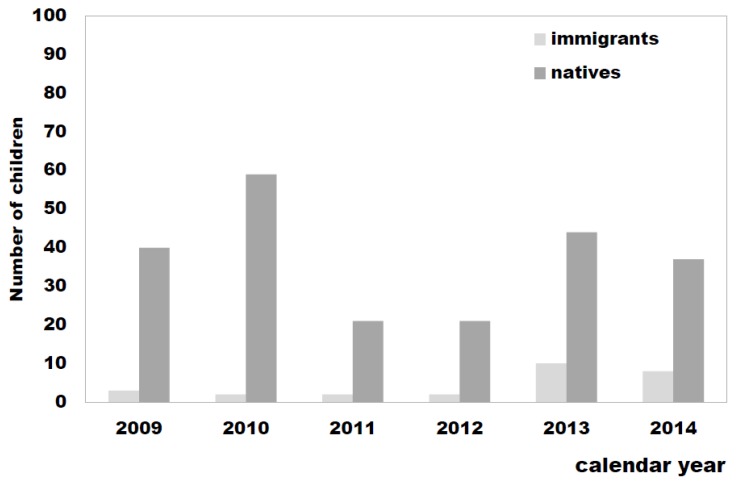
As shown in Figure 1, about 2/3 (67%) of the immigrants arrived in Sardinia more recently, during the years 2013 and 2014, confirming the reported trend towards a progressive increase in the number of immigrants from countries where TB is endemic, including East Europe and Africa.
Figure 1.
TB high-risk pediatric population observed at the referral Center for pediatric TB in the Northern Sardinia: number of native versus immigrant children stratified per calendar year (2009–2014).
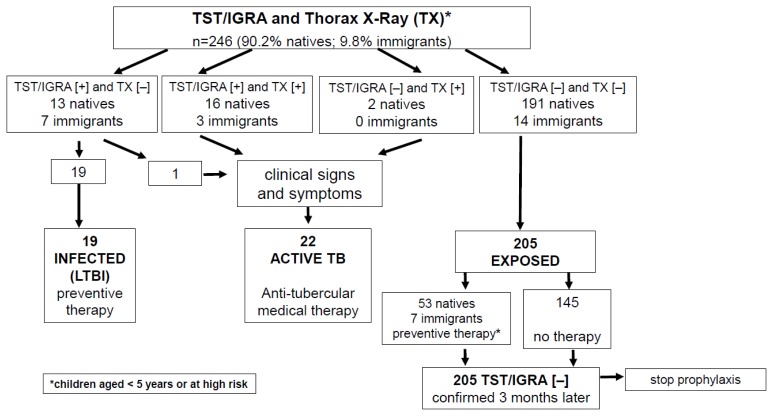
The study population included 111 (45.1%) children who were less than 5 years old, 117 (47.5%) 5–12 years old, and 18 (7.3%) over 12 years old. In our cohort, the majority of children (n=205; 83.3%) were found to be exposed to TB but not infected, 19 (7.7%) had evidence for LTBI, and 22 children (8.9%) had active TB (Table 2 and Figure 2). As our study involved a high-risk population within institutional settings, a higher prevalence of active TB was found compared to LTBI.
Table 2.
Classification of the study population into three categories of “exposed”, “LTBI” and “active TB” patients (n, %), depending on the TST/IGRA and Chest X-ray (TX) negative [−] or positive [+] results at the first evaluation and management.
| TOTAL OF CHILDREN STUDIED (n=246) | ||||
|---|---|---|---|---|
| EXPOSED (205; 83.3%) |
LTBI (19; 7.7%) |
ACTIVE TB (22; 8.9%) |
||
| TST/IGRA[−] TX [−] | TST/IGRA [+] TX [−] | TST/IGRA[−] TX [+] | TST/IGRA [+] TX [−] | TST/IGRA [+] TX [+] |
| 205 (100%) |
19 (100%) |
2 (9%) |
1 (4.5%) |
19 (86.3%) |
| MANAGEMENT | ||||
| 3 months of prophylaxis* | 9 months of prophylaxis | 6–12 months of anti-TB therapy | ||
| 60 (29.2%) |
19 (100 %) |
22 (100%) |
||
only children at high risk or aged < 5 years.
Figure 2.
Diagnostic and therapeutic flow-chart of our study population, subdivided into the three categories of latent TB (LTBI), active TB and TB exposed children depending on the TST/IGRA and chest-X Ray (TX) negative [−] or positive [+] results.
The exposed but not infected group consisted of 145 children (71%) who were not treated and 60 (29%) children who were at risk for developing TB and received isoniazid prophylaxis (Table 2 and Figure 2). Of those children, 53 (25.8%) were native Sardinians, and 7 (3.4%) were immigrants.
Among the 39 children who were TST-positive, 19 (49%) were diagnosed with LTBI because of negative TX and asymptomatic, while 20 (51%) showed active TB (Table 2 and Figure 2). Interestingly, of children who were TST positive, significantly higher proportions were immigrants compared to native Sardinians (42.5% versus 14%, p < 0.001), and there was a trend for immigrant children to have active TB (12.5% vs. 9%, p = 0.050).
In the LTBI group of 19 children, all but four were TST and IGRA positive. Of these, three (16%; 5, 9 and 11 years old) were TST and IGRA negative, and one (5%; 12 years old) was TST negative but IGRA positive.
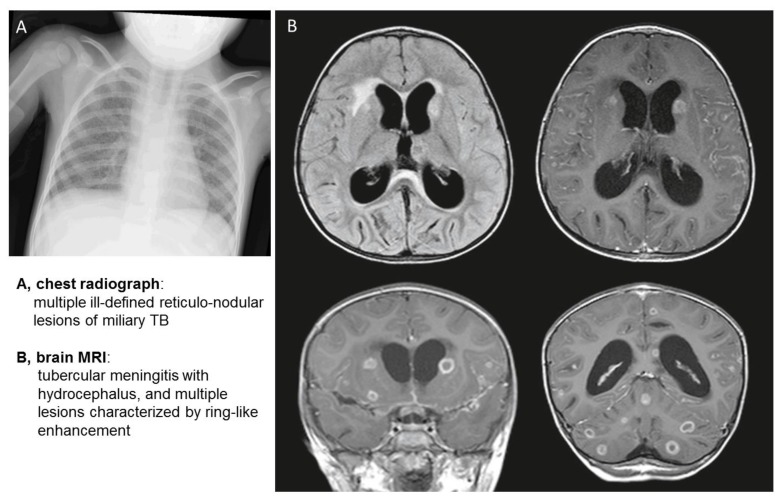
Among the 22 children with active TB, 10 (45.4%) children were less than 5 years old, 10 (45.4%) were 5–12 years old, and two (9%) were 14 years old. Half of all TB diagnoses (11/22; 50%) were made in 2009, 5/22 (22%) in 2013, 3/22 (13.6%) in 2010 and 2014 and none in 2011 and 2012. All but three children were TST and IGRA positive. Two of them (9%) tested negative at the first TST and IGRA evaluation (Table 3). One of these was a 9-year old boy under immunosuppressive treatment for juvenile idiopathic arthritis, who underwent TB investigation because of fever and cough. At the initial evaluation, TST and IGRA were negative, but the TX and the culture of gastric aspirate were positives, allowing the diagnosis of active TB (Table 3). Three months later, at the second evaluation, TST and IGRA turned positive. The second patient was a 2-year old girl with a few months history of fever of unknown origin, lack of appetite and irritability. Because of the initial negative laboratory tests, the diagnosis of TB went unrecognized until she presented with the severe clinical picture of disseminated TB, including pulmonary miliary, meningitis, bone and renal involvement (Table 3, Figure 3).
Table 3.
Clinical and radiological findings associated to TST and/or TX positivity at the initial evaluation in the 22 children with active TB.
| n. | Clinical and radiological findings | TST[+]TX [+] | TST[+] TX [−] | TST[−] TX [+] |
|---|---|---|---|---|
| 16 | Pulmonary involvement with hilar lymphadenopathy | 15 | 1* a | |
| 2 | pleurisy | 2 | ||
| 1 | pleurisy and axillary lymphadenopathy | 1 | ||
| 1 | pneumonia with calcifications | 1 | ||
| 1 | disseminated TB (miliary and meningitis) | 1* b | ||
| 1 | cervical lymphadenopathy | 1* |
MT etiology was confirmed by the culture of the gastric fluid aspirate and by PCR;
patient under immunosuppressant treatment for juvenile idiopathic arthritis;
child aged 2 years.
Figure 3.
Chest radiograph and brain MRI images of a 2-year-old girl with disseminated TB.
In the 22 children with active TB, pulmonary involvement with hilar lymphadenopathy was the most common clinical manifestation (n=16 patients, 72%), followed by pleurisy in two patients (13,6%), and one patient each (4.5%) had pleurisy and axillary lymphadenopathy, pneumonia with calcifications, cervical lymphadenopathy, or disseminated TB (miliary and meningitis) (Table 3, Figures 3–5).
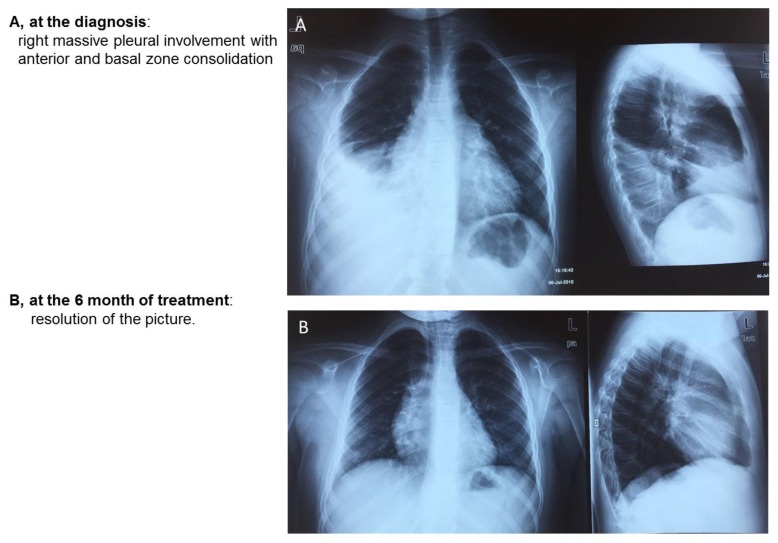
Figure 4.
Chest radiographs at two different time points of a 9-year-old girl with pleurisy.
Figure 5.
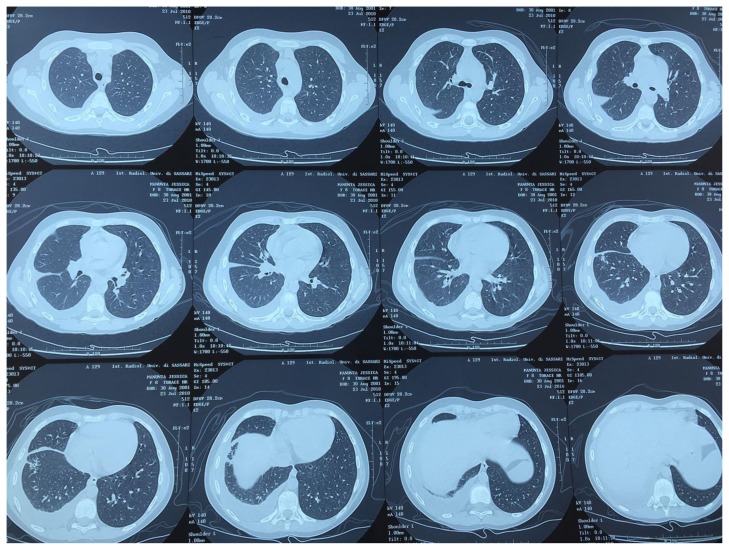
Chest TC images of 9-year-old girl with pleurisy at the time of TB diagnosis.
In one of two patients who presented with adenopathy as extra-pulmonary TB, the initial ultrasound imaging revealed cervical lymphoadenopathy with a concomitant involvement of the parotid gland, which is a known contraindication for surgical treatment (potential risk of causing VII cranial nerve lesion). This patient was a 3-year-old girl in whom the MT etiology was confirmed by culture of the gastric fluid aspirate and by the PCR of the caseous material collected from an external skin fistula that was drained from the colliquative node. The second patient who developed axillary adenopathy as secondary MDR-TB was an 8-year-old boy with Down syndrome, who was initially affected with tubercular pleurisy. According to the conventional therapy protocol, this child received treatment with rifampin, isoniazid, ethambutol and pyrazinamide for 18 months. At the fifth month of follow-up, he presented with axillary lymphoadenopathy. The culture of surgically excised lymphonodal tissue revealed multi-drug resistant MT.13,14 Anti-TB treatment with amikacin, ethionamide, levofloxacin, and pyrimethamine was successful, with complete recovery after one year of therapy.
Among the children who were symptomatic when they came to the Center, it was possible to identify the index case only in 6 children (50%), while the identification of the TB contact preceded the diagnosis of TB in all the 10 children referred to the Center by the Public Health Service. When known, the index case was an adult in all but one case (a school companion). In the majority (9/16 children), it was one of the parents, mostly the father, followed by an uncle in four cases, a grandparent in one, and a neighbor in another one.
Discussion
Recent data on pediatric TB indicate that immigration is an important risk factor for TB in countries where the incidence of TB is low.15–17 Our study is the first report on pediatric TB in Northern Sardinia, where the native children represented the large majority (9:1 ratio). However, immigrant children showed a trend toward a higher proportion of both TST positivity and active TB, even though the latter did not reach statistical significance likely due to the small sample size. Interestingly, the rate of immigrant children undergoing isoniazid prophylaxis was three-fold higher than that of native children. This is perhaps not surprising as these immigrant children were from Eastern Europe (i.e. Romania) and Africa (i.e. Morocco), areas where TB is endemic. For this reason, we argue that increased TB surveillance is necessary as the “boat migration” phenomenon is progressively increasing in Sardinia as well as in other European nations.
The results of our study showed a strong concordance between TST and IGRA tests, which appears to be not affected by a younger age. The higher percentage of discordant tests was noted among LTBI cases that presented with negative IGRA in spite of a positive TST (3 patients). All this suggests the utility of performing both the tests in all children, as it has been previously reported.18
The clinical study revealed that hilar lymphoadenopathy with pneumonia is the most common manifestation of TB in children. These were mostly the children referred because of household or occasional contacts with index cases. On the other hand, children in our study with the most advanced and severe TB manifestations were exclusively those with de novo diagnosis, confirming that contact tracing plays a key role in limiting the consequence of TB in children.
In addition, it important to note that of the children who received isoniazid prophylaxis, none developed active TB. At least one-third of these children were immigrants. Thus, pediatric TB prevention assumes a priority in public health.
Conclusions
In conclusion, our data show that:
pediatric TB is a public health issue in Northern Sardinia;
immigrant children have a high rate of LTBI;
the progressively increasing “boat migration” phenomenon requires immediate action on the TB surveillance level in current low incidence countries;
TB diagnosis cannot be excluded in children with initial negative TST and IGRA and in whom other diseases have been ruled out by appropriate investigations; a delay in TB diagnosis and treatment is potentially fatal;
the presence of MDR-TB should always be expected, especially in children living in high-risk families;
the household or occasional contacts of index cases referred by the local Public Health System significantly benefit from earlier diagnosis and treatment compared to the ex-novo active TB children.
Acknowledgements
We are grateful to Professor Giovanni Fadda for advice and helpful discussion and to Dr. Mark Soloski and Mary Blue for assistance with writing in the English language. This work was partially supported by the Autonomous Region of Sardinia.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
Author Contributions. The first two authors (Maria Grazia Clemente and Elena Dore) contributed equally and wrote the first draft of the paper. Lidia Abis and Paolina Olmeo performed the clinical study. Paola Molicotti and Stefania Zanetti were responsible for the microbiological culturing and molecular genetic assays for MT detection. Paolina Olmeo and Roberto Antonucci were co-senior authors.
References
- 1.Italian Ministry of Health. Guidelines for the control of tuberculosis on the proposal of the Minister of Health under Article 115, paragraph 1, letter b of Legislative Decree 31, March 1998, no. 112.
- 2.Bua A, Cubeddu M, Piras D, Delogu R, Zanetti S, Molicotti P. Tuberculosis screening among asylum seekers in Sardinia. J Public Health (Oxf) 2016 Jan;:24. doi: 10.1093/pubmed/fdv215. pii: fdv215. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Abubakar I, Griffiths C, Ormerod P. Diagnosis of active and latent tuberculosis: summary of NICE guidance. BMJ. 2012;345:e6828. doi: 10.1136/bmj.e6828. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global tuberculosis report 2016. World Health Organization; Geneva: 2016. http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 5.Tebruegge M, Salo E, Ritz N, Kampmann B On Behalf Of The Paediatric Tuberculosis Network European Trialsgroup Ptbnet. Inclusion of latent tuberculosis infection as a separate entity into the international classification of diseases. Thorax. 2013;68:588. doi: 10.1136/thoraxjnl-2012-202824. https://doi.org/10.1136/thoraxjnl-2012-202824. [DOI] [PubMed] [Google Scholar]
- 6.Buonsenso D, Sali M, Focarelli B, Onesimo R, Palucci I, Delogu G, et al. The tuberculosis spectrum: Translating basic research into pediatric clinical practice. Med Hypotheses. 2015 Oct 28; doi: 10.1016/j.mehy.2015.10.028. pii: S0306-9877(15)00410-7. [DOI] [PubMed] [Google Scholar]
- 7.Sali M, Buonsenso D, Goletti D, D’Alfonso P, Zumbo A, Fadda G, et al. Accuracy of QuantiFERON-TB Gold Test for Tuberculosis Diagnosis in Children. PLoS One. 2015 Oct 6;10(10):e0138952. doi: 10.1371/journal.pone.0138952. https://doi.org/10.1371/journal.pone.0138952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eurosurveillance Editorial Team. ECDC and WHO/Europe joint report on tuberculosis surveillance and monitoring in Europe. Euro Surveill. 2014;19(11) doi: 10.2807/ese.19.11.20741-en. pii: 20741. [DOI] [PubMed] [Google Scholar]
- 9.Durando P, Alicino C, Orsi A, Barberis I, Paganino C, Dini G, et al. Latent tuberculosis infection among a large cohort of medical students at a teaching hospital in Italy. Biomed Res Int. 2015;2015:746895. doi: 10.1155/2015/746895. https://doi.org/10.1155/2015/746895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiappini E, Bonsignori F, Orlandini E, Sollai S, Venturini E, Galli L, de Martino M. Increasing incidence of tuberculosis in Tuscan youth, 1997 to 2011. Pediatr Infect Dis J. 2013;32(11):1289–91. doi: 10.1097/INF.0b013e31829e7d81. https://doi.org/10.1097/INF.0b013e31829e7d81. [DOI] [PubMed] [Google Scholar]
- 11.Bua A, Molicotti P, Cannas S, Ruggeri M, Olmeo P, Zanetti S. Tuberculin skin test and QuantiFERON in children. New Microbiol. 2013;36( 2):153–6. [PubMed] [Google Scholar]
- 12.Molicotti P, Bua A, Mela G, Olmeo P, Ortu S, Sechi LA, et al. Performance of Quantiferon TB testing in a outbrak at a primary school. J Pediatr. 2008;152( 4):585–86. doi: 10.1016/j.jpeds.2007.12.014. https://doi.org/10.1016/j.jpeds.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Graham SM. Treatment of paediatric TB: revised WHO guidelines. Paediatr Respir Rev. 2011;12( 1):22–6. doi: 10.1016/j.prrv.2010.09.005. https://doi.org/10.1016/j.prrv.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Santiago B, Baquero-Artigao F, Mejías A, Blázquez D, Jiménez MS, Mellado-Pe-a MJ EREMITA Study Group. Pediatric drug-resistant tuberculosis in Madrid: family matters. Pediatr Infect Dis J. 2014;33(4):345–50. doi: 10.1097/INF.0000000000000111. https://doi.org/10.1097/INF.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 15.Pang J, Teeter LD, Katz DJ, Davidow AL, Miranda W, Wall K, et al. Tuberculosis Epidemiologic Studies Consortium. Epidemiology of tuberculosis in young children in the United States. Pediatrics. 2014;133(3):e494–504. doi: 10.1542/peds.2013-2570. https://doi.org/10.1542/peds.2013-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durando P, Sotgiu G, Spigno F, Piccinini M, Mazzarello G, Viscoli C, et al. Latent tuberculosis infection and associated risk factors among undergraduate healthcare students in Italy: a cross-sectional study. BMC Infect Dis. 2013;13:443. doi: 10.1186/1471-2334-13-443. https://doi.org/10.1186/1471-2334-13-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli L, Lancella L, Tersigni C, Venturini E, Chiappini E, Bergamini BM, et al. Pediatric Tuberculosis in Italian Children: Epidemiological and Clinical Data from the Italian Register of Pediatric Tuberculosis. Int J Mol Sci. 2016 Jun 17;17(6) doi: 10.3390/ijms17060960. pii: E960. https://doi.org/10.3390/ijms17060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garazzino S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, et al. SITIP IGRA Study Group. Performance of interferon-? release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J. 2014 Sep;33(9):e226–31. doi: 10.1097/INF.0000000000000353. https://doi.org/10.1097/INF.0000000000000353. [DOI] [PubMed] [Google Scholar]