Abstract
Gap-junctional communication (GJC) plays a central role in oocyte growth. However, little is known about the regulation of connexin 43 (Cx43)-based gap-junction channels in cumulus-oocyte complexes (COCs) during in vitro maturation. We show that rupture of COCs from mural granulosa cells up-regulates Cx43-mediated GJC and that gonadotropins signal GJC breakdown by recruiting Cx43 to lipid rafts when oocyte meiosis resumes. Oocyte calcein uptake through gap junctions increases during early in vitro oocyte maturation and remains high until 18 h, when it falls simultaneously with the oocyte germinal vesicle breakdown. Immunodetection of Cx43 and fluorescence recovery after photobleaching assays revealed that the increase of GJC is independent of gonadotropins but requires RNA transcription, RNA polyadenylation, and translation. GJC rupture, in contrast, is achieved by a gonadotropin-dependent mechanism involving recruitment of Cx43 to clustered lipid rafts. These results show that GJC up-regulation in COCs in in vitro culture is independent of gonadotropins and transcriptionally regulated. However, GJC breakdown is gonadotropin dependent and mediated by the clustering of Cx43 in lipid raft microdomains. In conclusion, this study supports a functional role of lipid raft clustering of Cx43 in GJC breakdown in the COCs during in vitro maturation.
Recovery of the cumulus-oocyte complex from the follicle induces an increased gap-junctional communication followed by a gonadotropin-dependant decrease involving Cx43 recruitment to the lipid raft.
Intercellular gap-junctional communication (GJC) is implicated in many physiological processes such as cardiac contractility and nervous system functions, and in pathological conditions such as cancer, atherosclerosis, and skin diseases (1, 2). It also plays a significant role in ovarian follicle physiology (3). Gap junctions are made of connexin hexamers and allow the transit of molecules less than 1000 Da. Eight different connexins have been detected in mammalian ovaries (4). Gap junction protein α 4 (known also as connexin 37)-deficient mouse ovaries have no Graafian follicles and are infertile (5). Moreover, GJC between the oocyte and neighboring granulosa cells was disrupted whereas communication among the granulosa cells remained functional, as shown by fluorescently labeled neurobiotin transfer (5). A study by Ackert et al. (6) using gap junction protein α 1 [GJA1, known also as connexin 43 (Cx43)]-deficient ovaries grafted into adult mice demonstrated that GJA1 inactivation interrupts folliculogenesis and prevents granulosa cells from forming more than one layer around the oocyte. From these and other studies, Cx43 has been proposed as a major mediator of granulosa-granulosa cell communication, whereas connexin 37 is a carrier of germ-somatic cell communication (7).
Gap-junctional exchange of small molecules between follicular somatic cells and oocytes has been reported for ATP, sodium, chloride, calcium ions, and cAMP (8, 9, 10, 11). The second messenger cAMP, which has a central role in granulosa, cumulus cell, and oocyte physiology (12, 13), has been shown to increase in the oocyte in a cumulus cell-dependent manner both in response to gonadotropins and the adenylyl cyclase activator forskolin, in the mouse (11, 14), rat (15), rabbit (16), and cow (17).
Fully grown oocytes are maintained in meiotic arrest in vivo and in vitro by high levels of cAMP. A central concept of mammalian oocyte maturation is that arrested oocytes in the ovary are stimulated to resume meiosis by the preovulatory gonadotropin surge, which leads to a concomitant loss of oocyte-cumulus cell GJC. One model of oocyte meiotic resumption proposes that rupture of GJC between granulosa/cumulus cells and the oocyte causes a decrease in cAMP transfer to the oocyte, thereby triggering meiotic maturation. Early studies showed that LH initiates GJC breakdown before the resumption of rat oocyte meiosis both in vivo and in a follicle culture system (15, 18). Later studies have shown that this breakdown depends on ERK (19, 20). Resumption of oocyte meiosis in vivo or in cultured follicles can be triggered by the gap-junction blocker, carbenoxolone (21). In hamsters, a decrease in metabolic communication between cumulus cells and the oocyte precedes and accompanies meiotic maturation (22); however, significant interruption of cAMP transfer to the oocyte was detected only after oocyte meiotic resumption (23). GJC in bovine cumulus-oocyte complexes (COCs) decreases by 50% before the onset of oocyte nuclear maturation (24). In the pig, GJC breakdown correlates with Cx43 hyperphosphorylation and oocyte nuclear maturation (25, 26). A causal relationship between GJC breakdown and oocyte nuclear maturation is still debated and is likely to be subject to interspecies differences.
Modulation of GJC in COCs plays a pivotal role for oocytes, but the nature of this role has been a puzzling and contentious issue. The present study was undertaken to investigate the dynamic changes and regulation of GJC in COCs during porcine oocyte meiotic resumption. The results demonstrate that after 4 h of culture, Cx43 up-regulation is caused by the mechanical rupture of the COCs with mural granulosa cells and is gonadotropin independent and transcriptionally regulated. Later during in vitro maturation (IVM), germ-somatic cell GJC is abruptly decreased in a gonadotropin-dependent manner concurrent with the resumption of oocyte meiosis. Concomitant with GJC breakdown and germinal vesicle breakdown (GVBD), Cx43 is clustered to lipid rafts in a gonadotropin-dependent manner.
Results
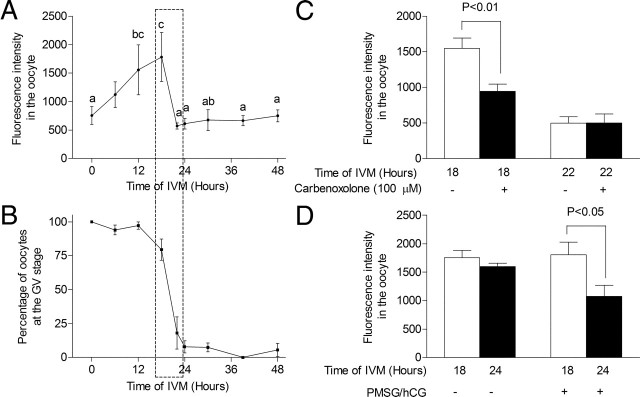
Initial experiments were designed to revisit GJC in COCs without using the traditional technique (i.e. injection of Lucifer Yellow into the oocyte) but by using another method recently designed by Thomas et al. (24) for bovine COCs. Figure 1A shows that oocyte calcein uptake increased continuously from the start of culture, reaching maximal levels between 12 and 18 h (P < 0.05). This high level of GJC fell precipitously between 18 and 22 h of culture (P < 0.05) and remained unchanged thereafter (Fig. 1A), supporting a major decrease in the ability of the oocyte to uptake calcein after 22 h of IVM. Interestingly, this loss of GJC coincided with oocyte GVBD, between 18 and 22 h of IVM (Fig. 1, A and B). Exposing COCs at 18 h to the GJC inhibitor carbenoxolone significantly prevented oocyte calcein uptake (P < 0.01) (Fig. 1C). Oocyte calcein uptake after 22 h of culture was no longer affected by exposure to carbenoxolone (Fig. 1C), supporting the contention that GJC between cumulus cells and the oocyte was already shut off, as shown in Fig. 1A. The supplementation of gonadotropins triggers the decrease in the capacity of the oocyte to take up calcein (P < 0.05), which was not observed when no gonadotropins were added (Fig. 1D). These lines of evidence suggest that GJC in the COC is initially increased followed by a gonadotropin-dependent decrease. The role of Cx43 was then further investigated.
Fig. 1.
Oocyte calcein uptake through GJC during oocyte maturation. A, After calcein-AM uptake by the COC, calcein fluorescence was measured in the oocyte after various times of IVM. B, After calcein measurement, meiotic resumption in the same oocytes was assessed by detection of GVBD. C, The effect on oocyte calcein fluorescence by exposing COC to the GJC inhibitor carbenoxolone (100 μm) before and during calcein transfer. D, The effect of gonadotropins treatment during IVM on oocyte calcein uptake. GV, Germinal vesicle; PMSG, pregnant mare serum gonadotropin.
Is Cx43 mediating the initial increase in GJC?
Because Cx43 has a major role in GJC between somatic cells in the ovarian follicle (4), further experiments were undertaken to assess its role during in vitro maturation. Immunodetection revealed that Cx43 is up-regulated in the COC after 4 h of IVM irrespective of the presence of gonadotropins (P < 0.05) (Fig. 2A). To identify the multiple bands detected, a protein sample from COCs cultured for 4 h was treated with alkaline phosphatase, whereupon the multiple bands merged into a single band, suggesting that these immunodetected bands represent phosphorylated forms of Cx43 (Fig. 2B). Treatment of COCs with cAMP-dependant protein kinase inhibitor H89 during the first 4 h of IVM led to an increase in Cx43 that remained at the unphosphorylated form (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), whereas protein kinase C inhibitor U0126 and MAPK kinase 1 inhibitor bisindolyl maleimide 1 had no effect (supplemental Fig. 2).
Fig. 2.
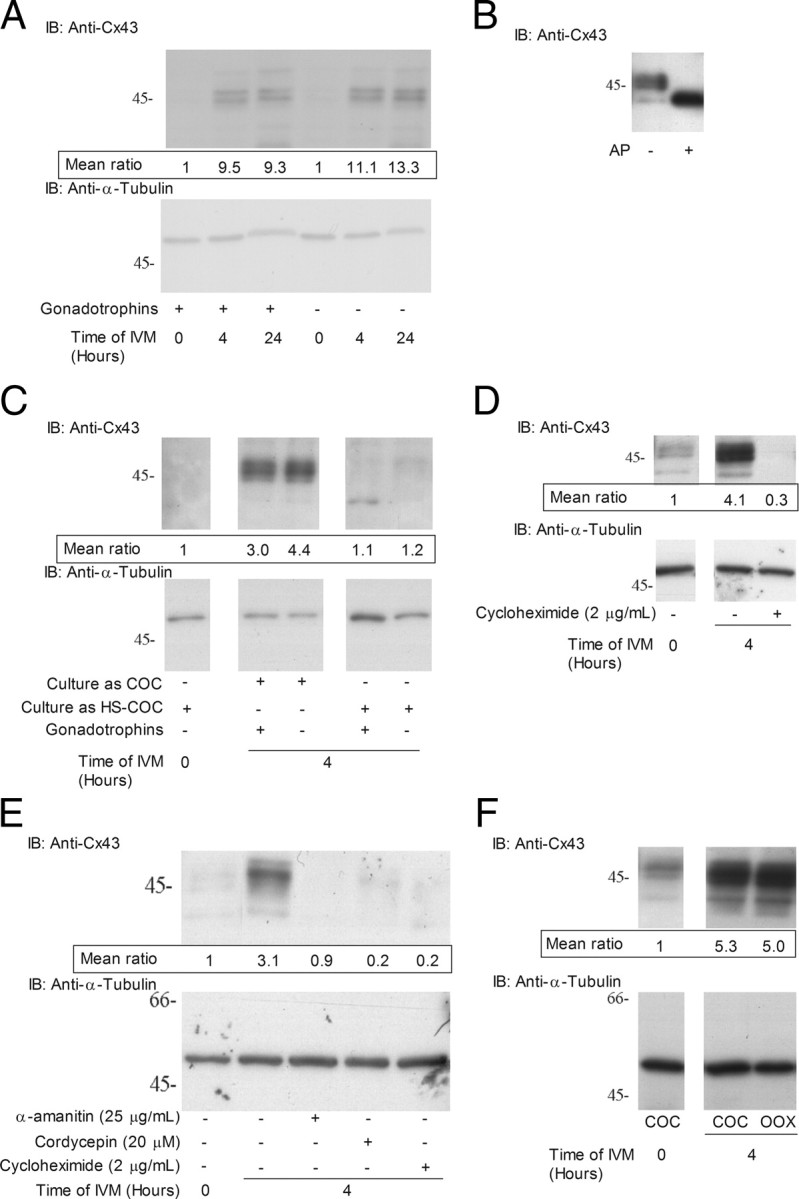
Regulation of Cx43 protein in the COC during IVM. A, Immunodetection of Cx43 in COC after 0, 4, and 24 h of IVM with or without gonadotropins. B, Effect of alkaline phosphatase treatment on immunodetection of Cx43 by Western blotting. Lane 1, cell extract from 10 COCs after 4 h of IVM. Lane 2, cell extract treated for 30 min with alkaline phosphatase. C, Contact with mural granulosa cells prevents Cx43 up-regulation in the COC as shown by hemisection (HS)-enclosed COC culture and immunoblotting for Cx43 and α-tubulin. D, Effect of the translation inhibitor cycloheximide (2 μg/ml) on immunodetection of Cx43 in COC after 4 h of IVM in medium containing follicular fluid. E, Effect of RNA synthesis, polyadenylation, and translation inhibitors, α-amanitin (25 μg/ml), cordycepin (20 μm), and cycloheximide (2 μg/ml), respectively, on Cx43 up-regulation in follicular fluid-free PVA-supplemented IVM medium. F, Up-regulation of Cx43 in oocytectomized (OOX) complexes after 4 h of IVM as detected by immunoblotting for Cx43 and α-tubulin. For all figures, upper panel is a representative experiment of samples immunoblotted for Cx43. Lower panel represents the same samples immunoblotted for α-tubulin. On the left is presented the molecular weight of a protein marker migrated with the samples expressed in kilodaltons. The values presented immediately under the Cx43 panel represent the mean densitometric ratio of Cx43/α-tubulin protein levels in at least three experiments relative to 0 h. AP, Alkaline phosphatase; IB, immunoblotting.
To further support that the rupture of COCs from the follicle triggers in vitro up-regulation, COCs were cultured as follicle hemisections (27) for 4 h with or without gonadotropins. The results showed that follicle hemisection-cultured COCs did not up-regulate Cx43 compared with control COCs (Fig. 2C). These data demonstrate that up-regulation of Cx43 is induced by the mechanical removal of the COC from the follicular environment.
Cx43 up-regulation in the in vitro matured COCs required de novo protein synthesis, because the translation inhibitor cycloheximide inhibited it after 4 h of culture (Fig. 2D). Cx43 up-regulation was also observed in COCs after 4 h of culture in follicular fluid-free medium but was inhibited by RNA synthesis and polyadenylation inhibitors α-amanitin and cordycepin, respectively (Fig. 2E). Because growth factors secreted from the oocyte have a particularly potent influence on cumulus cell function and gene expression (28, 29), it was important to exclude the oocyte as a potential stimulatory agent for Cx43 up-regulation. The results showed that removing the oocyte cytoplasm from the COC by microsurgery did not prevent the increase of Cx43 protein levels (Fig. 2F), supporting that the oocyte is unable to modulate Cx43 expression in the surrounding cumulus cells. These data are clearly suggesting that up-regulation of Cx43 is induced by the mechanical removal of the COC from the follicular environment, transcriptionally regulated, independent of both the oocyte and gonadotropins.
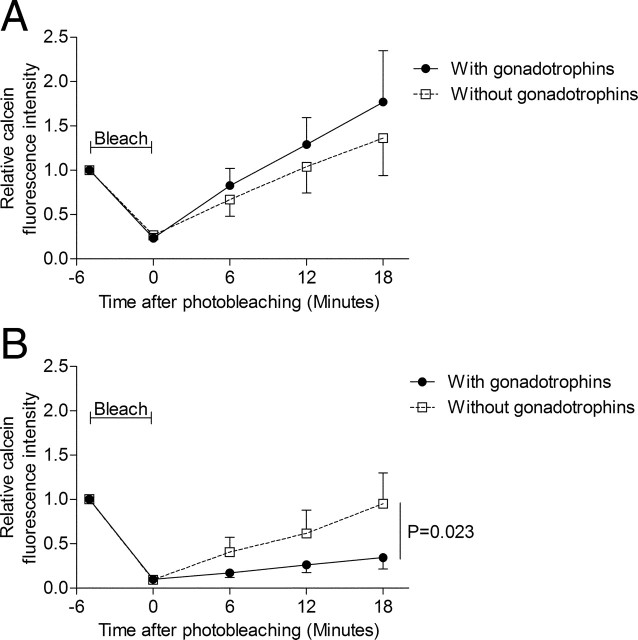
Fluorescence recovery after photobleaching (FRAP) assay measures the degree of GJC between cumulus cells. Our results revealed that untreated and gonadotropin-treated COCs had similar recovery (Fig. 3A). These results provide additional support for the notion of gonadotropin-independent up-regulation of GJC in the COC observed at Fig. 1 is at least partially mediated by an increase in Cx43 (Fig. 2) and of cumulus-cumulus GJC (Fig. 3A).
Fig. 3.
Functional characterization of GJC between cumulus cells by FRAP during IVM. A, The mean relative calcein fluorescence intensity in the bleached region after 4–8 h of IVM is presented for COCs cultured with (closed circles with solid line; n = 8) or without (open boxes with dashed line; n = 7) gonadotropins. B, The mean relative calcein fluorescence intensity recovery after 20–24 h of IVM in gonadotropin-treated (n = 6) or untreated (n = 6) COCs after a laser pulse bleaching period. The mean relative calcein fluorescence intensity in the bleached region is presented for COCs cultured with (closed circles with solid line) or without (open boxes with dashed line) gonadotropins. At 20–24 h of IVM, untreated cumulus cells recover significantly more fluorescence than gonadotropin-treated cumulus cells (P < 0.05). Statistical analysis was performed by ANOVA (P < 0.05).
Cx43 regulation during the closure of GJC between the cumulus cells and the oocyte
FRAP assays were performed on COCs after 20–24 h of IVM when GJC has ceased. As expected, the FRAP assays revealed that gonadotropin-stimulated COCs showed almost no fluorescence recovery (Fig. 3B), supporting the observed sharp decrease in oocyte calcein uptake (Fig. 1A). Interestingly, in the absence of gonadotropins, COCs displayed a significantly (3.4-fold) higher rate of FRAP than gonadotropin-treated COCs (P < 0.05) (Fig. 3B). These results suggest that the breakdown of GJC in between cumulus cells after 20–24 h is gonadotropin dependent. This gonadotropin-dependent cumulus-cumulus communication breakdown might be mediating the decrease in GJC between 18 and 22 h (Fig. 1A).
Role of the first 4 h of IVM on oocyte meiotic maturation
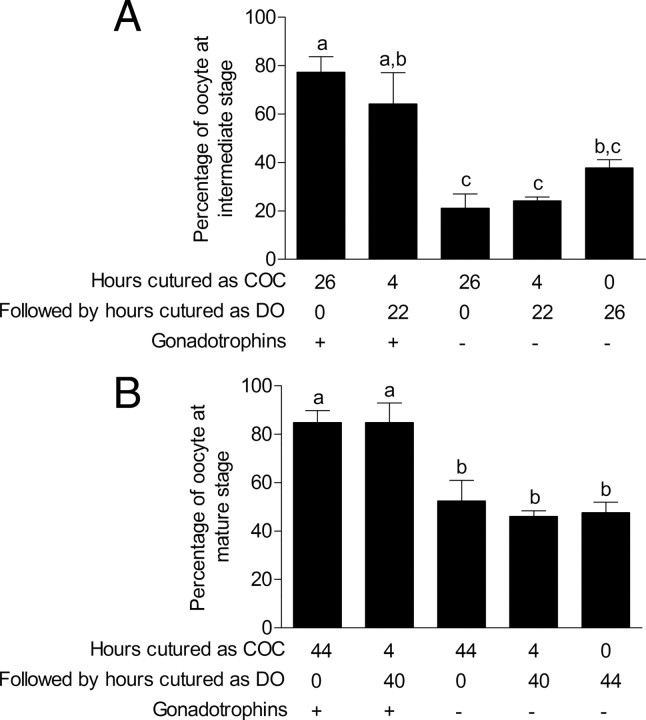
Cumulus cells and the oocytes are forming an electrophysiological syncytium in which both cell types are influencing each other (29). Thereby, the impact of cumulus cells on nuclear maturation was investigated. Oocytes were either cultured with or without gonadotropins and 1) as intact COC from 0–44 h; 2) as intact COCs for just 4 h and then denuded of cumulus cells and cultured for a further 40 h; or 3) cultured as denuded oocytes from 0–44 h. Oocytes cultured as COCs with gonadotropins for 4 or 26 h displayed higher percentage of metaphase I chromatin configurations than COCs without gonadotropins (Fig. 4A). After 44 h of IVM, both gonadotropin-stimulated intact COCs and COCs with cumulus cells for only 4 h of IVM displayed high percentage of metaphase II chromatin configuration (Fig. 4B). Absence of gonadotropin stimulation significantly reduced the number of oocytes reaching the metaphase II state (Fig. 4B). Although gonadotropin-stimulated and untreated COC showed similar GJC after 4 h of in vitro culture (Fig. 3A), the capacity of cumulus cells to promote the resumption and completion of oocyte meiosis was gonadotropin dependent. These data highlight that even if no difference in GJC is measured during the early hours of oocyte maturation with or without gonadotropins, the crucial role of cumulus cells and gonadotropins affects the capacity of the oocyte to complete nuclear maturation.
Fig. 4.
Effect of gonadotropins and cumulus cells on oocyte nuclear maturation. Assessment of oocyte nuclear status after 26 h (A) or 44 h (B) of IVM in the presence or absence of cumulus cells and gonadotropins. Results are presented as the percentage of oocytes at intermediate stage after 26 h or at mature stage after 44 h. Statistical analysis was performed by ANOVA (P < 0.05). Different superscripts indicate significant differences according to Bonferroni’s post hoc tests; DO, denuded oocytes.
Gonadotropin-mediated mechanisms of Cx43-mediated GJC closure
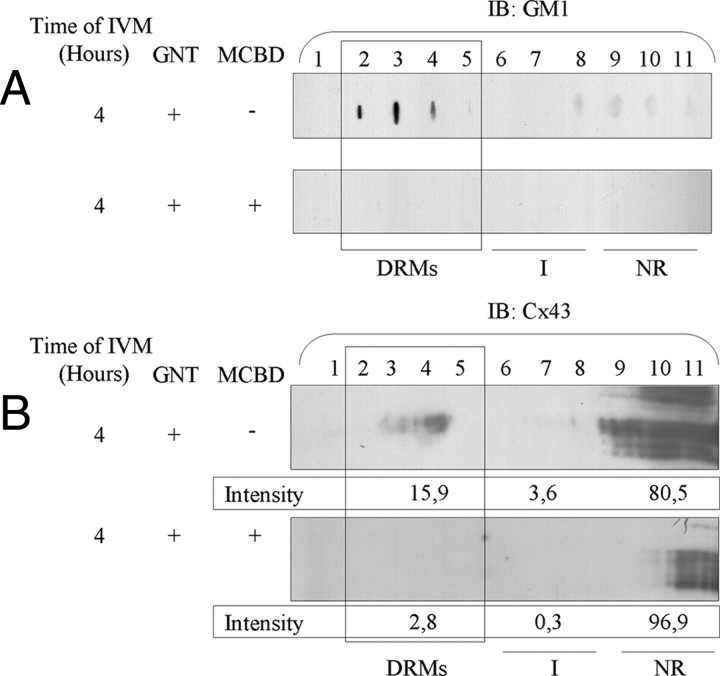
Because Cx43 is clustered to lipid rafts in many cell types (30, 31, 32, 33) and is sometimes associated with a decrease in GJC (34), it was hypothesized that lipid raft microdomains are actively involved in the GJC breakdown observed at 22 h of IVM. Therefore, the association of Cx43 on the cumulus cell membrane was investigated. Detergent-resistant membranes (DRMs) were recovered after Triton X-100 treatment followed by sucrose density gradient centrifugation. Their composition is thought to be representative of cell membrane lipid rafts (35). Therefore, DRMs were used as a model to study the trafficking of connexins in lipid rafts, those domains enriched in cholesterol and sphingolipids that are less fluid regions of the plasma membrane. Fractions 2–5 of the sucrose density gradient contain the DRMs. DRMs from each preparation (from ∼2000 COCs) were enriched in ganglioside GM1 (Fig. 5A), a well-known marker of lipid rafts (36). Those that had undergone 24 h of IVM were even more enriched (Fig. 5A). Cx43 was not detected in DRMs from freshly isolated COCs (Fig. 5B); however, it was observed that the association of Cx43 with DRMs increased with the progression of IVM. A small proportion of Cx43 was associated with DRMs after 4 h of IVM (Fig. 5B), and this Cx43 was phosphorylated, as assessed by treating fraction 4 with alkaline phosphatase, which converted the multiple immunodetected bands to a single nonphosphorylated Cx43 band (Fig. 5C). After 24 h of IVM (Fig. 5B), Cx43 association with DRMs was even more abundant. Again, alkaline phosphatase treatment revealed that DRM-associated Cx43 was mostly phosphorylated (Fig. 5C). These results clearly suggest that the composition of cumulus cell lipid rafts is changed during IVM. Because lipid rafts require cholesterol for their structure, the effect of cholesterol depletion by methyl-β-cyclodextrim (MBCD) was tested to validate our isolation of DRMs. Treatment of COCs for 30 min with 10 mm MBCD after 4 h of IVM show no GM1 in DRM using densitometric intensity analysis, supporting the loss of structural integrity of DRM (Fig. 6A). No signal was detected for Cx43, supporting a loss of Cx43 association with DRMs (Fig. 6B). These results demonstrate that DRMs are present and are perturbed by cholesterol depletion upon MBCD treatment.
Fig. 5.
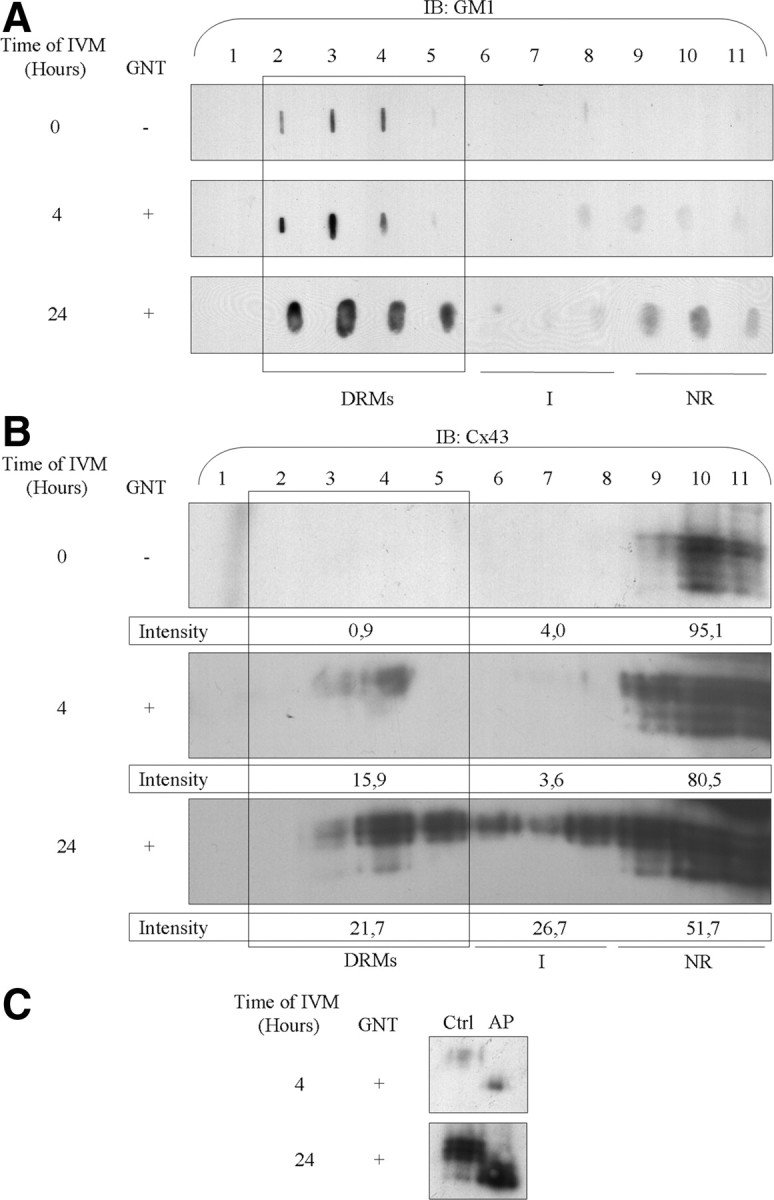
Modulation of DRM composition and association with Cx43 during IVM. Sucrose density gradient centrifugation was performed on freshly isolated COCs (A and B, first panel) and COCs recovered after 4 h (A and B, second panel) and 24 h of IVM with gonadotropins (A and B, third panel). Dot blot and hybridization with CTB revealed GM1 enrichment in the DRM-containing fractions of each preparation (A). Hybridization with an anti-Cx43 antibody revealed an increase in DRM-associated Cx43 as IVM progressed (B). Alkaline phosphatase treatment of fraction 4 from gradients performed after 4 and 24 h of IVM revealed that DRM-associated Cx43 is mostly in phosphorylated form (C). The values presented under each Cx43 panel represent the percentage of Cx43 distributed in the different fraction groups. AP, Alkaline phosphatase; Ctrl, control; IB, immunoblotting; I, intermediate fractions; NR, non-raft fractions; GNT, gonadotropins.
Fig. 6.
Effect of cholesterol extraction on DRM-associated Cx43 and GM1. After 4 h of IVM, a sucrose density gradient was performed after Triton X-100 extraction on control (upper panels) or MBCD-treated COC (lower panels). GM1 detection revealed a perturbed association of GM1 with DRMs upon MBCD treatment (A). Cx43 Western blotting also revealed that MBCD treatment decreased Cx43-DRM association (B). The values presented under each Cx43 panel represent the percentage of Cx43 distributed in the different fraction groups. IB, Immunoblotting; GNT, gonadotropins; NR, non-raft fractions; I, intermediate fractions.
To investigate the localization of Cx43 in the COC during gap-junctional breakdown, the quantity of Cx43 present at the cell surface was measured during IVM. To do so, surface proteins were biotinylated, isolated by neutravidin affinity, and measured by immunodetection. Figure 7A shows that there were similar levels of Cx43 after 4 and 20 h of IVM, in the latter, irrespective of the presence of gonadotropins. The presence of Cx43 at the cell surface during gap-junctional communication breakdown (observed in Fig. 1), suggests that the rupture in communication does not require an immediate Cx43 internalization. Lamin A/C was used as a negative control because it is present at the nuclear membrane and should remain free of biotin, as observed in Fig. 7B.
Fig. 7.
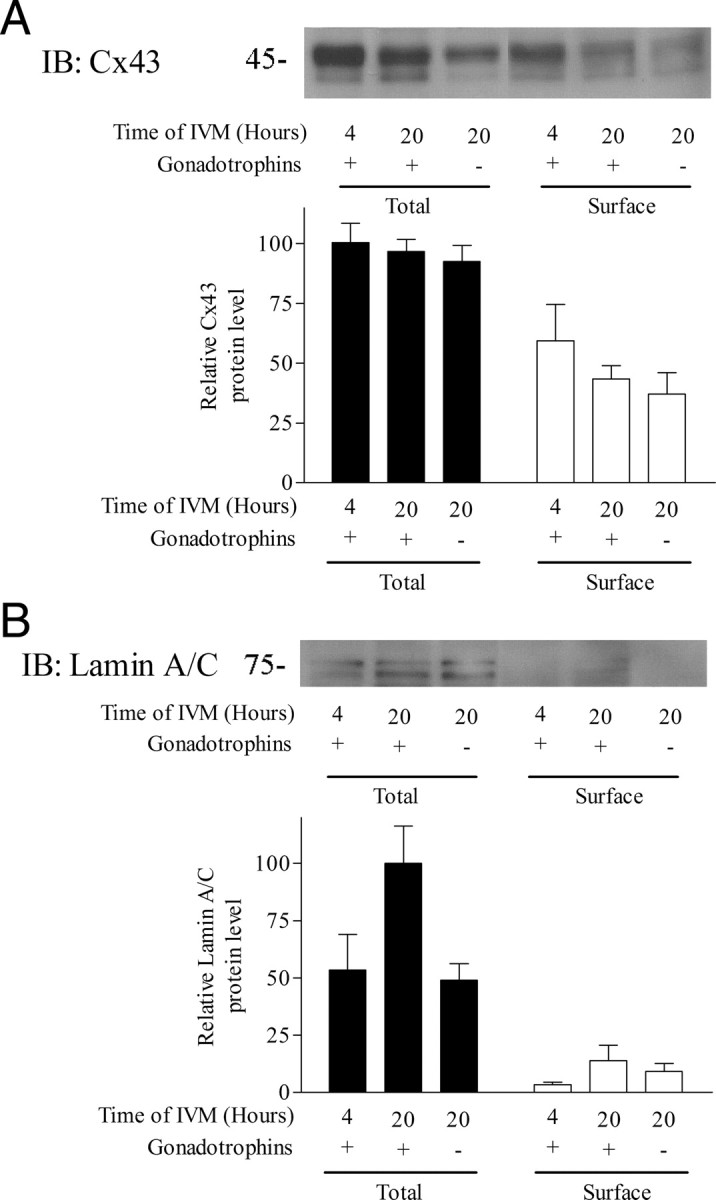
The localization of Cx43 after 4 and 20 h of IVM. After culture, proteins present at the cell surface of the COC were biotinylated, affinity purified, and immunodetected. A, Cx43 immunodetection revealed its presence at the cell surface after 4 and 20 h of culture with and without gonadotropins. The total lysate refers to 10 COCs, whereas the surface Cx43 is the biotinylated Cx43 found at the surface of about 100 COCs per treatment. B, Lamin A/C immunodetection was the negative control showing that a nuclear membrane protein remained unbiotinylated throughout the course of IVM. IB, Immunoblotting.
To assess the role of gonadotropins in the localization of Cx43 within cell membrane microdomains, DRMs were isolated from gonadotropin-free COCs after 24 h of IVM. Figure 8, A and B, shows the GM1 and Cx43 distribution after sucrose density gradient of COCs cultured for 24 h without gonadotropins. These figures show a lower percentage of Cx43 in the DRM and intermediate fractions compared with gonadotropin-treated COCs (Fig. 5, A and B). Cx43 association with DRM (fraction 4) was more abundant in gonadotropin-treated than untreated COCs after 24 h of IVM (Fig. 8C). Alkaline phosphatase treatment of these samples revealed that DRM-associated Cx43 was mostly phosphorylated (Fig. 8C). Similar results were obtained when analyzing fractions 5 (DRMs) and 7 (intermediate) (supplemental Fig. 3). These results suggest that Cx43 is still present at the cell surface upon gap-junctional communication breakdown but emphasizes the importance of Cx43 targeting to the DRM as a means to reduce both cumulus-cumulus and cumulus-oocyte gap-junctional communication.
Fig. 8.
Impact of gonadotropins on DRM-associated Cx43. Sucrose density gradient centrifugation was performed on COCs recovered after 24 h of IVM without gonadotropins. GM1 and Cx43 detection was performed on each fraction (A and B). Fraction 4 samples from gradients performed on COCs after 24 h of IVM with and without gonadotropins were analyzed by Western blotting with an anti-Cx43 antibody. Higher DRM-associated Cx43 in gonadotropin-treated COCs are observed compared with untreated COCs (C). Treatment of the same fractions with alkaline phosphatase (AP) revealed that DRM-associated Cx43s are mostly phosphorylated. IB, Immunoblotting; NR, non-raft fractions; I, intermediate fractions.
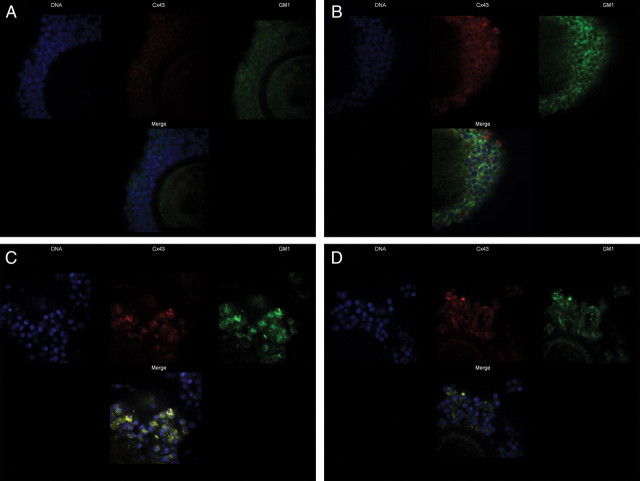
To assess the distribution of lipid rafts in the entire cells, GM1 and Cx43 were localized by confocal microscopy. Freshly isolated COCs show a very weak Cx43 signal and a diffuse distribution of GM1 in the cumulus cells (Fig. 9A). After 4 h of IVM with gonadotropins, Cx43 was distributed diffusely over cumulus cells and weakly colocalized with GM1 (Fig. 9B). After 24 h of IVM with gonadotropins, aggregation and recruitment of Cx43 within the clustered lipid rafts (GM1) were clearly observed (Fig. 9C), as compared with COCs without gonadotropins (Fig. 9D), supporting the clustering of Cx43 into lipid raft microdomains. These results further indicate a temporal change in Cx43 location and support that Cx43 is clustered to lipid raft microdomains in cumulus cells during IVM and in the presence of gonadotropins.
Fig. 9.
Distribution of Cx43 and the lipid raft marker GM1 in cumulus cell membranes during IVM. Freshly isolated COCs (A), COCs after 4 h of IVM with gonadotropins (B), and COCs after 24 h of IVM with (C) or without gonadotropins (D) were hybridized with anti-Cx43 antibody (red), cholera toxin β subunit (green), and 4′,6-diamidino-2-phenylindole (blue).
Discussion
Our data show that intercellular GJC is tightly regulated throughout in vitro maturation of the COC; initially, it is transcriptionally up-regulated by rupture from the mural granulosa cells and then is closed concomitant with GVBD in a gonadotropin-dependent manner that implicated lipid rafts. The rate of GJC-dependent oocyte calcein uptake increased from the start of maturation and remained high up to 18 h, decreased sharply between 18 and 22 h, and then remained low until the end of IVM. FRAP assays revealed that the early increase in GJC was concomitantly happening with a gonadotropin-independent Cx43 up-regulation. This Cx43 up-regulation could be prevented by culturing COCs in follicular hemisections, supporting the idea that mechanical rupture of the COC from its granulosa cell layer triggers Cx43 early up-regulation. The decrease in GJC after 18 h of IVM was gonadotropin dependant, as determined by oocyte calcein uptake and FRAP. Because total and surface-labeled Cx43 remained stable between 4 and 24 h of IVM, its association to membrane microdomains was studied. Gonadotropins triggered the clustering of lipid rafts and the colocation of Cx43 with these structures. The association of phosphorylated Cx43 with DRMs was increased as well by gonadotropins. These results suggest that gonadotropin-dependent GJC breakdown occurs initially by a functional inactivation of Cx43 channels involving lipid rafts, rather than by an immediate removal of Cx43 from the cell surface. Together, these results provide a new perspective on the dynamic regulation of GJC in the COC during the course of oocyte in vitro maturation, in particular by examining, for the first time, the potential involvement of lipid rafts in the regulation of oocyte-somatic cell gap junction communication.
The role of GJC breakdown in oocyte meiotic resumption
Previous studies on porcine COCs used a different technique to measure GJC and failed to detect the gonadotropin-independent increase before GVBD. Microinjection of Lucifer Yellow into the oocyte was used to detect cumulus cells coupled to the oocyte but gave little quantitative information on the degree of cell-to-cell communication (26, 37). Such an increase in oocyte-cumulus cell GJC has never been reported in other species studied. Rat COCs displayed an immediate decrease in GJC-dependent oocyte tritiated-uridine uptake after either in vitro LH or in vivo human choriogonadotropin (hCG) stimulation, and quantitative freeze-fracture electron microscopy revealed that cumulus-cumulus gap-junctional loss was even faster in vitro after FSH stimulation than in control COCs (15, 38). Using the same assay as the current study, it was reported that bovine COCs showed a transient 20–50% increase in GJC under specific cAMP pathway-stimulatory conditions [FSH + phosphodiesterase (PDE) type 4 inhibitor, forskolin + PDE type 3 or PDE type 4 inhibitors] but not in the absence of such stimuli (24, 39). A recent study using the same oocyte calcein uptake assay revealed that human COC GJC decreases rapidly during the first 6 h of IVM and that addition of PDE3 inhibitor and/or forskolin could delay GJC breakdown by 6 h (40). It could be argued that the rupture from the mural granulosa cells does not necessarily increase GJC in all mammals and may also depend on the stage of follicular growth. The physiological reason for this is unclear. However, it is worth noting that spontaneous oocyte GVBD is substantially slower in the pig than in any other mammals; it remains in complete meiotic arrest for 18 h during IVM, in contrast to rat, bovine, and human, which take 1, 6, and 12 h, respectively, to begin resuming meiosis (15, 24, 40, 41).
Norris et al. (20) have elegantly shown that GJCs are ruptured between the mural granulosa cells and the COCs by LH in cultured mouse ovarian follicle and that this rupture was followed by oocyte meiotic resumption. Interestingly, a GJC blocker like carbenoxolone (100 μm) was shown to mimic the effect of LH by inhibiting GJC and triggering oocyte meiotic resumption (20). Carbenoxolone treatment by intrabursal injection in live rat or on cultured ovarian follicle was shown to similarly trigger oocyte meiotic resumption (21). These two models strongly suggest that in vivo LH-stimulated oocyte maturation follows a decrease in GJC between mural granulosa cells and the COCs involving ERK (19, 20). However, the MAPK kinase inhibitor U0126 (at a dose that prevented Cx43 phosphorylation by ERK and prevented gap-junction breakdown) was unable to significantly change LH-induced follicle-enclosed oocyte maturation (20). This observation suggests that, in addition to the action of LH to release the inhibitory signal, there is a positive signal coming from the granulosa/cumulus cells that triggers oocyte maturation. In agreement with the results reported by Norris et al. (20), Downs and Chen (42) have recently shown that epidermal growth factor (EGF)-induced COC maturation could be inhibited by a gap-junction blocker, suggesting the presence of a positive signal. Our results are showing that gonadotropin-stimulated GJC breakdown does not seem to directly cause oocyte maturation. The results in Fig. 4 illustrate this paradigm: 1) mechanical GJC breakdown by denuding after 4 h does not affect oocytes maturation; 2) gonadotropins action on the cumulus cells provide a positive signal to the oocyte that allows high maturation rates. However, the nature of that signal remains to be determined. GJC-independent mechanisms leading to oocyte meiotic resumption in the ovarian follicle must be considered as well. Modulations of PDE3A activity in the oocyte, as seen in hCG-treated rat follicles, or factors signaling through an inhibitory G protein pathway (such as Insl3 and progesterone) could hypothetically play a part in a GJC-independent LH action on oocyte meiotic resumption (43, 44, 45).
Our study provides interesting observations on GJC dynamics in the COC after recovery from the ovary. It is interesting to note that the recovery of the COC from the ovary might mimic LH or carbenoxolone effect in the rodent models discussed above, where a decoupling between mural granulosa cells and the COC was followed by oocyte maturation. The reason for the initial increase after 4 h is under investigation. However, the observed decrease after 18 h of IVM could very likely be the event that, like LH or carbenoxolone in rodent, triggers porcine oocyte maturation in vivo, although this hypothesis remain to be tested. The fact that 1) oocyte maturation was occurring simultaneously with GJC breakdown (Fig. 1), and that 2) oocytes maturation occurred simultaneously even when cumulus cells were removed after 4 h (Fig. 4) led us to think that GJC breakdown is not the direct cause of in vitro porcine oocyte meiotic resumption.
The carefully orchestrated up-regulation followed by down-regulation of GJC within the COC, observed in the present study, is likely to have important nonmeiotic roles such as facilitating metabolic support to the oocyte, regulation of oocyte cytoplasmic maturation, and acquisition of developmental competence by the oocyte (39, 46, 47).
Cx43 posttranslational modifications affect its function
The effect of Cx43 phosphorylation in different cell types has been previously shown (48, 49). As in many cell types, GJC in porcine granulosa cells is enhanced by protein kinase A (PKA) phosphorylation but decreased by protein kinase C (PKC) (50). In rat granulosa cells, FSH promotes the phosphorylation of four serine residues in Cx43 (51). Further studies revealed that FSH-stimulated Cx43 phosphorylation was attributable to PKA (52). In a recent study using a follicle culture system, Norris and collaborators (20) reported that LH caused an ERK-dependant decrease in gap junction communication between granulosa cells and this before meiotic resumption. In rat kidney cells, casein kinase 1 was shown to promote Cx43 gap-junction assembly. Although casein kinase I and II activities have been reported in porcine follicle wall membrane, no study has explored their role in establishing GJC in the ovarian follicle (53, 54). FSH acts on GJC in two steps in the rat granulosa cell line GFSHR-17; 1) a rapid increase, within minutes (presumably by phosphorylation); and 2) after 6 h of culture, an increase in the Cx43 mRNA level (55). In our study, gonadotropins were not required for the initial up-regulation of GJC and Cx43. LH has been reported to inhibit Cx43 transcription and translation in rat follicles (56, 57). GJC closure is regulated by phosphorylation in the ovary as well as in other systems (48). Tyrosine phosphorylation has been particularly associated with Cx43-mediated GJC closure (58). Wayne et al. (59) have reported that FSH stimulates three distinct signaling cascades in rat primary granulosa cells, one being the small GTP-binding protein RAS by the Rous sarcoma oncogene family tyrosine kinase and EGF receptor. It has been shown gonadotropins trigger the up-regulation of the EGF-like peptides, epiregulin, amphiregulin, and β-cellulin, in pig granulosa and cumulus cells (60). Considering the reported role of EGF in the hyperphosphorylation, ubiquitination, internalization, and proteasome degradation of Cx43 in the rat liver epithelial cell line IAR20 (61), it could be interesting to assess whether the present gonadotropin-dependent decrease in GJC, concomitant with GVBD, could be triggered by the expression of EGF-like peptides. In support of this hypothesis, Shimada and collaborators (25, 62, 63) have reported that hyperphosphorylation of Cx43 in porcine cumulus cells upon GVBD was dependent on phosphatidylinositol 3-kinase and PKC, two kinases activated downstream of the EGF receptor.
Compartmentalization of Cx43 directs its function
Cx43 has been shown to partition into DRMs in many cell types (30, 31, 32, 33), although not always (64). The impact of the Cx43 phosphorylation state on its DRM association seems to be cell type dependent (30, 32, 33). The present study indicates that Cx43 recruitment to DRMs is more efficient after GVBD (Fig. 5B) whereas the global levels of Cx43 protein are stable (Fig. 2A). Moreover, phosphorylated Cx43 is more abundant in DRMs in the presence of gonadotropins (Fig. 8C). Some studies have reported that the interaction of activated PKCγ with Cx43 and caveolin-1 causes a redistribution of both proteins within lipid rafts, resulting in disassembly of the gap junction plaque (31). The hypothesis emerging from these results is that the redistribution within lipid rafts may be important for achieving equilibrium between single connexons and gap-junction plaques. An additional study reports a reduction in GJC that is not explained by a decrease in Cx43 protein levels, but by disrupting the interaction between Cx43 and ZO-1, a major connexin-interacting protein (34). The authors proposed that when newly synthesized Cx43 reaches the plasma membrane, it partitions into lipid rafts, where it does not form intercellular conduits. Cx43 would then be recruited from lipid rafts to gap junction plaques to participate in GJC. In the present study, we observed a partial colocalization of Cx43 with lipid rafts (Fig. 9B) and an association of Cx43 with DRMs in the first 4 h of IVM (Fig. 5B), when GJC is not yet optimal (Fig. 1A) but Cx43 protein levels were high (Fig. 2A). These data support the hypothesis that Cx43 reaching the cumulus cell membrane is sequestered in lipid rafts before joining the gap-junction plaque.
At steady state, the lipid rafts contain very few proteins and in order to be engaged in membrane functions, they have to cluster (65). Our results support a new role of lipid rafts in sequestering Cx43. This occurs after 24 h of oocyte maturation when GJC is suppressed, which is after commitment to GVBD. At that point in time, gonadotropin-dependent clustering of Cx43 into lipid rafts was observed (Fig. 9C), which support their involvement in membrane function. The strongest association of Cx43 with DRMs was also observed at 24 h, which is after GJC breakdown (Fig. 5B). However, we still observed a large amount of remaining Cx43 protein in the non-DRM fractions. Similar results were observed in the ROS 17/2.8 osteosarcoma cell line, in which a 85% reduction of GJC was associated with migration of only 40% of Cx43 in DRMs (34), supporting that a loss of GJC does not require a total association of Cx43 to the DRMs. The causal relationship of gap-junctional communication breakdown and recruitment of Cx43 to lipid rafts remains to be determined. The possibility that GJC breakdown is simply followed by recruitment of Cx43 to the DRM for recycling cannot be discarded. The association of flotillin-1 (supplemental Fig. 4) GM1 with DRMs after 24 h of IVM (Fig. 5A) suggests that lipid raft composition is evolving throughout oocyte maturation. Modifications in flotillin levels, localization, or insolubility in Triton X-100 are observed through differentiation among cell types, as preadipocytes and C2C12 myoblasts (66, 67). Flotillin-1 is involved in a clathrin-independent endocytic pathway that was reported to be implicated in internalization of GM1 (68). This role suggests that surface Cx43 present after 20 h of IVM could be targeted for endocytosis later in IVM.
In conclusion, we have described new mechanisms implicated in the complex regulation of germ-somatic cell GJC throughout in vitro maturation of the COC. The first hours of IVM are of paramount importance for appropriate oocyte maturation, representing a brief window of opportunity for cumulus cells to regulate, at least in part, via GJC, and the capacity of the oocyte to complete meiosis and acquire developmental competence. Indeed, the composition of oocyte maturation medium in terms of gonadotropins, energy sources, growth factors, and lipids, clearly affects oocyte-cumulus cell GJC, which, in turn, has implications for the developmental outcome of the oocyte and the ensuing embryo.
Materials and Methods
Chemicals and ovary collection
Unless otherwise stated, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Prepubertal pig ovaries were collected as previously described (69).
IVM and assessment of oocyte nuclear status
COCs were collected by puncturing 3- to 6-mm antral follicles and were prepared and cultured as described previously (70). COCs were washed in HEPES-buffered Tyrode medium containing 0.01% (wt/vol) polyvinyl alcohol (PVA-HEPES) (71). Groups of 10–50 COCs were then cultured in NunclonΔ four-well dishes in 500 μl standard porcine IVM medium: BSA-free NCSU23 medium containing 25 μm 2-mercaptoethanol, 0.1 mg/ml cysteine, 10% (vol/vol) 0.22 μm-filtered porcine follicular fluid, and gonadotropins at final concentrations of 2.5 IU/ml hCG, and 2.5 IU/ml Pregnant mare’s serum gonadotropin (Intervet; Whitby, Ontario, Canada). The inhibitors cycloheximide, α-amanitin, and cordycepin were used at working concentrations of 2 μg/ml, 25 μg/ml, and 20 μm, respectively.
Follicle hemisections were prepared by removing entire 3- to 6-mm antral follicles from the ovary with surgical scissors and eliminating most of the surrounding cortex (27). Five hemisections were cultured in 1 ml of IVM medium, and COCs were retrieved by scraping the follicular cells with a glass pipette into a Petri dish. Oocyte nuclear status was assessed by acetoorcein staining, as described previously (70). A minimum of 20 oocytes was fixed per treatment. Oocyte nuclear maturation status was classified as 1) GV stage oocyte displaying a germinal vesicle (GV arrested); 2) intermediate stage oocyte displaying GVBD and condensed chromatin or metaphase I-organized chromatin; or 3) mature stage oocyte displaying anaphase I, telophase I, or metaphase II DNA configuration.
GJC assay
The cumulus cell-oocyte GJC assay was designed to measure oocyte calcein uptake from the cumulus cells using a technique already described, with minor modifications (24). Ten COCs were cultured in IVM medium as described above. They were incubated for 15 min in the presence of calcein-AM (39,69-Di(O-acetyl)-29,79-bis[N,N-bis(carboxymethyl) amino methyl]-fluorescein, tetraacetoxy methyl ester; Molecular Probes, Eugene, OR), an acetoxymethyl ester derivative of the fluorescent indicator calcein. A 25-min period was allowed for dye to transfer from the cumulus to the oocyte. Oocytes were denuded of cumulus cells to terminate dye transfer. Fluorescence emission of calcein was then quantified in individual oocytes using a fluorophotometric-inverted microscope (Leica, Wetzlar, Germany). Inspeck Green fluorescent beads (6 μm) (Molecular Probes) were used as an external fluorescence standard for constant sensitivity between experiments. The values presented are the mean fluorescence intensities relative to control at 0 h of culture, arbitrarily set at 100 U. After measurement, the oocytes were fixed and their nuclear status was assessed. The time points for the GJC experiment and resumption of oocyte meiosis correspond to the IVM period after which COCs were retrieved for GJC measurement and do not account for the time required for the measurement itself (45–60 min).
Western blotting
All samples were loaded on a 12% polyacrylamide gel for electrophoresis and then transferred to a Hybond-P membrane (GE Healthcare, Baie d’Urfé, Québec, Canada).
Flotillin-1
The first hybridization was performed with a mouse antiflotillin-1 (BD Biosciences, Mississauga, Ontario, Canada) diluted 1:500. The membranes were hybridized with the second antibody, horseradish peroxidase-conjugated goat antimouse (Upstate Biotechnology, Charlottesville, VA) diluted 1:3000.
Cx43
The first hybridization was performed with a mouse anti-Cx43 (Chemicon, Temecula, CA) diluted 1:2000. The membranes were then hybridized with the second antibody, horseradish peroxidase-conjugated goat antimouse diluted 1:10,000 (Upstate).
α-Tubulin
Before the first hybridization for α-tubulin, membranes hybridized with anti-Cx43 were stripped with Restore Western blot stripping buffer (Pierce Biotechnology, Rockford, IL). The first hybridization was performed with a mouse anti-α-tubulin diluted 1:50,000. The membranes were hybridized with a horseradish peroxidase-conjugated goat antimouse diluted 1:20,000 (Upstate).
Lamin A/C
The first hybridization was performed using an antilamin A/C (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:200 followed by a second hybridization using a horseradish peroxidase-conjugated goat antimouse diluted 1:3000 (Upstate).
For phosphoprotein evaluation, samples were incubated at 37 C with 10 U of calf intestinal alkaline phosphatase (New England Biolabs, Ipswich, MA) and New England Biolabs reaction buffer 2 adjusted to 1×. Proteins were detected with an ECL detection kit and exposed on autoradiographic films (GE Healthcare). Images were analyzed using Quantity One software (Bio-Rad Laboratories Ltd., Mississauga, Ontario, Canada).
FRAP assay
After defined periods of IVM, COCs were loaded with 1 μm calcein-AM in IVM medium with 0.1 mg/ml PVA. COCs were mounted on glass slides in IVM medium-PVA. The FRAP assays were conducted on a Nikon Eclipse TE2000-E inverted confocal microscope. Bleaching was effected by laser pulses on a limited region of cumulus cells in the COCs for 5 min at ×90 magnification. The COCs were photographed under confocal microscopy at ×60 magnification before and immediately after calcein bleaching and every 6 min thereafter. Fluorescence intensity was quantified using ImageJ 1.37 version software (National Institutes of Health, Bethesda, MD). A relative fluorescence value was achieved by dividing the raw fluorescence measurement in the bleached area by the mean fluorescence in two regions adjacent to this area. This value was further divided by the fluorescence value of a region at the opposite end of the COCs to correct for bleaching caused by laser excitation.
Isolation of detergent-resistant membranes by sucrose-density gradient
DRMs are insoluble in nonionic detergents such as Triton X-100, whereas the bulk membrane is solubilized at 4 C. The composition of the DRMs is thought to be similar to that of lipid rafts in live cells (London and Brown, 2000). The term “rafts” is then not appropriate to describe these isolated membranes; therefore, they will be referred to as rafts only when no detergent treatment is performed, e.g. with confocal microscopy localization. Otherwise, when a Triton X-100 extraction is performed and followed by a sucrose density gradient, the term “DRM” will be used. DRMs were isolated as previously reported with minor modifications (72). Briefly, COCs were frozen and thawed eight times in ice-cold TNE (10 mm Tris-HCl, pH 7.5; 150 mm NaCl; 5 mm EDTA). The cells were further lysed in TNE containing 1% Triton X-100 for 30 min on ice. The lysates were mixed with an equal volume of 85% (wt/vol) sucrose and overlaid with 2.4 ml 35% (wt/vol) sucrose and 1 ml 5% (wt/vol) sucrose. Centrifugation was performed at 39,000 × g in a Beckman SW60Ti rotor at 4 C for 18 h using an OptimaXL-80K Beckman-Coulter centrifuge. Eleven fractions of 380 μl were collected starting from the top of the tube. Fraction 12 was washed twice in TNE before being resuspended in TNE and then subjected to ultrasonic treatment.
Monosialoganglioside GM1 detection by dot spot
Each sucrose density fraction (10 μl) was dotted on to Hybond-P membranes using a dot spot apparatus (Invitrogen, Carlsbad, CA). The membranes were incubated with horseradish peroxidase-conjugated cholera toxin B subunit diluted 1:3000.
Surface protein biotinylation
Surface protein biotinylation was performed using the Cell Surface Protein Isolation Kit from Pierce Biotechnology (Rockford, IL) according to the manufacturer’s instructions. After culture, COCs were washed in cold PBS (0.1 m sodium phosphate; 0.15 m sodium chloride, pH 7.2), and surface protein biotinylation was performed for 30 min at 4 C. The reaction was ended by adding quenching solution and incubating for 15 min on ice. COCs were washed and were lysed by sonication in lysis solution. The samples were centrifuged at 10,000 × g for 2 min, and the supernatant was recovered and incubated for 60 min at room temperature with immobilized neutravidin gel. After washing the gel with the wash solution, biotinylated proteins were eluted from the gel by adding SDS-PAGE sample buffer with 50 mm dithiothreitol. Biotinylated samples were run on SDS-PAGE, and Cx43 and lamin A/C were detected as described above.
Confocal microscopy
COCs were placed on a polylysine-coated slide (Erie Scientific Co., Portsmouth, NH). Oocytes were fixed in 4% paraformaldehyde for 30 min. The first hybridization was performed with a mouse anti-Cx43 (Chemicon) diluted 1:50 and, with an Alexa-conjugated cholera toxin subunit B (Molecular Probes), was diluted 1:1500. The second hybridization was with an fluorescein isothiocyanate-conjugated goat antimouse (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted 1:150. Images were acquired on a Nikon Eclipse TE2000-E inverted confocal microscope at ×90 magnification.
Statistical analyses
All values are presented with their corresponding sem of at least three replicates. Statistical analyses were performed using Prism 5.00 GraphPad for Windows (GraphPad Software, San Diego, CA; www.graphpad.com). Statistical significance was assessed by ANOVA followed by Bonferroni’s multiple comparison post hoc tests to identify individual differences between means. Probabilities of P < 0.05 were considered statistically significant.
Acknowledgments
We thank Marc-André Sirard for critical reading of the manuscript and Jeremy G. Thompson for very valuable discussions. We also thank Lesley J. Ritter for help with the gap-junctional communication assay, Isabelle Laflamme for professional technical assistance, and Richard Prince for collecting ovaries at the slaughterhouse.
Footnotes
This work was supported by grants from the Natural Science and Engineering Research Council of Canada (NSERC) and by the Canadian Institutes for Health Research in the Program for Oocyte Health (to F.J.R.). M.S. is supported by a NSERC Ph.D., scholarship and a Fonds Québécois de la Recherche sur la Nature et les Technologies travel grant to Australia. R.B.G. is supported by the National Health and Medical Research Council of Australia with a RD Wright Fellowship and through a Program Grant.
Disclosure Summary: The authors have no conflict of interest.
First Published Online February 19, 2009
M.S. and M.-C.G. contributed equally to this work.
Abbreviations: COC, Cumulus-oocyte complex; Cx43, connexin 43; DRM, detergent-resistant membrane; EGF, epidermal growth factor; FRAP, fluorescence recovery after photobleaching; GJC, gap-junctional communication; GVBD, germinal vesicle breakdown; hCG, human choriogonadotropin; IVM, in vitro maturation; MBCD, methyl-β-cyclodextrim; PDE, phosphodiesterase; PVA, polyvinyl alcohol.
References
- 1.Laird DW2006. Life cycle of connexins in health and disease. Biochem J 394:527–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesnil M2002. Connexins and cancer. Biol Cell 94:493–500 [DOI] [PubMed] [Google Scholar]
- 3.Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF2000. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 226:167–179 [DOI] [PubMed] [Google Scholar]
- 4.Kidder GM, Mhawi AA2002. Gap junctions and ovarian folliculogenesis. Reproduction 123:613–620 [DOI] [PubMed] [Google Scholar]
- 5.Simon AM, Goodenough DA, Li E, Paul DL1997. Female infertility in mice lacking connexin 37. Nature 385:525–529 [DOI] [PubMed] [Google Scholar]
- 6.Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM2001. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 233:258–270 [DOI] [PubMed] [Google Scholar]
- 7.Gittens JE, Kidder GM2005. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J Cell Sci 118:5071–5078 [DOI] [PubMed] [Google Scholar]
- 8.Arellano RO, Martínez-Torres A, Garay E2002. Ionic currents activated via purinergic receptors in the cumulus cell-enclosed mouse oocyte. Biol Reprod 67:837–846 [DOI] [PubMed] [Google Scholar]
- 9.Kelly A, West JD2002. Survival and normal function of glycolysis-deficient mouse oocytes. Reproduction 124:469–473 [DOI] [PubMed] [Google Scholar]
- 10.Mattioli M, Gioia L, Barboni B1998. Calcium elevation in sheep cumulus-oocyte complexes after luteinising hormone stimulation. Mol Reprod Dev 50:361–369 [DOI] [PubMed] [Google Scholar]
- 11.Bornslaeger EA, Schultz RM1985. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod 33:698–704 [DOI] [PubMed] [Google Scholar]
- 12.Conti M2002. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod 67:1653–1661 [DOI] [PubMed] [Google Scholar]
- 13.Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A2002. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 187:153–159 [DOI] [PubMed] [Google Scholar]
- 14.Salustri A, Petrungaro S, De Felici M, Conti M, Siracusa G1985. Effect of follicle-stimulating hormone on cyclic adenosine monophosphate level and on meiotic maturation in mouse cumulus cell-enclosed oocytes cultured in vitro. Biol Reprod 33:797–802 [DOI] [PubMed] [Google Scholar]
- 15.Sherizly I, Galiani D, Dekel N1988. Regulation of oocyte maturation: communication in the rat cumulus-oocyte complex. Hum Reprod 3:761–766 [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura Y, Nakamura Y, Ando M, Jinno M, Oda T, Karube M, Koyama N, Nanno T1992. Stimulatory role of cyclic adenosine monophosphate as a mediator of meiotic resumption in rabbit oocytes. Endocrinology 131:351–356 [DOI] [PubMed] [Google Scholar]
- 17.Thomas RE, Armstrong DT, Gilchrist RB2002. Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol 244:215–225 [DOI] [PubMed] [Google Scholar]
- 18.Dekel N, Lawrence TS, Gilula NB, Beers WH1981. Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol 86:356–362 [DOI] [PubMed] [Google Scholar]
- 19.Sela-Abramovich S, Chorev E, Galiani D, Dekel N2005. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146:1236–1244 [DOI] [PubMed] [Google Scholar]
- 20.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA2008. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N2006. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147:2280–2286 [DOI] [PubMed] [Google Scholar]
- 22.Racowsky C, Satterlie RA1985. Metabolic, fluorescent dye and electrical coupling between hamster oocytes and cumulus cells during meiotic maturation in vivo and in vitro. Dev Biol 108:191–202 [DOI] [PubMed] [Google Scholar]
- 23.Racowsky C1985. Effect of forskolin on the spontaneous maturation and cyclic AMP content of hamster oocyte-cumulus complexes. J Exp Zool 234:87–96 [DOI] [PubMed] [Google Scholar]
- 24.Thomas RE, Armstrong DT, Gilchrist RB2004. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod 70:548–556 [DOI] [PubMed] [Google Scholar]
- 25.Shimada M, Maeda T, Terada T2001. Dynamic changes of connexin-43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3-kinase during meiotic resumption in porcine oocytes. Biol Reprod 64:1255–1263 [DOI] [PubMed] [Google Scholar]
- 26.Isobe N, Maeda T, Terada T1998. Involvement of meiotic resumption in the disruption of gap junctions between cumulus cells attached to pig oocytes. J Reprod Fertil 113:167–172 [DOI] [PubMed] [Google Scholar]
- 27.Richard FJ, Sirard MA1996. Effects of follicular cells on oocyte maturation. I. Effects of follicular hemisections on bovine oocyte maturation in vitro. Biol Reprod 54:16–21 [DOI] [PubMed] [Google Scholar]
- 28.Gilchrist RB, Ritter LJ, Armstrong DT2004. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci 82–83:431–446 [DOI] [PubMed]
- 29.Gilchrist RB, Lane M, Thompson JG2008. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 14:159–177 [DOI] [PubMed] [Google Scholar]
- 30.Barth K, Gentsch M, Bläsche R, Pfüller A, Parshyna I, Koslowski R, Barth G, Kasper M2005. Distribution of caveolin-1 and connexin43 in normal and injured alveolar epithelial R3/1 cells. Histochem Cell Biol 123:239–247 [DOI] [PubMed] [Google Scholar]
- 31.Lin D, Zhou J, Zelenka PS, Takemoto DJ2003. Protein kinase Cγ regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci 44:5259–5268 [DOI] [PubMed] [Google Scholar]
- 32.Mograbi B, Corcelle E, Defamie N, Samson M, Nebout M, Segretain D, Fénichel P, Pointis G2003. Aberrant connexin 43 endocytosis by the carcinogen lindane involves activation of the ERK/mitogen-activated protein kinase pathway. Carcinogenesis 24:1415–1423 [DOI] [PubMed] [Google Scholar]
- 33.Schubert AL, Schubert W, Spray DC, Lisanti MP2002. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 41:5754–5764 [DOI] [PubMed] [Google Scholar]
- 34.Laing JG, Chou BC, Steinberg TH2005. ZO-1 alters the plasma membrane localization and function of Cx43 in osteoblastic cells. J Cell Sci 118:2167–2176 [DOI] [PubMed] [Google Scholar]
- 35.Brown DA, London E2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275:17221–17224 [DOI] [PubMed] [Google Scholar]
- 36.Harder T, Scheiffele P, Verkade P, Simons K1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 141:929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isobe N, Terada T2001. Effect of the factor inhibiting germinal vesicle breakdown on the disruption of gap junctions and cumulus expansion of pig cumulus-oocyte complexes cultured in vitro. Reproduction 121:249–257 [PubMed] [Google Scholar]
- 38.Wert SE, Larsen WJ1989. Meiotic resumption and gap junction modulation in the cultured rat cumulus-oocyte complex. Gamete Res 22:143–162 [DOI] [PubMed] [Google Scholar]
- 39.Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB2004. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod 71:1142–1149 [DOI] [PubMed] [Google Scholar]
- 40.Shu YM, Zeng HT, Ren Z, Zhuang GL, Liang XY, Shen HW, Yao SZ, Ke PQ, Wang NN2008. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum Reprod 23:504–513 [DOI] [PubMed] [Google Scholar]
- 41.Chian RC, Buckett WM, Tulandi T, Tan SL2000. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod 15:165–170 [DOI] [PubMed] [Google Scholar]
- 42.Downs SM, Chen J2008. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev 75:105–114 [DOI] [PubMed] [Google Scholar]
- 43.Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarka M, Morita H, Toppari J, Fu P, Wade JD, Bathgate RA, Hsueh AJ2004. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA 101:7323–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richard FJ, Tsafriri A, Conti M2001. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 65:1444–1451 [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luciano AM, Modina S, Vassena R, Milanesi E, Lauria A, Gandolfi F2004. Role of intracellular cyclic adenosine 3′,5′-monophosphate concentration and oocyte-cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol Reprod 70:465–472 [DOI] [PubMed] [Google Scholar]
- 47.Gilchrist RB, Thompson JG2007. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 67:6–15 [DOI] [PubMed] [Google Scholar]
- 48.Lampe PD, Lau AF2004. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solan JL, Lampe PD2005. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta 1711:154–163 [DOI] [PubMed] [Google Scholar]
- 50.Godwin AJ, Green LM, Walsh MP, McDonald JR, Walsh DA, Fletcher WH1993. In situ regulation of cell-cell communication by the cAMP-dependent protein kinase and protein kinase C. Mol Cell Biochem 127–128:293–307 [DOI] [PubMed]
- 51.Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T2002. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Lett 531:132–136 [DOI] [PubMed] [Google Scholar]
- 52.Yogo K, Ogawa T, Akiyama M, Ishida-Kitagawa N, Sasada H, Sato E, Takeya T2006. PKA implicated in the phosphorylation of Cx43 induced by stimulation with FSH in rat granulosa cells. J Reprod Dev 52:321–328 [DOI] [PubMed] [Google Scholar]
- 53.Cooper CD, Lampe PD2002. Casein kinase 1 regulates connexin-43 gap junction assembly. J Biol Chem 277:44962–44968 [DOI] [PubMed] [Google Scholar]
- 54.Lamm ML, Ekstrom RC, Maizels ET, Rajagopalan RM, Hunzicker-Dunn M1994. The effect of protein kinases on desensitization of the porcine follicular membrane luteinizing hormone/chorionic gonadotropin-sensitive adenylyl cyclase. Endocrinology 134:1745–1754 [DOI] [PubMed] [Google Scholar]
- 55.Sommersberg B, Bulling A, Salzer U, Fröhlich U, Garfield RE, Amsterdam A, Mayerhofer A2000. Gap junction communication and connexin 43 gene expression in a rat granulosa cell line: regulation by follicle-stimulating hormone. Biol Reprod 63:1661–1668 [DOI] [PubMed] [Google Scholar]
- 56.Granot I, Dekel N1994. Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem 269:30502–30509 [PubMed] [Google Scholar]
- 57.Kalma Y, Granot I, Galiani D, Barash A, Dekel N2004. Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation. Endocrinology 145:1617–1624 [DOI] [PubMed] [Google Scholar]
- 58.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF2001. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol 154:815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wayne CM, Fan HY, Cheng X, Richards JS2007. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957 [DOI] [PubMed] [Google Scholar]
- 60.Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M2007. Hormone-induced expression of tumor necrosis factor α-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology 148:6164–6175 [DOI] [PubMed] [Google Scholar]
- 61.Leithe E, Rivedal E2004. Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci 117:1211–1220 [DOI] [PubMed] [Google Scholar]
- 62.Oliva JL, Griner EM, Kazanietz MG2005. PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors 23:245–252 [DOI] [PubMed] [Google Scholar]
- 63.Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A2006. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta 1766:120–139 [DOI] [PubMed] [Google Scholar]
- 64.Darby PJ, Kwan CY, Daniel EE2000. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca(2+) handling. Am J Physiol Lung Cell Mol Physiol 279:L1226–L1235 [DOI] [PubMed]
- 65.Rajendran L, Simons K2005. Lipid rafts and membrane dynamics. J Cell Sci 118:1099–1102 [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Deyoung SM, Zhang M, Dold LH, Saltiel AR2005. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J Biol Chem 280:16125–16134 [DOI] [PubMed] [Google Scholar]
- 67.Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP1999. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 274:12702–12709 [DOI] [PubMed] [Google Scholar]
- 68.Glebov OO, Bright NA, Nichols BJ2006. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol 8:46–54 [DOI] [PubMed] [Google Scholar]
- 69.Sasseville M, Côté N, Vigneault C, Guillemette C, Richard FJ2007. 3′5′-Cyclic adenosine monophosphate-dependent up-regulation of phosphodiesterase type 3A in porcine cumulus cells. Endocrinology 148:1858–1867 [DOI] [PubMed] [Google Scholar]
- 70.Sasseville M, Côté N, Guillemette C, Richard FJ2006. New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev Biol 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petters RM, Wells KD1993. Culture of pig embryos. J Reprod Fertil Suppl 48:61–73 [PubMed] [Google Scholar]
- 72.Bouillon M, El Fakhry Y, Girouard J, Khalil H, Thibodeau J, Mourad W2003. Lipid raft-dependent and -independent signaling through HLA-DR molecules. J Biol Chem 278:7099–7107 [DOI] [PubMed] [Google Scholar]