Abstract
The temperature dependence of the yield of chlorophyll a fluorescence was measured at room temperatures in living algal cells and higher plant chloroplasts. 3-(3′,4′-Dichlorophenyl)-1, 1-dimethylurea was added to the samples during the measurements in order to eliminate the influence of photosynthetic photochemical reactions on the fluorescence yield.
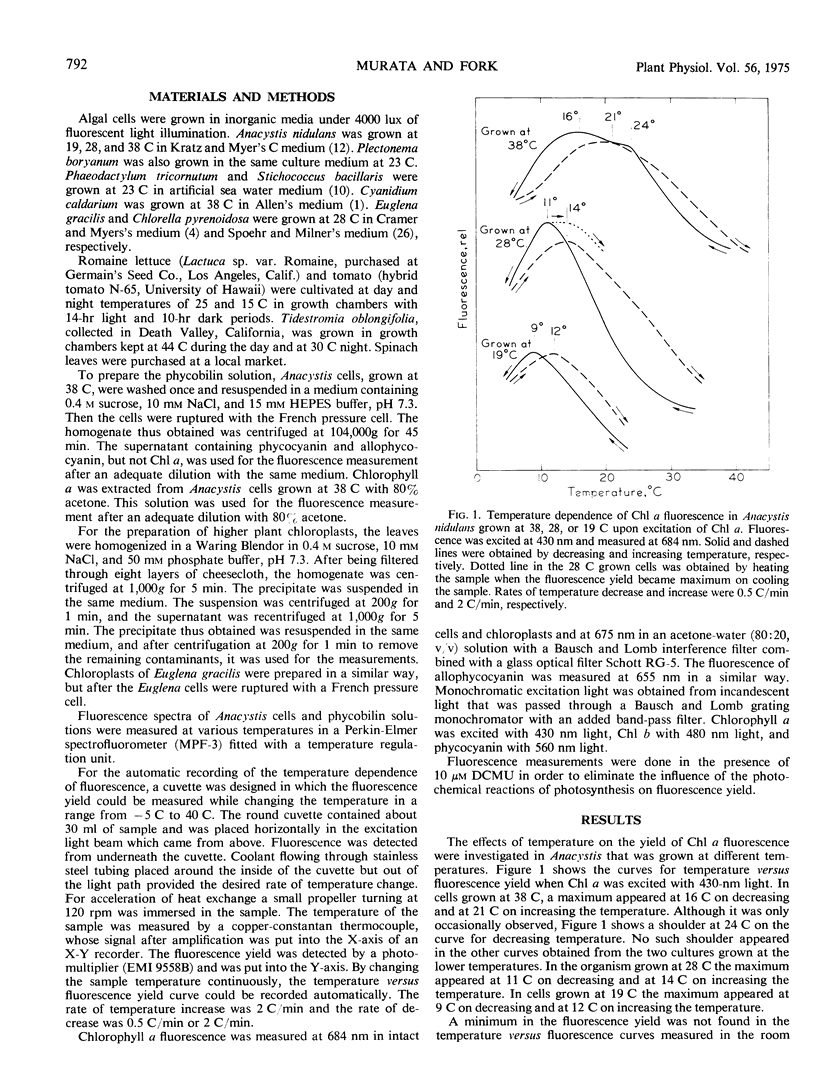
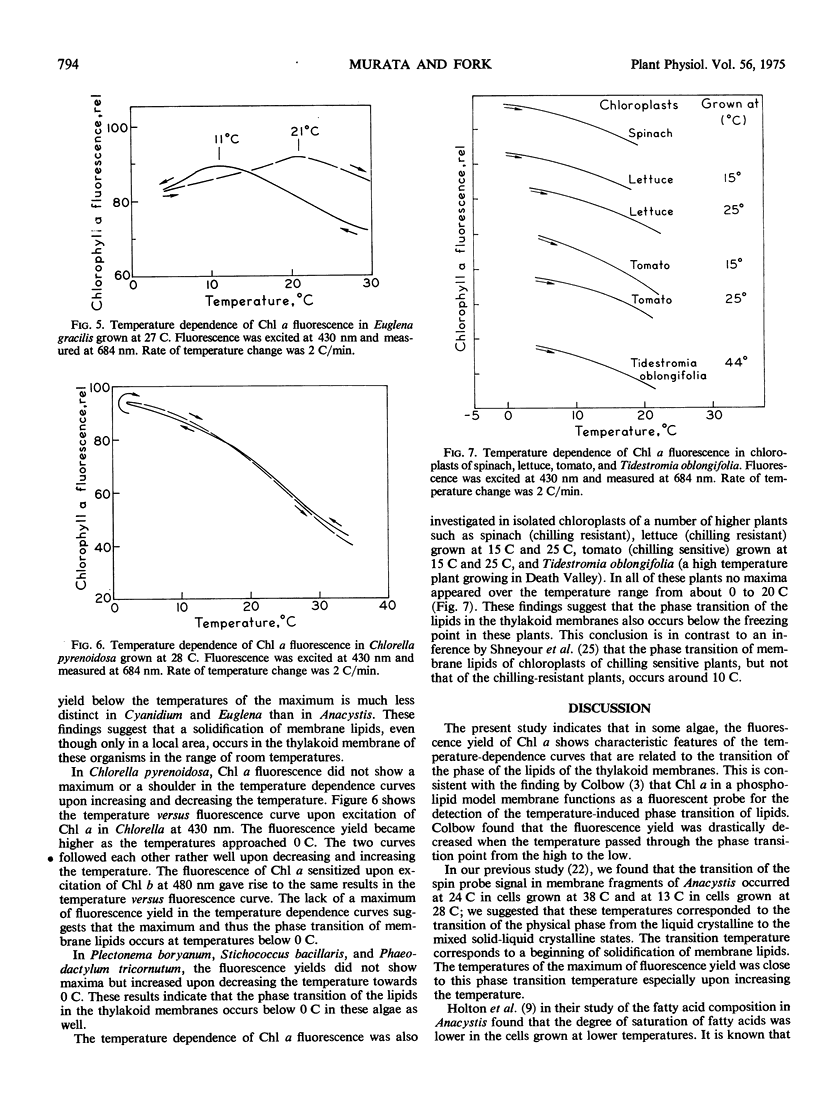
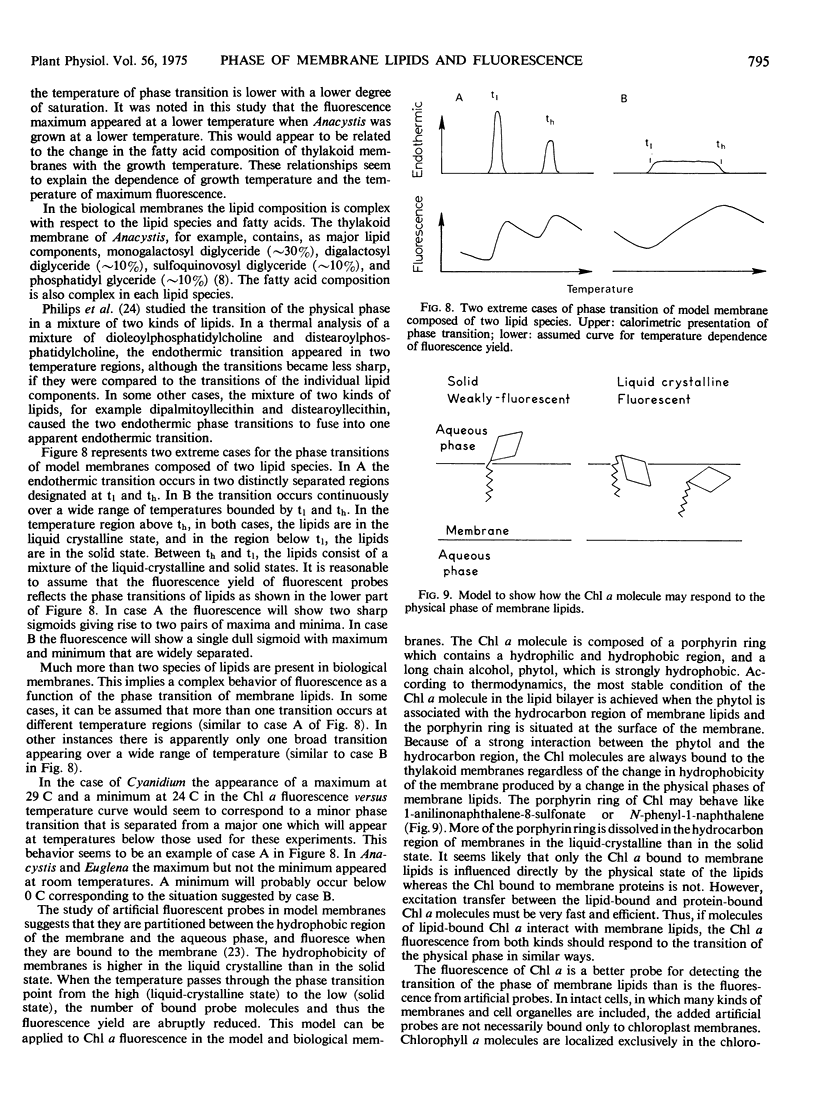
In Anacystis nidulans the maximum in the curve for the fluorescence yield versus temperature occurred near the temperatures at which the transition of physical phase of the membrane lipids changes from the liquid crystalline to the mixed solid-liquid crystalline states. These findings suggest that the occurrence of a fluorescence maximum in the temperature-dependence curve is an indication of the thermal transition of physical phase of membrane lipids. Similar but less distinct maxima were found in Cyanidium caldarium and Euglena gracilis. No maxima were seen in the curves for chlorophyll a fluorescence versus temperature in Chlorella pyrenoidosa, Plectonema boryanum, Stichococcus bacillaris Phaeodactylum tricornutum, or in chloroplasts of the higher plants, spinach, lettuce, tomato, and Tidestromia oblongifolia.
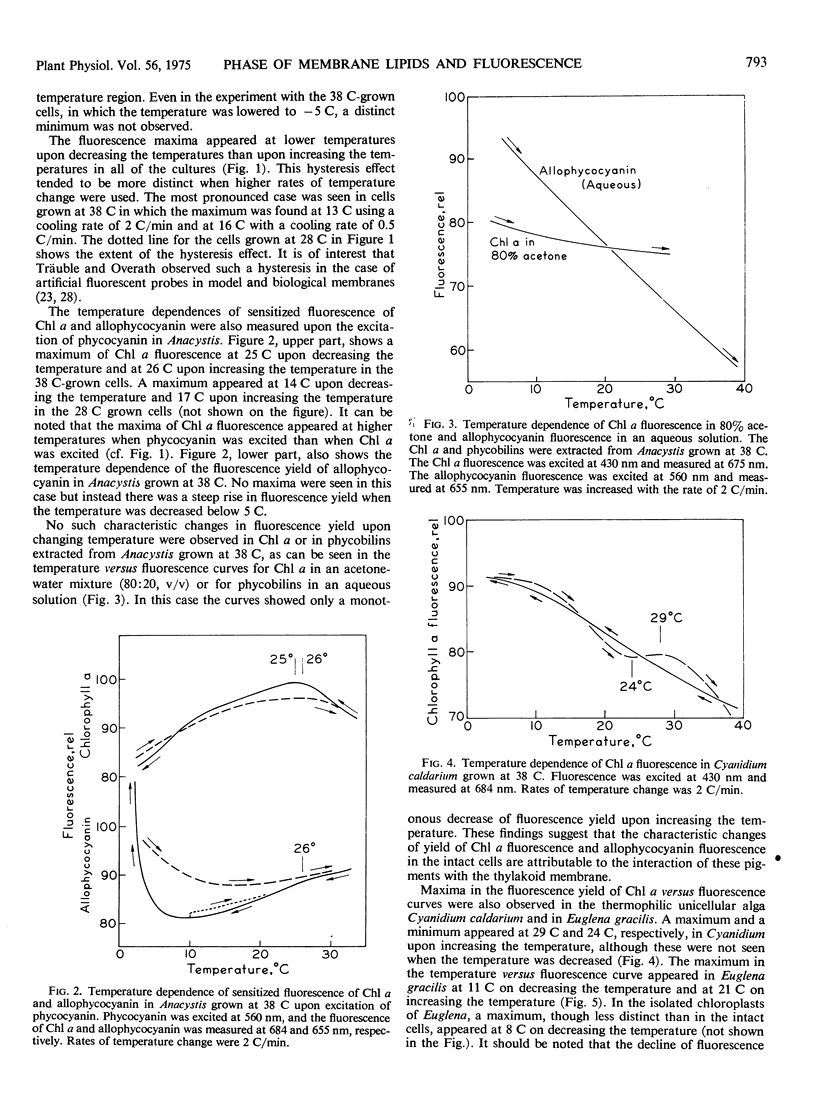
The fluorescence yield of allophycocyanin in Anacystis did not show such a maximum, but did show a steep increase with decreasing temperature below 5 C. the fluorescence yields of chlorophyll a and phycobilins extracted from Anacystis increased monotonously with decreasing temperature.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Colbow K. Chlorophyll in phospholipid vesicles. Biochim Biophys Acta. 1973 Aug 9;318(1):4–9. doi: 10.1016/0005-2736(73)90330-1. [DOI] [PubMed] [Google Scholar]
- Forrest H. S., VAN Baalen C., Myers J. Occurrence of Pteridines in a Blue-Green Alga. Science. 1957 Apr 12;125(3250):699–700. doi: 10.1126/science.125.3250.699. [DOI] [PubMed] [Google Scholar]
- KAUTSKY H., APPEL W., AMANN H. [Chlorophyll fluorescence and carbon assimilation. Part XIII. The fluorescence and the photochemistry of plants]. Biochem Z. 1960;332:277–292. [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. IV. Kinetics of chlorophyll a fluorescence in Porphyra yezoensis. Biochim Biophys Acta. 1970 Jun 30;205(3):379–389. doi: 10.1016/0005-2728(70)90104-0. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. V. Correlation of membrane structure to regulation of excitation transfer between two pigment systems in isolated spinach chloroplasts. Biochim Biophys Acta. 1971 Sep 7;245(2):365–372. doi: 10.1016/0005-2728(71)90155-1. [DOI] [PubMed] [Google Scholar]
- Murata N., Nishimura M., Takamiya A. Fluorescene of chlorophyll in photosynthetic systems. II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1966 May 12;120(1):23–33. doi: 10.1016/0926-6585(66)90273-1. [DOI] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Sugahara K. Control of excitation transfer in photosynthesis. 3. Light-induced decrease of chlorophyll a fluorescence related to photophosphorylation system in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):182–192. doi: 10.1016/0005-2728(69)90046-2. [DOI] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Raison J. K., Smillie R. M. The effect of temperature of the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta. 1973 Jan 18;292(1):152–161. doi: 10.1016/0005-2728(73)90259-4. [DOI] [PubMed] [Google Scholar]
- Spoehr H. A., Milner H. W. THE CHEMICAL COMPOSITION OF CHLORELLA; EFFECT OF ENVIRONMENTAL CONDITIONS. Plant Physiol. 1949 Jan;24(1):120–149. doi: 10.1104/pp.24.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Träuble H. Phasenumwandlungen in Lipiden. Mögliche Schaltprozesse in biologischen Membranen. Naturwissenschaften. 1971 Jun;58(6):277–284. doi: 10.1007/BF00624732. [DOI] [PubMed] [Google Scholar]
- Wraight C. A., Crofts A. R. Energy-dependent quenching of chlorophyll alpha fluorescence in isolated chloroplasts. Eur J Biochem. 1970 Dec;17(2):319–327. doi: 10.1111/j.1432-1033.1970.tb01169.x. [DOI] [PubMed] [Google Scholar]


