Abstract
Pseudokinase TRB3 is an inducible gene whose expression is regulated by stress response and insulin and associated with insulin resistance and metabolic syndrome. In this report, we have investigated the mechanism under which insulin regulates TRB3 gene expression and demonstrated that insulin induces TRB3 expression via C/EBPβ. We found that in Fao hepatoma and 3T3-L1 adipocytes, C/EBPβ expression induced by insulin preceded that of TRB3 and that mutation of the C/EBPβ binding site in TRB3 promoter abolished the responsiveness of the TRB3 gene to insulin. We further showed that ectopic expression of C/EBPβ augmented, whereas knockdown of C/EBPβ reduced, TRB3 expression induced by insulin. In addition, we presented data to show that insulin, through a similar mechanism under which insulin induces TRB3 expression, promotes the expression of genes such as ANAS, ATF3, BIP, and CHOP, which are typical stress-responsive genes. We also examined the impact of C/EBPβ expression on Akt activation and found that inaction of C/EBPβ not only augmented Akt activation but also obliterated the suppression of Akt activation due to prolonged insulin stimulation. We suggest, through induction of C/EBPβ in hepatic cells and adipocytes, that insulin induces the expression of stress-responsive genes, which may represent a novel insulin action.
The expression of metabolic stress responsive genes is associated with insulin action and modulates insulin signaling.
Mammalian tribbles homologs (TRB) are a newly recognized protein family including three isoforms: TRB1, TRB2, and TRB3. Members of TRB protein family are characterized by the presence of a domain homologous to protein kinase in the middle of molecule [kinase homology (KH) domain] (1). The KH domain of TRB lacks a functional ATP binding site and contains the variant amino acid residues that are essential for kinase catalytic activity. Thus, members of the TRB family are referred to as pseudokinase to reflect the view that they are not functional kinases (2). Despite lacking kinase activity, KH domain of TRBs still contain substrate-binding domain through which TRBs interact with different factors. By interacting with various factors, TRBs have been implicated in the regulation of numerous biological processes including cell growth, cell differentiation, and cellular metabolism. Tribbles, the only form of TRB in Drosophila, interacts with String/dCDC25 and Slbo/dC/EBP to regulate cell growth and migration during Drosophila development (3, 4). In mammals, TRBs interact with protein kinases Akt and MAPK, transcription factor CCAAT/enhancer-binding protein beta (C/EBPβ), E3 ligase constitutive photomorphogenic 1 (COP1). By interacting with Akt, TRB3 negatively regulates insulin signaling and, thereby, liver and muscle glucose metabolism (5, 6, 7, 8) and muscle differentiation (9). By interacting with C/EBPβ, both TRB2 and TRB3 are found to modulate adipocyte differentiation (10, 11), and TRB1 is found to modulate gene expression induced by cytokines in macrophages (12). By interacting with E3 ligase COP1, TRB3 has been found to modulate lipid metabolism in adipocytes (13). In addition, TRB3 also interacts with activation transcription factor 4 (ATF4) and E3 ligase SMAD-specific E3 ubiquitin protein ligase 1 (Smurf-1). By suppressing ATF4 transcriptional activity, TRB3 attenuates the stress response (14) and may protect ATF4-induced cell cytotoxicity (15), and by interacting with Smurf-1, TRB3 modulates bone morphogenetic protein (BMP) signaling and osteoblast differentiation (16).
TRB3 is an inducible gene whose expression is modulated by metabolic stresses including endoplasmic reticulum (ER) stress (17, 18) and nutrient stress (e.g. amino acid and glucose deprivation) (19, 20) and insulin (21, 22). In addition, elevated TRB3 expression is associated with insulin resistance (5) and metabolic syndromes (23), implying that regulation of TRB3 gene expression constitutes an important mechanism to modulate TRB3 function.
TRB3 expression induced during the stress response is mediated by ATF4 (17, 18), a basic leucine zipper transcription factor whose expression is specifically augmented by phosphorylation of eukaryotic initiation factor 2α (eIF2α) at Ser51 (24), the convergent point of different stresses including ER stress, amino acid deprivation, viral infection, and heme deficiency (25). The expression of TRB3 during the stress response provides a feedback mechanism to prevent overdose stress response under normal conditions and promotes cell apoptosis under overdosed stress response (26, 27).
The modulation of TRB3 expression by insulin is cell type specific. Insulin promotes TRB3 expression in hepatocytes and adipocytes (21), whereas it reduces TRB3 expression in C2C12 cells (9) and preadipocytes (21). Induction of TRB3 expression by insulin depends on phosphatidylinositol 3-kinase (PI3K) because inhibition (21) or inactivation (28) of PI3K in hepatic cells suppresses TRB3 expression. Strikingly, inactivation of Akt enhances TRB3 expression, suggesting that insulin transmits both positive and negative signals to regulate TRB3 expression (21).
Currently, the mechanism under which insulin promotes TRB3 expression is not well understood. However, negative and positive regulation of TRB3 expression by insulin implies that TRB3 expression induced by insulin may represent a balanced insulin signaling. Thus, further studying of the regulation of TRB3 expression by insulin is warranted. In this report, we have investigated the mechanism under which insulin promotes TRB3 expression and demonstrated that insulin promotes TRB3 expression through induction of C/EBPβ in both Fao hepatoma and 3T3-L1 adipocytes. Moreover, we showed that induction of C/EBPβ by insulin appears to represent a general mechanism by which insulin promotes the expression of stress-responsive genes.
Results
Insulin induces expression of C/EBPβ and TRB3 in Fao cells and 3T3-L1 adipocytes
Both ATF4 and C/EBPβ have been implicated in the regulation of TRB3 gene expression due to the presence of CCAAT enhancer and amino acid-responsive element (AARE) in TRB3 promoter (17, 18, 29). Insulin induces C/EBPβ expression in hepatocytes (30) and 3T3-L1 adipocytes (31) and is able to relieve the suppression of ATF4 expression by dexamethasone in mouse L6 cells (32), raising the possibility that insulin may regulate TRB3 gene expression through either ATF4 or C/EBPβ or both. To get an overall sense about the potential roles of ATF4 and C/EBPβ in the regulation of TRB3 expression by insulin, we examined the expression patterns of ATF4, C/EBPβ, and TRB3 in response to insulin. Shown in Fig. 1A, insulin treatment of Fao cells led to an incremental increase of the expression of both TRB3 (panel I) and C/EBPβ (panel II) proteins but with different time courses. The level of C/EPBβ protein increased within 1 h of insulin stimulation, whereas that of TRB3 protein did not increase until 2 h of insulin stimulation (compare panels I and II as well as right side of the figure), indicating that the expression of C/EBPβ preceded that of TRB3. On the other hand, the level of ATF4 remained relatively constant within 8 h of insulin treatment (panel III). The difference in C/EBPβ and TRB3 expression induced by insulin was not due to sample variation because a comparable amount of β-tubulin was detected in each sample (panel IV). We also examined the expression profiles of ATF4, C/EBPβ, and TRB3 in 3T3-L1 adipocytes. Similarly, insulin promoted the expression of TRB3 and C/EBPβ but not that of ATF4. Again, the expression of C/EBPβ preceded that of TRB3 (Fig. 1B).
Fig. 1.
A and B, Induction of the expression of C/EBPβ and TRB3 in Fao (A) and 3T3-L1 adipocytes (B) by insulin. Overnight serum-starved Fao cells (and 3T3-L1 adipocytes) were treated with 100 nm insulin at indicated times. Total cell lysates were then prepared for Western blot analysis with anti-TRB3 (I), anti-C/EBPβ (II), anti-ATF4 (III), and anti-β-tubulin (IV) antibodies respectively. C, Impact of PI3K inhibitor LY294002 and Akt inhibitor VIII on the expression of C/EBPβ and TRB3 in Fao cells. Fao cells were pretreated with either dimethylsulfoxide (vehicle, Veh), or 2.5 μm Akt inhibitor VIII (A8) or LY294002 (LY) followed by 6 h insulin (100 nm) treatment. Total cell lysates were then prepared for Western blot with anti-TRB3 (panel I), anti C/EBPβ (panel II), anti-phospho-Akt at Ser473 (panel III), and anti-Akt (panel IV) antibodies, respectively. In each figure, the right panel represented the quantitative presentation of the left panel, in which the expression of each protein at time zero was set as 1 after normalization to the amount of β-tubulin in each lane. All of these experiments were repeated at least three times with similar results. Only representative data are shown.
We have previously observed that pretreatment of Fao cells with PI3K inhibitor LY294002 obliterated, whereas that with Akt inhibitor VIII augmented TRB3 expression induced by insulin (21). We therefore tested the impact of these inhibitors on C/EBPβ expression. Shown in Fig. 1C, similar to their impact on TRB3 expression (compare panel I with panel II), Akt inhibitor VIII increased (panel II, compare lanes 1 with 3 or 2 with 4), whereas LY294002 suppressed C/EBPβ expression induced by insulin (panel II, compare lanes 2 and 4). The inhibitors used were effective because they blocked Akt activation (panel III) without impact on the total cellular levels of Akt (panel IV). Taken together, our data demonstrated that the expression of TRB3 is well correlated with that of C/EBPβ.
Induction of TRB3 promoter activity by insulin depends on the C/EBPβ binding site in the TRB3 promoter
Data in Fig. 1 argue that insulin likely regulates TRB3 gene expression through induction of C/EBPβ. To directly address the role of C/EBPβ in TRB3 expression induced by insulin, we generated several TRB3 promoter luciferase constructs including −115-TRB3-Luc, in which the region of +115–+287 is removed; mCAAT TRB3-luc, in which the C/EBP binding site is mutated; and mAARE TRB3-Luc in which AARE is mutated in TRB3 promoter luciferase reporter as previous described (29) (Fig. 2A). Transient transfection and luciferase assay in HepG2 cells revealed that the luciferase activity of TRB3 promoter luciferase reporter encompassing −1000–+287 bp of the TRB3 gene increased by about 2.5-fold after insulin treatment as previously reported (21). Removal of the region from +115–+287 (−155 TRB3-Luc) reduced the basal promoter activity by more than 20-fold and completely abolished the responsiveness of TRB3 promoter to insulin (Fig. 2B), suggesting that the sequence between +115 and +287 of TRB3 gene consists of cis-acting elements that are responsive to insulin stimulation. Mutating either the C/EBPβ binding site or AARE within this region led to a more than 5-fold decrease in basal TRB3 promote activity as previously reported (29). However, only mutating the CCAAT enhancer impaired the responsiveness of TRB3 promoter to insulin, indicating that CCAAT enhancer to which C/EBPβ binds mediates TRB3 expression induced by insulin.
Fig. 2.
A, Schematic presentation of TRB3 promoter TATA box, the CCAAT enhancer, and AARE are indicated. Numbers represent the position of each cis-acting element. Lowercase letters represent the mutated nucleotide in each cis-acting element. B, The impact of CCAAT enhancer and AARE on the responsiveness of TRB3 to insulin. HepG2 cells were transfected with TRB3 promoter luciferase reporter constructs indicated in A. At 24 h after transfection, the transfected cells were serum starved overnight. After 8 h insulin (100 nm) treatment, the total cell lysates were extracted for measuring luciferase activity. The experiments were carried out as triplicate and repeated twice. The final luciferase activity was normalized to β-galactosidase activity cotransfected with reporter plasmids. The error bars represent sd from three experiments. *, P < 0.04, Student’s t test; **, P < 0.05. C, The same as B except the TRB3 promoter activity was presented as fold changes in which the activity of each TRB3 promoter construct was set as 1 under the basal (no insulin treatment) condition. *, P < 0.021, Student’s t test; **, P < 0.012.
To provide the additional evidence that C/EBPβ regulates TRB3 expression, we examined how ectopic expression of C/EBPβ affected TRB3 expression in Fao cells. To this end, Fao cells were transduced with either GFP- (served as a control) or C/EBPβ-expressing pMIGR-1 retroviruses. Then, viral-infected Fao cells were treated with or without 100 nm insulin (3 and 6 h, respectively) for assessment of the role of C/EBPβ in TRB3 expression. Shown in Fig. 3A, insulin induced the expression of both TRB3 and C/EBPβ (compare lanes 1–3) as observed earlier. Ectopic expression of C/EBPβ whose level was about 2- to 3-fold higher than that of endogenous C/EBPβ (compare lanes 1 and 4) augmented TRB3 expression under basal (compare lanes 1 with 4) and insulin-stimulated conditions (compare lanes 2 with 5 or 6). The increase in TRB3 expression by C/EBPβ is specific because no change of the level of β-tubulin in the same samples was observed under any condition (bottom panel).
Fig. 3.
Impact of C/EBPβ expression on TRB3 gene expression A, Ectopic expression of C/EBPβ augments TRB3 expression. Fao cells were transduced with either GFP- or C/EBPβ-expressing pMIGR1 retrovirus, and 48 h later, the cells were treated with insulin at indicated time points and total cell lysates were prepared for Western blot with anti-TRB3 (top), anti-C/EBPβ (middle), and anti-β-tubulin (bottom) antibodies, respectively. B, Transient transfection and luciferase assay to show that C/EBPβ activates TRB3 promoters in HepG2 cells. The experiments were carried out in triplicate and repeated twice. The final luciferase activity was normalized to β-galactosidase activity cotransfected with reporter and effector plasmids. The error bars represent sd from three experiments. *, P < 0.003, Student’s t test; **, P < 0.011. C, Knockdown of C/EBPβ inhibits TRB3 expression induced by insulin Fao cells expressing either control (scrambled) or C/EBPβ shRNA via adenoviral transfer were treated with insulin at indicated times, and total cell lysates were prepared for Western blot with anti-TRB3 (top), anti-C/EBPβ (middle), and anti-β-tubulin (bottom) antibodies, respectively. D, ChIP assay to show that C/EBPβ binds TRB3 promoter Fao cells expressing control (scrambled) or C/EBPβ shRNA were treated with insulin, and ChIP assays were carried out as described in Materials and Methods. E, Transient transfection and luciferase assay to show that C/EBPβ shRNA suppresses TRB3 promoters in HepG2 cells. The experiments were carried out in triplicate and repeated twice. The final luciferase activity was normalized to β-galactosidase activity cotransfected with reporter and effector plasmids. The error bars represent sd from three experiments. *, P < 0.012; **, P < 0.021.
We also examined the impact of forcing expression of C/EBPβ on TRB3 promoter activity in transient transfection and luciferase assay (Fig. 3B). In these experiments, cotransfection of C/EBPβ expression vectors with TRB3 promoter luciferase reporter led to a more than 3-fold increase of TRB3 promoter-driven luciferase activity, which further increased upon insulin stimulation. The activation of TRB3 promoter activity by C/EBPβ requires a functional C/EBPβ binding site in TRB3 promoter because mutating the C/EBPβ binding site in the TRB3 promoter completely abolished the ability of C/EBPβ to activate TRB3 promoter luciferase reporter.
To further test the requirement of C/EBPβ for insulin to induce TRB3 expression, we examined the effect of C/EBPβ inactivation on TRB3 expression. To this end, Fao cells were transduced with either control (scrambled) or C/EBPβ small hairpin RNA (shRNA)-expressing adenoviruses. Then, the expression of TRB3 and C/EBPβ was assessed after these cells were stimulated with insulin for 3 and 6 h, respectively. Presented in Fig. 3C, the expression of both isoforms of C/EBPβ (LAP, liver-activating protein, and LIP, liver inhibitory protein) derived from alternative translation of C/EBPβ mRNA (33) were induced by insulin (panel II, compare lanes 1 and 2) as previously reported (30). Expression of C/EBPβ shRNA led to more than 90% reduction of C/EBPβ expression under basal and insulin-simulated conditions (panel II, compare lanes 1, 2, and 3 with 4, 5, and 6). Correspondingly, TRB3 expression induced by insulin was largely diminished (panel I, compare lane 1, 2, and 3 with 4, 5, and 6). In addition, we also examined the expression of TRB1 and TRB2, the other two members of the TRB protein family. No appreciable change of either protein in insulin-treated Fao cells was observed (panels III and IV). As a control, no changes of β-tubulin were observed (panel V).
To determine whether decreased TRB3 expression under C/EBPβ shRNA expression was correlated with binding of C/EBPβ to TRB3 gene, we carried out chromatin immunoprecipitation (ChIP) assays to assess the occupancy of C/EBPβ on TRB3 promoter. In agreement with the notion that C/EBPβ binds TRB3 promoter (29) and that insulin promotes the expression of C/EBPβ, TRB3 promoter was amplified by PCR from anti-C/EBPβ immunoprecipitates (Fig. 3D, top panel, compare lanes 1 and 2), and insulin stimulation increased the amount of TRB3 promoter in anti-C/EBPβ immunoprecipitates (top panel, compare lanes 2 and 4). In line with the idea that C/EBPβ expression is suppressed by C/EBPβ shRNA, no detectable amount of TRB3 promoter was amplified from anti-C/EBPβ immunoprecipitates of C/EBPβ shRNA-expressing Fao cells under current experimental conditions. The different amount of TRB3 promoter associated with anti-C/EBPβ immunoprecipitates was not due to the genomic DNA variation because comparable amount of TRB3 promoter was detected from the total cell homogenates (Fig. 3D, bottom panel). In addition, we also carried out transient transfection and luciferase assay to determine the impact of C/EBP shRNA on TRB3 promoter activity. As shown in Fig. 3E, C/EBPβ shRNA suppressed the basal TRB3 promoter activity by about 60% and almost completely abolished insulin-induced TRB3 promoter activity. The ability of C/EBPβ shRNA to suppress TRB3 promoter activity depends on the functional C/EBPβ binding site in TRB3 promoter because C/EBPβ shRNA had minimal effect on the activity of TRB3 promoter that harbors a mutated C/EBPβ binding site. Taken all together, our data demonstrate that insulin promotes TRB3 expression via induction of C/EBPβ.
Insulin, in parallel to its ability to induce TRB3 expression, promotes expression of a panel of stress-responsive genes
As demonstrated in preceding experiments, TRB3 expression is induced by insulin. Because TRB3 expression is also induced by the stress response (14, 17, 18), we wondered whether insulin also induces the expression of other genes whose expression is induced by the stress response. To this end, we assessed the expression of asparagine synthetase (ASNS), ATF3, Ig heavy chain binding protein (BIP), and C/EBP homolog protein (CHOP), the typical ER stress-responsive genes, in insulin-treated Fao cells by real time RT-PCR. Presented in Fig. 4A, TRB3 mRNA is increased by about 4- to 6-fold after insulin and thapsigargin treatments, respectively (Fig. 4A, panel v), in agreement with the finding that TRB3 protein expression is induced by insulin and ER stress. In parallel, the expression of ASNS, ATF3, BIP, and CHOP was also elevated after insulin and thapsigargin treatment (panels i–iii and v), which were further verified by conventional RT-PCR (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Fig. 4.
Insulin promotes the expression of stress-responsive genes. A, Real-time RT-PCR analysis of total RNA from Fao cells were treated with either 100 nm insulin (Ins) or 2 μm thapsigargin (Thg) or dimethylsulfoxide (−) for 4 h with specific primers to ASNS, BIP (also known as GRP78 for glucose-responsive protein 78 kDa), CHOP, and TRB3. Total RNAs were prepared for real-time RT-PCR analysis as described in Materials and Methods. B and C, 3T3-L1 adipocytes (B) and 3T3-L1 preadipocytes (C) were treated with either insulin (Ins) or thapsigargin (Thg) or dimethylsulfoxide (−) for 4 h. Total RNAs were then prepared for RT-PCR with specific primers to indicated genes. In all cases, the level of 36B4 served as control, and the level of each mRNA was set as 1 after normalization to the level of 36B4. Each bar represents the mean ± sd (n = 3). *, P < 0.015; **, P < 0.021.
To determine whether insulin induces stress-responsive genes in other types of cells, we examined the expression of above described genes in 3T3-L1 adipocytes and 3T3-L1 preadipocytes, respectively. Insulin treatment augmented the expression of all these genes in 3T3-L1 adipocytes (Fig. 4B) but not in 3T3-L1 preadipocytes (Fig. 4C). The inability of insulin to induce the expression of these genes in 3T3-L1 preadipocytes was not because these genes are silenced because their expression was induced by thapsigargin. Collectively, these data demonstrate that insulin promotes the expression of stress-responsive genes in cell type-specific manner.
Induction of stress-responsive genes by insulin is not due to the stress response
Because insulin promoted the expression of a set of stress-responsive genes, one might wonder whether insulin provokes ER stress, thereby inducing the expression of ER stress-responsive genes. During ER stress, PERK and IRE1 are activated due to sequestering of chaperone proteins. Activation of PERK leads to phosphorylation of eIF2α at Ser51 (34), whereas that of IRE1 promotes splicing of X-box binding protein 1 (Xbp-1), in which a short form of mRNA (XBP-1s) that encodes a transcriptional activator (XBP-1u) is generated from a long form of mRNA (XBP-1l) that encodes a transcription repressor (35). To determine whether insulin stimulates ER stress, we next examined the phosphorylation of eIF2α phosphorylation at Ser51 and splicing of Xbp1, two signature events of ER stress response (36), in insulin-stimulated Fao cells.
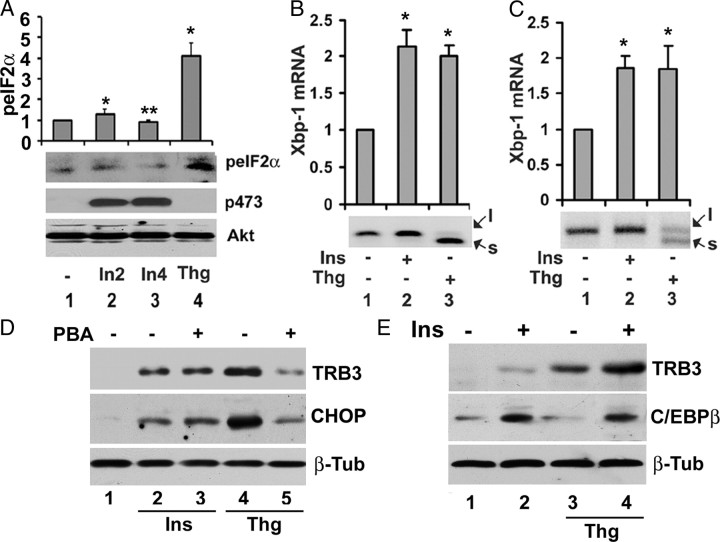
Presented in Fig. 5A, treatment of Fao cells with thapsigargin for 4 h led to a 4-fold increase in eIF2α phosphorylation at Ser51 (compare lanes 1and 4). In marked contrast, treatment of Fao cells with insulin for 2 and 4 h, respectively, had no appreciable effect on eIF2α phosphorylation at Ser51 (compare lanes 1, 2, and 3). Insulin used here was effective because it promoted Akt phosphorylation at Ser473 (middle panel).
Fig. 5.
Insulin does not provoke ER stress response. A, Insulin does not promote phosphorylation of eIF2α at Ser51. Serum-starved Fao cells were treated with either 100 nm insulin for 2 and 4 h (In2 and In4) or 2 μm thapsigargin for 4 h, and total cell lysates were prepared for Western blot with anti-phospho-eIF2a at Ser51, anti-phospho-Akt at Ser473, and anti-Akt antibodies, respectively; top, quantification of eIF2α phosphorylation at Ser 52 in which the level of eIF2α phosphorylation from untreated cells was set as one after normalization to the level of Akt. Each bar represents the mean ± sd (n = 3). *, P < 0.026; **, P < 0.05. B and C, In Fao cells (B) and 3T3-L1 adipocytes (C), insulin does not promote Xbp-1 splicing. Fao cells (or 3T3-L1 adipocytes) were treated with either 100 nm insulin or 2 μm thapsigargin for 4 h, and total RNA was prepared for RT-PCR analysis with specific primers for Xbp-1; top, quantification level of Xpb-1 mRNA (note that the level of Xbp-1 under thapsigargin treatment contains both forms of Xbp-1). Each bar represents the mean ± sd (n = 3). *, P < 0.016. D, Chemical chaperone has no impact on insulin-induced TRB3 and CHOP expression. Fao cells were pretreated with or without 10 mm 4-PBA for 12 h followed by insulin for 6 h. Total cell lysates were then prepared for Western blot with anti-TRB3 (top) anti-CHOP (middle), and β-tubulin (bottom) antibodies, respectively. E, Additive effects of insulin and ER stress on TRB3 and C/EBPβ expression. Fao cells were treated with 100 nm insulin, 2 μm thapsigargin, and insulin plus thapsigargin, respectively. Total cell lysates were prepared after 6 h treatment for Western blot analysis with anti-TRB3 (top), anti-C/EBPβ (middle), and anti-β-tubulin (bottom) antibodies, respectively.
Presented in Fig. 5, B (Fao cells) and C (3T3-L1 adipocytes), both insulin and thapsigargin modestly induces Xbp-1 expression (about 2-fold). Insulin has no impact on splicing of Xbp-1 judged by the fact that no appreciable amount of Xbp-1s was detected in insulin-treated cells (compare lanes 1 and 2). The lack of Xbp-1s in response to insulin was not due to experimental variation because the expression of Xbp-1s was readily detected in thapsigargin-treated Fao cells and 3T3-L1 adipocytes (compare lanes 1 and 3) under our experimental conditions.
To further determine that insulin-promoted expression of stress-responsive genes is not due to ER stress, we examined the impact of chemical chaperone phenylbutyric acid (PBA) on the expression of stress-responsive genes induced by insulin and thapsigargin. As shown in Fig. 5D, pretreatment of Fao cells with PBA strongly suppressed the expression of TRB3 and CHOP induced by thapsigargin (compare lanes 4 and 5), in agreement with the finding that PBA blocked ER stress-induced gene expression (37). However, similar treatment had no impact on that induced by insulin (compare lanes 2 and 3). Taken together, these data demonstrate that the signal transduction pathway under which insulin induces stress-responsive genes is distinguished from ER stress.
Because both insulin and the stress response induce stress response gene expression, it is interesting to know the potential relationship between insulin and the stress response to induce the expression of stress-responsive genes. To this end, Fao cells were treated with insulin, thapsigargin, and the combination of both for 6 h, respectively. Then, the levels of TRB3 and C/EBPβ were assessed by Western blot. Alone, each one of these stimuli promoted the expression of TRB3 (Fig. E, top panel, lanes 1–3). However, no induction of C/EPBβ by thapsigargin was observed (Fig. E, middle panel, lanes 1–3). A combination of insulin with thapsigargin further stimulated expression of TRB3 but not that of C/EBPβ (lanes 4). As a loading control, no changes of β-tubulin were observed. Taken together, these data demonstrated that there is an additive effect of insulin and ER stress to induce TRB3 expression. Furthermore, these data also provide additional evidence that the signal mechanism under which insulin promotes the expression of stress-responsive genes is distinguished from that of ER stress.
Induction of stress-responsive genes by insulin depends on C/EBPβ expression
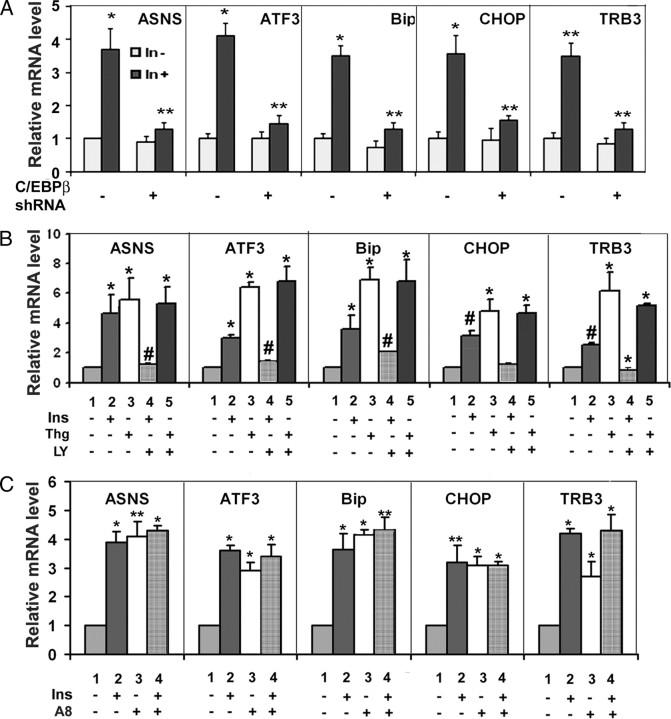
Because C/EBPβ was essential for TRB3 expression induced by insulin (Figs. 3 and 4), we next investigated the role of C/EBPβ in the expression of stress-responsive genes induced by insulin. Real-time RT-PCR analysis of total RNA prepared from control or C/EBPβ shRNA-expressing Fao cells treated with or without insulin revealed that the expression of these genes induced by insulin is largely diminished in C/EBPβ shRNA-expressing Fao cells (Fig. 6A), indicating that C/EBPβ expression is required for insulin to induce expression of stress-responsive genes.
Fig. 6.
Role of C/EBPβ in insulin-promoted expression of stress-responsive genes. A, C/EBPβ-dependent induction of stress-responsive gene expression by insulin control (scrambled) or C/EBPβ shRNA-expressing Fao cells treated with or without 100 nm insulin for 4 h. Total RNAs were prepared for real-time RT-PCR analysis with the level of 36B4 serving as control. For each gene, the level of untreated cells was set as 1 after normalization to 36B4. Each bar represents the mean ± sd (n = 3). *, P < 0.016; **, P < 0.009. B, PI3K-dependent induction of the expression of stress-responsive genes by insulin. Fao cells were pretreated with or without 10 μm PI3K inhibitors LY294002 (LY) for 45 min followed by 4 h 100 nm insulin (Ins) or 2 μm thapsigargin (Thg) treatment. Total RNAs were prepared for real-time RT-PCR analysis. C, Negative role of Akt in the expression of stress-responsive genes by insulin. Fao cells were pretreated with or without 2.5 μm Akt inhibitor VIII (A8) for 45 min followed by 4 h 100 nm insulin (Ins). Total RNAs were prepared for real-time RT-PCR analysis. In all cases, the level of 36B4 serves as control, and the level of each mRNA was set as 1 after normalization to the level of 36B4. Each bar represents the mean ± sd (n = 3). *, P < 0.019; **, P < 0.015.
Role of PI3K and Akt in insulin-induced expression of stress-responsive genes
In Fig. 1C, we showed that PI3K inhibitor LY294002 inhibited and Akt inhibitor VIII increased C/EBPβ expression induced by insulin. We therefore examined the impact of these inhibitors on the expression of ASNS, BIP, CHOP, and ATF3 induced by insulin. Shown in Fig. 6B, pretreatment of Fao cells with LY294002 suppressed the expression of all examined genes induced by insulin (Fig. 6B) (the real-time PCR is verified by conventional RT-PCR, and a similar result was also observed in differentiated 3T3-L1 adipocytes; see supplemental Fig. 1B), suggesting that induction of expression of stress-inducible genes by insulin is PI3K dependent. In additional studies, we also examined the effect of LY294002 on the expression of stress-responsive genes induced by thapsigargin. In contrast to its ability to block the expression of stress-responsive genes induced by insulin, LY294002 had no impact on thapsigargin-induced gene expression. Shown in Fig. 6C, in agreement with the notion that Akt inihbitor VIII augmented C/EBPβ expression, treatment of Fao cells with Akt inhibitor VIII enhanced the expression of stress-inducible genes. Again, the real-time RT-PCR was verified by conventional RT-PCR (supplemental Fig. 2C). Taken together, these data not only demonstrate the correlation of the expression of stress-responsive genes with that of C/EBPβ but also provide the additional evidence that the signaling transduction pathway initiated by insulin to induce the expression of stress-responsive gene is distinguished from that of ER stress.
Knockdown of C/EBPβ augments Akt activation induced by insulin
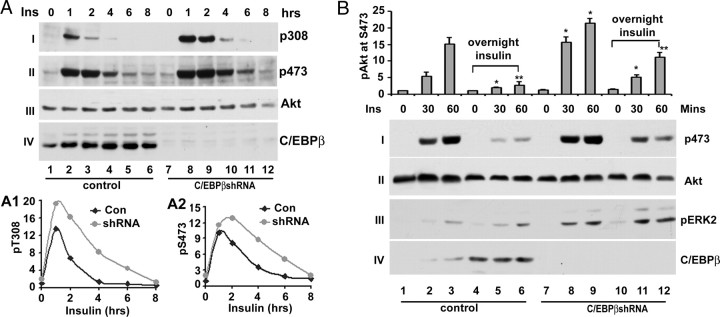
The expression of stress-responsive genes has been shown to modulate insulin signaling (36, 38, 39). Thus, it is interesting to know the effect of expression of stress-responsive genes induced by insulin on insulin signaling. Because C/EBPβ appears as the regulator of the expression of stress-responsive genes induced by insulin, we examined the effect of inactivation of C/EBPβ on Akt activation, the major downstream kinase in insulin signaling, induced by insulin. To this end, Fao cells expressing either control or C/EBPβ shRNA were treated with 100 μm insulin for different periods, and phosphorylation of at Thr308 and Ser473 were examined in Western blot with anti-phospho-Akt-specific antibodies. Shown in Fig. 7A, insulin treatment of Fao cells promoted the phosphorylation of Akt at both Thr308 and Ser473 (panels I and II, respectively). Knockdown of C/EBPβ had no apparent impact on the time course of Akt activation. However, it significantly augmented Akt phosphorylation at both sites (compare lanes 3–4 and 9–10 in panels I and II) without change of total cellular level of Akt (panel III). The C/EBPβ was successfully knocked down as judged by the fact that the expression of C/EBPβ was largely diminished in C/EBPβ shRNA-expressing Fao cells (panel IV).
Fig. 7.
The impact of C/EBPβ expression on Akt activation induced by insulin. A, Knockdown of C/EBPβ augments Akt activation in Fao cells. Control (scrambled) or C/EBPβ-expressing Fao cells were treated with 100 nm insulin for indicated periods. The total cell lysates were prepared for Western blot with anti-phospho-Akt at Thr308 (I) and at Ser473 (II), anti-Akt (III), and anti-C/EBPβ (IV) antibodies, respectively. These experiments were repeated three times with similar results. A1 and A2, Quantitative analysis of Akt phosphorylation at Thr308 and Ser473, respectively. In these analyses, the level of Akt phosphorylation after normalization to total cellular level of Akt is set as 1. B, Knockdown of C/EBPβ obliterates suppression of Akt activation by prolonged insulin stimulation. Control (scrambled) or C/EBPβ-expressing Fao cells were pretreated with 100 nm insulin for overnight followed by further insulin stimulation for the indicated times. The total cell lysates were prepared for Western blot analysis with anti-phospho-Akt at Ser473 (I), anti-Akt (II), anti-pERK2 (III), and anti-C/EBPβ (IV) antibodies, respectively. Top, Quantitative analysis of phosphorylation of Akt at Ser473. In these analyses, the level of Akt phosphorylation after normalization to the total cellular level of Akt is set as 1. Each bar represents the mean ± sd (n = 3). *, P < 0.023; **, P < 0.016.
Prolonged (long-term) insulin treatment or hyperinsulinemia causes insulin resistance (40, 41). We therefore examined whether the expression of stress-responsive genes induced by insulin contributes to this process. To this end, Fao cells expressing control (scrambled) or C/EBPβ shRNA were pretreated with or without insulin overnight. Then, the level of Akt phosphorylation at Ser473 was assessed in Western blot after these cells were further treated with fresh insulin for different time periods. Presented in Fig. 7B, treatment of Fao cells with insulin strongly induced Akt phosphorylation at Ser473 (lanes 1–3, panel i). Overnight treatment of Fao cells with insulin severely impaired the ability of insulin to promote Akt activation (lanes 1–3 and 4–6, panel I), in agreement with the notion that prolonged insulin treatment of hepatic cells promotes insulin resistance (40, 41). Similar to the above observation, knockdown of C/EPBβ enhanced Akt activation by more than 50% (compare lanes 2–3 and 8–9, top panel). Overnight treatment of C/EBPβ shRNA-expressing Fao cells also reduced Akt activation comparing with untreated cells (compare lanes 7–9 and 11–12, panel I). However, in insulin-pretreated C/EBPβ shRNA-expressing Fao cells, the level of Akt phosphorylation at Ser473 induced by insulin was about three times higher than that of control cells (compare lanes 5–6 and 11–12) and reached about 80% that of Akt in control cells (compare lanes 2–3 and 11–12), arguing that induction of C/EBPβ by insulin contributes to the insulin resistance induced by prolonged insulin stimulation. To determine whether the impact of C/EBPβ on insulin is specific on Akt activation, we examined phosphorylation of ERK2. Although knockdown of C/EBPβ leads to an overall enhancement of ERK2 phosphorylation (compare lanes 1–3 and 7–9, panel III), overnight insulin treatment had no adverse effect on insulin-promoted ERK2 activation (compare lanes 1–3 and 4–6 or 7–9 and 10–12, panel III). Similarly, we also observed that C/EBPβ shRNA effectively suppressed C/EBPβ expression (panel IV).
Discussion
In present study, we have first investigated the mechanism under which insulin promoted TRB3 gene expression. Based on the established roles of ATF4 and C/EBPβ in the regulation of TRB3 gene expression (17, 18, 29), we considered the possibility that induction of TRB3 expression by insulin depends on ATF4 and/or C/EBPβ. The findings that that expression of C/EBPβ but not that of ATF4 was induced by insulin in Fao cells and 3T3-L1 adipocytes and the expression of C/EBPβ induced by insulin preceded that of TRB3 argue that it is C/EBPβ that mediates the expression of TRB3 expression induced by insulin. Consistently, we found that 1) mutating CCAAT enhancer abolished TRB3 promoter activity induced by insulin (Fig. 2), 2) overexpression of C/EBPβ enhances TRB3 gene expression induced by insulin (Fig. 3A), and 3) knockdown of C/EBPβ reduced TRB3 gene expression induced by insulin (Fig. 3C).
Based on the notion that TRB3 expression is induced by insulin and metabolic stresses, we investigated the possibility that insulin might also modulate the expression of other stress-responsive genes. Supporting this view, we found that insulin induced the expression of ASNA, BIP, ATF3, and CHOP, all of which are typical stress-responsive genes. The induction of stress-responsive genes by insulin is not because of ER stress because insulin promoted neither phosphorylation of eIF2α at Ser51 nor splicing of Xbp-1, two signature events of ER stress response. In addition, chemical chaperone PBA suppressed the expression of stress-responsive genes induced by thapsigargin, but not that by insulin (Fig. 5D), also support this notion. Of interest, insulin induced the expression of stress-responsive genes in hepatic cells and adipocytes but not in preadipocytes (Fig. 3) and mouse embryonic fibroblasts (MEFs) (data not shown). Because the liver and adipose are the primary insulin-responsive tissues, it is plausible that promotion of the expression of stress-responsive genes by insulin is the additional insulin action in insulin-sensitive tissues.
It is reminded that although insulin induction of the expression of stress-responsive gene, the level of expression of stress-responsive genes induced by insulin is significantly lower than that induced by thapsigargin, which is known to induce the ER stress response and cell apoptosis at the dose (2 μm) used in this study (Fig. 4). The different levels of expression of these stress-responsive genes may explain why thapsigargin but not insulin promotes cell apoptosis (26, 27). At present, we have not investigated the signal event through which insulin promotes the expression of stress-responsive genes in 3T3-L1 adipocytes. However, recent studies showing that insulin-induced BIP and CHOP expression in 3T3-L1 adipocytes independent of phosphorylation of eIF2α at Ser51 (42) suggest that insulin promotes the expression of stress-responsive genes through a similar mechanism in both hepatocytes and adipocytes.
Based on the notion that C/EBPβ regulates TRB3 expression induced by insulin, we also examined the role of C/EBPβ in the expression of stress-responsive genes induced by insulin. Consistent with the view that expression of C/EBPβ is critical for insulin to induce TRB3 expression, inaction of C/EBPβ in Fao cells significantly impaired the expression of stress-responsive genes induced by insulin (Fig. 6A). C/EBPβ has been implicated in the expression of ASNS (43) and possibly other stress-responsive genes (44). Our finding that insulin induces expression of C/EBPβ and, thereby, stress-responsive genes is consistent with this view.
It is noteworthy that the recent studies of TRB3 by Bezy et al. (11) demonstrated that TRB3 interacted with C/EBPβ and inhibited C/EBPβ transcriptional activity during adipocyte differentiation. It is also found that TRB3 expression was decreased during adipocyte differentiation. At present, we have not determined whether TRB3 also inhibits C/EBPβ transcriptional activity in hepatic cells. Should this be the case, it will imply that induction of TRB3 by insulin also provides a feedback mechanism to prevent a higher level of expression of stress-responsive genes by insulin, a similar mechanism that is employed during the stress response (14).
Because the signal events through which insulin induces the expression of stress-responsive genes is distinguished from that of ER stress, we examined the roles of PI3K and Akt in the expression of stress-responsive genes induced by insulin. Similar to the observation that inhibition of PI3K impaired and inhibition of Akt enhances the expression of C/EBPβ and TRB3 induced by insulin, inhibition of PI3K blocked and that of Akt enhanced the expression of stress-responsive genes (Fig. 6). Because PI3K is upstream of Akt, these findings imply that insulin transmits both positive and negative signals to modulate expression of stress-responsive genes. One of the implications of this notion is that unbalanced insulin signaling will abnormally modulate the expression of stress-responsive genes. It is known that the expression of stress response genes is increased during insulin resistance. Based on our finding, it is tempting to think that the increase in the expression of stress-responsive genes during insulin resistance is in part due to impaired Akt activation.
TRB3 expression is associated with impaired Akt activation (5, 7, 8, 45). Thus, we examined the effect of C/EBPβ on insulin signaling in Fao cells. We found that silencing C/EBPβ enhanced phosphorylation of Akt at Thr308 as well as Ser473 (Fig. 7A). Moreover, our data also indicate that the expression of C/EBPβ induced by insulin likely contributes to the insulin resistance induced by prolonged insulin treatment or hyperinsulinemia as knockdown of C/EBPβ could partially reversed the suppression of Akt activation by prolonged insulin treatment (Fig. 7B). Previously, Wang et al. (46) reported that C/EBPβ-null mice exhibited enhanced Akt activation in muscle. Thus, it is possible that enhancement of Akt activation by inaction of C/EBPβ may represent a general action of C/EBPβ. The levels of both C/EBPβ protein and DNA-binding activity increased during insulin resistance (47). Thus, it is tempting to think that the increase of C/EBPβ expression and DNA-binding activity during insulin resistance may constitute a mechanism under which the expression of stress-responsive genes is elevated. This may provide an additional account for the fact that inactivation of C/EBPβ enhances insulin sensitivity (48).
In summary, we have demonstrated that insulin promotes the expression of TRB3 and other stress-responsive genes through C/EBPβ, which may represent a novel action of insulin. Currently, how insulin regulates C/EBPβ is not understood. Further studies are required to clarify this issue.
Materials and Methods
Reagents
Insulin, dexamethasone, 3-isobutyl-1-methylxanthine, 4-PBA, and anti-β-tubulin antibodies are from Sigma Chemical Co. (St. Louis, MO). Thapsigargin, kinase inhibitors, and protease inhibitors are from Calbiochem (La Jolla, CA). Anti-C/EBPβ, anti-C/EBPα, and anti-ATF4 rabbit antibodies are from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Akt, anti-phospho-Akt, anti-phospho-ERK2 at Thr202 /Tyr204, and anti-CHOP1 antibodies are from Cell Signaling Technology (Beverly, MA). The luciferase reagents are from Promega Corp. (Madison, WI). Rabbit anti-TRB1 antibodies were generated with recombinant His-tagged TRB1 (amino acids 1-172) as antigen. Rabbit anti-TRB2 antibodies were generated with recombinant His-tagged TRB2 (full length). The anti-TRB3 rabbit antibody has been described (5).
Plasmids
Mouse and rat C/EBPβ shRNA expression adenovirus is a gift from Dr. J. E. Friedman of the University of Colorado (Denver, CO) (48). The human C/EBPβ shRNA was generated by ourselves with oligonucleotide 5′-GATCCCCATCCATGGAAGTGGCCAACTTCAAGAGAGTTGGCCACTTCCATGGATTTTTTGGAA-3′, in which the targeting sequence of human C/EBPβ is underlined, as reported (49). The scrambled shRNA is from Addgene Co. (Cambridge, MA). To generate C/EBPβ-expressing pMIGR1, rat C/EBPβ cDNA was cloned into BglII and EcoRI of pMIGR1 retroviral vectors. The TRB3 promoter-luciferase reporter and its mutants have been described (21, 29).
Cell cultures
Fao cells were grown in RPMI 1640 supplemented with 10% (vol/vol) fetal bovine serum, 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). HepG2 cells were grown in DMEM supplemented with 10% (vol/vol) bovine serum, 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). For viral infection of Fao cells, 40% confluent Fao cells were incubated with adenovirus for 24 h. HEK293T cells and 3T3-L1 preadipocytes were grown in DMEM (high glucose) supplemented with 10% (vol/vol) bovine serum, 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). The differentiation of 3T3-L1 adipocytes was carried out as previously described (21). For treatment, the cells were serum starved overnight and treated with different reagents including 100 nm insulin or 2 μm thapsigargin as indicated in each figure.
Transient transfection and luciferase assay
The cells in 12-well dishes were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Typically, each transfection includes 100 ng reporter plasmid, 100 ng effector plasmid, and 25 ng pCMV-β galactosidase plasmid. At 24 h after transfection, the cells were serum starved overnight followed by treatment with 100 nm insulin for 6–8 h. Luciferase assays were carried out as previously described (5).
Western blotting
After indicated treatments, cells were washed twice with PBS and extracted with cell lysate buffer [20 mm Tris (pH 7.6), 250 mm NaCl, 0.5 mm EDTA, 0.5 mm dithiothreitol, 10 mm β-glycerophosphate, 10% glycerol, and protease inhibitors]. Equal amounts of protein (20–30 μg) were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). After blocking in 5% dry milk, the membranes were incubated with each primary antibody, followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Bio-Rad). The protein bands were visualized using the ECL detection system (Amersham Biosciences, Arlington Heights, IL). In all cases, blots were not reprobed. Instead, the artifact introduced by stripping the blots was avoided by running parallel blots. For quantification, the densities of bands were measure in Genetool.
ChIP was performed as previously described (29). Briefly, Fao cells were serum deprived for overnight and treated with insulin with or with or insulin for 4 h. Then, cells were harvested and fixed for 10 min with 1% fresh formaldehyde and lysed in SDS lysis buffer. The cells were then sonicated and precleared with protein G agarose/salmon sperm DNA. Immunoprecipitation was performed with anti-C/EBPβ antibodies. PCR was performed with primers corresponding to C/EBPβ forward, 5′-GGGCGTGTGGCTCCTAAG-3′, and C/EBPβ reverse, 5′-GGATCCCCGCCCGGCTGAT-3prime]. PCR was performed with QIAGEN (Valencia, CA) Master Mix (29 cycles) and visualized in 2% agarose gel.
RNA and PT-PCR
The total RNA preparation is with RNeasy kit from QIAGEN. The RT-PCR was carried out with RT-PCR kit from Ambion (Austin, TX) according to the manufacturer’s instructions. The primers correspond to mouse and rat cDNA for RT-PCR for each gene. The PCR program was as follows: 94 C for 30 sec, 54 C for 30 sec, and 72 C. The number of cycles ranged from 20–24 depending on the gene for getting a linear range of amplification. The real-time PCRs were carried with Option 2 Cycler (MJ Research, Waltham, MA) with the reagents from Applied Biosystems (Foster City, CA). Primers for RT-PCR were as follows: ASNS forward, 5′-GTGTCTGAGTGCGATGAAGA-3′, and reverse, 5′-GTAGCGCCTTGTGGTTGTAG-3; BIP (GRP78) forward, 5′-TGCGCAGGAC ATCAAGTC-3′, and reverse, 5′-CCATGTCAATGGTGAGAG-3′; CHOP forward, 5′-TAGTTGGCTGACTGAGGG-3′, and reverse, 5′-GACATGCGGTCGATCAGG-3′; TRB3 forward, 5′-CCATGTCAATGGTGAGAG-3′, and reverse, 5′-TAAGCCCCAGTCGAGTTC-3′; XBP-1 forward, 5′-AACTCCAGCTAGAAAATCAGC-3′, and reverse, 5′-CCATGGGAAGATGTTCTGGG-3′; and GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′, and reverse, 5′-TCCACCACCCTGTTGCGTA-3′. Most RT-PCR were verified by conventional RT-PCR (see supplemental figures).
Acknowledgments
We thank Dr. Jacob Friedman (University of Colorado) for C/EBPβ shRNA.
Footnotes
K.D. is the recipient of Thomas Lee Career Development Award from The American Diabetes Association.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 22, 2009
Abbreviations: AARE, Amino acid-responsive element; ASNS, asparagine synthetase; ATF, activation transcription factor; BIP, Ig heavy chain binding protein; C/EBP, CCAAT enhancer-binding protein; ChIP, chromatin immunoprecipitation; CHOP, C/EBP homolog protein; eIF2α, eukaryotic initiation factor 2α; ER, endoplasmic reticulum; KH, kinase homology; PBA, phenylbutyric acid; PI3K, phosphatidylinositol 3-kinase; shRNA, small hairpin RNA; TRB, tribbles homolog; Xbp-1, X-box binding protein 1.
References
- 1.Hegedus Z, Czibula A, Kiss-Toth E2007. Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell Signal 19:238–250 [DOI] [PubMed] [Google Scholar]
- 2.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR2006. Emerging roles of pseudokinases. Trends Cell Biol 16:443–452 [DOI] [PubMed] [Google Scholar]
- 3.Mata J, Curado S, Ephrussi A, Rorth P2000. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101:511–522 [DOI] [PubMed] [Google Scholar]
- 4.Rorth P, Szabo K, Texido G2000. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol Cell 6:23–30 [DOI] [PubMed] [Google Scholar]
- 5.Du K, Herzig S, Kulkarni RN, Montminy M2003. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300:1574–1577 [DOI] [PubMed] [Google Scholar]
- 6.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M2004. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat Med 10:530–534 [DOI] [PubMed] [Google Scholar]
- 7.Matsushima R, Harada N, Webster NJ, Tsutsumi YM, Nakaya Y2006. Effect of TRB3 on insulin and nutrient-stimulated hepatic p70 S6 kinase activity. J Biol Chem 281:29719–29729 [DOI] [PubMed] [Google Scholar]
- 8.Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ2006. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol 26:8217–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S, Du K2007. TRB3 modulates C2C12 differentiation by interfering with Akt activation. Biochem Biophys Res Commun 353:933–938 [DOI] [PubMed] [Google Scholar]
- 10.Naiki T, Saijou E, Miyaoka Y, Sekine K, Miyajima A2007. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPβ. J Biol Chem 282:24075–24082 [DOI] [PubMed] [Google Scholar]
- 11.Bezy O, Vernochet C, Gesta S, Farmer SR, Kahn CR2007. TRB3 blocks adipocyte differentiation through the inhibition of C/EBPβ transcriptional activity. Mol Cell Biol 27:6818–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto M, Uematsu S, Okamoto T, Matsuura Y, Sato S, Kumar H, Satoh T, Saitoh T, Takeda K, Ishii KJ, Takeuchi O, Kawai T, Akira S2007. Enhanced TLR-mediated NF-IL6 dependent gene expression by Trib1 deficiency. J Exp Med 204:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, Newgard C, Nelson M, Evans RM, Yates J, Montminy M2006. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 312:1763–1766 [DOI] [PubMed] [Google Scholar]
- 14.Jousse C, Deval C, Maurin AC, Parry L, Cherasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P2007. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 282:15851–15861 [DOI] [PubMed] [Google Scholar]
- 15.Ord D, Meerits K, Ord T2007. TRB3 protects cells against the growth inhibitory and cytotoxic effect of ATF4. Exp Cell Res 313:3556–3567 [DOI] [PubMed] [Google Scholar]
- 16.Chan MC, Nguyen PH, Davis BN, Ohoka N, Hayashi H, Du K, Lagna G, Hata A2007. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol 27:5776–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H2005. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ord D, Ord T2005. Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem Biophys Res Commun 330:210–218 [DOI] [PubMed] [Google Scholar]
- 19.Schwarzer R, Dames S, Tondera D, Klippel A, Kaufmann J2006. TRB3 is a PI 3-kinase dependent indicator for nutrient starvation. Cell Signal 18:899–909 [DOI] [PubMed] [Google Scholar]
- 20.Corcoran CA, Luo X, He Q, Jiang C, Huang Y, Sheikh MS2005. Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol Ther 4:1063–1067 [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Kato S, Du K2008. PI3K activates negative and positive signals to regulate TRB3 expression in hepatic cells. Exp Cell Res 314:1566–1574 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Han S, Kitamura T, Accili D2006. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest 116:2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi XP, Tan HW, Xing SS, Wang ZH, Tang MX, Zhang Y, Zhang W2008. Overexpression of TRB3 gene in adipose tissue of rats with high fructose-induced metabolic syndrome. Endocr J 55:747–752 [DOI] [PubMed] [Google Scholar]
- 24.Proud CG2005. eIF2 and the control of cell physiology. Semin Cell Dev Biol 16:3–12 [DOI] [PubMed] [Google Scholar]
- 25.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633 [DOI] [PubMed] [Google Scholar]
- 26.Lin KR, Lee SF, Hung CM, Li CL, Yang-Yen HF, Yen JJ2007. Survival factor withdrawal-induced apoptosis of TF-1 cells involves a TRB2-Mcl-1 axis-dependent pathway. J Biol Chem 282:21962–21972 [DOI] [PubMed] [Google Scholar]
- 27.Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, Piris MA, Iovanna JL, Guzman M, Velasco G2006. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 9:301–312 [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Aleman JO, Ueki K, Luo J, Asano T, Kaneto H, Stephanopoulos G, Cantley LC, Kahn CR2007. The p85α regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol Cell Biol 27:2830–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selim E, Frkanec JT, Cunard R2007. Fibrates upregulate TRB3 in lymphocytes independent of PPARα by augmenting CCAAT/enhancer-binding protein beta (C/EBPβ) expression. Mol Immunol 44:1218–1229 [DOI] [PubMed] [Google Scholar]
- 30.Kato S, Ding J, Pisck E, Jhala US, Du K2008. COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. J Biol Chem 283:35464–35473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDougald OA, Cornelius P, Liu R, Lane MD1995. Insulin regulates transcription of the CCAAT/enhancer binding protein (C/EBP) α, β, and δ genes in fully-differentiated 3T3-L1 adipocytes. J Biol Chem 270:647–654 [DOI] [PubMed] [Google Scholar]
- 32.Adams CM2007. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 282:16744–16753 [DOI] [PubMed] [Google Scholar]
- 33.Xiong W, Hsieh CC, Kurtz AJ, Rabek JP, Papaconstantinou J2001. Regulation of CCAAT/enhancer-binding protein-β isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucleic Acids Res 29:3087–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimball SR1999. Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol 31:25–29 [DOI] [PubMed] [Google Scholar]
- 35.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida H2007. ER stress and diseases. FEBS J 274:630–658 [DOI] [PubMed] [Google Scholar]
- 37.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregor MG, Hotamisligil GS2007. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48:1905–1914 [DOI] [PubMed] [Google Scholar]
- 39.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D2008. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab 7:520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge X, Yu Q, Qi W, Shi X, Zhai Q2008. Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic Res 42:582–591 [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Veledo S, Nieto-Vazquez I, de Castro J, Ramos MP, Bruderlein S, Moller P, Lorenzo M2008. Hyperinsulinemia induces insulin resistance on glucose and lipid metabolism in a human adipocytic cell line: paracrine interaction with myocytes. J Clin Endocrinol Metab 93:2866–2876 [DOI] [PubMed] [Google Scholar]
- 42.Miyata Y, Fukuhara A, Matsuda M, Komuro R, Shimomura I2008. Insulin induces chaperone and CHOP gene expressions in adipocytes. Biochem Biophys Res Commun 365:826–832 [DOI] [PubMed] [Google Scholar]
- 43.Siu F, Chen C, Zhong C, Kilberg MS2001. CCAAT/enhancer-binding protein-β is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem 276:48100–48107 [DOI] [PubMed] [Google Scholar]
- 44.Thiaville MM, Dudenhausen EE, Zhong C, Pan YX, Kilberg MS2008. Deprivation of protein or amino acid induces C/EBPβ synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription. Biochem J 410:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Simmen FA, Mehendale HM, Ronis MJ, Badger TM2006. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane. Role of TRB3 in inhibition of Akt/protein kinase B activation. J Biol Chem 281:11126–11134 [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Shao J, Muhlenkamp P, Liu S, Klepcyk P, Ren J, Friedman JE2000. Increased insulin receptor substrate-1 and enhanced skeletal muscle insulin sensitivity in mice lacking CCAAT/enhancer-binding protein β. J Biol Chem 275:14173–14181 [DOI] [PubMed] [Google Scholar]
- 47.Arizmendi C, Liu S, Croniger C, Poli V, Friedman JE1999. The transcription factor CCAAT/enhancer-binding protein β regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem 274:13033–13040 [DOI] [PubMed] [Google Scholar]
- 48.Schroeder-Gloeckler JM, Rahman SM, Janssen RC, Qiao L, Shao J, Roper M, Fischer SJ, Lowe E, Orlicky DJ, McManaman JL, Palmer C, Gitomer WL, Huang W, O'Doherty RM, Becker TC, Klemm DJ, Jensen DR, Pulawa LK, Eckel RH, Friedman JE2007. CCAAT/enhancer-binding protein β deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J Biol Chem 282:15717–15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomis RR, Alarcon C, Nadal C, Van Poznak C, Massague J2006. C/EBPβ at the core of the TGFβ cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell 10:203–214 [DOI] [PubMed] [Google Scholar]