Abstract
G protein-coupled receptors (GPCRs) are involved in the pathophysiology of a wide range of diseases and constitute an attractive therapeutic target. In the thyroid gland, TSH receptor (TSHR), a member of the GPCR family, is a major regulator of thyroid differentiation and function. Alterations in TSHR activity are often involved in the development of pathologies such as thyroid cancer and thyroid enlargement (goiter). Here we show that DREAM (downstream regulatory element antagonist modulator) modulates TSHR activity through a direct protein-protein interaction that promotes coupling between the receptor and Gαs. In transgenic mice, DREAM overexpression provokes a marked enlargement of the thyroid gland. Increased levels of DREAM protein were observed in human multinodular goiters, suggesting a novel etiopathogenic mechanism in nodular development in humans. Taken together, these findings identify a mechanism for the control of TSHR activity and provide a new approach for the study and treatment of thyroid pathologies associated with impaired TSHR function.
DREAM regulates TSHR activity and increased DREAM levels are associated with thyroid pathologies.
G protein-coupled receptors (GPCRs) are membrane proteins located on the cell surface from where they control cell physiology through the activation of various signaling cascades. Impaired function of specific GPCRs is strongly implicated in the pathophysiology of a wide range of conditions, including among others, pain, inflammation, obesity, hypertension, cardiovascular disease, or generalized anxiety disorder (1, 2, 3, 4, 5, 6, 7). Because of this and their accessibility to drugs, GPCRs are indisputably an attractive class of therapeutic targets. In the thyroid gland, TSH is the major regulator of thyroid growth and differentiation. TSH binds to the TSH receptor (TSHR) and induces coupling to G proteins (8, 9), of which heterotrimeric Gs protein mediates most of the TSHR signaling and activates a cAMP cascade that controls the expression of key genes in thyroid function (10).
TSHR is associated with many thyroid diseases (11). Inactivating mutations of TSHR are responsible for asymptomatic resistance to TSH and overt congenital hypothyroidism. Activating mutations of TSHR are found in autonomously functioning thyroid nodules, thyroid hyperplasia, and congenital hyperthyroidism (12, 13, 14, 15). Development of TSHR autoantibodies that bind to and stimulate TSHR activity causes Graves’ disease, which is one of the most prevalent human autoimmune diseases (16, 17). Furthermore, it has been shown that TSHR maintains a relatively high activity in the absence of TSH (18, 19), yet the molecular mechanism and the physiological relevance for this are unknown (20).
DREAM (downstream regulatory element antagonist modulator) is a neuronal calcium sensor preferentially expressed in the central nervous system and the thyroid gland (21). DREAM, also named KChIP-3 (K+ channel interacting protein-3), belongs to a group of structurally and functionally related proteins (KChIP-1 to -4) that interact with Kv4 potassium channels to regulate their membrane expression and gating (21, 22, 23). In the nucleus, DREAM is a calcium-, cAMP-, and phosphatidylinositol 3-kinase-sensitive transcriptional repressor that regulates transcription by binding to specific sites in target genes (21, 24, 25, 26, 27) or through the interaction with other nucleoproteins (28, 29). In neurons, DREAM represses basal expression of the prodynorphin gene, and DREAM knockout mice display a hypoalgesic phenotype, suggesting a critical role for DREAM in pain modulation (30). In thyroid follicular cells, DREAM modulates the transcriptional activity of thyroid transcription factor-1 (TTF-1) and represses thyroglobulin (Tg) gene expression (29).
In the present work, we show that DREAM functions as an intracellular TSHR ligand that controls receptor protein levels and promotes its coupling to Gαs protein. Analysis of transgenic mice and human patients support an etiopathological role of elevated levels of DREAM in human multinodular goiters.
Results
DREAM regulates thyroid growth and differentiation
DREAM regulates Tg expression in follicular cells (29). To further investigate DREAM function in the thyroid gland, we generated two independent lines of transgenic mice, LeuBTA7 and LeuBTA15, which express different levels of a dominant active DREAM mutant insensitive to both calcium and cAMP/protein kinase A (21, 25) (Fig. 1A). Histological analysis of 2- to 3-month-old mice revealed a nonnodular, diffuse, and homogeneous hyperplasia of the gland with small follicles lined by columnar epithelium in both transgenic lines (Fig. 1, B–E). Quantitative RT-PCR analysis of the glands confirmed this observation and showed an increase in the proliferation marker proliferating cell nuclear antigen (Fig. 1F). Analysis of thyroid glands from 9- to 12-month-old mice showed a marked enlargement of the transgenic gland [12.4 ± 2.1 mg (LeuBTA7) and 13.84 ± 1.6 mg (LeuBTA15)] compared with wild-type (4.4 ± 0.3 mg) that could be either diffuse, resembling a colloid goiter, or nodular, resembling human multinodular goiters (Fig. 2A). In the colloid goiter phenotype, many follicles showed a large lumen, irregular shape, colloid accumulation, and low cuboidal or flattened epithelium, which is a sign of hypoactivity (Fig. 2, compare B and E with C and F). Heterogeneity prevailed, and the glands showed also hyperplastic areas with follicles surrounded by a tall, active epithelium forming papillary infoldings protruding into the lumen (Fig. 2, D and G) or with small follicles lined with a tall epithelium resembling follicular adenomas (Fig. 2H), nodules (Fig. 2I), and highly hyperproliferative foci without follicular structure. Nodularity is the typical evolution of colloid goiters and was observed also in old mice. Some heterogeneity, however, was found between animals and within each thyroid gland, which is a common finding in thyroid pathologies and agrees with the variable phenotypes of the thyroid in response to the same stimulus (15, 31).
Fig. 1.
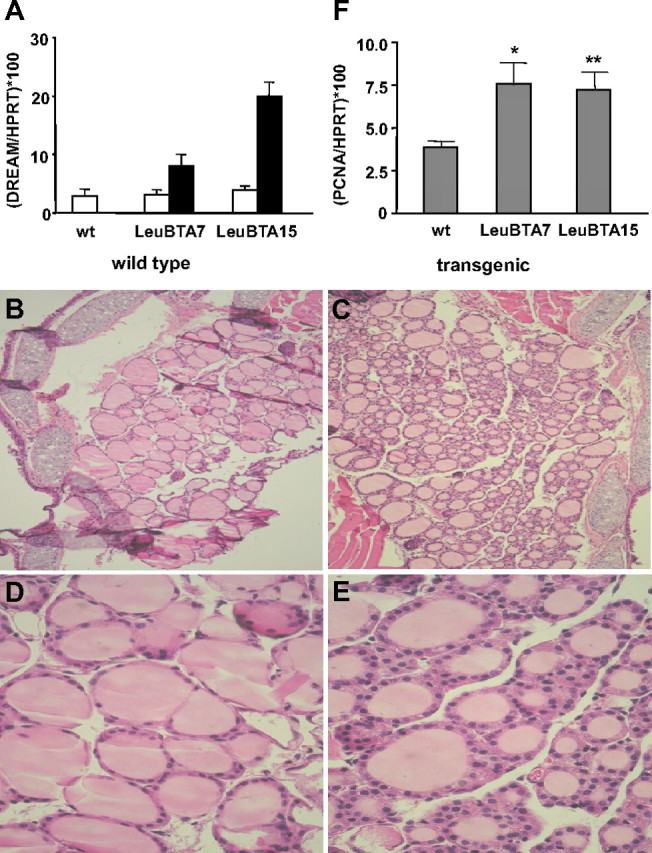
Early morphological and proliferative changes in young transgenic mice. A, Quantitative real-time PCR showing HPRT mRNA-normalized expression levels (mean ± sem) of endogenous (white bars) and mutant DREAM (black bars) in the thyroid of 2- to 3-month-old wild-type (wt) and LeuBTA7 and LeuBTA15 DREAM transgenic mice. B–E, Hematoxylin and eosin staining of paraffin sections from 2- to 3-month-old wild-type (B–D) and transgenic (C–E) mice. A representative example of the hyperproliferative phenotype observed in both transgenic lines is shown. Magnification, ×100 (B and C) and ×600 (D and E). F, Quantitative real-time PCR showing HPRT mRNA-normalized expression level (mean ± sem) of proliferating cell nuclear antigen in wild-type (n = 9), LeuBTA7 (n = 8), and LeuBTA15 (n = 11) mice. Significant differences from wild-type mice by unpaired Student’s t test are indicated: *, P = 0.0252; **, P = 0.0027.
Fig. 2.
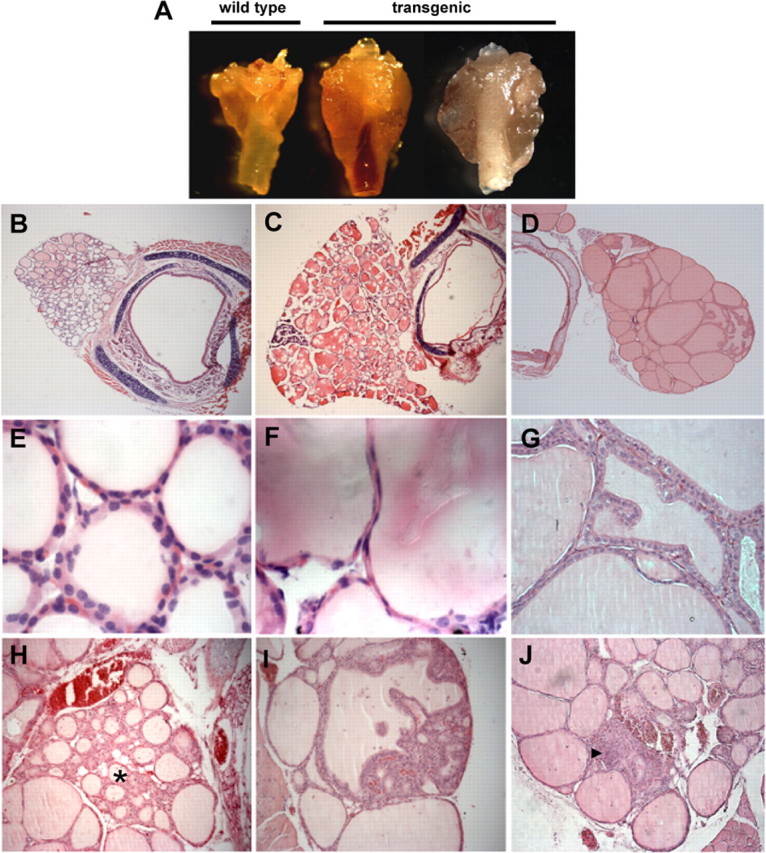
Thyroid histopathology in 9- to 12-month-old mice. A, Gross appearance of thyroid glands from wild-type (left) and transgenic mice with diffuse goiter (middle) or multinodular goiter (right). B–J, Hematoxylin and eosin staining of paraffin sections from wild-type (B and E) and transgenic (C, D, F–J) mice. C and F, Transgenic glands showing follicles with large lumens surrounded by flat epithelium. D and G, Hyperplastic areas with follicles surrounded by tall epithelium forming papillary infoldings protruding into the lumen. H, Adenomatous hyperproliferation (asterisk). Nodules with disorganized follicular structure (I) and nonfollicular hyperproliferative foci (arrowhead in J). Similar heterogeneity and pathological phenotypes were observed in both transgenic line LeuBTA7 (D, G, H, and J) and LeuBTA15 (A, C, F, and I). Magnification, ×50 (B–D), ×1000 (E and F), ×400 (G), and ×200 (H, I, and J).
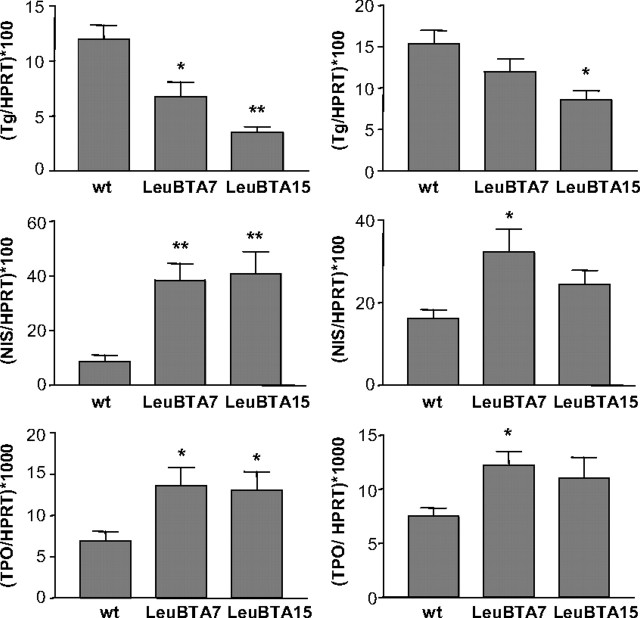
Analysis of several differentiation markers in 2- to 3-month-old mice showed a significant down-regulation of Tg mRNA and up-regulation of genes involved in thyroid hormone synthesis, such as the sodium-iodide symporter (NIS), and thyroid peroxidase (TPO) (Fig. 3). Similar results, although less pronounced, were observed in older mice (Fig. 3).
Fig. 3.
Thyroid differentiation markers in DREAM transgenic mice. Tg, NIS, and TPO mRNA levels were quantified by real-time quantitative PCR. Left panels correspond to young mice (2–3 months) from wild-type (n = 7), LeuBTA7 (n = 10), and LeuBTA15 (n = 10) transgenic lines. Right panels correspond to old mice (12 months) from wild-type (n = 6), LeuBTA7 (n = 6), and LeuBTA15 (n = 6) transgenic lines. Values are normalized by the content of HPRT mRNA. Data are mean ± sem. Significant differences from wild-type mice by unpaired Student’s t test are indicated. *, P < 0.05; **, P < 0.01.
As a result, the euthyroid state was maintained and no significant changes in T3, T4, and TSH serum levels were observed at any age in DREAM transgenic mice (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Increased cAMP signaling in the thyroid gland of DREAM transgenic mice
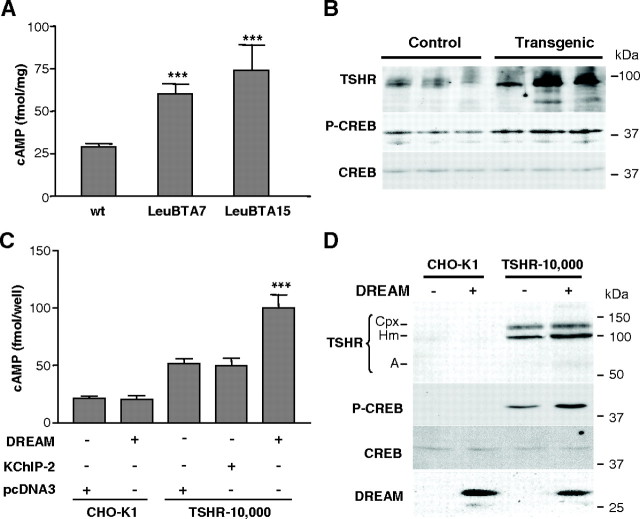
To investigate the mechanism underlying the observed phenotype, we focused on modifications in the cAMP cascade, which is known to be involved in the pathogenesis of several thyroid diseases. We found that transgenic thyroid glands contained significantly higher levels of TSHR and cAMP than wild-type glands (Fig. 4, A and B). As a consequence, levels of phosphorylated cAMP response element-binding protein (CREB) were also increased in transgenic glands, without change in total CREB protein (Fig. 4B and supplemental Fig. 2). Noteworthy, increased TSHR protein did not correlate with increased TSHR mRNA (supplemental Fig. 3), indicating that the increase in TSHR levels is not due to transcriptional activation of the TSHR gene.
Fig. 4.
DREAM overexpression activates the cAMP signaling pathway through TSHR. A, The levels of cAMP in thyroid glands from 9-month-old wild-type (Wt) (n = 6) or DREAM transgenic LeuBTA7 (n = 5) and LeuBTA15 (n = 4) mice were determined. Data are mean ± sem. Significant differences from wild-type mice by unpaired Student’s t test are indicated: ***, P < 0.001. B, Representative Western blot using whole-cell extracts from 9-month-old wild-type and transgenic thyroid glands (line LeuBTA15 is shown). Blots were incubated with specific antibodies against TSHR, phospho-CREB (P-CREB), and total CREB. C, Analysis of intracellular cAMP levels after transfection with DREAM, KChIP-2, or pcDNA3 in CHO-K1 or TSHR-10,000 cells as indicated. Significant differences from control (pcDNA3 in TSHR-10,000 cells) by unpaired Student’s t test are indicated: ***, P < 0.001. D, Western blot using whole-cell extracts from CHO-K1 or TSHR-10,000 cells after transfection with DREAM. Blots were incubated with specific antibodies against DREAM, TSHR, phospho-CREB, and total CREB. TSHR immunoreactive bands correspond to TSHR holoreceptor-bearing complex carbohydrate (Cpx) or high-mannose carbohydrate (Hm) and the A subunit (A).
To analyze the relation between DREAM overexpression and increased TSHR/cAMP signaling, we generated stable clones of thyroid PC Cl3 cells that overexpress wild-type DREAM, mutant DREAM insensitive to calcium (EF-mDREAM), or mutant DREAM insensitive to cAMP (Leu-mDREAM) (21, 28). These mutations correspond to those expressed simultaneously in DREAM transgenic mice. Unexpectedly, overexpression of wild-type DREAM induced an increase in intracellular TSHR, cAMP, and phospho-CREB similar to that observed with either DREAM mutant (supplemental Fig. 3, B–D). These results indicate that overexpression of DREAM protein, regardless of whether it is mutated or not, is responsible for the induction of the cAMP cascade observed in transgenic mice. The fact that DREAM mutations are not needed for TSHR/cAMP activation suggests a role for endogenous DREAM in tonic activation of TSHR in basal conditions.
DREAM-induced activation of the cAMP signaling pathway is mediated by TSHR
To link the effect of DREAM on cAMP signaling to TSHR, we transfected DREAM into TSHR-10,000 cells, a CHO-derived cell line, that stably overexpress TSHR (19) or into the original CHO-K1 cells. In basal conditions, TSHR-10,000 cells contain higher levels of cAMP and phospho-CREB (Fig. 4, C and D) than CHO-K1 cells due to the constitutive activity of TSHR in the absence of TSH (19). Expression of DREAM resulted in a significant increase in cAMP and phospho-CREB levels in TSHR-10,000 cells and no changes in CHO-K1 cells (Fig. 4, C and D). The effects of DREAM on TSHR and cAMP signaling were not reproduced by expression of KChIP-2 (Fig. 4C and supplemental Fig. 4), a closely related member of the DREAM/KChIP family that is expressed in the thyroid gland (29). These data indicate that activation of cAMP signaling is specific of DREAM and is mediated by TSHR.
DREAM interacts with TSHR
DREAM has been reported to interact with proteins in the nucleus and in the cytoplasm (32). Therefore, we next investigated whether DREAM is able to interact with TSHR. Using thyroid glands from wild-type and transgenic mice, we show that TSHR coimmunoprecipitates with DREAM (Fig. 5A). The interaction was confirmed using cell extracts from TSHR-10,000 cells transfected with DREAM-hemaglutinin (HA) (Fig. 5B).
Fig. 5.
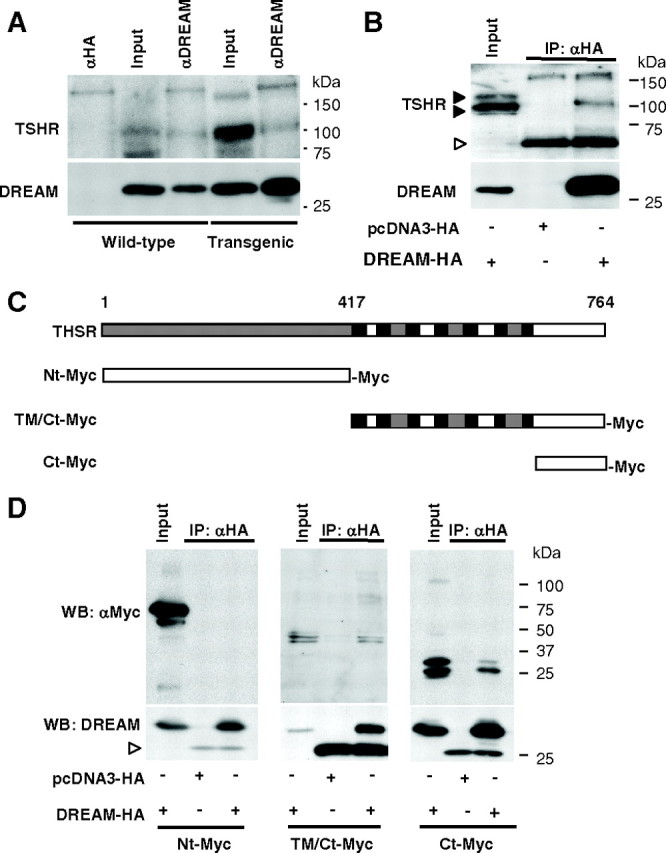
DREAM interacts with the Ct region of TSHR. A, Coimmunoprecipitation of DREAM and TSHR with a monoclonal antibody for DREAM in whole-cell extracts of thyroid glands from wild-type and transgenic mice (LeuBTA15). A nonrelated antibody (anti-HA) was used as a negative control. TSHR and DREAM were detected by Western blot. Note that transgenic mice contain higher levels of TSHR and total DREAM proteins, as expected. Equal amounts of total protein extracts were used in both cases. B, Coimmunoprecipitation of DREAM-HA and TSHR with an anti-HA antibody in whole-cell extracts from TSHR-10,000 cells transfected with the indicated plasmids. TSHR and DREAM were detected by Western blot. White arrowhead indicates Ig chain. C, Schematic representation of the TSHR protein and deletion fragments containing the indicated Nt, intracellular (TM/Ct), and Ct regions tagged with Myc. Extracellular domains (gray), transmembrane domains (black), and intracellular domains (white) are indicated. D, Coimmunoprecipitation of DREAM-HA and the Myc-tagged TSHR deletion fragments with an anti-HA antibody in whole-cell extracts from CHO-K1 cells transfected with the indicated plasmids. Western blots were developed with anti-Myc and with anti-DREAM as a control. Input corresponds to 5% of total protein. White arrowhead indicates Ig chain.
To further characterize the interaction between DREAM and TSHR, we used Myc-tagged TSHR fragments (Fig. 5C) transfected together with DREAM-HA in CHO-K1 cells. Immunoprecipitation showed that DREAM specifically interacts with the C-terminal (Ct) intracellular region of TSHR (Fig. 5D). Conversely, experiments using Myc-tagged DREAM fragments (Fig. 6A) revealed that the N-terminal (Nt) region of DREAM containing the first 90 amino acids (DREAMΔ1-90) is needed for the interaction with the receptor (Fig. 6B). Coimmunoprecipitation experiments using TSHR Ct-Myc and DREAMΔ1-90-HA constructs confirmed that the Ct region (amino acids 696-763) of the receptor is able to interact directly with the 1-90 fragment of DREAM (Fig. 6C).
Fig. 6.
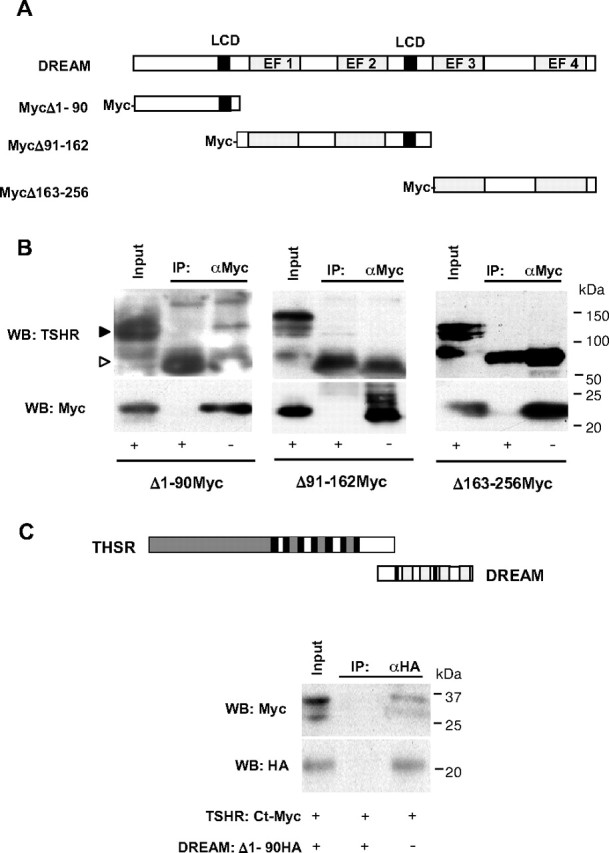
The Nt region of DREAM interacts with THSR. A, Schematic representation of DREAM protein and fragments containing the Nt (Δ1-90), middle (Δ91-162), and Ct (Δ163-256) regions tagged with Myc. B, Coimmunoprecipitation of DREAM fragments with anti-Myc antibody in whole-cell extracts from TSHR-10,000 cells transfected with indicated plasmids. Endogenous TSHR was detected by immunoblot. Input corresponds to 5% of total protein. White arrowhead indicates Ig chain. C, Coimmunoprecipitation of the Myc-tagged Ct fragment of TSHR, amino acids 696-763, and the HA-tagged 1-90 fragment of DREAM transfected in CHO-K1 cells as indicated. Schematic representation of the interaction between the TSHR intracellular Ct region and DREAM Nt region is shown. TSHR domains are as indicated in Fig. 5C; DREAM functional domains are indicated in A.
DREAM-derived peptides activate cAMP signaling
Previous work by others has shown that calreticulin interacts with and stabilizes TSHR (33). Overexpression of calreticulin in TSHR-10,000 cells, however, did not increase cAMP levels (supplemental Fig. 5), indicating that receptor stabilization and increased cAMP signaling are independent events. To further investigate the molecular basis for this effect, we checked whether DREAM fragments can directly activate the receptor as intracellular agonists. Expression in TSHR-10,000 cells showed that amino acids 43–90 of DREAM are involved in the activation of TSHR (Fig. 7A). To narrow down the residues directly responsible, we analyzed the effect of three overlapping peptides, P1, P2, and P3, that cover this region (Fig. 7A). Exposure of TSHR-10,000 cells to P1 specifically activated the cAMP cascade and increased phospho-CREB levels without increasing TSHR protein levels (Fig. 7, B and C). Similar results were observed in PC Cl3 thyroid cells (data not shown). P1 also increased cAMP levels in the human ML-1 line of poorly differentiated thyroid carcinoma cells (Fig. 7D) that contain low levels of TSHR (34) but not in NPA cells (Fig. 7E), a cell line originally described as papillary thyroid cancer (35), which does not express detectable levels of THSR (36). Using TSHR-10,000 cells, coimmunoprecipitation showed that both DREAM and P1, like TSH (10), enhanced the coupling between TSHR and Gαs (Fig. 7F). These data indicate that the P1 peptide, which neither stabilizes nor increases TSHR levels is still able to induce the cAMP cascade, strongly supporting DREAM as an intracellular TSHR ligand.
Fig. 7.
DREAM-derived peptides enhance TSHR activity. A, Increased cAMP levels in TSHR-10,000 cells after transfection with DREAM or DREAM fragments, schematically represented next to each bar. Data are mean ± sem. Significant differences from control (pcDNA3) by unpaired Student’s t test are indicated: **, P < 0.01; ***, P < 0.001. The sequence of three overlapping peptides (P1, P2, and P3) that cover amino acids 43-90 in DREAM is also shown. B, TSHR-10,000 cells were incubated with DREAM-derived peptides P1, P2, and P3 or a nonrelated peptide as negative control. Cells transfected with DREAM (DR) were included as positive control. Western blot with TSHR, phospho-CREB (P-CREB), and total CREB is shown. C–E, Effect on cAMP levels in TSHR-10,000 cells (C), thyroid follicular carcinoma ML-1 cells (D), and papillary carcinoma NPA cells (E) after incubation with DREAM-derived peptides (2 μm), a control nonrelated peptide (2 μm) or TSH (0.5 mU/ml, 10 min) used as a positive control. Data are mean ± sem. Significant differences from control by unpaired Student’s t test are indicated: ***, P < 0.001. F, Coimmunoprecipitation of TSHR and Gαs with an anti-TSHR antibody using whole-cell extracts from TSHR-10,000 cells transfected with DREAM (DR, upper panel), the P1 peptide (lower panel), and the corresponding controls, empty vector, or nonrelated peptide, respectively. Coimmunoprecipitation with a nonrelated antibody (anti-HA) was used as a negative control. Treatment with TSH (0.5 mU/ml, 10 min) was used as a control. Input corresponds to 1% of total extracts.
Endogenous DREAM regulates TSHR activity
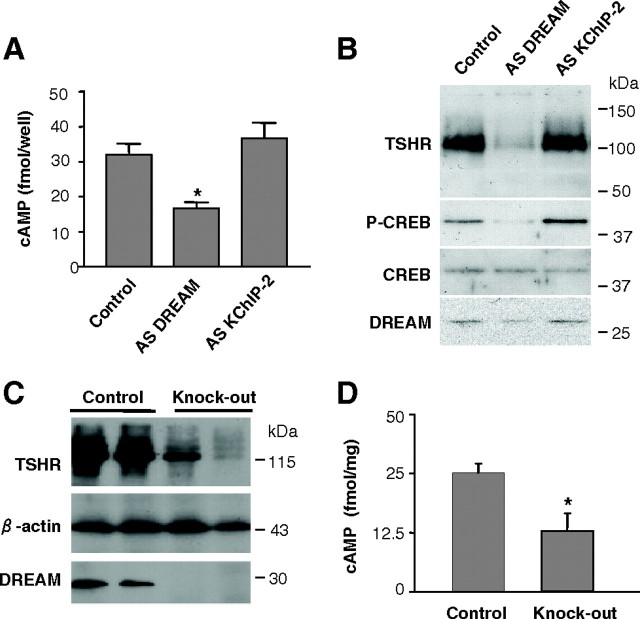
To further substantiate a physiological role of endogenous DREAM in TSHR function, we transiently knocked down DREAM expression in PC Cl3 thyroid cells using a previously characterized antisense vector (37). Reduction by 50–60% of the endogenous DREAM levels resulted in decreased TSHR, cAMP, and phospho-CREB levels, without change in total CREB (Fig. 8, A and B). This effect was specific for DREAM knock-down because it was not observed using an antisense vector for KChIP-2, in keeping with the lack of effect of KChIP-2 on the receptor (see Fig. 4C and supplemental Fig. 4). Analysis of thyroid glands from DREAM knockout mice (30) confirmed the antisense knock-down data and showed reduced levels of TSHR and cAMP (Fig. 8, C and D). Consistently, thyroid glands from DREAM knockout mice showed unstructured follicular pattern and cellular hypoactivity (supplemental Fig. 7). Complete characterization of the thyroidal phenotype in DREAM knockout mice is currently under investigation (Zannini, M. S., personal communication). Taken together, these data support a physiological role for endogenous DREAM in the control of TSHR activity in thyroid cells.
Fig. 8.
Endogenous DREAM controls TSHR signaling. A, Determination of cAMP levels in PC Cl3 thyroid cells 4 d after transfection with specific antisense (AS) vectors for DREAM, KChIP-2, or control pCDNA3. Data are mean ± sem of two independent experiments carried out in triplicate. Significant difference from control by unpaired Student’s t test is indicated: *, P = 0.0122. B, Western blot analysis of PC Cl3 thyroid cells 4 d after transfection as in A. The immunoblot was sequentially probed with antibodies for TSHR, phopho-CREB (P-CREB), total CREB, and DREAM. C, Western blot using whole-cell extracts from DREAM knockout and control thyroid glands. Two different knockout and control mice are shown. Blots were incubated with specific antibodies against DREAM, TSHR, and β-actin. D, In vivo analysis of cAMP levels in thyroid glands from DREAM knockout (n =7) and control mice (n = 6). Significant difference from control by unpaired Student’s t test is indicated: *, P = 0.035.
Analysis of DREAM and TSHR in human multinodular goiters
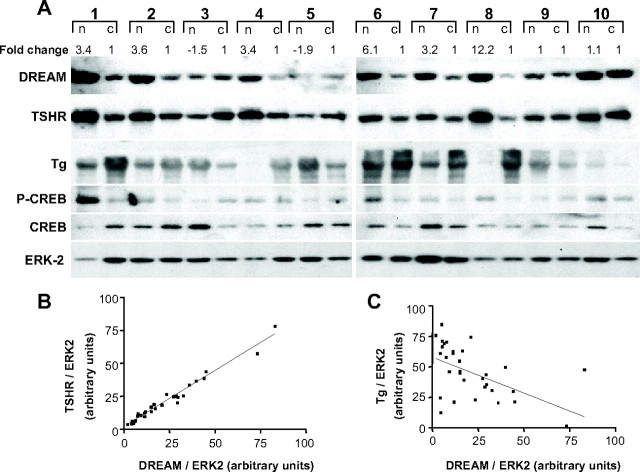
To investigate the pathophysiological relevance of the DREAM-TSHR interaction, we carried out Western blot analysis of nodules from human multinodular goiters using the surrounding normal tissue as control (Fig. 9). We found a higher than 2-fold increase in DREAM protein levels in nodular samples in 10 of 16 multinodular goiters. Representative examples (patients 1, 2, 4, 6, 7, and 8) are shown in Fig. 9A (for complete patient data set, see supplemental Table 1). Changes in DREAM levels were paralleled by TSHR, and statistical analysis showed a positive correlation between DREAM and TSHR proteins, supporting the notion that DREAM is able to modulate TSHR levels in the human thyroid gland (Fig. 9A). The increase in TSHR protein was not associated with an increase in TSHR mRNA (data not shown). A positive correlation was also observed between DREAM and phospho-CREB, suggesting that DREAM affects the cAMP signaling pathway. By contrast, a negative correlation was found between DREAM and Tg (Fig. 9C), which agrees with the transcriptional repression of the Tg promoter by DREAM in vitro (29) and in transgenic mice (see Fig. 3). No statistically significant correlations between DREAM and ERK-2, β-actin, and CREB were found in the same samples (Fig. 9A and data not shown). Together, these data suggest that up-regulation of endogenous DREAM may contribute to the development of thyroid nodules.
Fig. 9.
Increased levels of DREAM are associated with human multinodular goiters. A, Western blot analysis of human multinodular goiter (n) and control (c) tissue. Fold change for the DREAM protein is indicated. Immunodetection of DREAM, TSHR, Tg, ERK-2, phospho-CREB (P-CREB), and total CREB is shown. Only the 115-kDa band of TSHR, corresponding to the uncleaved form containing high-mannose carbohydrate, was clearly detected. B, Quantification of immunoreactive bands shows a positive correlation (***, P < 0.0001; Pearson r2 = 0.974) between DREAM and TSHR, whereas a negative correlation (**, P = 0.002; Pearson r2 = 0.2858) was found between DREAM and Tg (see panel C).
Discussion
Binding of TSH to its receptor controls thyroid function mainly through activation of the cAMP signaling pathway (10, 38, 39). Changes in TSHR activity due to point mutations or in response to auto-antibodies modify the cAMP cascade and have clinical consequences (11). In addition, TSHR displays a relatively high basal activity in the absence of TSH. The physiological significance and the existence of endogenous mediators of such spontaneous activity are not well understood (18, 19). In the present work, we show that DREAM is an endogenous intracellular effector of TSHR function that activates cAMP signaling.
DREAM transgenic thyroid glands showed increased cAMP and phospho-CREB levels, induction of the cAMP-regulated genes NIS and TPO, hyperproliferation, and goiter development. DREAM transgenic mice, however, do not show significant changes in T3, T4, or TSH levels as described in other models in which cAMP is constitutively activated (40). Maintenance of the euthyroid state could be related to the repression of Tg observed in DREAM transgenic glands (29), although the exact mechanism has not been established.
Calcium-binding proteins of the neuronal calcium sensor superfamily have been shown to regulate the activity of several cytosolic and membrane proteins. Thus, visinin-like protein-1 modulates the activity of guanylyl cyclase B as well as the surface expression and sensitivity for agonists of nicotinic receptors (22, 41, 42, 43). Ca-binding protein 4 regulates calcium influx in photoreceptor synaptic terminals through its interaction with the Cav1.4 channel (44). Likewise, DREAM has been associated with trafficking of Kv4 potassium channels to the plasma membrane and regulation of channel gating (22, 45), and like visinin-like protein-1 and other neuronal calcium sensor family members (46), DREAM regulates the membrane binding and kinase activity of G protein-coupled receptor kinase (47). The effect of DREAM on TSHR activity is specific and is mediated through the Nt TSHR-interacting region of DREAM. This domain is not present in KChIP2, otherwise a highly conserved member of the DREAM/KChIP family. Thus, KChIP2 does not regulate TSHR activity, and its expression in the thyroid gland could be related to transcriptional control in combination with DREAM (29). Previous work has shown that, like DREAM, calreticulin interacts and stabilizes TSHR. Calreticulin, however, did not induce the cAMP cascade, indicating that protein stabilization is not enough to explain the TSHR activation elicited by DREAM. Likewise, the DREAM-derived P1 peptide directly activates TSHR, promotes its coupling to Gαs and increases cAMP levels without stabilizing the receptor. Therefore, the activation elicited by DREAM is an independent effect not related to TSHR levels.
TSHR signaling is modulated by a number of posttranslational modifications, including phosphorylation by GRKs and Nt glycosylation (48, 49) changes that determine receptor desensitization and proper folding and membrane expression, respectively. In addition, TSHR function is regulated by oligomerization (50) and interaction with the membrane-associated PDZ protein hScrib (51), which determine intracellular trafficking and promotes receptor recycling blocking endocytosis, respectively. Whether DREAM interferes at these levels is presently unknown, but a Ca2+-dependent interaction between DREAM and the PDZ-containing protein PSD-95 has been observed in neurons (Naranjo, J. R., unpublished observations).
Our results show that DREAM interacts with the Ct cytosolic domain of TSHR. This region shares an approximately 70% homology with the Ct region of other glycoprotein-hormone receptors such as the FSH receptor and LH/chorionic gonadotropin receptor (10). Low molecular weight agonists or antagonists for these receptors have the potential to become oral therapeutics for infertility or contraception treatment, respectively (52). These studies have given rise to the discovery of molecules that bind to the TSHR transmembrane region and display partial agonist or antagonist activity although too moderate to be clinically useful (52, 53). Further experiments will be required to investigate the therapeutic value of DREAM and DREAM-derived peptides in pathologies associated with impaired TSHR, FSH receptor, or LH/chorionic gonadotropin receptor function. Activation or blockade of GPCRs by cell-penetrating peptides has been previously described (54) and was found to be of potential therapeutic value (55, 56). Theses peptides (named pepducins) derive from the intracellular transmembrane loops of GPCRs and, like DREAM, require the Ct of the receptor to activate G proteins.
Taken together, our data from cultured follicular cell lines, thyroid glands from transgenic mice, and human multinodular goiters reveal a new molecular mechanism that links deregulated DREAM expression with thyroid enlargement and nodular development.
Materials and Methods
Plasmids
Plasmids for wild-type DREAM, DREAM mutants, and expression vectors for antisense DREAM and KChIP-2 have been previously described (25, 37). TSHR and DREAM deletion fragments were cloned in the pCS2+Myc expression vector using the ClaI and the NcoI-XhoI sites, respectively. Calreticulin cDNA (a gift from Dr. M. Michalak, Canada) was subcloned in a pcDNA3-HA vector. Sequences of PCR primers for the DREAM and TSHR fragments are given in supplemental information. The plasmids were verified by sequencing on both strands.
Animals
The proximal bovine Tg promoter (40, 57) was used to target the dominant active DREAM mutant insensitive to calcium and cAMP (LeuEFmDREAM) to the thyroid gland. The transgenic cassette was microinjected into the pronuclei of one-cell embryos (C57BL/6 × CBA F1) using standard techniques. Transgenic progeny were identified by qualitative PCR of tail DNA using specific primers (supplemental information). Founder males were backcrossed to C57BL/6 females to generate lines that were maintained as heterozygotes. DREAM knockout mice (30) were kindly provided by J. M. Penninger. Female mice were analyzed in all experiments using wild-type littermates as controls. Experiments were approved and conducted according to institutional review board guidelines.
Cells
CHO-K1 cells stably transfected with human TSHR (TSHR-10,000 cells) (19) and the original CHO-K1 cell line were kindly provided by Dr. B. Rapoport. The cells were maintained in Ham’s F12 medium supplemented with Glutamax, fetal bovine serum (5%), and penicillin/streptomycin. Rat thyroid follicular PC Cl3 cells were cultured in DMEM/F12 (1:1) medium with Glutamax and supplemented with 5% calf serum and a six-growth-factor complement including TSH (0.5 mU/ml), insulin (10 μg/ml), somatostatin (10 ng/ml), hydrocortisone (10 nm), transferrin (5 μg/ml), and glycyl-histidyl-lysine (10 ng/ml). When cells were incubated in the absence of TSH, the calf serum concentration was reduced to 0.2%. Transfections were carried out with the JetPEI transfection reagent. For stable transfection, PC Cl3 cells received 5 μg plasmid DNA expressing either wild-type DREAM, EFmDREAM, EFLmDREAM, or empty pcDNA3 vector. Cells were selected after 3 wk with 300 μg/ml G418. ML1 and NPA cell lines, kindly provided by Dr. K. Törnquist, were cultured as described (58).
Peptide delivery
Peptides were delivered using the PULSin protein delivery reagent (Polyplus transfection) using the manufacturer’s protocol. For cAMP assays, peptides were added to 12-well plates (final concentration 2 μm), and for Western blots, peptides were added to 35-mm dishes (final concentration 2 or 3 μm). Peptide penetration into cells was analyzed by immunofluorescence using rhodamine-labeled peptides and showed that more than 90% of cells incorporated the peptide after 4 h incubation (not shown). Therefore, cells were analyzed after 4 h incubation with peptides.
cAMP assay
cAMP concentration in cells and thyroid gland extracts was measured using the Biotrak enzyme immunoassay system (GE Healthcare, Piscataway, NJ). Cells were plated in 12-well plates (1.7 × 105 PC Cl3 cells or 1.25 × 105 TSHR-10,000 cells per dish) and collected after 24 h (PC Cl3 stable clones) or 24 h after transient transfection (TSHR-10,000 cells). Proliferation profiles were not significantly different among control and DREAM-transfected cultures, and therefore cAMP values were normalized per well. In the experiments with DREAM-derived peptide, cells were collected after 4 h incubation with the peptide.
Histology
Thyroid glands were fixed in 4% paraformaldehyde in PBS overnight at 4 C, dehydrated through ethanol series, cleared in xylene, embedded in paraffin, and sectioned at 5 μm. For histological examinations, serial sections from transgenic and wild-type mice were stained with Harry’s hematoxylin and eosin.
Quantitative real-time RT-PCR
Total RNA from thyroid glands was prepared using Trizol. After reverse transcription, quantitative PCR was performed using specific primers and TaqMan probes. The sequences of primers and probes are given in supplemental information.
Coimmunoprecipitation and Western blot
For coimmunoprecipitation experiments, whole-cell extracts were prepared from thyroid glands, PC Cl3 cells, or transfected cells by incubation in Nonidet P-40 (NP40) lysis buffer [50 mm Tris (pH 8.0), 150 mm NaCl, 1% NP40, and protease inhibitor cocktail]. Extracts were precleared with protein G-Sepharose for 1 h and incubated overnight at 4 C with a monoclonal antibody against DREAM (28) or with anti-HA (sc-7392; Santa Cruz Biotechnology, Santa Cruz, CA). Immune complexes were captured for 2 h with protein G-Sepharose in lysis buffer containing 5% BSA, and beads were washed three times in lysis buffer. The same protocol using protein A-Sepharose was used for immunoprecipitation of TSHR and Myc-fusion proteins with goat and rabbit polyclonal antibodies, respectively. Protein complexes were eluted with SDS sample buffer and analyzed by immunoblot. For membrane protein preparations cells were lysed in buffer [20 mm Tris (pH 7.5), 0.32 m sucrose, 0.2 mm EDTA, and 0.5 mm EGTA and protease inhibitor cocktail]. After sonication, cells were centrifuged at 50,000 rpm and pellets resuspended in GTED buffer [20% glycerol, 10 mm Tris (pH 7.5), 1 mm EDTA, and 1 mm dithiothreitol]. Cell extracts were resolved in SDS-PAGE and transferred to polyvinylidene difluoride membranes. Rabbit polyclonal anti-DREAM has been described (37). The polyclonal antibody for TSHR (sc-7816; Santa Cruz) is directed against the N-terminal region and recognizes the full-length and the A subunit of the receptor. Antibodies against ERK-2 (sc-153), Gαs (sc-823), and c-Src (sc-19) were from Santa Cruz; phospho-CREB (9191S) and total CREB (9192) from Cell Signaling (Beverly, MA); Myc (ab9106-100) from Abcam (Cambridge, MA); and β-actin (A-5441) from Sigma Chemical Co. (St. Louis, MO). Blots were developed by enhanced chemiluminescence and quantified using the NIH Image software.
Human sample analysis
Human samples were obtained after informed consent and formally approved by the Ethical Committee at the Federico II University in Naples, Italy. All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki. After surgical removal, samples were immediately frozen in liquid nitrogen. Samples were lysed in NP40 lysis buffer and analyzed by Western blot as indicated above.
Statistics
The correlation analysis and Student’s t test applied for two group comparisons were done using the Prism statistical software. Values with P < 0.05 were considered significant. For cAMP assays, the number of independent experiments were at least three (n ≥ 3) carried out in triplicate.
Acknowledgments
We acknowledge Dr. B. Pintado and Dr. A. Gutiérrez-Adán for preparation of transgenic mice. We thank P. González and Dr. E. Lopez for assistance and Dr. L. Nitch and C. Mark for critical reading of the manuscript.
Footnotes
This work was supported by grants from Ministerio de Ciencia e Innovación and Communidad Autonoma de Madrid (to J.R.N. and B.M.) and from Fondo de Investigaciones de la Seguridad (to M.R. and B.M.)
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 19, 2009
Abbreviations: CREB, cAMP response element-binding protein; Ct, C-terminal; DREAM, downstream regulatory element antagonist modulator; GPCR, G protein-coupled receptor; HA, hemagglutinin; KChIP-3, K+ channel interacting protein-3; NIS, sodium-iodide symporter; NP40, Nonidet P-40; Nt, N-terminal; Tg, thyroglobulin; TPO, thyroid peroxidase; TSHR, TSH receptor.
References
- 1.Daull P, Jeng AY, Battistini B2007. Towards triple vasopeptidase inhibitors for the treatment of cardiovascular diseases. J Cardiovasc Pharmacol 50:247–256 [DOI] [PubMed] [Google Scholar]
- 2.Mori MA, Araújo RC, Reis FC, Sgai DG, Fonseca RG, Barros CC, Merino VF, Passadore M, Barbosa AM, Ferrari B, Carayon P, Castro CH, Shimuta SI, Luz J, Bascands JL, Schanstra JP, Even PC, Oliveira SM, Bader M, Pesquero JB2008. Kinin B1 receptor deficiency leads to leptin hypersensitivity and resistance to obesity. Diabetes 57:1491–1500 [DOI] [PubMed] [Google Scholar]
- 3.Ong KL, Wong LY, Cheung BM2008. The role of urotensin II in the metabolic syndrome. Peptides 29:859–867 [DOI] [PubMed] [Google Scholar]
- 4.Xapelli S, Agasse F, Ferreira R, Silva AP, Malva JO2006. Neuropeptide Y as an endogenous antiepileptic, neuroprotective and pro-neurogenic peptide. Recent Pat CNS Drug Discov 1:315–324 [DOI] [PubMed] [Google Scholar]
- 5.Fujiki N, Nishino S2007. Neuropeptides as possible targets in sleep disorders: special emphasis on hypocretin-deficient narcolepsy. CNS Neurol Disord Drug Targets 6:45–62 [DOI] [PubMed] [Google Scholar]
- 6.Roecker AJ, Coleman PJ2008. Orexin receptor antagonists: medicinal chemistry and therapeutic potential. Curr Top Med Chem 8:977–987 [DOI] [PubMed] [Google Scholar]
- 7.Taborga M, Corcoran KE, Fernandes N, Ramkissoon SH, Rameshwar P2007. G-coupled protein receptors and breast cancer progression: potential drug targets. Mini Rev Med Chem 7:245–251 [DOI] [PubMed] [Google Scholar]
- 8.Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G1996. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA 93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allgeier A, Laugwitz KL, Van Sande J, Schultz G, Dumont JE1997. Multiple G-protein coupling of the dog thyrotropin receptor. Mol Cell Endocrinol 127:81–90 [DOI] [PubMed] [Google Scholar]
- 10.Vassart G, Dumont JE1992. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- 11.Davies TF, Ando T, Lin RY, Tomer Y, Latif R2005. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest 115:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paschke R, Ludgate M1997. The thyrotropin receptor in thyroid diseases. N Engl J Med 337:1675–1681 [DOI] [PubMed] [Google Scholar]
- 13.Duprez L, Parma J, Van Sande J, Rodien P, Sabine C, Abramowicz M, Dumont JE, Vassart G1999. Pathology of the TSH receptor. J Pediatr Endocrinol Metab 12(Suppl 1):295–302 [PubMed] [Google Scholar]
- 14.Corvilain B, Van Sande J, Dumont JE, Vassart G2001. Somatic and germline mutations of the TSH receptor and thyroid diseases. Clin Endocrinol (Oxf) 55:143–158 [PubMed] [Google Scholar]
- 15.Krohn K, Führer D, Bayer Y, Eszlinger M, Brauer V, Neumann S, Paschke R2005. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev 26:504–524 [DOI] [PubMed] [Google Scholar]
- 16.Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM1998. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev 19:673–716 [DOI] [PubMed] [Google Scholar]
- 17.Davies T, Marians R, Latif R2002. The TSH receptor reveals itself. J Clin Invest 110:161–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Sande J, Swillens S, Gerard C, Allgeier A, Massart C, Vassart G, Dumont JE1995. In Chinese hamster ovary K1 cells dog and human thyrotropin receptors activate both the cyclic AMP and the phosphatidylinositol 4,5-bisphosphate cascades in the presence of thyrotropin and the cyclic AMP cascade in its absence. Eur J Biochem 229:338–343 [PubMed] [Google Scholar]
- 19.Chazenbalk GD, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B1996. Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology 137:4586–4591 [DOI] [PubMed] [Google Scholar]
- 20.Kakinuma A, Chazenbalk G, Filetti S, McLachlan SM, Rapoport B1996. Both the 5′ and 3′ noncoding regions of the thyrotropin receptor messenger ribonucleic acid influence the level of receptor protein expression in transfected mammalian cells. Endocrinology 137:2664–2669 [DOI] [PubMed] [Google Scholar]
- 21.Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR1999. DREAM is a Ca2+-regulated transcriptional repressor. Nature 398:80–84 [DOI] [PubMed] [Google Scholar]
- 22.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ2000. Modulation of A-type potassium channels by a family of calcium sensors. Nature 403:553–556 [DOI] [PubMed] [Google Scholar]
- 23.Holmqvist MH, Cao J, Hernandez-Pineda R, Jacobson MD, Carroll KI, Sung MA, Betty M, Ge P, Gilbride KJ, Brown ME, Jurman ME, Lawson D, Silos-Santiago I, Xie Y, Covarrubias M, Rhodes KJ, Distefano PS, An WF2002. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc Natl Acad Sci USA 99:1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrión AM, Mellström B, Naranjo JR1998. Protein kinase A-dependent derepression of the human prodynorphin gene via differential binding to an intragenic silencer element. Mol Cell Biol 18:6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledo F, Carrión AM, Link WA, Mellström B, Naranjo JR2000. DREAM-αCREM interaction via leucine-charged domains derepresses downstream regulatory element-dependent transcription. Mol Cell Biol 20:9120–9126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanz C, Mellstrom B, Link WA, Naranjo JR, Fernandez-Luna JL2001. Interleukin 3-dependent activation of DREAM is involved in transcriptional silencing of the apoptotic Hrk gene in hematopoietic progenitor cells. EMBO J 20:2286–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link WA, Ledo F, Torres B, Palczewska M, Madsen TM, Savignac M, Albar JP, Mellström B, Naranjo JR2004. Day-night changes in downstream regulatory element antagonist modulator/potassium channel interacting protein activity contribute to circadian gene expression in pineal gland. J Neurosci 24:5346–5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledo F, Kremer L, Mellström B, Naranjo JR2002. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J 21:4583–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas M, Mellström B, Naranjo JR, Santisteban P2004. Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J Biol Chem 279:33114–33122 [DOI] [PubMed] [Google Scholar]
- 30.Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM2002. DREAM is a critical transcriptional repressor for pain modulation. Cell 108:31–43 [DOI] [PubMed] [Google Scholar]
- 31.Meissner W1971. In: Werner SC, Ingbar SH, eds. Pathology of the thyroid. New York: Harper & Row
- 32.Burgoyne RD2007. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci 8:182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siffroi-Fernandez S, Giraud A, Lanet J, Franc JL2002. Association of the thyrotropin receptor with calnexin, calreticulin and BiP. Efects on the maturation of the receptor. Eur J Biochem 269:4930–4937 [DOI] [PubMed] [Google Scholar]
- 34.Schönberger J, Bauer J, Spruss T, Weber G, Chahoud I, Eilles C, Grimm D2000. Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J Mol Med 78:102–110 [DOI] [PubMed] [Google Scholar]
- 35.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR2008. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WW, Lee B, Kim SJ, Jin J, Moon DH, Lee H2003. Kinetics of iodide uptake and efflux in various human thyroid cancer cells by expressing sodium iodide symporter gene via a recombinant adenovirus. Oncol Rep 10:845–849 [PubMed] [Google Scholar]
- 37.Savignac M, Pintado B, Gutierrez-Adan A, Palczewska M, Mellström B, Naranjo JR2005. Transcriptional repressor DREAM regulates T-lymphocyte proliferation and cytokine gene expression. EMBO J 24:3555–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC, Davies TF, Zannini MS, De Felice M, Di Lauro R2002. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA 99:15462–15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF2002. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA 99:15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledent C, Dumont JE, Vassart G, Parmentier M1992. Thyroid expression of an A2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. EMBO J 11:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brackmann M, Schuchmann S, Anand R, Braunewell KH2005. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci 118:2495–2505 [DOI] [PubMed] [Google Scholar]
- 42.Ren XQ, Cheng SB, Treuil MW, Mukherjee J, Rao J, Braunewell KH, Lindstrom JM, Anand R2005. Structural determinants of α4β2 nicotinic acetylcholine receptor trafficking. J Neurosci 25:6676–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L, Jeanclos EM, Treuil M, Braunewell KH, Gundelfinger ED, Anand R2002. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist sensitivity of the α4β2 nicotinic acetylcholine receptor. J Biol Chem 277:41872–41878 [DOI] [PubMed] [Google Scholar]
- 44.Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K2004. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci 7:1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS2003. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem 278:36445–36454 [DOI] [PubMed] [Google Scholar]
- 46.Iacovelli L, Sallese M, Mariggiò S, de Blasi A1999. Regulation of G-protein-coupled receptor kinase subtypes by calcium sensor proteins. FASEB J 13:1–8 [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Gomez A, Mellström B, Tornero D, Morato E, Savignac M, Holguin H, Aurrekoetxea K, González P, González-García C, Ceña V, Mayor Jr F, Naranjo JR2007. G protein-coupled receptor kinase 2-mediated phosphorylation of downstream regulatory element antagonist modulator regulates membrane trafficking of Kv4.2 potassium channel. J Biol Chem 282:1205–1215 [DOI] [PubMed] [Google Scholar]
- 48.Nagayama Y2005. Animal models of Graves’ hyperthyroidism. Endocr J 52:385–394 [DOI] [PubMed] [Google Scholar]
- 49.Frenzel R, Krohn K, Eszlinger M, Tönjes A, Paschke R2005. Sialylation of human thyrotropin receptor improves and prolongs its cell-surface expression. Mol Pharmacol 68:1106–1113 [DOI] [PubMed] [Google Scholar]
- 50.Calebiro D, de Filippis T, Lucchi S, Covino C, Panigone S, Beck-Peccoz P, Dunlap D, Persani L2005. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet 14:2991–3002 [DOI] [PubMed] [Google Scholar]
- 51.Lahuna O, Quellari M, Achard C, Nola S, Méduri G, Navarro C, Vitale N, Borg JP, Misrahi M2005. Thyrotropin receptor trafficking relies on the hScrib-βPIX-GIT1-ARF6 pathway. EMBO J 24:1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC2008. A low molecular weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology 149:5945–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jäschke H, Neumann S, Moore S, Thomas CJ, Colson AO, Costanzi S, Kleinau G, Jiang JK, Paschke R, Raaka BM, Krause G, Gershengorn MC2006. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR). J Biol Chem 281:9841–9844 [DOI] [PubMed] [Google Scholar]
- 54.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A2002. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA 99:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covic L, Misra M, Badar J, Singh C, Kuliopulos A2002. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med 8:1161–1165 [DOI] [PubMed] [Google Scholar]
- 56.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A2005. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med 11:661–665 [DOI] [PubMed] [Google Scholar]
- 57.Ledent C, Parmentier M, Vassart G1990. Tissue-specific expression and methylation of a thyroglobulin-chloramphenicol acetyltransferase fusion gene in transgenic mice. Proc Natl Acad Sci USA 87:6176–6180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balthasar S, Samulin J, Ahlgren H, Bergelin N, Lundqvist M, Toescu EC, Eggo MC, Törnquist K2006. Sphingosine 1-phosphate receptor expression profile and regulation of migration in human thyroid cancer cells. Biochem J 398:547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]