Abstract
Sleep problems can boost inflammation and may jeopardize interpersonal functioning, risks that may be magnified in couples. This observational study examined the effects of self-reported recent sleep duration on couples’ inflammation, inflammatory responses to a problem discussion, interpersonal behavior, and use of emotion regulation strategies (emotion expression, cognitive reappraisal) during conflict. People who slept less had higher stimulated interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) production after the marital problem discussion than those who slept more. However, using emotion expression and cognitive reappraisal strategies during conflict protected couples who slept less from inflammatory reactivity. Specifically, people’s short sleep did not relate to inflammatory increases when they expressed their own feelings more or when their partner reappraised or expressed their emotions more. When both partners slept less, couples interacted in a more hostile way than when at least one partner slept more. These data point to the combination of short sleep and marital conflict as a novel path to heightened inflammation, a risk that partners’ emotion regulation strategies may counteract. The study also highlights the role of short sleep in more negative or punishing marital behavior.
Keywords: sleep duration, inflammation, inflammatory response, couples, marital conflict, emotion regulation
1. Introduction
Sufficient sleep is critical for maintaining health and well-being. Short sleep is implicated in premature mortality as well as cardiovascular disease, cancer, obesity, and diabetes (Cappuccio et al., 2011; Cappuccio et al., 2010). In fact, an expert consensus panel recommended 7–9 hours of sleep each night to preserve health in adulthood (Consensus Conference et al., 2015).
Short sleep upregulates inflammatory signaling, a key pathway to many chronic conditions. For example, just one night of 4-hour sleep deprivation was sufficient to increase messenger RNA transcription and stimulated monocyte production of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α, Irwin et al., 2006), as well as nuclear factor-κB (NF-κB) activation (Irwin et al., 2008). Similarly, restricting sleep to 6 hours per night for one week resulted in heightened resting plasma IL-6 and TNF-α (Vgontzas et al., 2004), suggesting that the inflammatory effects of minor sleep debt may accumulate rapidly across days. On the other hand, a meta-analysis did not find effects for experimental sleep deprivation or subjective sleep duration on IL-6, indicating that short sleep alone may not increase inflammation (Irwin et al., 2016).
Nevertheless, short sleep duration may independently exacerbate inflammatory responses to stressors. Experimentally sleep-deprived people mount exaggerated sympathetic responses to stressful tasks compared to well-rested people (Liu et al., 2015; O’Leary et al., 2015). Because sympathetic activation heightens inflammation (Pongratz and Straub, 2014), increased inflammatory responsiveness may follow short sleep in daily life as well. Inflammatory sensitization to psychosocial stressors may reflect a novel path from short sleep to chronic illness, as inflammatory responses can contribute to long-term health risks beyond the effects of resting inflammation (Pace et al., 2006).
For couples, sleep problems can provoke hostility (Gordon and Chen, 2014; Hasler and Troxel, 2010). In a diary study, men reported more problematic interactions with their wives after a night of disturbed sleep (Hasler and Troxel, 2010). Likewise, couples who slept poorly over 14 nights reported more daily marital conflict than those who slept better (Gordon and Chen, 2014). Poorer sleep quality also increased negative affect and reduced positive affect during a marital problem discussion (Gordon and Chen, 2014). Moreover, just one partner’s poor sleep affected not only his or her own mood and empathic accuracy (i.e. the ability to interpret the other partner’s emotions) but also impacted the partner’s affect and accuracy (Gordon and Chen, 2014). Consistent with sleep quality’s effects on couples, people who were sleep-deprived for 36 hours were less sociable in a decision-making game with an anonymous partner, and they were less likely to trust their teammate (Anderson and Dickinson, 2010). Thus, shortened sleep also sets the stage for soured social interactions.
Emotion regulation strategies are the behaviors we use to manage and change our emotions. Common strategies include emotion expression (conveying feelings with words and nonverbal gestures) and cognitive reappraisal, i.e., thinking about an upsetting situation from a different perspective. These regulation strategies play a role in the aftermath of sleep loss, but whether short sleep disturbs emotion regulation is unclear. On the one hand, short sleep may increase both inflammation and interpersonal conflict by disrupting emotion regulation. For instance, total sleep deprivation reduced neural communication between emotion-relevant brain regions, resulting in poorer discrimination of emotion cues (Simon et al., 2015; Yoo et al., 2007). Emotion expression may also suffer with shortened sleep: following sleep deprivation, people were less facially expressive and used more negative emotion words and fewer positive words than after a full night of sleep (McGlinchey, 2011; Minkel et al., 2011). On the other hand, some studies suggest that a person’s emotion regulation strategies remain unchanged after sleep loss and, therefore, protect against its negative consequences. For example, after sleep restriction, people who frequently used cognitive reappraisal paid less attention to negative-emotion faces than did infrequent reappraisers (Cote et al., 2015). Because regulation strategies directly affect interpersonal communication, conflict, and intimacy between partners (Laurenceau et al., 2005), the question of whether they are reduced by or buffer the effects of short sleep is important; the two hypotheses have not been tested together in a single sample.
Sleep is dyadic for many adults: 70% share a bed with their significant other (National Sleep Foundation, 2013). Nevertheless, there is a surprising dearth of studies on short sleep and inflammation in couples. Having a bed partner impacts sleep quantity and quality (Troxel et al., 2007), and beyond the effects of their own sleep problems, people whose partners slept poorly reported poorer health and well-being (Strawbridge et al., 2004). Inflammatory changes may account for this trend: partners’ short sleep may serve to boost one another’s next-day inflammation and heighten inflammatory responses to conflict, a novel mechanism of short sleep’s health effects. Given that partners’ poor sleep has also led to increases in negative mood and lower empathic accuracy (Gordon and Chen, 2014), it is important to examine whether short sleep translates to more hostile, less warm behavior during conflict. Furthermore, both partners’ emotion regulation use during conflict may modulate the effects of sleep on inflammation.
The first objective of our study was to examine the effects of husbands’ and wives’ self-reported recent sleep duration on both partners’ inflammation and inflammatory reactivity to marital conflict, a potent stressor. We hypothesized that those with shorter recent sleep would have higher morning inflammation and inflammatory reactivity to conflict than people who had slept more, assessed via stimulated IL-6 and TNF-α. We also predicted that people whose partners had slept less would have higher next-morning and post-conflict inflammation, above and beyond the effects of their own sleep duration. Our second objective was to test the effects of both partners’ short sleep on their behavior and emotion regulation during conflict. We hypothesized that both partners’ short sleep would relate to more negative and less positive behavior during conflict. Finally, we tested emotion expression and cognitive reappraisal strategies both as outcomes of sleep and buffers of the sleep-inflammation tie, expecting that more expression and reappraisal would relate to lower post-conflict inflammation or would attenuate short sleep’s effects on post-conflict inflammation.
2. Method
2.1 Participants
Couples were recruited for a parent study of immune responses to high-fat meals (Kiecolt-Glaser et al., 2015b). An initial online screen and follow-up in-person screen determined eligibility. Couples married fewer than 3 years and those who had sensory impairments that would interfere with study completion were excluded. Couples were not considered if either partner had a chronic health problem including anemia or diabetes (HbA1c > 6.5), smoked, abused substances, or used prescription medication other than birth control (n = 5) or levothyroxine (n = 3). Participants fit our exercise criteria if they engaged in a minimum of 2 hours of vigorous activity per week for those with a BMI of < 24.99 (normal weight) and 5 hours per week for BMI > 25 (overweight or obese).
In the online screen, potential participants completed the 16-item version of the Couples Satisfaction Index; the full version was given at the end of the first visit (Funk and Rogge, 2007). Happier couples were overrepresented among applicants, consistent with evidence that recruiting unhappy couples is a challenge for marital research in general (Bradbury and Karney, 1993). Accordingly, in terms of both inclusion and scheduling, we prioritized dissatisfied couples to represent the full range of marital discord. We also spent considerable time and effort to recruit people who were healthy but overweight to address aims relevant to the parent study’s meal component. A total of 350 interested individuals were excluded because either they or their spouse did not meet our stringent health criteria.
The sample consisted of 86 participants (43 couples). Participants were 38.2 years old on average (SD = 8.2, range = 24−61) and primarily White (81%). None of the participants took sleep medication. All couples were married, and the average length of marriage was 11.5 years (SD = 6.6, range = 3 − 27). Table 1 provides additional sample characteristics.
Table 1.
Sample description
| N(%) or Mean(SD) | Range | |
|---|---|---|
| Age | 38 (8.2) | 24–61 |
| BMI | 32.1 (5.8) | 19.5–46 |
| College educated | 58 (67.4%) | |
| Employed full-time | 60 (70%) | |
| Marital satisfaction (CSI) | 124 (33) | 7–160 |
| Years married | 11.5 (6.6) | 3–27 |
| Chronic sleep problems (PSQI) | 4.9 (2.5) | 1–14 |
| Hours of prior two nights’ sleep | 6.7 (1.0) | 3.5–9 |
Note. Higher CSI scores indicate greater marital happiness. Higher PSQI scores indicate more past-month sleep problems. CSI = Couples Satisfaction Index; PSQI = Pittsburgh Sleep Quality Index
2.2 Data Collection Procedure
Participants completed two full-day study visits at the Clinical Research Center (CRC), a hospital research unit. During this double-blind randomized crossover study, couples ate a high saturated fat meal at the beginning of one visit and a high oleic sunflower oil meal at the beginning of the other (in random order to test the parent study’s key aims). Couples were told to avoid alcohol and caffeine use within 1 day prior and strenuous physical activity within 2 days prior to both study visits. Participants were also instructed to stop taking aspirin, vitamins (except multivitamins), antioxidants, and any other dietary supplements for 7 days prior to each admission. On the day before each visit, participants received three standardized meals from the CRC’s metabolic kitchen, reducing any variability in inflammation associated with recent food intake.
At each admission, both members of a couple arrived at 7:30 a.m. after fasting for 12 hr, and a catheter was inserted into each person’s arm. Following a short relaxation period, each member of the couple had 20 min to eat the high saturated fat or high oleic sunflower oil meal; the husband and wife received the same meal and both were required to eat the entire meal.
Couples also engaged in a marital problem discussion on the morning of each visit. To initiate the discussion, an experimenter conducted a 10- to 20-min interview to identify the most contentious topics for both partners. These topics were selected from an inventory each spouse completed about their relationship problems. Couples were then asked to discuss and try to resolve one or more marital issues that the experimenter judged to be the most conflict-producing (e.g., money, communication, or in-laws). The research team remained out of sight while videotaping the subsequent 20-min problem discussion.
Blood samples of interest for the current study were collected upon arrival and approximately 1 hour after the conflict discussion given the time course of inflammatory reactivity to stressors (Steptoe et al., 2007). This research was approved by the Ohio State University (OSU) Institutional Review Board; participants provided written informed consent before participating.
2.3 Self-Report Measures
2.3.1 Past two nights’ sleep duration
During an activity recall task at both study visits, each participant reported their average hours of sleep in the past two nights. This 48-hour recall measure was adapted from a reliable and well-validated 7-day recall (Blair et al., 1985). The mean sleep duration of the prior two nights fell below the recommended 7–9 hours (M = 6.7, SD = 1.0, range = 3.5 − 9), indicating short sleep on average (Consensus Conference et al., 2015). This measure was selected to index acute sleep duration as opposed to chronic sleep problems.
2.3.2 Self-reported emotion regulation during conflict
Participants reported their use of emotion regulation strategies during the problem discussion (adapted for conflict discussion from Thoits, 1991). Five summed items indexed expression of feelings on a 4-point scale, with higher scores indicating more expression and less suppression (e.g. “I let my feelings out” and “I tried to hide my feelings,” reverse-scored). Another five items tapped into use of active cognitive strategies to manage emotions during the conflict discussion, e.g. reframing, problem-solving, perspective-taking, all of which are henceforth collectively referred to as reappraisal for the sake of brevity. Example items included “I tried to see things from my spouse’s point of view” and “I reminded myself how much worse things could be.”
2.3.3 Chronic sleep problems
At their first visit, participants completed the Pittsburgh Sleep Quality Index (PSQI), which assesses subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the past month (Buysse et al., 1989). Higher scores indicate greater sleep problems. The PSQI has good internal consistency and discriminant validity (Buysse et al., 1989). This past-month sleep measure served as a covariate to separate the role of acute sleep patterns from longer-term problems, which may affect inflammation and behavior through different mechanisms (Beattie et al., 2015).
2.3.4 Marital quality
Administered at the first full-day visit, the 32-item Couple Satisfaction Index (CSI) assessed marital satisfaction (Funk and Rogge, 2007). Developed using item response theory, the CSI can discriminate between satisfied and dissatisfied couples with greater precision than other commonly used marital scales (Funk and Rogge, 2007). Marital satisfaction was included as a covariate to account for the effects of general relationship quality on inflammation (Robles et al., 2014).
2.3.5 Depressive symptoms
Participants completed the Center for Epidemiological Studies Depression Scale (CES-D) at each visit. The CES-D has been used extensively as a brief measure of depressive symptomatology (Radloff, 1977). Studies have shown acceptable test-retest reliability and excellent construct validity (Basco et al., 1997). Depression was included as a covariate given its role in sleep disturbance, inflammation, and conflict behavior (Kiecolt-Glaser et al., 2015a; Kiecolt-Glaser and Newton, 2001).
2.4 Conflict Behavior
Marital disagreement discussions were coded using the Rapid Marital Interaction Coding System (RMICS), which discriminates well between distressed and nondistressed couples (Heyman, 2004). Distressed marriages are characterized by negative affect, conflictual communication, and poor listening skills (Kiecolt-Glaser and Newton, 2001). Accordingly, the negative composite index summed four RMICS codes: psychological abuse (e.g., disgust, contempt, belligerence, as well as nonverbal behaviors like glowering), distress-maintaining attributions (e.g., “You’re only being nice so I’ll have sex with you tonight” or “You were being mean on purpose”), hostility (e.g., criticism, hostile voice tone, or rolling the eyes dramatically), and withdrawal (behaviors that suggest pulling back from the interaction or not listening). Unhappy couples also exhibit fewer positive behaviors (Kiecolt-Glaser and Newton, 2001). The positive behavioral composite consisted of: acceptance (e.g., attempts at active listening, expressing concern), relationship-enhancing attributions (e.g., “You’re short with me because you’ve had a hard day”), self-disclosure (e.g., any expression of personal feelings or wishes not considered negative or hostile toward the partner), humor (e.g. playful joking, teasing, or sarcasm), and constructive problem discussion (e.g., “Let’s stop eating out so often” or “I think you’re right about that”).
Holley and Gilford’s G was used to quantify interrater agreement for the RMICS positive and negative behaviors (Xu and Lorber, 2014). Interrater agreement was high, with a value of .97 averaged over G indices for negative behaviors and .87 averaged over positive behaviors. Negative and positive scores were converted to percentages to account for person-to-person differences in the number of speech turns during discussion.
2.5 Cytokine Assays
PBMCs were treated with 1.0 μg/ml lipopolysaccharide (LPS) for 24 hours to measure the production of IL-6 and TNF-α (Calder, 2001). After 24 hours, the cells were pelleted by centrifugation (2000 rpm for 5 minutes) and the supernatant removed, aliquoted and frozen at -80C until assayed. All samples, including each person’s control cell cultures incubated in media alone, were run at the same time for each subject. Sensitivity was 0.7 pg/mL for stimulated IL-6 and 1.0 pg/mL for stimulated TNF-α. The intra-assay coefficient of variation for IL-6 was 2.76%, and the inter-assay coefficient of variation was 13.57%; corresponding values for TNF-α were 3.40% and 11.91%.
2.6 Analytic Approach
Research questions were addressed using linear mixed models, which allowed explicit modeling of the within-subject correlations across visits and accounted for couple-level correlation. Cytokine data were natural-log (ln) transformed to better approximate normality of residuals. Residual plots were also inspected for outliers. Removal of one identified residual outlier did not change results. The Kenward-Roger degrees of freedom adjustment was used to control type I error (Kenward and Roger, 1997). Continuous variables were grand-mean-centered for ease of interpretation.
Hypothesized effects were tested in a two-model sequence. The reduced model contained fixed and random effects relevant to the parent study’s nested design—meal types, nested within subjects, nested within couples. Specifically, random subject-specific intercepts modeled the within-person correlation and the couple-level correlation was captured using a couple-specific random intercept. Both meal type (high-fat or not) and visit (first or second) were included as fixed effects. Own and partner recent sleep were included as predictors simultaneously to capture the unique effects of one while adjusting for the other. Cytokine models also included a random effect for assay plate to account for plate-to-plate variability and increase precision of estimates (Browne et al., 2013; Kiecolt-Glaser et al., 2015b).
The fully adjusted model contained conceptually important covariates in addition to all fixed and random effects in the reduced model. Gender was included to account for husband-wife differences in behavior and inflammatory responses to conflict. Own and partner chronic sleep problems (PSQI) served to separate the role of acute sleep patterns from longer-term sleep problems, which may affect inflammation and behavior through different mechanisms (Beattie et al., 2015). Own or partner marital satisfaction was added to parse out the effects of general relationship quality on inflammation from sleep-specific effects (Robles et al., 2014). Due to their high correlation (r = .83), own and partner satisfaction were entered separately to avoid collinearity problems. Specifically, one’s own marital satisfaction was used to test the effect of own recent sleep; partner marital satisfaction to examine the effect of partner sleep; and own marital satisfaction to test interactions including one’s own sleep. Own and partner depressive symptoms served as covariates given the role of depression in sleep disturbance, inflammation, and conflict behavior (Kiecolt-Glaser et al., 2015a; Kiecolt-Glaser and Newton, 2001). Additionally, age and BMI were covariates in cytokine analyses, and morning cytokine levels were included in models with post-conflict cytokines as outcome. In a secondary step, negative and positive behavior during the discussion were added as covariates to test whether behavior accounted for the relationship between sleep and inflammation. Own and partner behavior were entered separately to prevent collinearity problems (rown, partner negative = .79; rown, partner positive = .78).
Main effects of both partners’ prior two nights’ sleep duration were of primary interest. The interaction between own and partner recent sleep was also tested in each model. Gender was also tested as a moderator of sleep effects. Non-significant interactions were removed, and significant interactions were investigated using simple slopes at approximately one standard deviation above and below the mean. All analyses were conducted in SAS version 9.4 (Cary, NC).
Given the equivocal evidence for the role of emotion regulation as outcome or moderator of short sleep’s negative effects (Schwarz et al., 2013; Yoo et al., 2007), we explored the effect of sleep on reappraisal and emotion expression as well as the moderating effect of these strategies on links between sleep and post-conflict inflammation.
3. Results
According to zero-order correlations, partners’ recent sleep duration were modestly related to each other but not redundant (r = .233, p = .003). Those who reported fewer hours of sleep in the prior two nights also experienced more sleep problems in the past month, as measured by the PSQI (r = −.439, p < .0001). Partners’ past-month sleep problems were not significantly correlated with each other (r = .020, p = .78).
3.1 Recent sleep duration and inflammation
3.1.1 Morning inflammation
Short sleep did not relate to one’s own morning inflammation in reduced models (ps > .34). In the reduced model, people whose partner slept less had higher morning IL-6 production than those whose partner had slept more (B = −0.091, SE = 0.035, p = .01). The effect of sleep on the partner’s TNF-α trended in the same direction but was not statistically significant (B = −0.068, SE = 0.041, p = .10). After adjusting for additional covariates, effects of recent sleep on the partner’s morning baseline inflammation were no longer significant (for IL-6, p = .11; for TNF-α, p = .28). Interactions between both partners’ sleep were also not significant, and effects did not differ by gender (ps > .21).
3.1.2 Post-conflict inflammation
In the reduced model (see Table 2), people with shorter sleep produced more IL-6 (B = −0.057, SE = 0.021, p = .01) and TNF-α (B = −0.056, SE = .023, p = .01) after conflict than people who had slept more. Effects on the partner’s post-conflict inflammation were nonsignificant in reduced models (ps > .11). In the fully adjusted model, effects of sleep on one’s own IL-6 (B = −0.057, SE = 0.024, p = .02) and TNF-α production (B = − 0.057, SE = 0.026, p = .03) remained significant. That is, those who slept 1 hour less than average had 6.0% higher IL-6 and TNF-α production post-conflict.
Table 2.
Recent sleep duration predicting post-conflict log-transformed stimulated cytokines
| Ln(IL-6) Reduced | Ln(IL-6) Fully Adjusted | Ln(TNF-α) Reduced | Ln(TNF-α) Fully Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Intercept | 3.283 | 0.551 | < .0001 | 3.382 | 0.602 | < .0001 | 0.481 | 0.312 | 0.127 | 0.530 | 0.394 | 0.18 |
| Baseline cytokine | 0.705 | 0.049 | < .0001 | 0.704 | 0.050 | < .0001 | 0.925 | 0.043 | < .0001 | 0.909 | 0.047 | < .0001 |
| Visit | −0.019 | 0.035 | .59 | −0.014 | 0.035 | .69 | 0.008 | 0.036 | .83 | 0.010 | 0.037 | .79 |
| Meal | 0.003 | 0.035 | .92 | 0.001 | 0.035 | .97 | −0.006 | 0.036 | .87 | −0.008 | 0.037 | .82 |
| Gender | 0.073 | 0.037 | .05 | −0.025 | 0.039 | .52 | ||||||
| Age | −0.002 | 0.003 | .46 | −0.0003 | 0.003 | .93 | ||||||
| BMI | −0.001 | 0.004 | .76 | 0.003 | 0.004 | .50 | ||||||
| Own satisfaction | 0.0005 | 0.0008 | .57 | 0.0007 | 0.0009 | .41 | ||||||
| Own depression | 0.002 | 0.004 | .69 | −0.0009 | 0.004 | .84 | ||||||
| Partner depression | −0.007 | 0.004 | .08 | −0.003 | 0.004 | .44 | ||||||
| Own chronic sleep | −0.007 | 0.011 | .56 | 0.002 | 0.012 | .84 | ||||||
| Partner chronic sleep | 0.020 | 0.011 | .07 | 0.023 | 0.012 | .05 | ||||||
| Own prior two night sleep | −0.057 | 0.021 | .01 | −0.057 | 0.024 | .02 | −0.056 | 0.023 | .01 | −0.057 | 0.026 | .03 |
| Partner prior two night sleep | 0.036 | 0.022 | .11 | 0.035 | 0.024 | .16 | 0.034 | 0.023 | .14 | 0.040 | 0.026 | .13 |
Note. Because interactions between own and partner recent sleep duration were non-significant, as were gender interactions, these terms were trimmed from final models and are not listed. All models included subject-level intercepts, couple-specific effects, and random plate effects.
Interactions between both partners’ recent sleep were not significant for either IL-6 or TNF-a (ps > .42). Effects did not differ by gender (ps > .19). Furthermore, including either partner’s conflict behavior as a covariate did not alter the statistical significance of recent sleep on one’s own post-conflict inflammation.
3.2 Recent sleep duration and behavior during problem discussion
No main effects emerged for recent sleep on either partner’s problem-discussion behavior in reduced models (ps > .09) or fully adjusted models (ps > .45). However, in reduced models (Table 3), interactions between partners’ sleep were statistically significant predictors of positive (p = .01) and negative (p = .003) behavior. These interactions remained significant in fully adjusted models both for positive (p = .01) and negative behavior (p = .01). As shown in Figure 1, when their partner also slept less, people who slept fewer hours behaved less positively compared to people who had slept more (partner sleep = 5.5 average hours; Slope = 0.029, SE = 0.013, p = .02). On the other hand, recent sleep did not significantly predict positive behavior when partners had slept an adequate amount (partner sleep = 7.5 average hours; Slope = − 0.022, SE = 0.015, p = .12). Likewise, when their partner also slept fewer hours, those who had slept less behaved more negatively compared to those who slept more (partner sleep = 5.5 average hours; Slope = −0.025, SE = 0.012, p = .03). The effect of sleep on negative behavior was not significant for those whose partners had slept a normal amount (7.5 average hours; Slope = 0.021, SE = 0.014, p = .13). These conditional effects of both partners’ sleep on conflict behavior were not moderated by gender (ps > .35).
Table 3.
Recent sleep duration and behavior during marital problem discussion
| % Negative behavior Reduced | % Negative behavior Fully Adjusted | % Positive behavior Reduced | % Positive behavior Fully Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Intercept | 0.104 | 0.018 | < .0001 | 0.114 | 0.018 | <.0001 | 0.860 | 0.019 | < .0001 | 0.847 | 0.019 | <.0001 |
| Visit | −0.009 | 0.011 | .40 | −0.014 | 0.011 | .21 | 0.014 | 0.012 | .24 | 0.017 | 0.012 | .14 |
| Meal | −0.016 | 0.011 | .14 | −0.010 | 0.011 | .38 | 0.010 | 0.012 | .40 | 0.007 | 0.012 | .59 |
| Gender | −0.020 | 0.011 | .08 | 0.026 | 0.012 | .03 | ||||||
| Own satisfaction | −0.0006 | 0.0004 | .20 | 0.0006 | 0.0004 | .21 | ||||||
| Own depression | 0.003 | 0.002 | .08 | −0.001 | 0.002 | .49 | ||||||
| Partner depression | 0.005 | 0.002 | .005 | −0.003 | 0.002 | .05 | ||||||
| Own chronic sleep | 0.0003 | 0.006 | .95 | −0.007 | 0.006 | .25 | ||||||
| Partner chronic sleep | −0.002 | 0.005 | .66 | −0.003 | 0.006 | .61 | ||||||
| Own prior two night sleep | −0.002 | 0.009 | .84 | 0.003 | 0.009 | .78 | 0.005 | 0.010 | .60 | −0.002 | 0.010 | .84 |
| Partner prior two night sleep | 0.005 | 0.009 | .57 | 0.012 | 0.009 | .21 | −0.001 | 0.009 | .91 | −0.011 | 0.010 | .29 |
| Own X partner two night sleep | 0.027 | 0.009 | .003 | 0.023 | 0.009 | .01 | −0.028 | 0.010 | .005 | −0.026 | 0.010 | .009 |
Note. Because gender interactions were nonsignificant, these terms were trimmed from final models and are not listed. All models included subject-level intercepts and couple-specific effects
Figure 1.
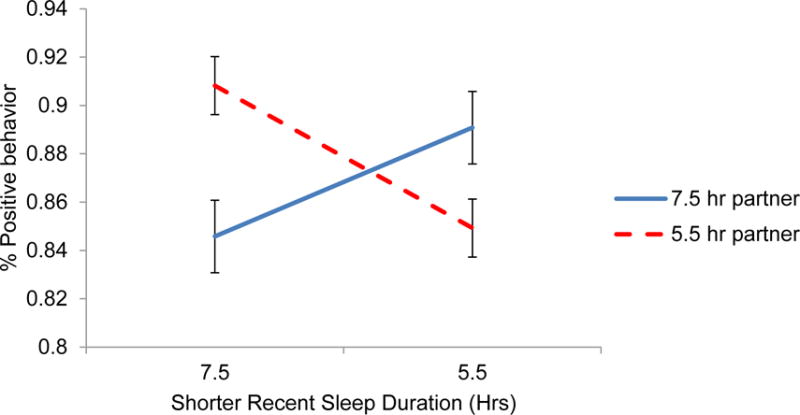
One’s own shorter sleep duration in hours predicting percent of positive behavior displayed during the marital problem discussion, conditional on the partner’s sleep duration. The simple slope of sleep and positive behavior for those whose partner slept 7.5 hours (solid line) was not significant (p = .12). The sleep-behavior simple slope for those whose partner slept 5.5 hours (dashed line) was significant (p = .02).
3.3 Recent sleep duration and regulation during problem discussion
3.3.1 Regulation as outcome of recent sleep duration
In reduced models, sleep did not relate to one’s own emotion expression or reappraisal strategies during conflict (ps > .21). However, the reduced model indicated that people’s shorter sleep significantly predicted their partner’s use of less reappraisal during conflict (B = 0.44, SE = 0.207, p = .04). In reduced models, the interaction of both partners’ sleep did not predict their emotion expression or reappraisal during conflict (ps > .59), but gender did significantly moderate the effect of sleep on the partner’s reappraisal (B = −0.880, SE = 0.409, p = .03). Specifically, self-reported reappraisal during conflict was lower in women whose husbands had slept less (Slope =0.845, SE = 0.281, p = .003), but wives’ sleep was not related to husbands’ reappraisal (p = .91). However, after adjusting for covariates, neither partner’s sleep significantly predicted use of emotion expression or reappraisal strategies during conflict (ps > .44). Interactions were also non-significant in fully adjusted models (ps > .11).
3.3.2 Regulation as moderator of recent sleep duration and post-conflict inflammation
In the reduced model, the effect of recent sleep on post-conflict IL-6 was significantly moderated by people’s own emotion expression (B = 0.027, SE = 0.011, p = .02). The effect remained significant in the fully adjusted model (B = 0.025, SE = 0.011, p = .03). As shown in Panel A of Figure 2, those who expressed less during the problem discussion had higher post-conflict IL-6 production after having slept less compared to people who slept more (Slope = −0.101, SE = 0.031, p = .001). Specifically, low expressers who slept 1 hour less than average had 11% higher IL-6 after conflict. However, high expressers were buffered from the tie between their shorter sleep and higher IL-6 production (p = .52).
Figure 2.
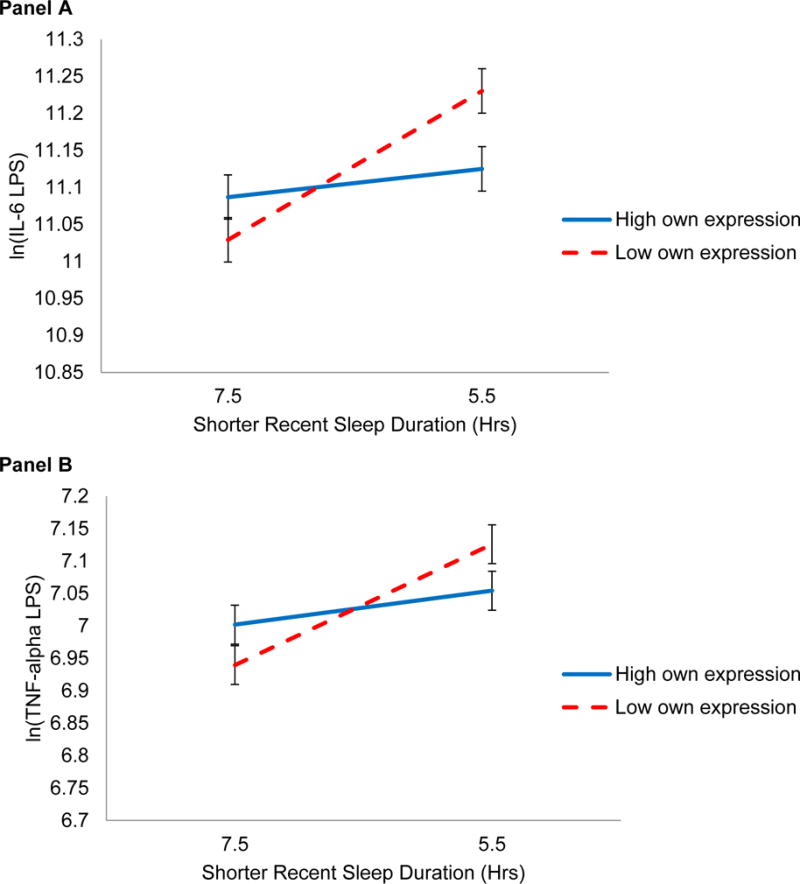
One’s own shorter sleep duration in hours predicting log-transformed LPS-stimulated IL-6 (Panel A) and TNF-α (Panel B) after the marital problem discussion, conditional on one’s own emotion expression during discussion. Panel A shows a significant interaction for IL-6 (p = .03). The simple slope of shorter sleep and IL-6 for those who expressed 1 SD more than average (solid line) was not significant (p = .52). The sleep-inflammation simple slope for those who expressed 1 SD less than average (dashed line) was significant (p = .001). Like IL-6 patterns in Panel A, Panel B shows a nonsignificant simple slope between sleep and TNF-α at high emotion expression (solid line, p = .41). The sleep-inflammation tie was significant at low levels of expression (p = .005), but the difference in slopes was not statistically significant (p = .09).
The same protective effect arose for partner emotion expression in the link between own sleep and IL-6 in the reduced model (B = 0.033, SE = 0.014, p = .02) and remained significant in the fully adjusted model (B = 0.035, SE 0.014, p = .02). That is, partners’ low expression left individuals vulnerable to the effect of their short sleep on IL-6 (Slope = −0.106, SE = 0.031, p = .001). Specifically, people with less expressive partners who also slept 1 hour less than average had 11% higher IL-6. On the other hand, people with highly expressive partners were protected from the proinflammatory effects of their own short sleep (p = .80).
In the reduced model, the buffering effect of one’s own emotion expression on sleep and TNF-α arose as a trend (B = 0.020, SE = 0.012, p = .08), consistent with patterns seen in IL-6. As shown in Panel B of Figure 2, this trend remained after controlling for important additional covariates in the fully adjusted model (Binteraction = 0.020, SE = 0.012, p = .09; Slopelow expression = − 0.093, SE = 0.032, p = .005; phigh expression = .41). Partner expression did not moderate the effect of sleep on TNF-α (p = .37).
In the reduced model, partner reappraisal moderated the effect of sleep duration on IL-6 at a trend level (B = 0.015, SE = 0.008, p = .07). As shown in Figure 3, this effect was statistically significant after controlling for important covariates in the fully adjusted model (B = 0.018, SE = 0.008, p = .03), such that those whose partners used less reappraisal had greater IL-6 production after sleeping less (Slope = −0.090, SE = 0.029, p = .002). People with low-reappraising partners who slept 1 hour less than average also had 9% higher IL-6 production. However, those whose partners reappraised more during the discussion did not see significant sleep-related differences in post-conflict IL-6 (p = .98). In reduced and adjusted models, own reappraisal did not moderate the link between sleep and IL-6, nor did own or partner reappraisal moderate effects on TNF-α (ps > .62). Moderating effects of expression and reappraisal did not differ by gender (ps > .46).
Figure 3.
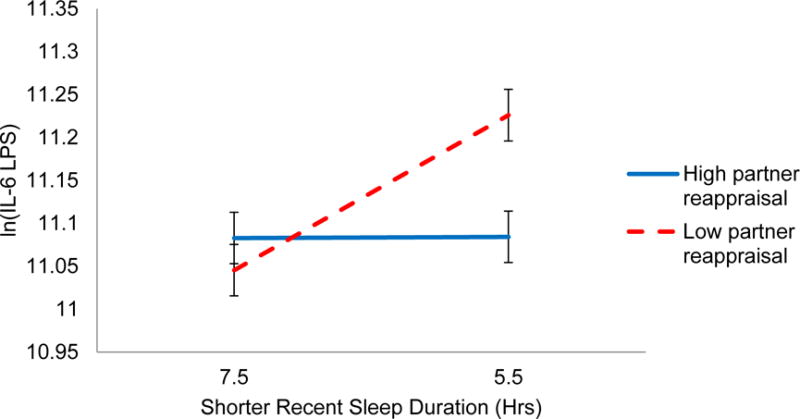
One’s own shorter sleep duration in hours predicting log-transformed LPS-stimulated IL-6 after the marital problem discussion, conditional on one’s partner’s reappraisal during discussion. The simple slope of shorter sleep and IL-6 for those whose partners reappraised 1 SD more than average (solid line) was not significant (p = .98). The sleep-inflammation simple slope for those whose partners reappraised 1 SD less than average (dashed line) was significant (p = .002).
3.4 Ancillary analyses: Testing chronic sleep problems (PSQI) as predictors
Both partners’ past-month sleep problems (PSQI scores) were included as covariates in all previous adjusted analyses. As shown in Tables 2 and 3, past-month sleep problems were not significant predictors of any outcomes in the models that included recent sleep duration. However, since recent and past-month sleep were moderately correlated (r = −.44), we conducted exploratory analyses to assess the effect of past-month sleep on outcomes, excluding recent sleep duration as predictors. Parallel to the models that used recent sleep, we tested the interaction between both partners’ past-month sleep problems and moderation by emotion regulation variables. People’s past-month sleep problems did not significantly relate to their own outcomes (ps > .28), but were related to their partner’s higher morning TNF-α (B = 0.050, SE = 0.023, p = .03), higher post-conflict TNF-α (B = 0.026, SE = 0.012, p = .03) and higher post-conflict IL-6 (B = 0.020, SE = 0.010, p = .05). The link between chronic sleep problems and the partner’s post-conflict IL-6 was conditional on partner reappraisal (B = −0.009, SE = 0.004, p = .01). That is, when people did not reappraise much, the effect of their poor sleep on their partner’s post-conflict inflammation was significant (B = 0.045, SE = 0.014, p = .002), but when they made greater use of reappraisal strategies, the effect was not significant (p = .92). The effect of sleep problems on their partner’s post-conflict TNF-α was not moderated by either partner’s emotion regulation (ps > .40). In summary, more chronic sleep problems related to the partner’s higher morning and post-conflict inflammation, whereas shorter recent sleep duration related to people’s own post-conflict inflammation. The protective effects of emotion regulation were evident for chronic sleep problems only in the case of partner reappraisal and IL-6—a less consistent pattern than for recent sleep duration. Unlike recent sleep, links between chronic sleep problems and conflict behavior were non-significant.
4. Discussion
People who slept fewer hours in the past two nights had higher inflammatory responses following marital conflict than those who slept more, despite the fact that baseline inflammation levels did not differ. Couples’ recent sleep also related to their marital problem discussion: when both partners had slept less, they behaved more negatively and less positively during conflict. However, they were protected from short sleep’s behavioral effects if one of the partners had sufficient sleep in the two prior nights. Furthermore, despite shorter sleep, couples who made greater use of emotion expression and cognitive reappraisal did not show increased stimulated cytokine production. These findings suggest that the risks of short sleep in daily life lie in heightened inflammatory sensitivity to stressors rather than elevated next-morning inflammation, and that these risks depend on both partners’ sleep and emotion regulation strategies.
Among our sample of healthy adult couples, sleep duration from the previous two nights spanned a wide range (3.5–9 hours), and almost half fell below the 7-hour threshold recommended to maintain health and well-being (Consensus Conference et al., 2015). Thus, the prevalence of naturally-occurring short sleep made this sample well-suited to extend the growing literature on the inflammatory risks of sleep problems (Irwin et al., 2016). Those who slept fewer hours had higher IL-6 and TNF-α after the marital problem discussion compared to their more rested counterparts. These results suggest that recent nights without sufficient sleep may promote a person’s inflammatory responses to interpersonal conflict and perhaps other stressors. Previous studies have demonstrated the effects of sleep-loss-related stressor reactivity (i.e., via the sympathetic nervous system, SNS) to challenging computer-based cognitive tasks after extreme sleep deprivation (Liu et al., 2015; O’Leary et al., 2015). Furthermore, examining responsiveness to marital conflict around a preexisting disagreement, a personally meaningful stressor, enhanced the external validity of the sleep-reactivity association. Interpersonal tensions are among the most common stressors in daily life and are particularly noxious for well-being compared to stressors of a non-interpersonal nature (Almeida, 2005). Thus, effects observed in this study, the ties between short sleep and greater stressor reactivity, are relevant to many people’s daily lives. Moreover, this finding extends the reach of short sleep’s effects from sympathetic reactivity, to inflammatory responses to stressors, a key pathway in the development of many chronic conditions.
As an observational study of recent sleep duration and inflammation, adjusting for potential confounds was important for interpretation. Effects of acute sleep duration were independent of both partners’ chronic sleep problems, marital satisfaction, and depression, thus helping to isolate the roles of acute sleep and conflict. In our sample, PSQI scores and acute sleep duration were moderately correlated but not redundant (r = −.44). Thus, we considered including PSQI scores as a covariate to serve as a strong test for acute sleep duration’s effects. Shorter sleep duration also related to increased inflammation regardless of how positive or negative the problem discussion was.
Cross-sectional studies have shown relationships between partners’ chronic sleep problems and self-reported physical health and well-being (Strawbridge et al., 2004). Yet, after accounting for the effects of the person’s own sleep, such a link did not emerge between partners’ recent sleep duration and pre-conflict inflammation, nor did partners’ short sleep exacerbate the effects of one’s own sleep. Hence, partners’ recent short sleep may impact inflammation through disturbance to one’s own sleep, or may simply be overshadowed by the effect of one’s recent sleep.
Unlike the significant effects for acute sleep on post-conflict inflammation, there was no link between short-term sleep duration and morning baseline cytokine production. A recent meta-analysis found that greater sleep disturbance, not self-reported sleep duration or experimental sleep deprivation, was associated with higher IL-6 (Irwin et al., 2016). Null effects for next-morning inflammation have been reported after one night of total sleep deprivation (IL-6, Frey et al., 2007), four days of two 2-hour naps (IL-6 and TNF-α, Shearer et al., 2001), seven nights of 6-hour sleep (null TNF-α effects in women, Vgontzas et al., 2004), and twelve nights of 4-hour sleep (TNF-α, Haack, 2007). Further, Irwin and colleagues (2008) showed that morning inflammatory increases following one night of 4-hour sleep loss resolved after a single night of undisturbed sleep. Taken together, the inflammatory risk of acute sleep duration may lie primarily in a heightened sensitivity to stressors rather than elevated next-morning inflammation. Upstream markers of transcriptional activation may also be more sensitive to acute sleep patterns (e.g. Irwin et al., 2006).
The effect of recent sleep on marital interaction depended on both partners: people were more negative and less positive with their spouse when both they and their partner had slept less than the recommended amount. That is, one’s own short sleep alone did not automatically lead to negative behavior, even though the conflict presented an opportunity. This finding expands on neuroimaging evidence showing that people misinterpret social cues and inadequately convey emotions after sleep loss (Schwarz et al., 2013; Yoo et al., 2007); indeed, a well-rested partner may help to neutralize disagreements despite the other’s sleep-related vulnerability to conflict. Likewise, this pattern extends previous couples data demonstrating that lower quality, less efficient sleep leads to more troubled marital interactions marked by a reduced ability to judge the other person’s emotions (Gordon and Chen, 2014; Hasler and Troxel, 2010). In short, the joint effect of both partners’ sleep on behavior underscores the necessity of a dyadic approach to understand the social and behavioral consequences of short sleep duration.
We found that emotion regulation strategies were not directly linked to short sleep, but instead protected against its negative consequences. This finding corroborates the literature identifying regulation as a buffer rather than an outcome of sleep (Cote et al., 2015; Schwarz et al., 2013). It is also the first study, to our knowledge, showing that emotion regulation use alters ties between sleep and inflammation. According to the process model of emotion regulation (Sheppes and Gross, 2011), cognitive reappraisal can inhibit emotional responses and, therefore, lead to reduced physiological reactivity and more adaptive social outcomes (John and Gross, 2004). Prior work has shown that reappraisal can reduce one’s partner’s blood pressure reactivity (Butler, 2003), consistent with our finding that this strategy buffered the partner’s heightened inflammation.
Expressing feelings more and holding back less protected both partners from increased inflammation following short sleep and conflict. Research has demonstrated that couples’ emotion expression may be health-promoting while emotion suppression may be stressful and unhealthy (Robles et al., 2014). Our data provide a novel mechanism that may explain these links via sleep and inflammation. Specifically, intimacy-building emotion expression (Laurenceau et al., 2005) appeared to neutralize elevations in IL-6. On the other hand, neither reappraisal nor expression protected against higher post-conflict TNF-α levels following shorter sleep. Just as some studies have shown inconsistent responses to acute psychological stressors for TNF-α compared to IL-6 (Steptoe et al., 2007), this cytokine may likewise respond differently than IL-6 to psychological buffering after elevation.
Secondary analyses revealed that chronic sleep problems were important for the partner’s inflammation and inflammatory responsiveness to conflict, when both partners’ acute sleep duration were removed as predictors. Half of our sample exceeded the clinical cutoff for poor sleep quality (PSQI ≥ 5), so the nonsignificant relationship between chronic sleep problems and a person’s own inflammation was not due to restricted range. However, the fact that participants were screened and excluded for many poor-sleep-related chronic health problems (e.g., diabetes, heart disease) may have reduced this effect. On the other hand, sleep problems may affect the partner in ways that cannot easily be controlled, through sharing a bed and daily life together. Dyadic work has shown that when one partner has a health problem, the other is at greater risk for developing it as well (Kiecolt-Glaser and Wilson, in press); conflict-based inflammatory responsiveness to partners’ chronic sleep problems may be one mechanism of couples’ illness contagion. Future work should follow couples longitudinally to determine whether inflammatory responsiveness mediates the transmission of chronic sleep problems and inflammation-based disease.
Unlike recent sleep duration, poorer chronic sleep predicted higher morning baseline TNF-α and IL-6 for the partner. A meta-analysis also found subjective sleep disturbance to be a significant predictor of inflammation levels (Irwin et al., 2016). When people used reappraisal strategies, their chronic sleep problems did not relate to the partner’s inflammation, but emotion regulation did not otherwise predict lower inflammatory risk. Accordingly, chronic sleep problems may be more systemic and less susceptible to emotion regulation efforts in the moment. In sum, couples’ chronic sleep problems may also be associated with post-conflict inflammation and regulation strategies, but these take a different form compared to couples’ recent sleep duration. Chronic sleep disturbances may be more likely to create systemic day-to-day dysfunction that could trigger stressor spillover from other parts of life to the relationship (Vedaa et al., 2016). Because acute periods of short sleep are even more common than chronic sleep dysfunction, we believe the recent sleep duration findings have particularly widespread implications for couples’ long-term health.
Though the present study extends the sleep-inflammation literature in new directions, aspects of our sleep measurement have limitations. Specifically, participants reported their sleep duration. Though self-reported sleep duration predicts important health outcomes (Mullington et al., 2009), objective measurement of sleep with polysomnography represents the gold standard. Likewise, although the sheer number of hours is a critical feature of acute sleep, many other features could arise as important for inflammatory processes—e.g. sleep efficiency, quality, and disturbances.
In conclusion, the current study’s findings suggest that shorter recent sleep relates to elevated inflammatory responses to couple conflict, a common stressor. Using emotion expression and cognitive reappraisal during conflict effectively guarded against heightened inflammation following short sleep. Couples behaved more negatively and less positively only when both partners had slept less. This study contributes to a growing literature identifying mechanisms that link sleep to long-term health risks (Krietsch et al., 2014). Findings also point to the value of a dyadic approach for understanding the dynamics of sleep and inflammation.
Highlights.
Couples’ shorter sleep duration related to higher stimulated cytokine production after marital conflict.
People who slept less behaved more negatively and less positively only when their partner had also slept less.
One’s own and one’s partner’s use of emotion regulation strategies during conflict buffered short-sleep-related inflammatory reactivity.
Acknowledgments
This work was supported in part by NIH grants R01 CA186720, K05 CA172296, and T32 DE014320, as well as a Pelotonia Postdoctoral Fellowship from the Ohio State University Comprehensive Cancer Center and American Cancer Society Postdoctoral Fellowship Grant 121911-PF-12-040-01-CPPB.
References
- Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Curr Dir Psychol Sci. 2005;14:64–68. [Google Scholar]
- Anderson C, Dickinson DL. Bargaining and trust: the effects of 36-h total sleep deprivation on socially interactive decisions. J Sleep Res. 2010;19:54–63. doi: 10.1111/j.1365-2869.2009.00767.x. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. American Psychological Association; Washington D C: 1997. pp. 207–245. [Google Scholar]
- Beattie L, Kyle SD, Espie CA, Biello SM. Social interactions, emotion and sleep: A systematic review and research agenda. Sleep Med Rev. 2015;24:83–100. doi: 10.1016/j.smrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Bradbury TN, Karney BR. Longitudinal study of marital interaction and dysfunction: Review and analysis. Clinical Psychology Review. 1993;13:15–27. [Google Scholar]
- Browne RW, Kantarci A, LaMonte MJ, Andrews CA, Hovey KM, Falkner KL, Cekici A, Stephens D, Genco RJ, Scannapieco FA, Van Dyke TE, Wactawski-Wende J. Performance of Multiplex Cytokine Assays in Serum and Saliva among Community-Dwelling Postmenopausal Women. PLoS ONE. 2013;8:e59498. doi: 10.1371/journal.pone.0059498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA. The social consequences of expressive suppression. Emotion. 2003;3:48–67. doi: 10.1037/1528-3542.3.1.48. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calder PC. N-3 polyunsaturated fatty acids, inflammation and immunity: Pouring oil on troubled waters or another fishy tale? Nutrition Research. 2001;21:309–341. [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consensus Conference P, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015;38:1161–1183. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote K, Jancsar C, Hunt B. Event-related neural response to emotional picture stimuli following sleep deprivation. Psychology & Neuroscience. 2015;8:102–113. [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Funk JL, Rogge RD. Testing the ruler with item response theory: increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. J Fam Psychol. 2007;21:572–583. doi: 10.1037/0893-3200.21.4.572. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Chen S. The role of sleep in interpersonal conflict: Do sleepless nights mean worse fights? Social Psychological and Personality Science. 2014;5:168–175. [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated Inflammatory Markers in Response to Prolonged Sleep Restriction Are Associated With Increased Pain Experience in Healthy Volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Troxel WM. Couples’ nighttime sleep efficiency and concordance: evidence for bidirectional associations with daytime relationship functioning. Psychosom Med. 2010;72:794–801. doi: 10.1097/PSY.0b013e3181ecd08a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman RE. Rapid Marital Interaction Coding System (RMICS) In: Kerig PK, Baucom DH, editors. Couple Observational Coding Systems. Lawrence Erlbaum Associates; Nahwah, New Jersey: 2004. pp. 67–94. [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang MG, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. J Pers. 2004;72:1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihoodsample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am J Psychiatry. 2015a;172:1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology. 2015b;52:239–250. doi: 10.1016/j.psyneuen.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ. Lovesick: How couples’ relationships influence health. Annual Review of Clinical Psychology. 17 doi: 10.1146/annurev-clinpsy-032816-045111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krietsch KN, Mason AE, Sbarra DA. Sleep Complaints Predict Increases in Resting Blood Pressure Following Marital Separation. Health Psychology. 2014;33:1204–1213. doi: 10.1037/hea0000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenceau JP, Barrett LF, Rovine MJ. The interpersonal process model of intimacy in marriage: a daily-diary and multilevel modeling approach. J Fam Psychol. 2005;19:314–323. doi: 10.1037/0893-3200.19.2.314. [DOI] [PubMed] [Google Scholar]
- Liu JC, Verhulst S, Massar SA, Chee MW. Sleep deprived and sweating it out: the effects of total sleep deprivation on skin conductance reactivity to psychosocial stress. Sleep. 2015;38:155–159. doi: 10.5665/sleep.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EL, Talbot LS, Chang K-H, Kaplan KA, Dahl RE, Harvey AG. The Effect of Sleep Deprivation on Vocal Expression of Emotion in Adolescents and Adults. Sleep. 2011;34:1233–1241. doi: 10.5665/SLEEP.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J, Htaik O, Banks S, Dinges D. Emotional expressiveness in sleep-deprived healthy adults. Behav Sleep Med. 2011;9:5–14. doi: 10.1080/15402002.2011.533987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. International Bedroom Poll 2013 [Google Scholar]
- O’Leary ÉD, Howard S, Hughes BM, James JE. Salivaryα-Amylase Reactivity to Laboratory Social Stress With and Without Acute Sleep Restriction. Journal of Psychophysiology. 2015;29:55–63. [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Research & Therapy. 2014;16:1–12. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. APPL PSYCH MEAS. 1977;1:385–401. [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: a meta-analytic review. Psychol Bull. 2014;140:140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JF, Popp R, Haas J, Zulley J, Geisler P, Alpers GW, Osterheider M, Eisenbarth H. Shortened night sleep impairs facial responsiveness to emotional stimuli. Biol Psychol. 2013;93:41–44. doi: 10.1016/j.biopsycho.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Gross JJ. Is timing everything? Temporal considerations in emotion regulation. Pers Soc Psychol Rev. 2011;15:319–331. doi: 10.1177/1088868310395778. [DOI] [PubMed] [Google Scholar]
- Simon EB, Oren N, Sharon H, Kirschner A, Goldway N, Okon-Singer H, Tauman R, Deweese MM, Keil A, Hendler T. Losing Neutrality: The Neural Basis of Impaired Emotional Control without Sleep. J Neurosci. 2015;35:13194–13205. doi: 10.1523/JNEUROSCI.1314-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Shema SJ, Roberts RE. Impact of spouses’ sleep problems on partners. 2004;27:3. doi: 10.1093/sleep/27.3.527. [DOI] [PubMed] [Google Scholar]
- Thoits PA. Patterns in coping with controllable and uncontrollable events. In: Cummings EM, Greene AL, Karraker KH, editors. Life-span developmental psychology: Perspectives on stress and coping. Lawrence Erlbaum Assoc.; Hillsdale, New Jersey: 1991. pp. 235–258. [Google Scholar]
- Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Medicine Reviews. 2007;11:389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedaa Ø, Krossbakken E, Grimsrud ID, Bjorvatn B, Sivertsen B, Magerøy N, Einarsen S, Pallesen S. Prospective study of predictors and consequences of insomnia: personality, lifestyle, mental health, and work-related stressors. Sleep Medicine. 2016;20:51–58. doi: 10.1016/j.sleep.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Xu S, Lorber MF. Interrater Agreement Statistics With Skewed Data: Evaluation of Alternatives to Cohen’s Kappa. J Consult Clin Psychol. 2014 doi: 10.1037/a0037489. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep–a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]


