Abstract
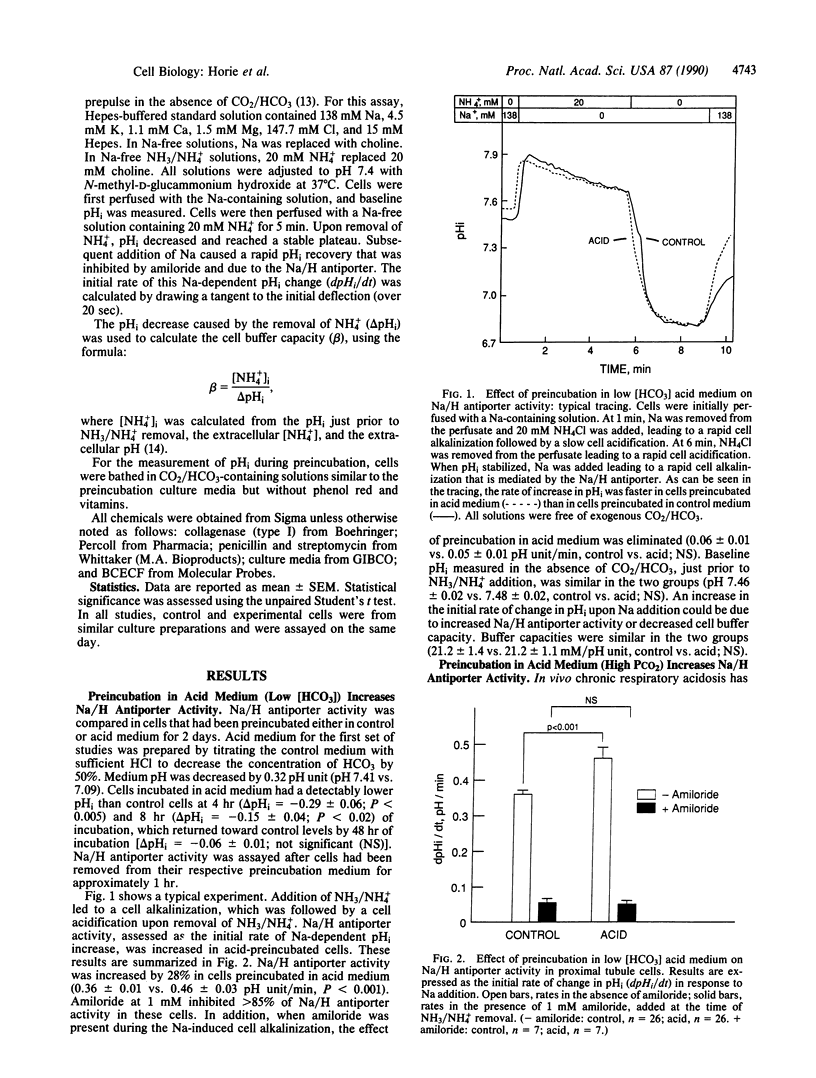
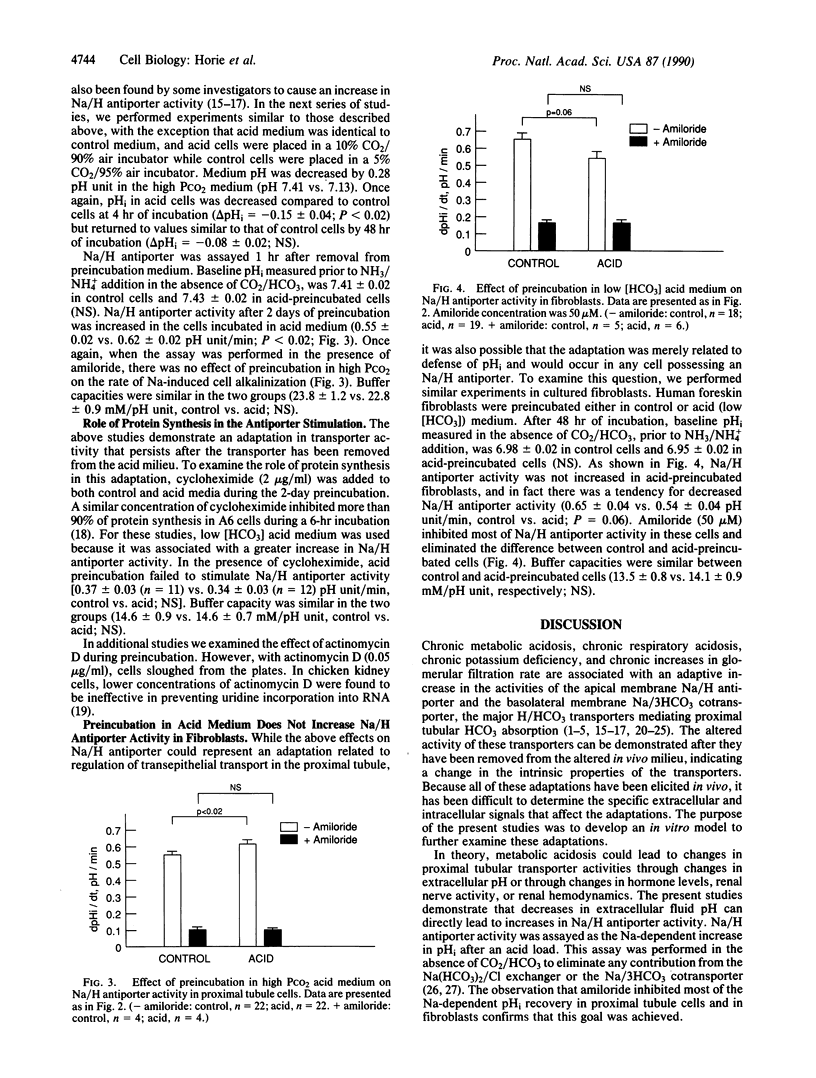
Chronic acidosis in vivo leads to an increase in proximal tubule Na/H antiporter activity that persists when the transporter is studied out of the acidotic environment. It is presently not clear whether a decrease in extracellular fluid pH alone is sufficient to elicit this adaptation. The present studies examined the effect of acid preincubation on Na/H antiporter activity in cultured proximal tubule cells. Antiporter activity was examined after a 2-day preincubation in control or acid medium, 1 hr after removal from the preincubation fluid. Na/H antiporter activity was assayed as the initial rate of Na-dependent alkalinization after cell acidification in the absence of CO2/HCO3. Preincubation in low [HCO3] acid medium or in high PCO2 acid medium led to increases in amiloride-sensitive Na/H antiporter activity. This adaptation was inhibited by addition of cycloheximide to the preincubation medium. Preincubation of fibroblasts in low [HCO3] acid medium did not lead to increased Na/H antiporter activity but rather caused a small inhibition. These studies demonstrate an adaptation in Na/H antiporter activity elicited by a low pH of the extracellular fluid, which is dependent on protein synthesis, and may be unique to certain H/HCO3-transporting epithelia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W. R., Koretsky A. P., Weiner M. W. 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol. 1986 Nov;251(5 Pt 2):F904–F910. doi: 10.1152/ajprenal.1986.251.5.F904. [DOI] [PubMed] [Google Scholar]
- Akiba T., Rocco V. K., Warnock D. G. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987 Aug;80(2):308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Chambers M. Basolateral membrane Cl/HCO3 exchange in the rat proximal convoluted tubule. Na-dependent and -independent modes. J Gen Physiol. 1987 Apr;89(4):581–598. doi: 10.1085/jgp.89.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Reuss E., Weber M. R. Electrophysiological studies on primary cultures of proximal tubule cells. Am J Physiol. 1986 Sep;251(3 Pt 2):F490–F498. doi: 10.1152/ajprenal.1986.251.3.F490. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. D., Alavi N., Livingston D., Hiller S., Taub M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J Cell Biol. 1982 Oct;95(1):118–126. doi: 10.1083/jcb.95.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. E., Hruska K. A., Klahr S., Hammerman M. R. Increased Na+-H+ exchange in brush border vesicles from dogs with renal failure. Am J Physiol. 1982 Sep;243(3):F293–F299. doi: 10.1152/ajprenal.1982.243.3.F293. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Klahr S., Hammerman M. R. Metabolic acidosis and parathyroidectomy increase Na+-H+ exchange in brush border vesicles. Am J Physiol. 1983 Aug;245(2):F217–F222. doi: 10.1152/ajprenal.1983.245.2.F217. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Badie-Dezfooly B., Lowe A. G., Hamzeh A., Wells J., Salehmoghaddam S. Stimulation of Na+/H+ antiport is an early event in hypertrophy of renal proximal tubular cells. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1736–1740. doi: 10.1073/pnas.82.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry C., Grinnell F. Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. J Cell Biol. 1987 Apr;104(4):1097–1103. doi: 10.1083/jcb.104.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty J. G., Agarwal N., Reilly R. F., Adelberg E. A., Slayman C. W. Pharmacologically different Na/H antiporters on the apical and basolateral surfaces of cultured porcine kidney cells (LLC-PK1). Proc Natl Acad Sci U S A. 1988 Sep;85(18):6797–6801. doi: 10.1073/pnas.85.18.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., Seifter J. L., Brenner B. M. Adaptation of Na+-H+ exchange in renal microvillus membrane vesicles. Role of dietary protein and uninephrectomy. J Clin Invest. 1984 Dec;74(6):1979–1987. doi: 10.1172/JCI111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R., Berry C. A., Alpern R. J., Rector F. C., Jr Regulation of cell pH by ambient bicarbonate, carbon dioxide tension, and pH in the rabbit proximal convoluted tubule. J Clin Invest. 1988 Feb;81(2):381–389. doi: 10.1172/JCI113330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R. Mechanisms of adaptation to chronic respiratory acidosis in the rabbit proximal tubule. J Clin Invest. 1989 Mar;83(3):890–896. doi: 10.1172/JCI113973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo P. G., Goelet P., Castellucci V. F., Morgan J., Kandel E. R., Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986 Dec 5;234(4781):1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Hafezi A., Kaunitz J. D., Trizna W., Fine L. G. pH gradient-dependent increased Na+-H+ antiport capacity of the rabbit remnant kidney. Am J Physiol. 1985 Jul;249(1 Pt 2):F90–F98. doi: 10.1152/ajprenal.1985.249.1.F90. [DOI] [PubMed] [Google Scholar]
- Noronha-Blob L., Sacktor B. Inhibition by glucocorticoids of phosphate transport in primary cultured renal cells. J Biol Chem. 1986 Feb 15;261(5):2164–2169. [PubMed] [Google Scholar]
- Preisig P. A., Alpern R. J. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988 Oct;82(4):1445–1453. doi: 10.1172/JCI113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Ruiz O. S., Arruda J. A., Talor Z. Na-HCO3 cotransport and Na-H antiporter in chronic respiratory acidosis and alkalosis. Am J Physiol. 1989 Mar;256(3 Pt 2):F414–F420. doi: 10.1152/ajprenal.1989.256.3.F414. [DOI] [PubMed] [Google Scholar]
- Sakhrani L. M., Badie-Dezfooly B., Trizna W., Mikhail N., Lowe A. G., Taub M., Fine L. G. Transport and metabolism of glucose by renal proximal tubular cells in primary culture. Am J Physiol. 1984 Jun;246(6 Pt 2):F757–F764. doi: 10.1152/ajprenal.1984.246.6.F757. [DOI] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Talor Z., Yang W. C., Shuffield J., Sack E., Arruda J. A. Chronic hypercapnia enhances Vmax of Na-H antiporter of renal brush-border membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F394–F400. doi: 10.1152/ajprenal.1987.253.3.F394. [DOI] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]
- Verrey F., Schaerer E., Zoerkler P., Paccolat M. P., Geering K., Kraehenbuhl J. P., Rossier B. C. Regulation by aldosterone of Na+,K+-ATPase mRNAs, protein synthesis, and sodium transport in cultured kidney cells. J Cell Biol. 1987 May;104(5):1231–1237. doi: 10.1083/jcb.104.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay P., Gougoux A., Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981 Oct;241(4):F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]



