Abstract
BACKGROUND
Alcohol use among adolescents is widespread and a growing concern due to long-term behavioral deficits, including altered Pavlovian behavior, that potentially contribute to addiction vulnerability. We tested the hypothesis that adolescent intermittent ethanol (AIE) exposure alters Pavlovian behavior in males and females as measured by a shift from goal-tracking to sign-tracking. Additionally, we investigated GLT-1, an astrocytic glutamate transporter, as a potential contributor to a sign-tracking phenotype.
METHODS
Male and female Sprague-Dawley rats were exposed to AIE (5g/Kg, intragastric) or water intermittently 2 days on, 2 days off from postnatal day (P) 25 to 54. Around P70, animals began 20 daily sessions of Pavlovian conditioned approach, where they learned that a cue predicted non-contingent reward delivery. Lever pressing indicated interaction with the cue, or sign-tracking, and receptacle entries indicated approach to the reward delivery location, or goal-tracking. To test for effects of AIE on nucleus accumbens excitatory signaling, we isolated membrane subfractions and measured protein levels of the glutamate transporter GLT-1 after animals completed behavior as a measure of glutamate homeostasis.
RESULTS
Females exhibited elevated sign-tracking compared to males with significantly more lever presses, faster latency to first lever press, and greater probability to lever press in a trial. AIE significantly increased lever pressing while blunting goal tracking, as indicated by fewer cue-evoked receptacle entries, slower latency to receptacle entry, and lower probability to enter the receptacle in a trial. No significant Sex-by-Exposure interactions were observed in sign- or goal-tracking metrics. Moreover, we found no significant effects of Sex or Exposure on membrane GLT-1 expression in the nucleus accumbens.
CONCLUSIONS
Females exhibited enhanced sign-tracking compared to males, while AIE decreased goal-tracking compared to control exposure. Our findings support the hypothesis that adolescent binge ethanol can shift conditioned behavior from goal- to cue-directed in Pavlovian conditioned approach, especially in females.
Keywords: Goal tracker, sign tracker, binge, western blot, conditioned Pavlovian approach
Introduction
Alcohol use among adolescents is widespread, with over 35% reporting regular consumption (Bachman et al., 2014). More importantly, about 15% of all teenagers in the United States reported heavy drinking or binge-drinking episodes (Bachman et al., 2014). Binge-level alcohol consumption is associated with elevated risk for numerous adverse outcomes, including increased risky behavior, drug consumption, physiological damage, and potential injury or death. However, equally concerning are the potential long-term effects of adolescent binge alcohol consumption, such as cognitive deficits and substance abuse susceptibility (e.g., Brown et al., 2000b). The brain is rapidly developing during adolescence, including the prefrontal cortex which is tasked with executive control (Luciana, 2013). These long-term consequences may be due to alcohol altering the specific neural development that occurs during adolescence, which continues well into early adulthood (e.g., Vetreno et al., 2016). However, little longitudinal data are available in humans, making it difficult to determine whether these adult deficits are due to adolescent exposure to high levels of alcohol, or to coincident genetic or environmental factors.
In contrast to the causal ambiguity in human studies, longitudinal preclinical studies allow scientists to isolate the effects of adolescent alcohol exposure on behavior and neurobiology in adulthood (Crews et al., 2016). To date, such studies have shown that adolescent intermittent ethanol (AIE) exposure leads to impaired reversal learning (Coleman et al., 2014), enhanced cue-driven behavior (McClory and Spear, 2014), increased ethanol-induced conditioned place preference (Pascual et al., 2012), greater ethanol self-administration [(Pascual et al., 2009, Toalston et al., 2015), but see (Slawecki and Betancourt, 2002)], and resistance to extinction of ethanol self-administration (Gass et al., 2014, Toalston et al., 2015).
Repeated presentation of a cue-associated non-contingent reward can lead to a strong cue bias, as the cue may acquire incentive salience (Berridge and Robinson, 2003). Here we used a Pavlovian conditioned approach (PCA) paradigm with adult male and female rats that previously received 14 binge-level doses of ethanol that spanned the adolescent period. In this paradigm, an otherwise neutral cue reliably precedes reward delivery. Some animals develop a conditioned response to approach the cue despite its non-contingent relationship to the reward; these are termed “sign trackers.” Other animals more predictably approach the reward delivery location during the cue presentation; these are termed “goal trackers.” AIE has previously been shown to enhance sign tracking in males (McClory and Spear, 2014), but the effect in females is unknown. There exists a stark gap in research investigating sex differences on behavior in adulthood as a result of adolescent ethanol exposure. Therefore, we chose to focus this study on potential sex differences in AIE-induced effects on PCA.
Most studies investigating the neurochemical mechanisms of sign-tracking behavior have focused on dopaminergic mechanisms (e.g., Flagel et al., 2007). Another possibility is glutamate signaling, which is regulated by astrocytes and can be altered by addictive drugs such as cocaine (Kalivas, 2009). Astrocytes express many of the same neurotransmitter receptors as neurons, rendering their function potentially vulnerable to drug exposure such as AIE. AIE can alter the morphology (Risher et al., 2015) and number (Koss et al., 2012) of astrocytes. GLT-1 glutamate transporters are primarily expressed on astrocytes and regulate synaptic levels of glutamate, as they are the primary mode of glutamate clearance from the synapse after vesicular release from the presynaptic terminal (Danbolt, 2001, Tzingounis and Wadiche, 2007). Previous studies have shown that repeated exposure to drugs of abuse leads to decreased levels of GLT-1 in the nucleus accumbens (NAcc) (Alajaji et al., 2013, Reissner et al., 2015, Shen et al., 2014, Sondheimer and Knackstedt, 2011). Adolescent alcohol exposure has been shown to increase basal glutamate levels in the NAcc (Carrara-Nascimento et al., 2011), suggesting deficient GLT-1 function or expression. Further, pharmacological intervention that restores levels of NAcc GLT-1 after drug-induced deficits results in less cue-evoked reinstatement of conditioned reinforcers (Shen et al., 2014, Sondheimer and Knackstedt, 2011). These reports suggest that GLT-1 levels are inversely related to cue sensitivity in instrumental responding; as such, we propose that GLT-1 expression may correlate with cue sensitivity in Pavlovian responding. Therefore, we hypothesized that AIE induces a persistent decrease in NAcc GLT-1 expression, contributing to changes in excitatory signaling and altering conditioned behavior to a cue-predictive reward.
Materials and Methods
Subjects and adolescent alcohol exposure
All experimental procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee. Male and female Sprague-Dawley rats were reared from in-house breeders and were maintained in a temperature- and humidity-controlled vivarium with a 12:12 hour light cycle, lights on at 0700. By postnatal day (P) 3, litters were culled to 8–10 pups with 4 or 6 males. Same-sex littermates were weaned to 2 per cage on P21. Adolescent ethanol was administered as previously described (Vetreno et al., 2014). Briefly, beginning on P25, rats were weighed and received 5g/Kg ethanol (IG, 25% v/v in water; AIE) or the equivalent volume of water (CON) once per day on a two-days-on, two-days-off regimen through P54 for a total of 16 doses.
Pavlovian conditioning
At ~P67 (specifically, P64 to P70), rats were given a bottle of 20% sucrose in water for 1 hour in their home cage to familiarize to the reward solution. On the same day, rats were placed into behavioral chambers (Med Associates, St. Albans, VT) for receptacle training. The behavioral chambers were 12″ L × 12.5″ W × 11.5″ H. A house light located near the chamber ceiling and opposite the reward receptacle remained illuminated during the entire session. The reward receptacle was recessed and entries were recorded by photobeam breaks. Receptacle training was performed to familiarize the rats with non-contingent reward delivery into the receptacle. 100μL of 20% sucrose was delivered to the reward receptacle in variable 120–230s intervals. Reward receptacles were checked after the session for any remaining sucrose solution to verify the animal consumed the reward.
At ~P70 (specifically, P67 to P73), rats began PCA training (Palmatier et al., 2014). Sessions were conducted daily, 5 days/week, for 4 weeks. Rats were placed into the behavior box and allowed to habituate for 5 minutes. Trials began with a 30s presentation of a conditioned stimulus (CS+) in the form of an illuminated cue light immediately above an extended lever. Termination of the CS+ consisted of extinguishing the cue light and retracting the lever. CS+ termination coincided with delivery of 100μL 20% sucrose to the reward receptacle. After a variable inter-trial interval (90–210s), a new trial began. Each daily session consisted of 15 trials. Behavioral measures (lever presses, receptacle entries) and conditioning events (CS+ onset, sucrose delivery) were digitally recorded by the instrumentation (Med Associates, St. Albans, VT).
Behavioral data analysis
Behavioral metrics included lever presses, reward receptacle entries, elevation score, latency to lever press, latency to receptacle entry, probability of lever press in a session, and probability to receptacle entry in a session. Lever presses were measured only during CS+ presentation, as lever extension only occurred as part of the CS+. Receptacle entries were the total number of receptacle entries in a session. Elevation score was calculated as the number of receptacle entries during a CS+ presentation less the number of receptacle entries 30s prior; this metric more accurately represented conditioned responses by accounting for differences in activity outside of the CS+ presentation (Besheer et al., 2004). Latencies were measured as the time to lever press or receptacle entry after CS+ onset, with a maximum of the 30s CS+ cue duration. The probability to lever press or receptacle entry in a trial was the number of trials where the behavior occurs divided by 15 (total number of trials), with a probability of 1 indicating the behavior occurring in all trials. CS+ and unconditioned stimulus (US) trials were determined by the first conditioned response in a trial. Within a trial, if an animal pressed the lever first it was recorded as a CS+ trial, if the animal entered the reward receptacle first it was recorded as a US trial, and if the animal did not respond during a trial nothing was recorded.
A tracking score modified from a previous publication (Yager and Robinson, 2015) was calculated to determine goal-tracking, intermediate, and sign-tracking phenotypes. In place of receptacle entries, we used elevation score. As a negative or positive elevation score is indicative of a decrease or increase in cue-specific conditioned responding for the reward receptacle, respectively, the denominator of the conditioned responding component of the tracking score employs the absolute value of the elevation score so as to be representative of a total level conditioned responding. In turn, a negative elevation score in the numerator of this component would appropriately amplify any preference for the cue over the reward receptacle. Further, we found that probability scores for females reached their maximum levels despite AIE exposure history. Therefore, we felt that CS+ and US trials in lieu of probability would be a more effective behavioral component in our study. We calculated tracking scores using the following formula:
An animal with a score between −1.0 and −0.3 is considered a goal-tracker, between −0.29 and +0.29 considered intermediate, and between +0.3 and +1.0 indicates a sign-tracker.
Lever presses, reward receptacle entries, elevation score, latency to lever press, latency to receptacle entry, probability of lever press in a trial, and probability to receptacle entry in a trial were all analyzed using 3-way ANOVA with session as a repeated measure and sex and adolescent exposure as between-subjects factors. We calculated Pearson’s correlation coefficients for elevation score against lever presses to determine any effects of sex or adolescent exposure on potential relationships between cue- and receptacle-approach behaviors.
Quantification of GLT-1 in the nucleus accumbens
24 hours after the final training session, rats were euthanized by decapitation, brains were removed, and the NAcc was dissected. A crude membrane subfraction (P2) was prepared from the freshly dissected NAcc tissue (Knackstedt et al., 2010). Crude membrane P2 pellets were resuspended in 30μL of 1x RIPA buffer supplemented with 1% SDS and 1:100 Halt protease inhibitor cocktail containing EDTA (Thermo Scientific, Waltham, MA) and centrifuged 12,000 × g for 10 min at 4°. Supernatant was recovered, and protein content was determined using the BCA Assay (Thermo Scientific). Twelve microgram of protein from each sample were separated onto two 7.5% or 12.5% BioRad Criterion gels (Bio-Rad Laboratories, Hercules, CA), and transferred onto PVDF membranes. In a separate experiment, a protein loading dose response curve was generated to verify our loading was within the linear range by increasing the amount of P2 membrane protein loaded from 3 to 15μg. Membranes were blocked with Odyssey blocker in TBS (LI-COR, Lincoln, NE) and probed with primary antibodies against GLT-1 (Millipore #AB1783, 1:1000; Merck Millipore, Billerica, MA) and calnexin (Enzo #ADI-SPA-860-D, 1:4000; Enzo Life Sciences, Inc., Farmingdale, NY) overnight at 4°. Fluorescent secondaries 800CW anti-guinea pig and 680RP anti-rabbit from LI-COR were used at 1:15,000 for 1.5 h at room temperature. Westerns were imaged and band densities quantified on a LI-COR Odyssey Fc imaging system. Protein content was measured as the ratio of GLT-1 to the protein-loading control calnexin for each sample.
Quantification of blood ethanol concentration (BEC)
To assess BEC, a separate group of 23 male and 26 female Sprague-Dawley rats were bred and weaned as described above. On P35, animals received 5g/Kg ethanol (IG, 25% v/v in water or the equivalent volume of water 15, 30, 60, or 90 minutes before euthanasia when tail and trunk blood samples were collected. Tail blood was collected by cutting the tip of the tail with a razor blade and depositing the blood into a heparin-lined tube. Immediately following tail blood collection, animals were rapidly decapitated for collection of trunk blood. Samples were immediately placed on ice, centrifuged at 1,700×g for 4 minutes, then plasma was collected and stored at −80 degrees C until analysis. BEC was measured using a 5μL plasma sample in an Analox-AM1 (Analox Technologies, Atlanta, GA, USA). Statistical analyses of BEC were performed using a blood source-by-sex-by-time 3-way ANOVA. One trunk-blood sample was eliminated from analysis as an outlier using Grubbs’ test for statistical outliers.
Results
Weights and Blood Ethanol Concentrations
Body weight on days of AIE exposure was measured and analyzed using day-by-exposure 2-way ANOVA with day as a repeated measure and exposure a between-subjects factor. Males and females were analyzed separately due to expected weight disparity between sexes. There was no main effect of AIE in the female group (F1,22=0.574, p=0.459) nor a significant day-by-exposure interaction (F15,30=0.803, p=0.674). Males exhibited no main effect of AIE (F1,22=2.8, p=0.108), however there was a significant day-by-exposure interaction (F15,330=4.5, p<0.01) indicating a smaller increase in body weight over exposure days as a response to AIE. Though body weight lagged in AIE exposed males during adolescent exposure, no significant differences were observed when comparing weight on the first day of behavior testing (Male: t22=0.702; Female: t22=0.982), suggesting no long-term effects of AIE on body weight.
BEC was measured following IG ethanol in a separate group of adolescent rats on P35. Male animals weighed 118.4 ± 4.2 g (mean ± SEM), females weighed 99.6 ± 3.0 g. We collected tail- and trunk-blood at 15, 30, 60, and 90min time points from males and females. Peak BEC, 242±15.4mg/dL for males and 234±17.7mg/dL for females, occurred at 60min post-gavage for both sexes in trunk and tail blood samples (Table 1). The 3-way ANOVA of blood source-by-sex-by-time revealed that BEC varied based on the source of the blood and the time point at which the blood was taken. There was a significant blood source-by-time interaction (F3,39 = 12.669, p<0.001), and post-hoc analysis indicated that BEC in tail blood was lower than trunk blood samples at the 15, 30, and 60min time points. In addition, BEC in tail blood samples increased significantly from the 15 to the 60min time point. BEC in trunk blood samples taken at 30 and 90min were significantly lower than those from samples taken at 60min. No differences emerged between trunk- or tail-blood across sex. There was a blood source-by-sex interaction (F1,39 = 9.178, p=0.004), but post-hoc analysis using Tukey’s HSD indicated that tail and trunk blood samples differed within sex but not between sexes.
Table 1.
Blood ethanol concentrations from trunk and tail blood following 5g/Kg I.G. ethanol in adolescent rats.
| Malea | Femalea | |||
|---|---|---|---|---|
|
| ||||
| Trunk | Tail | Trunk | Tail | |
| 15 minutesb,c,e | 201.1 ± 21.7 | 165.5 ± 18.1 | 184.8 ± 5.6 | 149.4 ± 5.1 |
| 30 minutesb,c,d,e | 190.9 ± 6.7 | 161.4 ± 9.2 | 174.2 ± 15.4 | 164.3 ± 16.7 |
| 60 minutesb | 242.0 ± 15.4 | 217.8 ± 15.8 | 234.3 ± 17.7 | 232.6 ± 20.3 |
| 90 minutesc,d | 183.0 ± 21.7 | 178.9 ± 19.8 | 186.6 ± 11.8 | 179.9 ± 11.1 |
Data are presented as mg/dL ± SEM.
significant difference between trunk and tail blood within sex, collapsed across time points, p<0.01;
significant difference between tail and trunk blood at this time point, collapsed across sex, p<0.05;
significant difference in tail blood at this time point from tail blood taken at 60 minutes, collapsed across sex, p<0.05;
significant difference in trunk blood at this time point from trunk blood taken at 60 minutes, collapsed across sex, p<0.05;
significant difference in tail blood at this time point from trunk blood taken at 60 minutes, collapsed across sex, p<0.05.
Sign- and Goal-Tracking
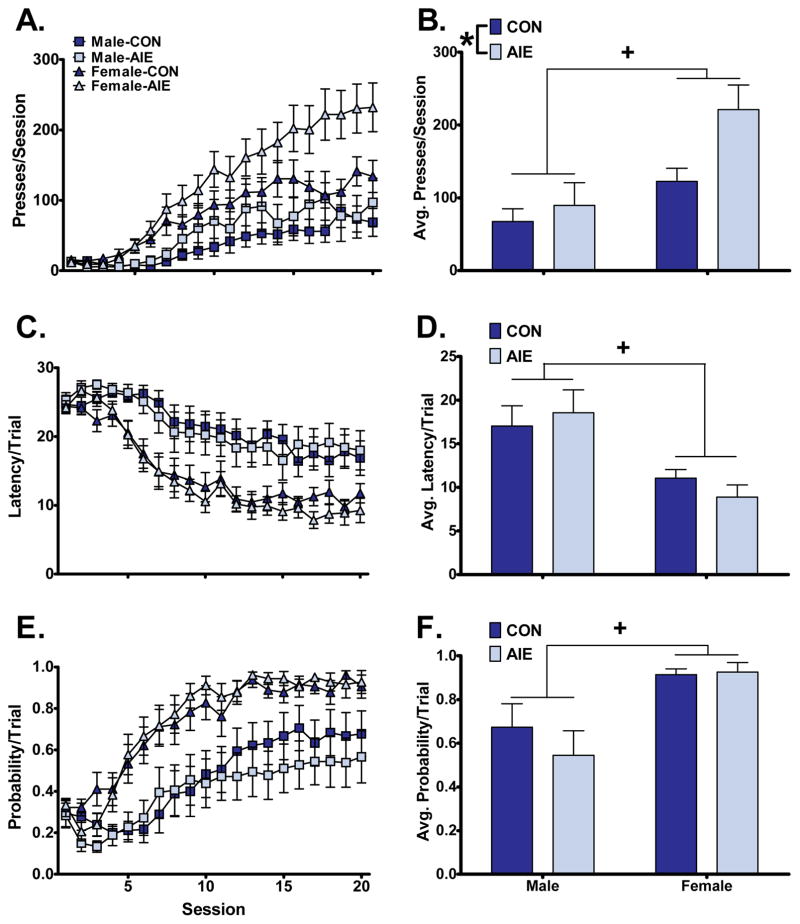
Sign-tracking was measured as lever pressing over 20 training sessions. The final five sessions were averaged and analyzed for effects of sex and AIE history (Figure 1). We found a strong effect of sex on sign-tracking, with females exhibiting elevated lever presses per session, faster latency to lever press, and increased probability of lever pressing in a trial compared to males. We observed an isolated effect of AIE history to increase lever pressing in both sexes (Figure 1). Analysis of lever presses revealed significant main effects of sex (F1,44=12.67, p<0.001) and AIE exposure (F1,44=5.31, p<0.05). Moreover, we found significant main effects of sex on latency to lever press (F1,44=16.29, p<0.001) and lever-press probability (F1,44=14.27, p<0.001). In contrast, there was no significant main effect of AIE on either latency to lever press or lever-press probability (F1,44=0.03 and F1,44=0.50, respectively). Furthermore, analyses revealed no significant sex-by-exposure interactions for any sign-tracking metric (lever presses: F1,44=2.14; latency to lever press: F1,44=0.91; lever-press probability: F1,44=0.74).
Figure 1.
Females exhibited elevated sign-tracking compared to males across multiple behavioral metrics, while AIE increased lever pressing. Left: sign-tracking across all 20 training sessions as measured by lever presses (A), latency to lever press (C), and probability to lever press (E). Right: the average of the last 5 sessions for lever presses (B), latency to lever press (D), and probability to lever press (F). (+, p<0.01 between sexes, *, p<0.05 between exposures).
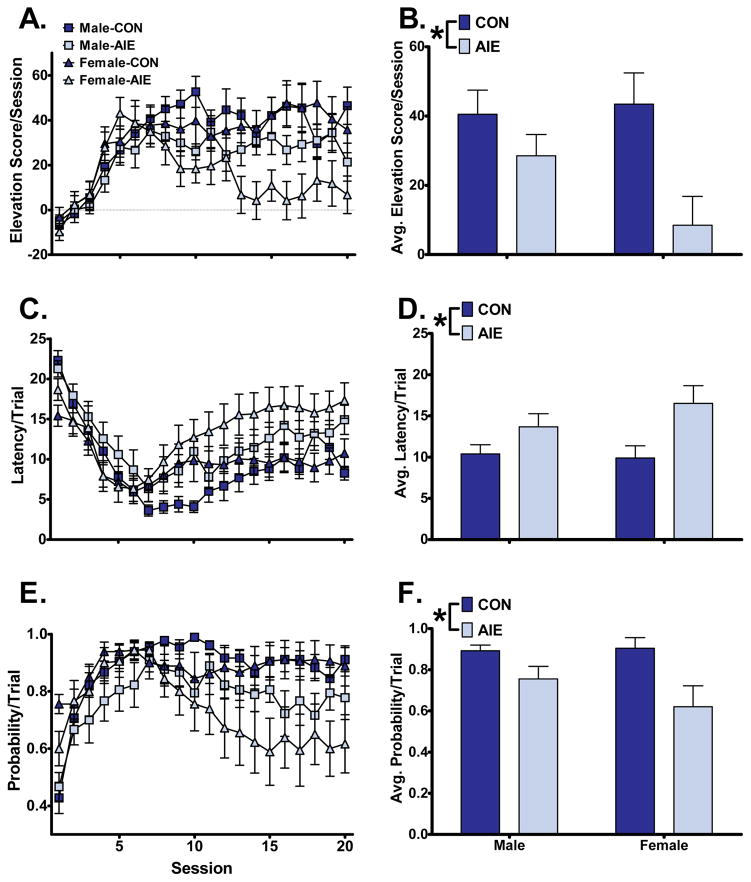
Goal-tracking was measured as reward receptacle entries during the CS+. As with sign-tracking, we performed 2-way sex-by-exposure ANOVA on the average of the last five sessions for elevation score, latency to receptacle entry, and probability of receptacle entry. In contrast to sign-tracking observations, AIE largely affected goal-tracking metrics (Figure 2). Main effects of AIE exposure emerged for elevation score (F1,44=9.26, p<0.01), receptacle latency (F1,44=9.16, p<0.01) and receptacle probability (F1,44=10.14, p<0.01). We observed no significant main effects of sex on goal-tracking (elevation score: F1,44=1.23; receptacle latency: F1,44=0.52; receptacle probability: F1,44=0.87) or sex-by-exposure interactions (elevation score: F1,44=2.23; receptacle latency: F1,44=1.05; receptacle probability: F1,44=1.25).
Figure 2.
AIE decreased all behavioral metrics of goal-tracking. Left: goal-tracking across all 20 training sessions as measured by elevation score (A), latency to receptacle entry (C), and probability to receptacle entry (E). Right: the average of the last 5 sessions for elevation score (B), latency to receptacle entry (D), and probability to receptacle entry (F). (*, p<0.05 between exposures).
In an additional analysis, we investigated individual behavioral variability to address the potential contribution of the female estrous cycle to our observed effects. Specifically, if the 4-day estrous cycle altered PCA behavior, then we would expect females to display more day-to-day variability in behavior than males (Prendergast et al., 2014). To assess this, the coefficient of variance (i.e. standard deviation divided by the mean) for the last five sessions in all behavioral metrics for each animal were calculated and then averaged within groups (Table 3). We found that in most behavioral metrics females exhibited less day-to-day variability than males. Thus, it is unlikely that estrous cycle contributed to behavioral variability in this study.
Table 3.
Average coefficients of variance of behavioral metrics in male and female rats.
| Sign-Tracking | Goal-Tracking | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Lever Presses | Lever Latency | Lever Probability | Elevation Score | Receptacle Latency | Receptacle Probability | |
| Male-CON | 59.7 | 15.4 | 40.6 | 44.2 | 35.1 | 11.0 |
| Male-AIE | 67.7 | 17.6 | 48.5 | 49.1 | 32.7 | 24.9 |
| Female-CON | 29.3 | 24.7 | 10.9 | 28.9 | 25.1 | 14.7 |
| Female-AIE | 18.8 | 22.9 | 8.2 | 46.7 | 25.5 | 35.4 |
Data presented as % standard deviation/mean.
Sign- and goal-tracking metrics were compiled to generate a tracking score to determine the impact of AIE on inducing a sign-tracking phenotype in our experimental groups (Table 2). We observed an overall increase in the number of sign-trackers when administered AIE, 20.84% of CON exposed and 45.85% of AIE exposed. However, AIE had no effect on the number of male sign-trackers, 16.67% of both CON and AIE exposed males were sign-trackers. Conversely, females were largely impacted by AIE exposure, 25% of CON females and 75% of AIE females were sign-trackers.
Table 2.
PCA tracking scores in male and female rats after adolescent alcohol or control exposure.
| Male | Female | |||
|---|---|---|---|---|
|
| ||||
| CON | AIE | CON | AIE | |
| GT | 25 | 41.67 | 8.33 | 0 |
| Int. | 58.33 | 41.67 | 66.67 | 25 |
| ST | 16.67 | 16.67 | 25 | 75 |
Data are presented as % animals within each group. Goal Tracker (GT), tracking score < −0.3; Intermediate (Int.), tracking score −0.29 to +0.29; Sign Tracker (ST), tracking score > 0.3.
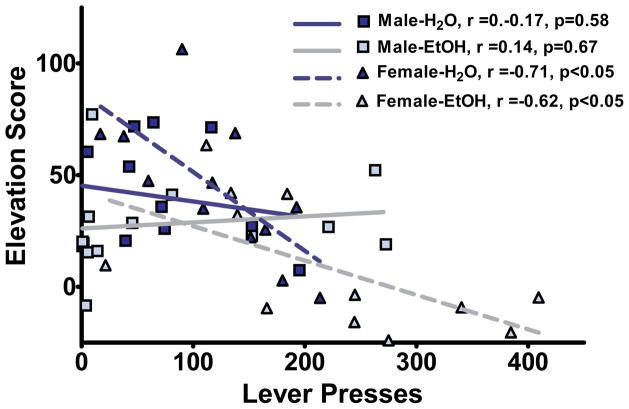
To determine if sign- and goal-tracking were independent behaviors or highly correlated, and if this relationship was changed by AIE exposure, we plotted individual elevation scores against lever presses (Fig. 3) and calculated Pearson correlations. We observed significant correlations in the female groups (Female-CON: r = −0.706, p<0.05; Female-AIE: r = −0.623, p<0.05), whereas neither male CON nor AIE groups exhibited significant correlations (Male-CON: r = −0.177; Male-AIE: r = 0.138). These results suggest that in males sign- and goal-tracking are independent behaviors whereas they are inversely correlated in females. Further, these relationships, or lack thereof, are unaltered by AIE.
Figure 3.
Females, but not males, exhibit a correlation between sign- and goal-tracking. Using lever pressing and elevation score as metrics for correlation, both CON and AIE females express significant sign- and goal-tracking behavioral correlations, with no correlation observed in either male group.
Membrane Expression of GLT-1
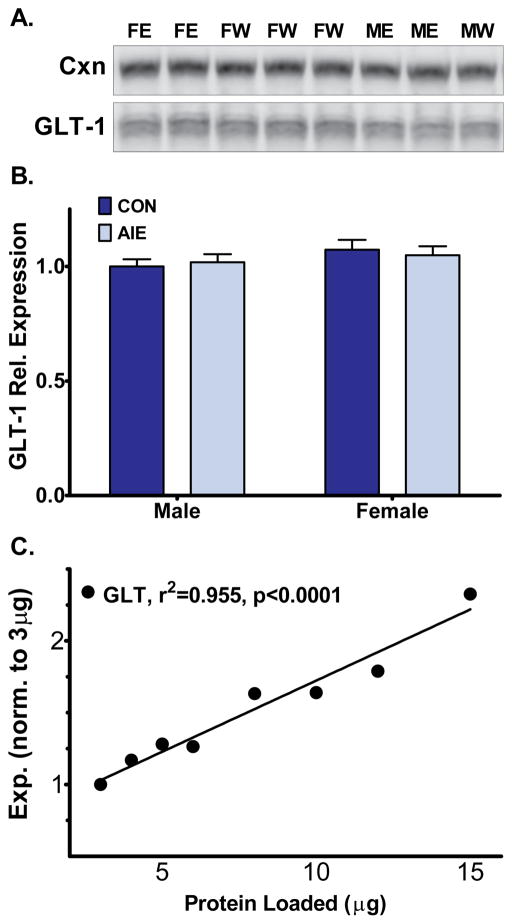
We tested GLT-1 membrane levels in the NAcc of AIE and CON animals, as a decrease would suggest greater NAcc excitability and glutamatergic signaling, potentially providing a physiological basis for AIE-induced behavioral effects. Contrary to our hypothesis, no changes to GLT-1 membrane expression were observed (Figure 4). Initially, we performed protein separation on a 7.5% PAGE gel. We observed a non-significant trend toward an overall increase in GLT-1 in females over males, statistical analyses revealed no main effect of sex (F1,44=2.31), AIE exposure (F1,44=0.03), nor a significant sex-by-exposure interaction (F1,44=1.25) on GLT-1 membrane expression. As a replication, and to obtain a clearer GLT-1 blot, we performed gel separation with remaining available sample in a 12.5% gel (shown in Figure 4); one male-AIE subject was not included in the second analysis due to insufficient remaining protein to load onto a second gel. From the 12.5% gel, we also observed no statistical significance in membrane GLT-1 expression for a main effect of sex (F1,43=1.91), AIE exposure (F1,43=0.01), nor a significant sex-by-exposure interaction (F1,43=0.31) on GLT-1 membrane expression. We verified that a change in expression of GLT-1 could be detected using our blotting conditions by generating a protein loading dose response curve for GLT-1 that ranged from 25% to 125% of the amount loaded from the experimental animals (Fig. 4C). The dose response curve showed linearity (r2= 0.955, P<0.0001), supporting our finding that GLT-1 membrane expression is not affected by sex nor AIE exposure.
Figure 4.
Membrane expression of GLT-1 was similar across sex and AIE history. (A) Representative bands from 12.5% gel GLT-1 western blot (MW, male water; ME, male ethanol; FW, female water; FE, female ethanol); (B) Expression levels from 12.5% gel of GLT-1 normalized to the Male-Water group, represented in arbitrary units; (C) Protein loading dose response curve indicated that expression of GLT-1 in behavior subjects (A and B) was well within the linear section of the protein loading curve.
Discussion
Binge ethanol drinking during adolescence may lead to adult behavioral deficits such as cognitive decline (Brown et al., 2000a) and addiction vulnerability (e.g. Brown et al, 2000b). The ability to manipulate ethanol levels and control for age of exposure onset in rodent models affords researchers the ability to more accurately determine how adolescent ethanol, rather than genetic or social predispositions, contribute to adult outcomes. We hypothesized that AIE disrupts normal cue-reward association as measured by PCA. In particular, we posited that animals pre-exposed to AIE would develop a strong preference for the CS+, despite the non-contingent nature of reward delivery. We observed that AIE reduced all indices of goal-tracking and increased the number of lever presses in both males and females. Multiple studies show that exposure to abused and addictive drugs promotes a sign-tracking phenotype (McClory and Spear, 2014, Palmatier et al., 2013, Peters and De Vries, 2014, Uslaner et al., 2006, Yager and Robinson, 2015). Therefore, we compiled metrics of sign- and goal-tracking to generate a tracking score to determine tracking phenotypes, sign- or goal-tracking, of our experimental groups. From this we found that AIE had no effect on sign-tracking phenotype in males. Consistent with this, significant inverse correlations between cue and goal interactions were not observed in males, regardless of AIE exposure history. Conversely, AIE produced a large shift towards a sign-tracking phenotype in females, possibly a result of the significant inverse correlation between cue and goal interactions in females. Our findings in males are in contrast to the previous report by McClory and Spear (2014), where significant increases in sign-tracking in males exposed to AIE were observed. Additionally, we observed larger effects of AIE in goal-tracking metrics in males when compared to sign-tracking metrics, whereas McClory and Spear (2014) observed more pronounced effects on sign-tracking metrics. This disparity in effects on behavior by AIE is likely due to differing PCA procedures. In particular, we provided a 30s reward-predictive cue prior to reward delivery (Palmatier et al., 2013), as opposed to a much shorter 8s cue (McClory and Spear, 2014). Nevertheless, in both studies we observed a significant influence of AIE on approach behavior, and the present study extends these findings by describing sex differences in AIE effects.
Sign-tracking, as indexed by metrics of CS+ interaction, was elevated in females compared to males, independent of AIE. This finding was unexpected, based on previous reports suggesting no sex difference in approach to a reward-predictive cue (Anderson and Spear, 2011, Pitchers et al., 2015). However, the Pitchers et al. (2015) study suggested stronger approach behavior in females, though not exclusively to a reward-predictive cue as it also occurred in response to a non-predictive cue (CS−). In the present study, we did not provide a CS− option for approach; therefore, these results may reflect non-specific behavior, though one could argue that a significant decrease in latency to cue-interaction indicates strong motivation for the CS+. Our findings suggest that females may be more likely to attribute salience to reward-specific cues. Moreover, sign-tracking and goal-tracking were correlated only in females, suggesting that both AIE and water-exposed females individually preferred either the CS+ or the reward location, whereas males as a group exhibited no obvious pattern of CS+ associated behavior. The sex difference observed here was not likely due to differences during the AIE exposure period, as BEC levels were similar in males and females after gavage ethanol administration during adolescence. Notably, females tended to exhibit less day-to-day variability in behavior than males, suggesting that estrous cycle does not strongly influence conditioned responding and consistent with the broader literature (Becker and Koob, 2016).
Adolescents report lower sensitivity to binge levels of ethanol consumption compared to adults (Day et al., 2013, Spear, 2014), leading to a prevalence of high consumption over a short time period. For example, >5% of high school seniors and up to 20% of 18–24 year-olds have reported consuming 15 or more drinks in a single occasion (Patrick et al., 2013, Schuckit et al., 2014). As such, we employed a binge dosing that resulted in high BEC. Moreover, steady-state exposure to ethanol such as occurs with an ethanol vapor chamber or forced diet protocol may not be appropriately translational for adolescent exposure studies (Crews et al., 2016). We did not make comparisons between AIE exposure and adult ethanol exposure on PCA behavior. A previous study comparing AIE and adult ethanol effects on PCA behavior reported no effect of adult ethanol exposure on PCA behavior to sucrose reward in males (McClory and Spear, 2014). Therefore, we chose to focus our study on sex effects on AIE-induced behavioral changes. However, this should not discount potential sex effects on adult ethanol exposure and subsequent behavior, which could be the focus of future experiments given the results we observed in the present study.
Despite high sign-tracking behavior in females and an AIE-induced shift toward sign tracking, neither sex nor AIE history associated with a change in GLT-1 expression. Decreases in astrocytic GLT-1 expression in the NAcc have been observed after administration of addictive substances (Alajaji et al., 2013, Knackstedt et al., 2010), leading to a state of hyperexcitability that is thought to contribute to drug use and relapse (Kalivas, 2009). Changes in GLT-1 expression and glutamate transport have been reported following exposure to alcohol in some paradigms (Das et al., 2015, Sari et al., 2013), but not others (Griffin et al., 2015, Pati et al., 2016). Thus, it was of interest to us to determine whether the exposure regimen here would lead to lower membrane expression of GLT-1 in the NAcc after AIE, potentially altering conditioned responses. Our negative GLT-1 results were surprising in light of a previous report that adolescent ethanol exposure increases basal extracellular glutamate, as measured by microdialysis (Carrara-Nascimento et al., 2011), of which GLT-1 is the primary regulator (Danbolt, 2001, Pati et al., 2016, Tzingounis and Wadiche, 2007). The lack of effect of AIE on GLT-1 membrane expression does not necessarily exclude changes to excitatory signaling or glutamate homeostasis as a contributing factor to the neurobiological consequences of AIE, as various pre- and post-synaptic neuronal mechanisms also contribute to glutamate sampled by microdialysis (Baker et al., 2002). Moreover, other reports support a model of hyperexcitability following AIE. In particular, AIE-induced immune gene induction activates microglia and astrocytes, leading to a TNFα-evoked inhibition of glutamate reuptake (Zou and Crews, 2005). Despite working through the same transporter, TNFα-induced decreases in glutamate uptake would not necessarily be reflected in changes to GLT-1 protein expression.
A change to mesolimbic dopamine circuit activity is an alternate mechanism that may contribute to the findings reported here. A distinct difference in dopamine receptor expression is associated with the development of sign- or goal-tracking phenotypes. Specifically, whereas sign-trackers express higher levels of NAcc D1 receptor mRNA, goal-trackers express higher NAcc D2 receptor mRNA, tyrosine hydroxylase mRNA in the ventral tegmental area (VTA), and VTA dopamine transporter mRNA (Flagel et al., 2007). These data suggest that sign-trackers trend towards higher dopamine-associated excitability when compared to goal-trackers, as has been suggested in pharmacological studies (Chow et al., 2016, Saunders and Robinson, 2012). While it is well known that acute ethanol increases tonic and phasic dopamine in the NAcc (e.g., Robinson et al., 2009) acute ethanol in adolescents evokes considerably higher dopamine compared to adults as measured by microdialysis (Philpot and Kirstein, 2004). Indeed, more recent studies have shown that adolescent ethanol exposure results in elevated phasic dopamine release in response to a reward-predictive cue in adulthood (Spoelder et al., 2015). Altogether, it is likely that AIE-induced hyperexcitability is not restricted to a single signaling mechanism, but rather arising from multiple pathways across circuits that contribute to conditioned behavior.
Binge drinking is the number one cause of alcohol-related deaths (Stahre et al., 2014), and approximately a third of late adolescents/emerging adults engage in this behavior (Johnston et al., 2011). Animal studies suggest that adolescent brains are more vulnerable to the neurotoxic effects of binge alcohol, leading to behavioral deficits that persist into adulthood (Crews et al., 2016, Spear and Varlinskaya, 2010). However, persistent effects of adolescent binge drinking in humans are difficult to isolate, as adolescent binge drinking is associated with continued binge drinking in adulthood (Degenhardt et al., 2013, Reich et al., 2015) and with pre-existing neurobiological and personality differences (Whelan et al., 2014). While the adult neurocognitive deficits associated with adolescent binge alcohol exposure in humans remain to be fully delineated, the present findings suggest that adolescent exposure itself is sufficient to induce persistent changes in reward associated behavior. Moreover, our findings point to the importance of considering sex-differences in the sequelae of adolescent alcohol exposure.
Acknowledgments
The authors thank Hannah Jaggers, Blake Riley, Lynde Wangler, and Jiaxuan Xu for valuable technical assistance. Dr. Tatiana Shnitko provided valuable discussion and insight. This research was funded by the National Institutes of Health (P60 AA011605, U24 AA020024, U01 AA019972, R00 DA031790). ACM was supported on T32 AA007573.
References
- ALAJAJI M, BOWERS MS, KNACKSTEDT L, DAMAJ MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology (Berl) 2013;228:419–26. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON RI, SPEAR LP. Autoshaping in adolescence enhances sign-tracking behavior in adulthood: impact on ethanol consumption. Pharmacol Biochem Behav. 2011;98:250–60. doi: 10.1016/j.pbb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHMAN JG, O’MALLEY PM, SCHULENBERG JE, JOHNSTON LD, BRYANT AL, MERLINE AC EBOOK LIBRARY. The Decline of Substance Use in Young Adulthood Changes in Social Activities, Roles, and Beliefs. Hoboken: Taylor and Francis; 2014. [Google Scholar]
- BAKER DA, XI ZX, SHEN H, SWANSON CJ, KALIVAS PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER JB, KOOB GF. Sex differences in animal models: focus on addiction. Pharmacological reviews. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE KC, ROBINSON TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- BESHEER J, PALMATIER MI, METSCHKE DM, BEVINS RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172:108–17. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- BROWN SA, TAPERT SF, GRANHOLM E, DELIS DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000a;24:164–71. [PubMed] [Google Scholar]
- BROWN SA, TAPERT SF, TATE SR, ABRANTES AM. The role of alcohol in adolescent relapse and outcome. J Psychoactive Drugs. 2000b;32:107–15. doi: 10.1080/02791072.2000.10400216. [DOI] [PubMed] [Google Scholar]
- CARRARA-NASCIMENTO PF, GRIFFIN WC, 3RD, PASTRELLO DM, OLIVE MF, CAMARINI R. Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol. 2011;45:451–60. doi: 10.1016/j.alcohol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOW JJ, NICKELL JR, DARNA M, BECKMANN JS. Toward isolating the role of dopamine in the acquisition of incentive salience attribution. Neuropharmacology. 2016;109:320–331. doi: 10.1016/j.neuropharm.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN LG, JR, LIU W, OGUZ I, STYNER M, CREWS FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–51. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREWS FT, VETRENO RP, BROADWATER MA, ROBINSON DL. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev. 2016;68:1074–1109. doi: 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANBOLT NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- DAS SC, YAMAMOTO BK, HRISTOV AM, SARI Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY AM, CELIO MA, LISMAN SA, JOHANSEN GE, SPEAR LP. Acute and chronic effects of alcohol on trail making test performance among underage drinkers in a field setting. J Stud Alcohol Drugs. 2013;74:635–41. doi: 10.15288/jsad.2013.74.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEGENHARDT L, O’LOUGHLIN C, SWIFT W, ROMANIUK H, CARLIN J, COFFEY C, HALL W, PATTON G. The persistence of adolescent binge drinking into adulthood: findings from a 15-year prospective cohort study. BMJ open. 2013;3:e003015. doi: 10.1136/bmjopen-2013-003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAGEL SB, WATSON SJ, ROBINSON TE, AKIL H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- GASS JT, GLEN WB, JR, MCGONIGAL JT, TRANTHAM-DAVIDSON H, LOPEZ MF, RANDALL PK, YAXLEY R, FLORESCO SB, CHANDLER LJ. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39:2570–83. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN WC, RAMACHANDRA VS, KNACKSTEDT LA, BECKER HC. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON LD, O’MALLEY PM, BACHMAN JG, SCHULENBERG JE EDUCATIONAL RESOURCES INFORMATION CENTER (U.S.) & UNIVERSITY OF MICHIGAN INSTITUTE FOR SOCIAL RESEARCH. College Students & Adults Ages. II. S.l: ERIC Clearinghouse; 2011. Monitoring the Future National Survey Results on Drug Use, 1975–2010; pp. 19–50. [Google Scholar]
- KALIVAS PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- KNACKSTEDT LA, MELENDEZ RI, KALIVAS PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSS WA, SADOWSKI RN, SHERRILL LK, GULLEY JM, JURASKA JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCIANA M. Adolescent brain development in normality and psychopathology. Dev Psychopathol. 2013;25:1325–45. doi: 10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLORY AJ, SPEAR LP. Effects of ethanol exposure during adolescence or in adulthood on Pavlovian conditioned approach in Sprague-Dawley rats. Alcohol. 2014;48:755–63. doi: 10.1016/j.alcohol.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMATIER MI, KELLICUT MR, BRIANNA SHEPPARD A, BROWN RW, ROBINSON DL. The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav. 2014;126:50–62. doi: 10.1016/j.pbb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMATIER MI, MARKS KR, JONES SA, FREEMAN KS, WISSMAN KM, SHEPPARD AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2013;226:247–59. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- PASCUAL M, BOIX J, FELIPO V, GUERRI C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- PASCUAL M, DO COUTO BR, ALFONSO-LOECHES S, AGUILAR MA, RODRIGUEZ-ARIAS M, GUERRI C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–19. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- PATI D, KELLY K, STENNETT B, FRAZIER CJ, KNACKSTEDT LA. Alcohol consumption increases basal extracellular glutamate in the nucleus accumbens core of Sprague-Dawley rats without increasing spontaneous glutamate release. Eur J Neurosci. 2016;44:1896–905. doi: 10.1111/ejn.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRICK ME, SCHULENBERG JE, MARTZ ME, MAGGS JL, O’MALLEY PM, JOHNSTON LD. Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr. 2013;167:1019–25. doi: 10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS J, DE VRIES TJ. Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology (Berl) 2014;231:447–53. doi: 10.1007/s00213-013-3258-7. [DOI] [PubMed] [Google Scholar]
- PHILPOT R, KIRSTEIN C. Developmental differences in the accumbal dopaminergic response to repeated ethanol exposure. Ann N Y Acad Sci. 2004;1021:422–6. doi: 10.1196/annals.1308.056. [DOI] [PubMed] [Google Scholar]
- PITCHERS KK, FLAGEL SB, O’DONNELL EG, WOODS LC, SARTER M, ROBINSON TE. Individual variation in the propensity to attribute incentive salience to a food cue: influence of sex. Behav Brain Res. 2015;278:462–9. doi: 10.1016/j.bbr.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRENDERGAST BJ, ONISHI KG, ZUCKER I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- REICH RR, CUMMINGS JR, GREENBAUM PE, MOLTISANTI AJ, GOLDMAN MS. The temporal “pulse” of drinking: Tracking 5 years of binge drinking in emerging adults. Journal of abnormal psychology. 2015;124:635–647. doi: 10.1037/abn0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSNER KJ, GIPSON CD, TRAN PK, KNACKSTEDT LA, SCOFIELD MD, KALIVAS PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20:316–23. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RISHER ML, SEXTON HG, RISHER WC, WILSON WA, FLEMING RL, MADISON RD, MOORE SD, EROGLU C, SWARTZWELDER HS. Adolescent Intermittent Alcohol Exposure: Dysregulation of Thrombospondins and Synapse Formation are Associated with Decreased Neuronal Density in the Adult Hippocampus. Alcohol Clin Exp Res. 2015;39:2403–13. doi: 10.1111/acer.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON DL, HOWARD EC, MCCONNELL S, GONZALES RA, WIGHTMAN RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–96. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARI Y, SREEMANTULA SN, LEE MR, CHOI DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci. 2013;51:779–87. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAUNDERS BT, ROBINSON TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUCKIT MA, SMITH TL, DANKO GP, BUCHOLZ KK, AGRAWAL A, DICK DM, NURNBERGER JI, KRAMER J, HESSELBROCK M, SAUNDERS G, HESSELBROCK V. Predictors of subgroups based on maximum drinks per occasion over six years for 833 adolescents and young adults in COGA. J Stud Alcohol Drugs. 2014;75:24–34. doi: 10.15288/jsad.2014.75.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN HW, SCOFIELD MD, BOGER H, HENSLEY M, KALIVAS PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014;34:5649–57. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAWECKI CJ, BETANCOURT M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- SONDHEIMER I, KNACKSTEDT LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225:252–8. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2014;41:51–9. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP, VARLINSKAYA EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Developmental psychobiology. 2010;52:236–43. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOELDER M, TSUTSUI KT, LESSCHER HM, VANDERSCHUREN LJ, CLARK JJ. Adolescent Alcohol Exposure Amplifies the Incentive Value of Reward-Predictive Cues Through Potentiation of Phasic Dopamine Signaling. Neuropsychopharmacology. 2015;40:2873–85. doi: 10.1038/npp.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHRE M, ROEBER J, KANNY D, BREWER RD, ZHANG X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOALSTON JE, DEEHAN GA, JR, HAUSER SR, ENGLEMAN EA, BELL RL, MURPHY JM, MCBRIDE WJ, RODD ZA. The reinforcing properties of ethanol are quantitatively enhanced in adulthood by peri-adolescent ethanol, but not saccharin, consumption in female alcohol-preferring (P) rats. Alcohol. 2015;49:513–8. doi: 10.1016/j.alcohol.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZINGOUNIS AV, WADICHE JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- USLANER JM, ACERBO MJ, JONES SA, ROBINSON TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–4. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- VETRENO RP, BROADWATER M, LIU W, SPEAR LP, CREWS FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One. 2014;9:e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VETRENO RP, YAXLEY R, PANIAGUA B, CREWS FT. Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addiction biology. 2016;21:939–53. doi: 10.1111/adb.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN R, WATTS R, ORR CA, ALTHOFF RR, ARTIGES E, BANASCHEWSKI T, BARKER GJ, BOKDE AL, BÜCHEL C, CARVALHO FM. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGER LM, ROBINSON TE. Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology (Berl) 2015;232:3149–60. doi: 10.1007/s00213-015-3962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU JY, CREWS FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Research. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]