Abstract
Based on their content of prolactin receptors, osteosarcoma cells were predicted to be responsive to prolactin (PRL), but whether PRL would be beneficial or contribute to pathogenesis was unclear. 1,25(OH)2 vitamin D3 [1α,25(OH)2D3] has antiproliferative effects on osteosarcoma cells, and a complex interregulatory situation exists between PRL and 1α,25(OH)2D3. Using osteosarcoma cells, Western blot, real time RT-PCR, and promoter-luciferase assays, we have examined the interaction between PRL and 1α,25(OH)2D3 and demonstrated that physiological concentrations of PRL block increased osteocalcin and vitamin D receptor (VDR) expression in response to 1α,25(OH)2D3. This blockade was shown to be the result of lack of nuclear accumulation of the VDR in response to 1α,25(OH)2D3. Although inhibition of proteasomic degradation with MG132 had no effect on the VDR itself in a 30-min time frame, it relieved the blockade by PRL. Analysis of ubiquitinated proteins brought down by immunoprecipitation with anti-VDR showed PRL regulation of a 250-kDa protein-VDR complex. P250 was identified as the breast cancer tumor suppressor gene product, BRCA1, by Western blot of the VDR immunoprecipitate and confirmed by immunoprecipitation with anti-BRCA1 and blotting for the VDR in the absence and presence of PRL. Knockdown of BRCA1 inhibited nuclear translocation of the VDR and the ability of 1α,25(OH)2D3 to induce the VDR. This, to our knowledge, is the first demonstration of a role for BRCA1 in nuclear accumulation of a steroid hormone and the first demonstration that PRL has the potential to affect the cell cycle through effects on BRCA1.
A BRCA1-vitamin D receptor (VDR) complex is undone by prolactin and required for VDR nuclear accumulation and 1,25 dihydroxyvitamin D3 functionality in osteosarcoma cells.
Osteosarcomas are aggressive, bone-forming mesenchymal tumors, primarily of the young (reviewed in Ref. 1). They are associated with periods of physiological or pathological rapid growth and are typically located in the metaphyseal regions of long bones (1). Human osteosarcoma cell lines (MG-63 and Saos-2) have been shown by others to express the prolactin (PRL) receptor (PRLR) (2), at least at the mRNA level. We therefore initiated a study aimed at determining the effects of PRL on osteosarcoma cells with the intent of determining whether PRL was beneficial or contributed to pathogenesis of this disease.
PRL is a single-chain polypeptide hormone with roles in the promotion of cell proliferation/survival and differentiation (3, 4, 5). PRL is produced in a variety of posttranslationally modified forms (reviewed in Ref. 6), but the two most abundant forms in most species are the unmodified and monophosphorylated polypeptide (reviewed in Ref. 7). In most cell types and tissues, the unmodified hormone stimulates cell proliferation, whereas the phosphorylated hormone (or a molecular mimic of the phosphorylated form) inhibits this effect and promotes cell differentiation (7). It was possible therefore that the two types of PRL might have very different effects on osteosarcoma cells. However, in previous studies of primary rat osteoblasts in vitro and of rat pup bone formation in vivo, bone cells seem to be the exception to the rule in that both forms of PRL have different degrees of the same effect, which was to reduce osteoblast alkaline phosphatase activity and bone formation (8). Nevertheless, because osteosarcoma cells frequently behave differently from normal osteoblasts (e.g. see Ref. 9), it was important to examine both major forms of PRL for their effect on osteosarcoma cells.
The molecular mimic of phosphorylated PRL (10) substitutes an aspartate residue for the normally phosphorylated serine (11), becoming S179D PRL. This molecule has been used extensively both in vitro and in vivo, and its attributes have been described in two recent reviews (7, 12). From an experimental point of view, an important attribute is that, unlike the naturally phosphorylated hormone, it cannot be dephosphorylated and thereby converted to the unmodified hormone during the course of an experiment.
1α,25(OH)2vitamin D3 [1α,25(OH)2D3] is the major regulator of calcium homeostasis and protects the organism from calcium deficiency via effects on the intestine, kidney, parathyroid gland, and bone. The vitamin D receptor (VDR) is present in osteoblasts and affects osteoblast functions including proliferation, apoptosis, mineralization, and expression of bone-specific proteins and growth factors (13, 14, 15, 16, 17, 18, 19, 20). In addition, 1α,25(OH)2D3 modulates Ca2+ and Cl− ion channel activities coupled to exocytosis through rapid nongenomic mechanisms (21). 1α,25(OH)2D3 also up-regulates expression of PRL mRNA in pituitary cells (22) and the receptor in osteosarcoma cells (2), and conversely S179D PRL has been shown to increase VDR expression in mammary and prostate cell lines (23, 24). These and other data suggest that there is a complex interaction between 1α,25(OH)2D3 and PRL, an interaction that we have examined in the current study.
In a number of tumor cell types, 1α,25(OH)2D3 decreases cell proliferation and promotes differentiation (reviewed in Ref. 25), although concentrations of 10–100 nm are often required. However, in osteosarcoma cells, administration of 1α,25(OH)2D3 has not proven very effective even at these supraphysiological concentrations, a result that has been attributed to insufficient normal retinoid X receptor for nuclear import (26).
In the current study, we have examined the effect of both major forms of PRL on expression and function of the VDR in osteosarcoma cells. In the process, we have discovered that PRL blocks nuclear accumulation and genomic function of the VDR and hence would compound the reduced efficacy of 1α,25(OH)2D3 in these cells. In addition, we report that the block on nuclear accumulation is achieved by regulating an interaction between the VDR and the breast cancer tumor suppressor gene product, BRCA1.
Results
Both long-form and short-form PRL receptors are expressed in Ros 17/2.8 osteosarcoma cells
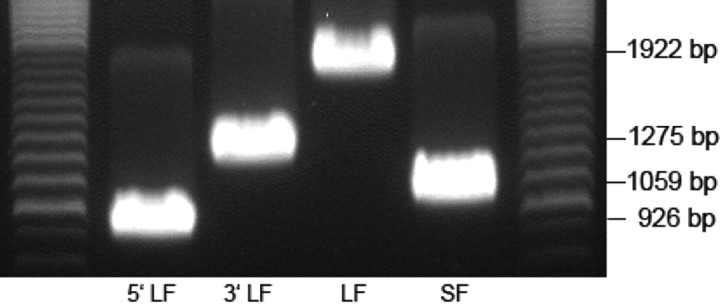
Ros 17/2.8 is a rat osteosarcoma cell line that retains most of the osteoblastic phenotype (27). To determine suitability as a model for investigating the effects of PRL on osteosarcoma, we cloned the PRLR from these cells. Results illustrated that both forms of the rat PRLR exist in this cell line. Figure 1 shows the long-form cDNA at 1922 bp and the short form at 1059 bp. Moreover, sequencing showed identity with rat receptors cloned from the ovary (GenBank nos NM_001034111 and NM_012630).
Fig. 1.
Cloning of long and short-form PRLRs from Ros 17/2.8 osteosarcoma cell. The gel shows the 5′-long-form (LF) (926 bp) and 3′-LF (1275 bp) part and the complete LF PRLR (1922 bp) and the complete short-form (SF) PRLR (1059 bp).
PRL can inhibit 1α,25(OH)2D3-induced increase in VDR protein level
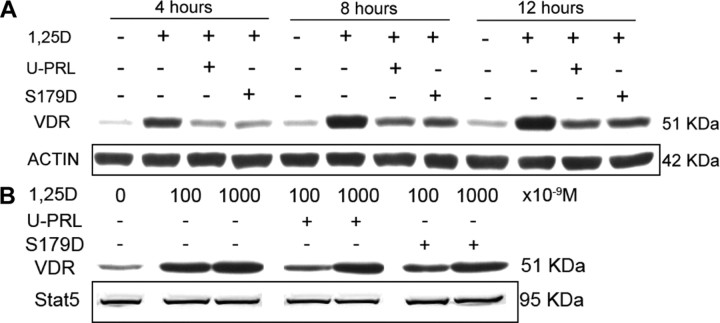
Preliminary experiments showed that 1α,25(OH)2D3 caused a significant increase in mRNA for the VDR in 2 h (data not shown), and so we chose 4 h for the first time point examining effects on protein levels. VDR protein level in Ros 17/2.8 cells was greatly increased by incubation in 1 nm 1α,25(OH)2D3 (Fig. 2A). However, when cells were coincubated in the same concentration of 1α,25(OH)2D3 and 200 ng/ml unmodified PRL (U-PRL) or the mimic of phosphorylated PRL (S179D PRL), this induction was blocked (Fig. 2A). The induction and blockade of same with both forms of PRL were obvious in as little as 4 h (Fig. 2A). To assess the relative potencies of the induction by 1α,25(OH)2D3 and its blockade by PRL, several additional concentrations of 1α,25(OH)2D3 (100 or 1000 nm) were tested against 200 ng/ml (8.7 nm) PRL. Results showed that 100 nm 1α,25(OH)2D3 could not stimulate the increase in VDR protein level in the presence of 200 ng/ml PRL, but 1000 nm 1α,25(OH)2D3 could overcome the inhibitory effect of PRL on VDR accumulation (Fig. 2B). Thus on a molar basis, the PRL effect was more substantial. To determine whether this interaction occurs in other osteosarcoma cell lines, we performed similar experiments in the human MG-63 line. In these cells, both U-PRL and S179D PRL once again inhibited 1α,25(OH)2D3-induced VDR accumulation, but a concentration of PRL (≥100 ng/ml) was required to overcome the effects of 1 nm 1α,25(OH)2D3, whereas in Ros cells an effect was observed at 1 ng/ml (data not shown). Because MG-63 cells were less sensitive to PRL, it was also possible to demonstrate this effect in the presence of serum, thereby confirming that it was in no way a stress response to the removal of serum.
Fig. 2.
Effect of PRL on the inductive effect of 1α,25(OH)2D3 on VDR protein levels. Ros 17/2.8 osteosarcoma cells were cultured in DMEM medium with 10% FBS and 1% PS and then incubated in the absence of FBS for 24 h before treatment. A, Ros 17/2.8 cells were treated with 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml unmodified hPRL (U-PRL) or the mimic of phosphorylated prolactin (S179D PRL) or diluent [control (CONT)] for 4, 8, or 12 h. B, Ros 17/2.8 cells were treated with 100 or 1000 nm 1α,25(OH)2D3 or this plus different concentrations of U-PRL or S179D PRL or diluent for 8 h. Western blots were carried out, and actin, total ERK1/2, or total Stat5 (none of which changed with treatment) are shown as the loading controls. 1,25D, 1α,25(OH)2D3; S179D, mimic of phosphorylated PRL.
In pilot experiments where amounts were compared with β-actin, total ERK 1/2 and signal transducer and activator of transcription (STAT)5 protein levels were found to be unaltered by any of the treatments examined in this study (for example see supplemental figure published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). To be able to make exact correlations by maximizing the number of blots and reprobes that could be performed on the same extract, total ERK or Stat5 are therefore used either together with (or instead of) actin and a nonspecific band as loading controls in the various figures throughout the manuscript.
Short- and long-term effects of PRL on 1α,25(OH)2D3-preinduced VDR protein level
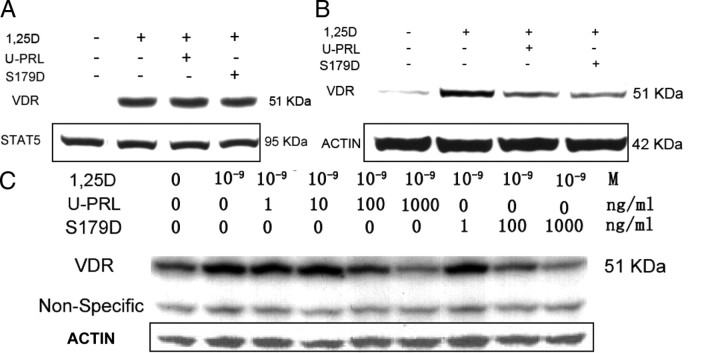
Next, we investigated whether pretreatment with 1α,25(OH)2D3 would prevent the effects of PRL. With 1 nm 1α,25(OH)2D3 pretreatment for 12 h before PRL treatment, followed by coincubation of the cells in the presence of both 1α,25(OH)2D3 and PRL, results showed that PRL did not change the VDR protein level within the 8-h coincubation (Fig. 3A). Thus, once the VDR has accumulated in response to 1α,25(OH)2D3, PRL does not promote the degradation within this time frame. Pretreatment with 1α,25(OH)2D3 and further cotreatment with 1α,25(OH)2D3 and PRL for 72 h, however, reduced the VDR protein level (Fig. 3B). Similar results were obtained with the MG-63 cells using this protocol, but a similar degree of reduction in VDR was obtained with 5-fold the amount of PRL (Fig. 3C), once again illustrating that MG-63 cells are not as sensitive to PRL as the Ros 17/2.8 cells. Because the time frame of the response to PRL did not suggest induced degradation of VDR protein, the possibility that PRL may inhibit the 1α,25(OH)2D3-induced transcription of the VDR gene was examined.
Fig. 3.
Effects of PRL on 1α,25(OH)2D3-preinduced VDR protein level. Ros 17/2.8 osteosarcoma cells were cultured in DMEM with 10% FBS and 1% PS and then incubated in the absence of FBS for 24 h before treatment. A, Pretreatment with 1 nm 1α,25(OH)2D3 for 12 h followed by 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml U-PRL or S179D PRL or diluent for 8 h. B, Pretreatment with 1 nm 1α,25(OH)2D3 for 12 h followed by daily refreshed 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml U-PRL or S179D PRL or diluent for 72 h. C, MG-63 cells preincubated with or without 1 nm 1α,25(OH)2D3 for 24 h and then treated with 1 nm 1α,25(OH)2D3 or this plus different concentrations of U-PRL or S179D PRL or diluent for 72 h. Western blots were performed and total Stat5 or actin or a nonspecific band (all of which do not change with treatment) were used as loading controls. Abbreviations as for previous figure.
PRL decreases 1α,25(OH)2D3-induced accumulation of VDR mRNA and activation of the VDR response element (VDRE)-containing osteocalcin promoter (OCP)
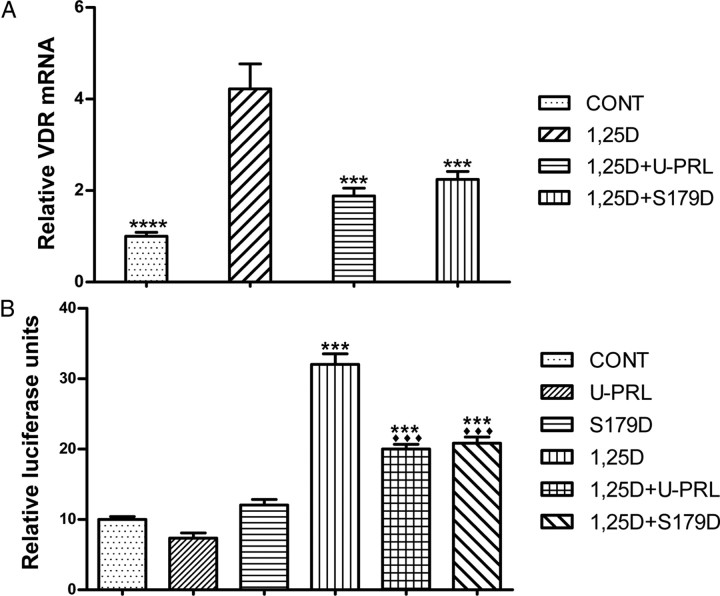
Quantitative real-time RT-PCR detected the relative abundance of VDR mRNA. The results demonstrated that incubation of Ros 17/2.8 cells for 7.5 h in the presence of 1 nm 1α,25(OH)2D3 resulted in a 4-fold increase compared with control. Combined synchronous treatment with 1α,25(OH)2D3 and PRL showed a VDR mRNA of less than 2-fold control (Fig. 4A). This experiment further supports the idea that the down-regulation of VDR protein by PRL is likely through an effect on levels of mRNA.
Fig. 4.
PRL effect on 1α,25(OH)2D3-induced transcription of both VDR (A) and osteocalcin (B) genes. Ros 17/2.8 osteosarcoma cells were cultured in DMEM with 10% FBS and 1% PS and then incubated in the absence of FBS for 24 h before treatment with 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml U-PRL or S179D PRL or diluent [control (CONT)] for 7.5 h. mRNA was quantified by real-time RT-PCR to produce panel A. ***, P < 0.001; ****, P < 0.0001 compared with 1,25D-treated cells. For panel B, cells were treated as above until the end of the incubation in the absence of FBS. They were then transfected with the hOCP/Renilla luciferase plasmid (hOCP/pMLuc-1) and/or control plasmids in the absence of FBS and PS for 12 h, followed by the treatment with daily refreshed 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml U-PRL or S179D PRL or diluent (CONT) for 48 h. A Dual-Luciferase Reporter Assay System was used for the luciferase assay. ***, P < 0.001 compared with CONT; ♦♦♦, P < 0.001 compared with 1,25D-treated. Abbreviations as for previous figures.
Because PRL reduced the 1α,25(OH)2D3-induction of VDR protein, less VDR would be available for activation of genes containing VDREs. To test this, we cloned the human (h) OCP promoter, which contains a VDRE sequence that can be greatly stimulated by 1α,25(OH)2D3 through the ligand-activated VDR-retinoid X receptor (RXR) heterodimer (28). In the construct, expression of a Renilla luciferase reporter was under the control of the OCP. The luciferase assay showed that although PRL itself did not significantly affect the transcription of osteocalcin in the absence of 1α,25(OH)2D3, it could suppress the 1α,25(OH)2D3-stimulated activation of the OCP (Fig. 4B). This result further substantiates the idea that the availability of 1α,25(OH)2D3-activated VDR-RXR heterodimer for nuclear binding to a VDRE (including that on the VDR gene) is reduced in the presence of PRL.
Nuclear translocation/accumulation of VDR is blocked by PRL
To stimulate transcription of VDR-responsive genes, 1α,25(OH)2D3 binds to the VDR, resulting in nuclear translocation/accumulation of the activated VDR. Although we have illustrated that PRL reduces 1α,25(OH)2D3-induced VDR through a reduction in the VDR mRNA level, this does not explain how PRL has this effect. During 1α,25(OH)2D3-induced transcription, the nuclear translocation of activated VDR is a very important step. Obviously, VDR-mediated transcription would be inhibited if the nuclear translocation of the VDR were blocked. To test whether PRL affected nuclear translocation of the VDR, we starved Ros 17/2.8 cells for 48 h before hormone treatment and then incubated the cells in 1α,25(OH)2D3 or 1α,25(OH)2D3 plus PRL for 30 min. This short time period was chosen in order not to confound the result by reduced levels of VDR. Separate extraction of protein from cytoplasm and nucleus was confirmed by staining for total Stat5 and the nuclear protein, TATA-binding protein (TBP), respectively. Even though PRL results in Stat5 phosphorylation and nuclear translocation of phosphorylated Stat5, the proportion of Stat5 phosphorylated is very low, and its loss from the total is not detectable. Figure 5 shows that, as expected, 1α,25(OH)2D3 reduces the cytoplasmic, and increases the nuclear, localization of the VDR. Incubation in PRL reduced the loss from the cytoplasm and decreased the gain in the nucleus. This was true whether U-PRL, S179D PRL, or a commercial recombinant human (h) PRL (used as an additional control for any potential nonrepesentative effects of the in-house preparations) was used. These data illustrate that 1α,25(OH)2D3 can induce a rapid nuclear localization of the VDR and that PRL can block this, a result that would reduce VDR-mediated transcription of the VDR and osteocalcin genes.
Fig. 5.
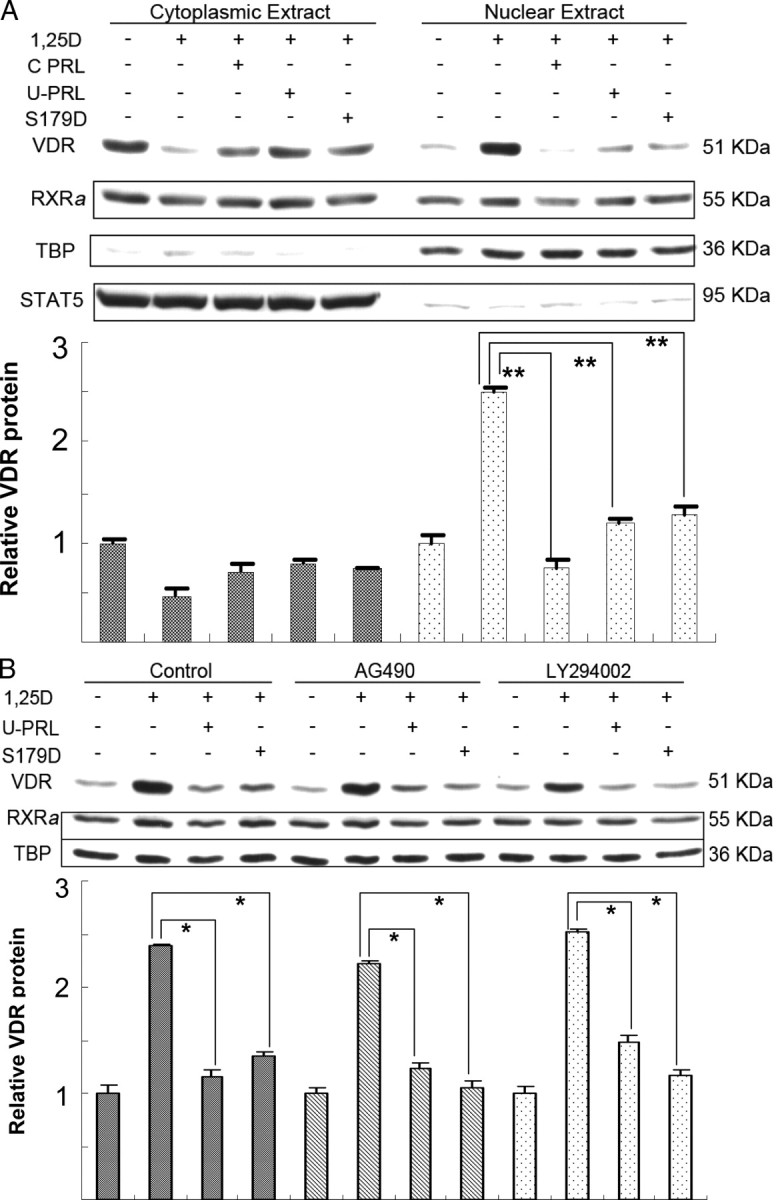
1α,25(OH)2D3-induced nuclear translocation of VDR is blocked by PRL. Ros 17/2.8 osteosarcoma cells were cultured in DMEM with 10% FBS and 1% PS and then incubated in the absence of FBS for 48 h before treatment with 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml commercial recombinant hPRL (C PRL) or U-PRL or S179D PRL or diluent for 30 min. Subcellular fractionation and protein extraction were performed using NE-PER reagents. Ten percent of either the nuclear protein or cytosolic protein was loaded per lane. Western blots were performed using TBP as a marker of nuclear protein and total Stat5 as a marker of cytosolic protein. For panel B, cells were incubated in 50 μm AG490 or LY294002 for the last 2 h of the 48-h preincubation without FBS and then were processed as for panel A except that the inhibitors continued to be present, this time at 25 μm. Quantification from multiple experiments was normalized to cells without hormone treatment. RXRa, Retinoid X receptor α; other abbreviations as for previous figures. *, P < 0.03; **, P ≤ 0.01.
Most functions of the VDR require heterodimerization with an RXR. The VDR recruits RXR to form heterodimers and uses importin α to mediate nuclear import in response to 1α,25(OH)2D3 (29). We therefore investigated whether the subcellular distribution of RXRα was also affected by PRL. As also shown in Fig. 5, an effect on the RXRα was not consistently observed.
The mechanisms by which PRL exerts intracellular effects include activation of the Janus kinase 2-Stat5, MAPK kinase-MAPK and Src-phosphatidylinositol 3-kinase-Akt pathways (reviewed in Ref. 30). We therefore investigated the possible signaling pathways involved in the effect of PRL on nuclear translocation of the VDR by using the inhibitors, AG490, U0126, PP1, LY294002, and the myristoylated protein kinase C peptide inhibitor, which inhibit Janus kinase 2, MAPK kinase 1/2, Src, phosphatidylinositol 3-kinase, and protein kinase C activity, respectively. Each inhibitor was used at 50 μm, and the cells were preincubated in the inhibitor for 2 h before incubation in 1α,25(OH)2D3 or this plus one or other form of PRL for 30 min. Although internal controls showed each inhibitor to be active (data not shown), none affected the ability of PRL to inhibit nuclear accumulation of the VDR in response to ligand. The results with AG490 and LY294002 are shown (Fig. 5B) by way of example.
Ubiquitin-proteasome pathway in blockade of VDR nuclear translocation
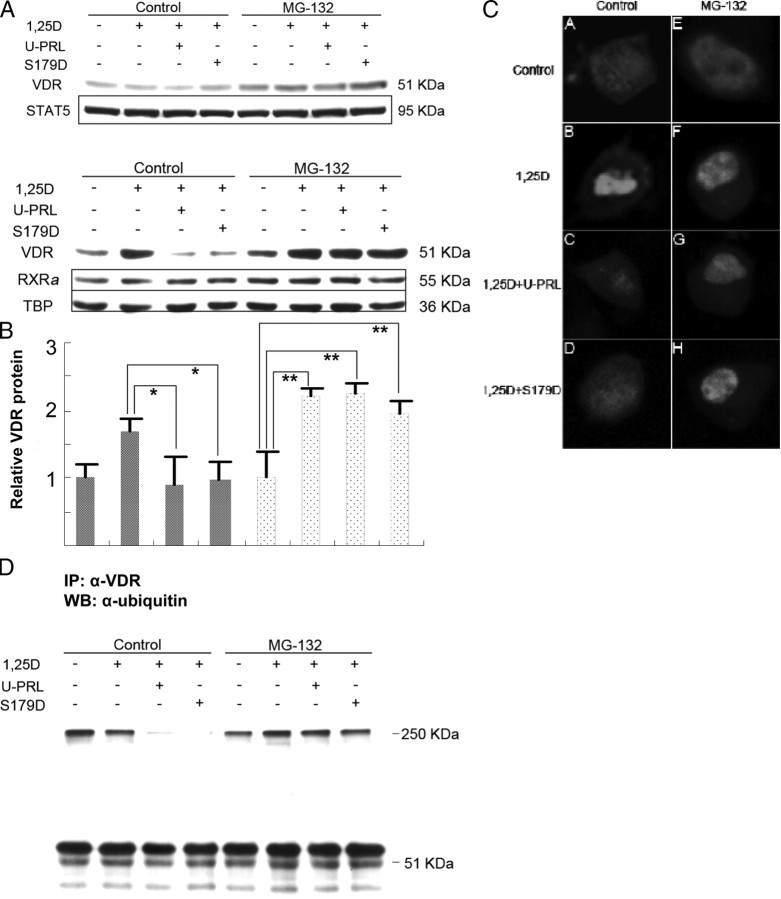
The VDR is largely degraded in proteasomes (31), and 1α,25(OH)2D3 has been shown to stabilize and increase nuclear VDR by blocking this pathway for degradation (32). As shown above, it takes more than 8 h for PRL to reduce the amount of VDR once VDR has already been induced and stabilized by ligand before PRL treatment (Fig. 3). Thus, although promotion of degradation of the VDR may, in some measure, contribute to a long-term effect of PRL, it seemed unlikely as an explanation of the 30-min effect. Nevertheless, we investigated the effects of PRL on the subcellular distribution and total amount of VDR in the presence of the proteasome inhibitor MG-132. Ros 17/2.8 cells were pretreated for 2 h with MG-132 and then incubated in the continued presence of MG-132 in 1α,25(OH)2D3 or 1α,25(OH)2D3 and PRL for 30 min. The result shows that although MG-132 treatment can slightly increase the total protein level of VDR in this time frame, the basal level in the presence of MG-132 is not affected by the 30-min treatment with 1α,25(OH)2D3 or 1α,25(OH)2D3 plus PRL (Fig. 6A). Because neither 1α,25(OH)2D3 nor PRL induced a significant change in total VDR protein within 30 min, the accumulation of VDR in the nucleus (Fig. 6B) was essentially only caused by 1α,25(OH)2D3-induced nuclear import/retention. When nuclear and cytoplasmic fractions were analyzed separately, the results interestingly indicated that the PRL blockade of nuclear import/accumulation was relieved by MG-132 treatment (Fig. 6B).
Fig. 6.
Short-term effect of MG-132 on the PRL inhibition of nuclear accumulation of the VDR. Ros 17/2.8 osteosarcoma cells were cultured in DMEM with 10% FBS and 1% PS and then incubated in the absence of FBS for 48 h. MG-132 (50 μm) was added 2 h before the end of this period. Cells were then incubated in 25 μm MG-132 and 1 nm 1α,25(OH)2D3 or this plus 200 ng/ml U-PRL or S179D PRL or diluent for 30 min. Whole-cell lysates are shown in panel A and nuclear extracts in panel B. Quantification from multiple experiments was normalized to cells without hormone treatment. For panel C, cells were transfected with pECFP-hVDR in the absence of FBS for 24 h and then incubated for a further 24 h in the absence of FBS. For the cells receiving MG-132, this was added as before: 2 h before the end of the total 48-h period in the absence of FBS and during the 30-min incubations. At the end of the 30-min incubations, cells were fixed in 4% formaldehyde in Dulbecco’s PBS at room temperature for 15 min, mounted in antifade, and observed by confocal fluorescence microscopy. The same samples as in panel A were used for the immunoprecipitation shown in panel D. From whole-cell lysate 500 μg protein per lane was used for immunoprecipitation with anti-VDR polyclonal antibody and then Western blotting with antiubiquitin monoclonal antibody. *, P < 0.05; **, P < 0.0004; 1,25D, 1α,25(OH)2D3; IP, immunoprecipitation; U-PRL, unmodified PRL; WB, Western blot.
To further verify this result, we constructed a vector in which hVDR was fused to enhanced cyan fluorescent protein (pECFP-hVDR), and transfected this construct into Ros 17/2.8 cells. The transfected cells were then treated as described for Fig. 6, panels A and B, and subsequently observed using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY). The images show that the VDR is distributed fairly evenly throughout the cell in the absence of 1α,25(OH)2D3 (Fig. 6C–A), and it localizes and accumulates in the nucleus in the presence of 1α,25(OH)2D3 at the 30-min time point (Fig. 6C–B). Coincubation in 1α,25(OH)2D3 and PRL shows the VDR still mainly localized throughout the cell (Fig. 6C–C and C–D). Upon treatment with MG-132, this situation is changed, and nuclear translocation/accumulation can take place in the presence of PRL (Fig. 6, C–G and C–H). MG-132 itself does not promote or block the nuclear localization of VDR (Fig. 6C–E) nor does it interfere with 1α,25(OH)2D3-stimulated nuclear translocation/accumulation (Fig. 6C–F). Collectively, these data allow the conclusion that PRL blocks nuclear accumulation of the VDR through an effect on another factor regulated by proteasomic degradation.
One likely possibility was that this factor was associated with the VDR. We therefore immunoprecipitated VDR protein and determined which associated proteins were scheduled for likely degradation in a proteasome by staining the Western blot with antiubiquitin antibody. The degree of ubiquitination of proteins in and around the correct size for the VDR was unaffected by treatment with PRL, a finding consistent with the conclusions above. In other words, degradation of the VDR was not being affected. However, a band running with an approximate mass of 250 kDa that coimmunoprecipitated with the VDR was affected by the presence of PRL (Fig. 6D). PRL reduced the ubiquitination and/or association of this ubiquitinated protein with the VDR. Coincubation in the proteasome inhibitor MG-132 restored the amount of this p250 associated with the VDR, thereby suggesting that PRL actually promoted degradation of this protein (Fig. 6D). This, in turn, suggested that the p250 was necessary for nuclear import/accumulation of the VDR.
Identification of p250
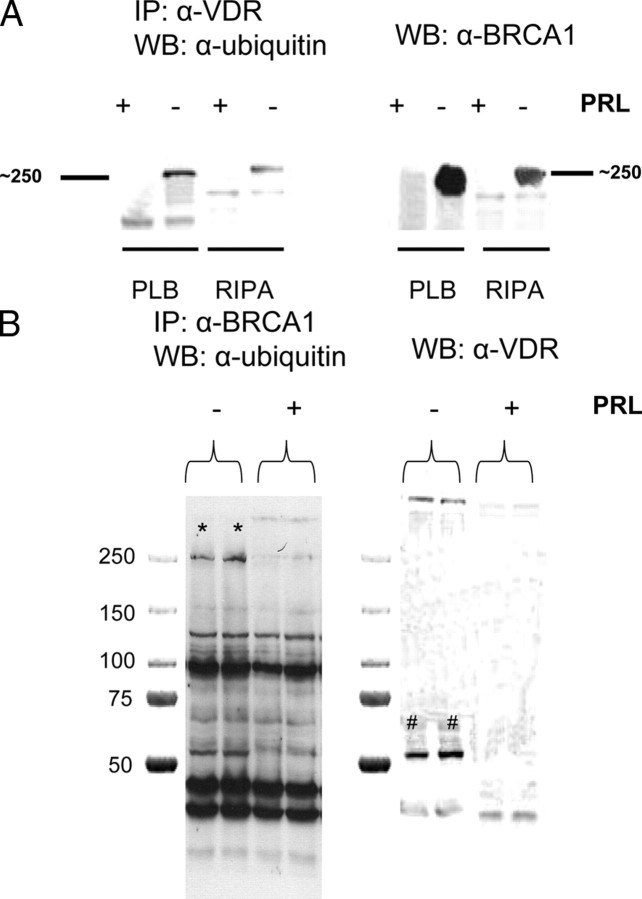
A number of different approaches were taken to identify the p250 ubiquitinated protein, finally resulting in the demonstration that it was the breast cancer tumor suppressor gene product, BRCA1. As previously shown, immunoprecipitation with anti-VDR results in coimmunoprecipitation of a ubiquitinated p250 in the absence, but not the presence, of PRL (Fig. 7A). When this blot is stripped and reprobed with anti-BRCA1, a protein around 250 kDa is seen to be BRCA1. The band size with anti-BRCA1, however, looks different from that seen with anti-ubiquitin unless one looks carefully on the anti-ubiquitin blot to see the lesser ubiquitinated bands below p250. To confirm the identity of the p250 protein, the immunoprecipitation protocol was reversed, using anti-BRCA1 to immunoprecipitate. With this approach, one can see a band of the correct mass for ubiquitinated VDR and BRCA1 and that both are absent when the cells are incubated in PRL (Fig. 7B). Staining with anti-VDR confirmed the identity of the VDR band and clearly showed that it was co-immunoprecipitated with BRCA1 in the absence, but not presence of PRL (Fig. 7B). Thus, we can conclude that PRL treatment regulates an association between BRCA1 and the VDR and infer that this association is required for nuclear accumulation of the VDR. To test this directly, we developed a cell line in which BRCA1 was knocked down by the expression of short hairpin RNA (ShRNA).
Fig. 7.
Identification of p250 as BRCA1. Ros 17/2.8 osteosarcoma cells were cultured and incubated in the absence of FBS as before and then treated with U-PRL or diluent for 30 min. Cells were extracted with either Promega passive lysis buffer (PLB) or radioimmune precipitation assay buffer, and the protein extract was then subjected to immunoprecipitation (IP) with either anti-VDR (α-VDR) for panel A or anti-BRCA1 for panel B. After transfer to nitrocellulose, Western blots with antiubiquitin were performed. Filters were then stripped, reblocked, and reprobed with either anti-BRCA1 (panel A) or anti-VDR (panel B). WB, Western blot.
Knockdown of BRCA1
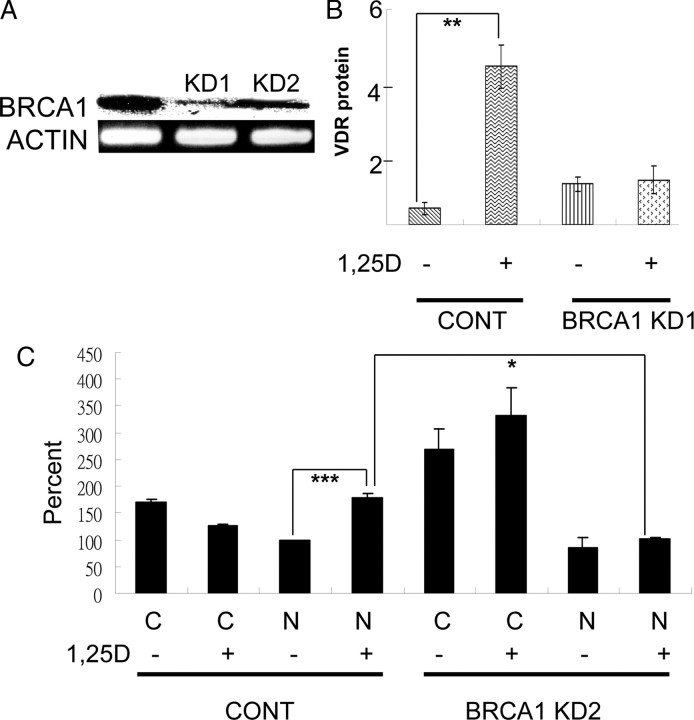
Figure 8A compares the level of expression of BRCA1 among the control, plasmid-transfected cell line and those expressing the ShRNA (KD1 and KD2). After 42 cycles of PCR, a very faint band for the BRCA1 mRNA amplicon was seen in the BRCA1 KD1 knockdown cells. We have reversed the image to better demonstrate the faint band. Knockdown of BRCA1 in these cells completely eliminated the ability of 1α,25(OH)2D3 to induce the VDR in 8 h (Fig. 8B). However, basal levels of the VDR were elevated in these cells, presumably as a result of some non-ligand-dependent compensatory mechanism. This was also seen in the second cell line (BRCA1 KD2) which was used to examine the effect of BRCA1 knockdown on nuclear translocation. Figure. 8C shows that knockdown of BRCA1 inhibits the ability of 1α,25(OH)2D3 to induce nuclear localization of the VDR. This figure also shows that the increase in basal VDR seen in the knockdown cells is in the cytoplasmic fraction.
Fig. 8.
Effect of knockdown of BRCA1. Ros 17/2.8 cells were transfected with either a control plasmid or a plasmid expressing ShRNA for BRCA1 and then selected for puromycin resistance. Panel A shows the result of RT-PCR for BRCA1 in two cell lines produced (KD1 and KD2). The image for the BRCA1 amplicon is reversed so that it is possible to see the band in KD1. Both lines were cultured and incubated in the absence of FBS as before and then treated with 1α,25(OH)2D3 for 8 h (panel B, which shows KD1) or 30 min (panel C, which shows KD2). Separate nuclear and cytoplasmic extracts were made from the KD2 cells. Panels B and C show quantification of Western blots (n = 4). For panel C, the results were normalized to the value for the nuclear fraction in the control cells in the absence of 1,25D to be able to combine analyses. This value was set at 100%. Quantification also allowed for the relative proportions of the nuclear and cytoplasmic extracts applied to the gel and was normalized to β-actin. N, Nucleus; C, cytoplasm; CONT, control; 1,25D, 1α,25(OH)2D3. *, P < 0.03; **, P < 0.01; ***, P < 0.001.
Discussion
1α,25(OH)2D3 has been shown to increase the level of its receptor in a variety of cultured cell types. Under some conditions, the increase of VDR protein is associated with elevated VDR mRNA expression (e.g. Ref. 33), whereas under other conditions/in other cell types 1α,25(OH)2D3 elevates VDR protein without altering VDR mRNA levels (e.g. Ref. 34). Our experimental results in Ros 17/2.8 and MG-63 osteosarcoma cells are consistent with the first set of reports. In the osteosarcoma cells, PRL inhibited this effect at physiological concentrations, although the Ros 17/2.8 cells were more sensitive.
The ability of PRL to affect levels of the VDR was demonstrated to be an effect primarily on the steady state level of VDR mRNA. The VDR distributes in both cytoplasm and nucleus in the absence of ligand, and 1α,25(OH)2D3 binding induces nuclear accumulation (e.g. Ref. 35). Although the VDR was once considered a receptor that mostly resided in the nucleus, more recent evidence suggests that it shuttles continuously between cytoplasm and nucleus (36). Nuclear accumulation therefore can be the result of either increased import or decreased export. Nuclear import of proteins is usually mediated by a cluster of basic residues in the primary sequence of the imported protein. Classical nuclear localization signals (NLS) are comprised of the sequence K(K/R)X(K/R), which is recognized by the adapter protein, importin α (37, 38). Cargo-loaded importin α binds to importin β, which, in turn, mediates the import of the complex through nuclear pore complexes (reviewed in Ref. 39). So far, three NLS in the VDR have been reported at amino acids 49–55, 76–102, and 158–173 (35, 40, 41). Some ligand-independent importation occurs, a process mediated through importin 4 (42), but ligand-dependent import, which is what we are concerned with here, is accomplished by exposure of the NLS at 158–173 by a conformational change upon ligand binding (42).
The VDR functions in the nucleus as a VDR-RXR heterodimer. The RXR can promote nuclear accumulation of the unliganded VDR by influencing both nuclear import and retention (36), but it is unlikely to serve as a dominant factor for nuclear localization of the VDR because the RXR-VDR heterodimer is imported into the nucleus predominantly via the VDR. This import is mediated by importin α and triggered by 1α,25(OH)2D3 (29). RXR also partners with other nuclear receptors and so normally would be present in large excess of the amount needed to accompany the VDR. Our results did not show a consistent effect of 1α,25(OH)2D3 on nuclear accumulation of the RXR, a result consistent with the movement of only a small proportion of cellular RXR. The small amount moved could also be related to an abnormality in the RXR (26).
In addition to reducing total VDR accumulation in response to 1α,25(OH)2D3, treatment of osteosarcoma cells with PRL also resulted in reduced nuclear accumulation of the VDR. Theoretically, this could have been the result of reduced nuclear import or accelerated export, resulting in a decrease in activation of a promoter strongly governed by liganded VDR. Because the promoter region of the VDR itself has a VDRE (43), reduced nuclear VDR would result in reduced total VDR levels in the longer term, as observed.
Studies have shown that several members of the nuclear receptor superfamily, including the glucocorticoid, estrogen, progesterone, androgen, thyroid hormone, retinoic acid, retinoid X, and vitamin D receptors undergo proteasome-mediated proteolysis (reviewed in Ref. 44). Analysis of the VDR sequence revealed a strong positive proline-, glutamate-, serine-, and threonine-enriched region (45) in the ligand-binding domain and so the effect of PRL on the amount of nuclear VDR could have been via an effect on proteasomic degradation of the VDR itself. However, the experiments indicate that the total VDR protein level is not reduced by PRL within 30 min, whereas the effect on nuclear accumulation was evident in this time frame. Nevertheless, the proteasome inhibitor MG-132 relieved inhibition of VDR nuclear accumulation by PRL in the 30-min time frame. Thus, proteasomic degradation was involved, but not proteasomic degradation of the VDR. Instead, coimmunoprecipitation showed that a VDR-associated factor (∼250 kDa) was involved. This p250 was partially ubiquitinated and associated with the VDR until the cells were stimulated with PRL. Western blotting with anti-BRCA1 and reverse immunoprecipitation showed that this VDR-associated p250 was BRCA1. The importance of BRCA1 to 1α,25(OH)2D3 -induced nuclear translocation, rather than intranuclear stabilization, was implied by inhibition of nuclear translocation with BRCA1 knockdown in the 30-min experiments and the fact that the increased basal levels of VDR that occur in the BRCA1 KD cells are present in the cytosol rather than the nucleus. The lack of nuclear translocation results in an inability of 1α,25(OH)2D3 to stimulate VDR expression in the BRCA1 knockdown cells.
BRCA1 is a ubiquitin ligase and can self ubiquitinate (reviewed in Ref. 46). Treatment of the cells with MG132 inhibited the ability of PRL to undo the VDR-BRCA1 association. This suggests that the proteasomic degradation of either BRCA1 itself or some other protein necessary for complex formation is promoted by PRL. BRCA1 is known to bind a number of other nuclear proteins and plays a role in cell cycle control and the regulation of DNA damage repair and hence functions as a tumor suppressor (46). There are two BRCA1 binding sites on the p21 promoter (47). Liganded VDR activates the p21 promoter and therefore also acts to regulate the cell cycle (reviewed in Ref. 48).
1α,25(OH)2D3 or analogs of 1α,25(OH)2D3 have been proposed as potential therapeutics for osteosarcoma because of their prodifferentiative effects. Assuming that PRL has effects in primary tumors similar to those described here, increased levels of PRL would therefore reduce the response of patients with osteosarcoma to administered 1α,25(OH)2D3 or analogs. Both major forms of PRL seem to have the same effect in this regard in osteosarcoma cells, and so total levels of PRL would be more important than the ratio of one form of PRL to the other. What makes normal osteoblasts (8) and now osteosarcoma cells different from other cell types in the similarity of their response to the two different forms of PRL is currently unknown. Although common to the two osteosarcoma cell lines examined, this effect of PRL is not duplicated in primary osteoblasts, which by contrast increase expression of the VDR in response to PRL (data not shown). What key factor accounts for this difference will be the subject of future investigation. What we do know is that it is not the result of an abnormal receptor or expression of only one form of the receptor, both of which hypotheses we laid to rest by cloning the receptors present in the osteosarcoma cells.
In conclusion, we have demonstrated that PRL inhibits the ability of 1α,25 (OH)2D3 to up-regulate its own receptor in osteosarcoma cells. This is achieved by the prevention of VDR nuclear accumulation. Nuclear accumulation is dependent on maintenance of a complex between BRCA1 and the VDR, and this complex is undone by PRL.
Materials and Methods
Cell culture and treatment
Cells were plated in DMEM with 10% fetal bovine serum (FBS) plus 1% penicillin-streptomycin (PS) and cultured at 37 C in a humidified atmosphere of 5% CO2. Before treatment, cells were incubated in serum-free DMEM/1% PS media for 24 or 48 h (see figure legends). The hormones and inhibitors used in the experiments are listed below. 1α,25(OH)2D3 (DM-200) and PP1 (EI-275) were from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, PA), and the 1α, 25(OH)2D3 was stored as a stock in ethanol at −20 C protected from light. AG490 (658401) and MG-132 (474790) were from EMD Biosciences, Inc. (San Diego, CA). LY294002 (ALX-270-038-M005) was from Alexis Biochemicals (San Diego, CA), and U0126 (no. 9903) was from Cell Signaling Technology, Inc. (Beverly, MA). Both unmodified (U-PRL) and the mimic of phosphorylated (S179D) PRLs were prepared as previously described (10). Myristoylated protein kinase C peptide inhibitor (V5691) was from Promega Corp. (Madison, WI). The concentration of inhibitors used was 50 μm for preincubation and 25 μm during hormone treatment.
Western blotting
For whole-cell lysates, cells were collected in a 1.5-ml Eppendorf tube and usually lysed in 1× Passive Lysis Buffer (E194A, Promega Corp.) (see Fig. 7 for exception) by a combination of high-speed vortexing and subsequent ultrasonic disruption. Equal amounts of protein (40–50 μg protein, depending on the experiment) were loaded per lane. NE-PER Reagents (78833; Pierce Chemical Co., Rockford, IL) were employed for the nuclear and cytoplasmic fractionation, and in this case 5–10 μg nuclear protein was loaded in different experiments. Antibodies against VDR (C-20, sc-1008), RXRα (D-20, sc-553), TBP (N-12, sc-204), Stat5 (C-17, sc-835), p-Stat5 (Tyr 694, sc-11761-R), p-ERK1/2 (E-4, sc-7383), and BRCA1 (H-100) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Ubiquitin (P4D1, no. 3936) and p44/42 MAPK (no. 9102) antibodies were from Cell Signaling Technology, Inc. Antibody for β-actin (A5441) was from Sigma Chemical Co. (St. Louis, MO). Goat antirabbit (170-6515) and antimouse (170-6516) IgG horseradish peroxidase-conjugated antibodies were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA). SuperSignal West Pico Chemiluminescent Substrate (34080, Pierce Chemical Co.) was used for detection of peroxidase activity. The specificity of each antibody was confirmed in pilot analyses that tested isotype control antibodies as well as incubations in the peroxidase-conjugated antibodies alone. Membranes were stripped with Restore Western Blot Stripping Buffer (Pierce Chemical Co.) for 30 min at 37 C. For Westerns that were quantified, incubations and exposures were optimized to keep development in the linear range.
Reverse transcription reaction
Total cellular RNA was extracted with TRIzol reagent (15596-026; Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized from total RNA by employing SuperScript III Reverse Transcriptase (18080-044; Invitrogen) and following the protocol in the product sheet.
Real-time PCR
The reaction was performed on an ABI Prism 7700 Sequence Detection System by utilizing 2× SYBR Green PCR Master Mix (4309155; Applied Biosystems, Foster City, CA) according to the product manual. The data analysis was carried out as described in User Bulletin 2 of ABI PRISM 7700 Sequence Detection System.
Conventional PCR for BRCA1
The primers used were 5′-ACA CCC GAA CTT ACC AGA-3′ (sense) and 5′-TAA CTA TCC ACT TTC CTC C-3′ (antisense) to produce an amplicon of 1385 bp. Conditions included 5 min at 94 C followed by 42 cycles of 94 C for 30 sec, 58 C for 40 sec, 68 C for 2 min, and 72 C for 2 min. Primers for β-actin were 5′-AGA GCA AGA GAG GCA TCC TCA CCC TGA AGT-3′ (sense) and 5′-GGA GCA ATG ATC TTG ATC TTC ATT GTG CTG-3′, which were amplified under the same conditions.
Cloning of PRLRs
Two pairs of primers were used for cloning the long form PRLR: the sense primer 5′-CTG TGA AGG GAG CCT CTG ATA CAT TGC-3′ and the antisense primer 5′-AGC AGC TCT TCA GAC TTG CCC TT-3′ for the 5′-end; the sense primer 5′-AAA GTA TCT TGT CCA GAC TCG CTG-3′ and the antisense primer 5′-AGC CAA TCG TTC CAT AAG TCT AGC-3′for the 3′-end. These two PCR products were put together as the templates, and another PCR was run by using the primer pair 5′-ACA GGT ACC AAG GGA GCC TCT GAT ACA TTG C-3′ (sense)/5′-CCT GAA TTC GCC AAT CGT TCC ATA AGT CTA GC-3′ (antisense) to get the full-length long-form PRLR. For the short-form PRLR, the primers 5′-ACA GGT ACC AAG GGA GCC TCT GAT ACA TTG C-3′ (sense)/5′-CCT GAA TTC CAT TTG TTG CGA TGG GGT AGA-3′ (antisense) were employed. Insertion of the full-length PRLR into pcDNA3.1(+) vector (V79020, Invitrogen) was accomplished by using KpnI (R4344) and EcoRI (R4014, Promega Corp.) restriction sites.
Construct of reporter gene
Female human genomic DNA (G1521, Promega Corp.) served as the template, the primer pair of 5′-GGA TCC GCA GGG TCA GGA GGA GAA T-3′ (sense) and 5′-AAG CTT CAG CCT CCA GCA CTG TTT A-3′ (antisense) and PCR Master Mix (M7502, Promega Corp.) were used for cloning the hOCP. The PCR product was ligated into pMLuc-1 Renilla luciferase vector (71116-3; EMD Biosciences, San Diego, CA) by utilizing BamHI (R4024) and HindIII (R4044, Promega) restriction sites.
For construction of pECFP-hVDR wt, the full-length human wild-type VDR (residues 3-427) was generated by PCR and inserted into the pECFP-Nuc vector (6904-1; CLONTECH, Mountain View, CA) by utilizing XhoI (R4164) and XbaI (R4184, Promega) restriction sites. This also removed the nuclear localization signal of the vector. All cloned sequences were analyzed by University of California Riverside Genomic Center.
Transient transfection and luciferase assay
Cells were cultured in a 96-well plate. Each well was transfected with a total of 0.2 μg DNA using Lipofectamine 2000 transfection reagent (11668-019; Invitrogen) by following the protocol in the product manual. pGL3-Control vector (E1741, Promega) was used as the transfection efficiency control. Cotransfection of pMLuc-1 vector (71116-3; EMD Biosciences, San Diego, CA) and pGL3-Enhancer vector (E1771; Promega) was used as a negative control for luciferase activity. Dual-Luciferase Reporter Assay System (E1910; Promega) was used to analyze the activities of firefly and Renilla luciferases. The relative activity of the Renilla luciferase was assessed by normalizing the Renilla luciferase driven by hOCP to the firefly luciferase encoded by the pGL3-Control vector.
Cell treatments
Details of each experimental treatment are given in the appropriate figure legends or for nonpresented results are given in Results. All results presented used serum-free conditions to eliminate both PRL and 1α,25(OH)2D3 from the medium. All experiments were repeated a minimum of three times with the same results. Statistical analysis for quantitative measures was by ANOVA with posttests and corrections for multiple comparisons against a single control group.
Immunoprecipitation
Treated and nontreated cells (subconfluent cell monolayers) were lysed with 3 ml ice-cold radioimmune precipitation assay buffer and incubated at 4 C for 10 min or extracted as before in passive lysis buffer. The suspension was then further disrupted by repeated passage through a 22-gauge needle. Cellular debris was pelleted by centrifugation at 10,000 × g for 10 min at 4 C. The supernatant containing soluble protein was transferred to a fresh tube on ice and precleared by adding 1.0 μg of the appropriate control IgG (rabbit IgG for the anti-VDR and anti-BRCA1 and mouse IgG for the antiubiquitin) together with 20 μl of suspended (25% vol/vol) protein A/G agarose (Santa Cruz Biotechnology) and incubating at 4 C for 30 min. The agarose was then pelleted and the supernatant (∼1000 μg) total cellular protein) was subjected to immunoprecipitation with the appropriate antibody (2 μg of each per tube per reaction, 3 μg for the BRCA1 antibody) overnight at 4 C with gentle constant rotation. Protein A/G-agarose suspension (20 μl per tube) was then added to retrieve the immunocomplexes. Immunocomplex pellets were washed three times with 300 μl PBS. Proteins in the pellet were then solubilized in 40 μl of 2× electrophoresis sample buffer for reducing SDS-PAGE.
Stable BRCA1 knockdown cell lines
BRCA1 knockdown was achieved using ShRNA-expressing plasmids purchased from Superarray Bioscience (Frederick, MD). Transfected cell selection was with 10 μg/ml puromycin.
NURSA Molecule Pages:
Coregulators: VDR.
Footnotes
This work was supported in part by Grant 10PB-0127 from the Prostate Cancer Foundation and California Breast Cancer Research Program (to A.M.W.).
Disclosure Statement: The authors have nothing to declare.
First Published Online December 12, 2008
Abbreviations: ECFP, Enhanced cyan fluorescent protein; FBS, fetal bovine serum; NLS, nuclear localization signals; OCP, osteocalcin promoter; 1α,25(OH)2D3, 1α,25(OH)2vitamin D3; PRL, prolactin; PRLR, PRL receptor; PS, penicillin-streptomycin; RXR, retinoid X receptor; ShRNA, short hairpin RNA; STAT, signal transducer and activator of transcription; TBP, TATA-binding protein; VDR, vitamin D receptor; VDRE, VDR response element.
References
- 1.Huvos A1991. Bone tumors: diagnosis, treatment and prognosis. Philadelphia: WB Saunders
- 2.Bataille-Simoneau N, Gerland K, Chappard D, Basle MF, Mercier L1996. Expression of prolactin receptors in human osteosarcoma cells. Biochem Biophys Res Commun 229:323–328 [DOI] [PubMed] [Google Scholar]
- 3.Doppler W1994. Regulation of gene expression by prolactin. Rev Physiol Biochem Pharmacol 124:93–130 [DOI] [PubMed] [Google Scholar]
- 4.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- 5.Morales P, Carretero MV, Geronimo H, Copin SG, Gaspar ML, Marcos MA, Martin-Perez J1999. Influence of prolactin on the differentiation of mouse B-lymphoid precursors. Cell Growth Differ 10:583–590 [PubMed] [Google Scholar]
- 6.Lorenson MY, Walker AM2001. Structure-function relationships in prolactin. In: Horseman N, ed. Prolactin. Boston, MA: Kluwer Academic Publishers; 189–217
- 7.Walker AM2007. S179D Prolactin: antagonistic agony! Mol Cell Endocrinol 276:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coss D, Yang L, Kuo CB, Xu X, Luben RA, Walker AM2000. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am J Physiol Endocrinol Metab 279:E1216–E1225 [DOI] [PubMed]
- 9.Scheven BA, Marshall D, Aspden RM2002. In vitro behavior of human osteoblasts on dentin and bone. Cell Biol Int 26:337–346 [DOI] [PubMed] [Google Scholar]
- 10.Chen TJ, Kuo CB, Tsai KF, Liu JW, Chen DY, Walker AM1998. Development of recombinant human prolactin receptor antagonists by molecular mimicry of the phosphorylated hormone. Endocrinology 139:609–616 [DOI] [PubMed] [Google Scholar]
- 11.Wang YF, Liu JW, Mamidi M, Walker AM1996. Identification of the major site of rat prolactin phosphorylation as serine 177. J Biol Chem 271:2462–2469 [DOI] [PubMed] [Google Scholar]
- 12.Walker AM2006. Therapeutic potential of S179D prolactin—from prostate cancer to angioproliferative disorders: the first selective prolactin receptor modulator. Expert Opin Invest Drugs 15:1257–1267 [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen JP, van Driel M, van den Bemd GJ, Pols HA2001. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr 11:199–226 [PubMed] [Google Scholar]
- 14.van Driel M, Pols HA, van Leeuwen JP2004. Osteoblast differentiation and control by vitamin D and vitamin D metabolites. Curr Pharm Des 10:2535–2555 [DOI] [PubMed] [Google Scholar]
- 15.Norman AW, Okamura WH, Bishop JE, Henry HL2002. Update on biological actions of 1α,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3 Mol Cell Endocrinol 197:1–13 [DOI] [PubMed] [Google Scholar]
- 16.Stern PH1990. Vitamin D and bone. Kidney Int Suppl 29:S17–S21 [PubMed]
- 17.Franceschi RT, Romano PR, Park KY1988. Regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D3 in human osteosarcoma cells. J Biol Chem 263:18938–18945 [PubMed] [Google Scholar]
- 18.Owen TA, Aronow MS, Barone LM, Bettencourt B, Stein GS, Lian JB1991. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology 128:1496–1504 [DOI] [PubMed] [Google Scholar]
- 19.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- 20.Javed A, Gutierrez S, Montecino M, van Wijnen AJ, Stein JL, Stein GS, Lian JB1999. Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D-responsive transcription and contribute to chromatin organization. Mol Cell Biol 19:7491–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanello LP, Norman AW2004. Rapid modulation of osteoblast ion channel responses by 1α,25(OH)2-vitamin D3 requires the presence of a functional vitamin D nuclear receptor. Proc Natl Acad Sci USA 101:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wark JD, Tashjian Jr AH1983. Regulation of prolactin mRNA by 1,25-dihydroxyvitamin D3 in GH4C1 cells. J Biol Chem 258:12118–12121 [PubMed] [Google Scholar]
- 23.Wu W, Chen YH, Ueda E, Tan D, Bartolini P, Walker AM2006. Different forms of prolactin have opposing effects on the expression of cell cycle regulatory proteins in differentiated mammary epithelial cells. Oncol Res 16:75–84 [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Zanello L, Walker AM2007. S179D prolactin sensitizes human prostate cancer cells such that physiological concentrations of 1,25 dihydroxy vitamin D3 result in growth inhibition and cell death. Prostate 67:1498–1506 [DOI] [PubMed] [Google Scholar]
- 25.Beer TM, Myrthus A2004. Calcitriol in cancer treatment: from the lab to the clinic. Mol Cancer Ther 3:373–381 [PubMed] [Google Scholar]
- 26.Prufer K, Schroder C, Hegyi K, Barsony J2002. degardation of RXRs influences sensitivity of rat osteosarcoma cells to the antiproliferative effects of calcitriol. Mol Endocrinol 16:961–976 [DOI] [PubMed] [Google Scholar]
- 27.Majeska RJ, Rodan SB, Rodan GA1980. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology 107:1494–1503 [DOI] [PubMed] [Google Scholar]
- 28.Morrison NA, Shine J, Fragonas JC, Verkest V, McMenemy ML, Eisman JA1989. 1,25-Dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science 246:1158–1161 [DOI] [PubMed] [Google Scholar]
- 29.Yasmin R, Williams RM, Xu M, Noy N2005. Nuclear import of the retinoid X receptor, the vitamin D receptor, and their mutual heterodimer. J Biol Chem 280:40152–40160 [DOI] [PubMed] [Google Scholar]
- 30.Clevenger CV, Kline JB2001. Prolactin receptor signal transduction. Lupus 10:706–718 [DOI] [PubMed] [Google Scholar]
- 31.Masuyama H, MacDonald PN1998. Proteasome-mediated degradation of the vitamin D receptor (VDR) and a putative role for SUG1 interaction with the AF-2 domain of VDR. J Cell Biochem 71:429–440 [PubMed] [Google Scholar]
- 32.Li XY, Boudjelal M, Xiao JH, Peng ZH, Asuru A, Kang S, Fisher GJ, Voorhees JJ1999. 1,25-Dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol Endocrinol 13:1686–1694 [DOI] [PubMed] [Google Scholar]
- 33.Mahonen A, Pirskanen A, Maenpaa PH1991. Homologous and heterologous regulation of 1,25-dihydroxyvitamin D3 receptor mRNA levels in human osteosarcoma cells. Biochim Biophys Acta 1088:111–118 [DOI] [PubMed] [Google Scholar]
- 34.Arbour NC, Prahl JM, DeLuca HF1993. Stabilization of the vitamin D receptor in rat osteosarcoma cells through the action of 1,25-dihydroxyvitamin D3 Mol Endocrinol 7:1307–1312 [DOI] [PubMed] [Google Scholar]
- 35.Hsieh JC, Shimizu Y, Minoshima S, Shimizu N, Haussler CA, Jurutka PW, Haussler MR1998. Novel nuclear localization signal between the two DNA-binding zinc fingers in the human vitamin D receptor. J Cell Biochem 70:94–109 [PubMed] [Google Scholar]
- 36.Prufer K, Barsony J2002. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol 16:1738–1751 [DOI] [PubMed] [Google Scholar]
- 37.Kalderon D, Roberts BL, Richardson WD, Smith AE1984. A short amino acid sequence able to specify nuclear location. Cell 39:499–509 [DOI] [PubMed] [Google Scholar]
- 38.Fanara P, Hodel MR, Corbett AH, Hodel AE2000. Quantitative analysis of nuclear localization signal (NLS)-importin α interaction through fluorescence depolarization. Evidence for auto-inhibitory regulation of NLS binding. J Biol Chem 275:21218–21223 [DOI] [PubMed] [Google Scholar]
- 39.Cyert MS2001. Regulation of nuclear localization during signaling. J Biol Chem 276:20805–20808 [DOI] [PubMed] [Google Scholar]
- 40.Michigami T, Suga A, Yamazaki M, Shimizu C, Cai G, Okada S, Ozono K1999. Identification of amino acid sequence in the hinge region of human vitamin D receptor that transfers a cytosolic protein to the nucleus. J Biol Chem 274:33531–33538 [DOI] [PubMed] [Google Scholar]
- 41.Luo Z, Rouvinen J, Maenpaa PH1994. A peptide C-terminal to the second Zn finger of human vitamin D receptor is able to specify nuclear localization. Eur J Biochem 223:381–387 [DOI] [PubMed] [Google Scholar]
- 42.Miyauchi Y, Michigami T, Sakaguchi N, Sekimoto T, Yoneda Y, Pike JW, Yamagata M, Ozono K2005. Importin 4 is responsible for ligand-independent nuclear translocation of vitamin D receptor. J Biol Chem 280:40901–40908 [DOI] [PubMed] [Google Scholar]
- 43.Zella A, Kim S, Shevde NK, Pike JW2007. Enhancers located in the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3 J Steroid Biochem Mol Biol 103:435–439 [DOI] [PubMed] [Google Scholar]
- 44.Nawaz Z, O'Malley BW2004. Urban renewal in the nucleus: is protein turnover by proteasomes absolutely required for nuclear receptor-regulated transcription? Mol Endocrinol 18:493–499 [DOI] [PubMed] [Google Scholar]
- 45.Rechsteiner M, Rogers SW1996. PEST sequences and regulation by proteolysis. Trends Biochem Sci 21:267–271 [PubMed] [Google Scholar]
- 46.Yarden RI, Papa MZ2006. BRCA1 at the crossroad of multiple cellular pathways: approaches for therapeutic interventions. Mol Cancer Ther 5:1396–1404 [DOI] [PubMed] [Google Scholar]
- 47.Somasundaram K, Zhang H, Zeng YX, Houvras Y, Peng Y, Zhang H, Wu GS, Licht JD, Weber BL, El-Deiry WS1997. Arrest of the cell cycle by the tumor-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature 389:187–190 [DOI] [PubMed] [Google Scholar]
- 48.Colston KW, Hansen CM2002. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer 9:45–59 [DOI] [PubMed] [Google Scholar]