Abstract
Liver X receptors, LXRα and LXRβ, are nuclear receptors belonging to the large family of transcription factors. After activation by oxysterols, LXRs play a central role in the control of lipid and carbohydrate metabolism as well as inflammation. The role of LXRα has been extensively studied, particularly in the liver and macrophages. In the liver it prevents cholesterol accumulation by increasing bile acid synthesis and secretion into the bile through ATP-binding cassette G5/G8 transporters, whereas in macrophages it increases cholesterol reverse transport. The function of LXRβ is still under investigation with most of the current knowledge coming from the study of phenotypes of LXRβ−/− mice. With these mice new emerging roles for LXRβ have been demonstrated in the pathogenesis of diseases such as amyotrophic lateral sclerosis and chronic pancreatitis. The present review will focus on the abnormalities described so far in LXRβ−/− mice and the insight gained into the possible roles of LXRβ in human diseases.
Studies on the phenotype of LXRβ−/− mice have revealed important roles for LXRβ in controlling not only lipid metabolism but also water transport.
Nuclear receptors are transcription factors that act as intracellular receptors for small biologically active molecules such as lipids and steroid hormones. In contrast to extracellular receptors that bind peptide ligands and activate a cascade of cytoplasmic kinases, nuclear receptors are intracellular, have lipophilic ligands, and directly regulate the expression of target genes by binding to specific DNA sequences (response elements) in the promoters of target genes. Each response element consists of a consensus sequence (AGGTCA) of single or double elements in a direct, everted, or inverted repeat involving binding of nuclear receptors as monomers, homodimers, or heterodimers (1).
Forty-eight members of the nuclear receptor superfamily have been identified in humans. Nuclear receptors share a canonical structure, composed of functionally distinct domains (Fig. 1A): the N-terminal activation function 1 domain, highly variable in sequence and length (2, 3); the very conserved DNA-binding domain (DBD) that contains two zinc fingers, involved not only in DNA binding but also in receptor dimerization (4); and the C-terminal ligand-binding domain (LBD) with a key role in ligand binding, nuclear localization, receptor dimerization, and interaction with coactivators and corepressors (5, 6). Between the DBD and LBD is the hinge domain that provides flexibility between these two domains. Activation function 2 is part of LBD the different conformations of which are dictated by the structure of the ligand bound in the ligand binding pocket and accordingly are recognized by either coactivators or corepressors.
Fig. 1.
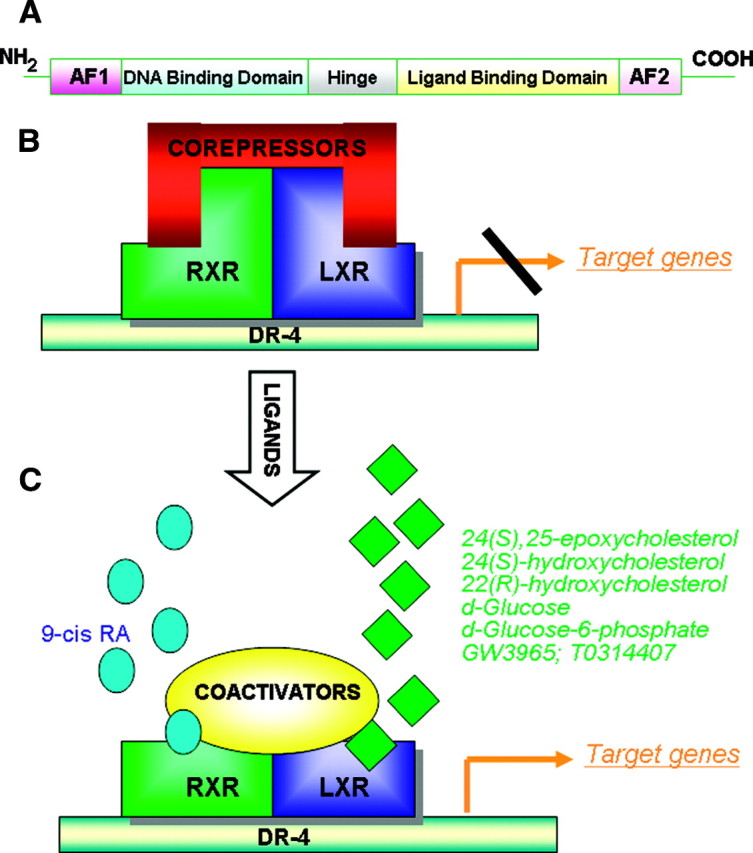
A, The canonical structure of nuclear receptors. B, LXR is interacting with corepressors in absence of ligands. C, With ligands, LXR binds to coactivators and promotes the transcription of target genes. AF2, Activation function 2; DR-4, direct repeat 4; RA, retinoic acid.
Over the past 15 yr, thanks to the highly conserved sequence homology of the DBD of nuclear receptors, it has been possible to discover many previously unknown nuclear receptors. Therefore, endocrinology has been fascinatingly reversed (7): the characterization of cloned receptor sequences took place before the identification of natural specific ligands and even before the discovery of any functional role and tissue distribution of many of these nuclear receptors. This reverse endocrinology has, as its final goal, the identification of pharmaceutical compounds that can selectively modulate the activity of nuclear receptors and be a promising treatment for diseases in which nuclear receptors or their target genes are involved.
Liver X Receptors (LXRs)
Liver X receptors, LXRα (nuclear receptor 1H3) and LXRβ (nuclear receptor 1H2), belong to the family of ligand-activated transcription factors with a key role in the control of lipid metabolism and inflammation.
Initially the LXRs were considered orphans because they were cloned on the basis of sequence homology with other receptors, and their ligands were unknown. They have since been adopted by oxygenated cholesterol metabolites that at physiological concentrations are able to bind to and activate these receptors. The most potent ligands for LXRs are 22-hydroxycholesterol, 24(S)-hydroxycholesterol, 24(S), 25-epoxycholesterol, and 27-hydroxycholesterol (8). Recently, high concentrations of d-glucose have been reported to activate LXRs in vitro (9) although the mechanism is still controversial (10). In addition, two nonsteroidal synthetic compounds, GW3965 and T0314407, have been identified as potent and orally active agonists for both LXRs (11, 12). Although subtype-specific ligands have not been synthesized, a subset of natural bile acids has been reported to activate LXRα (13) whereas N-acylthiadiazolines have selectivity for LXRβ but with modest potency (14).
Levels of LXRα mRNA are high in the liver, adipose tissue, adrenal glands, intestine, kidney, macrophages, and lung, whereas LXRβ mRNA is widely distributed throughout the body with high levels in the developing brain (15, 16).
LXRs form obligate heterodimers with retinoid X receptor (RXR) (17). The LXR/RXR complex, which can be activated by ligands of either partner, binds to LXR-responsive elements (LXREs) consisting of direct repeat 4 of the core sequence 5′-AGGTCA-3′ separated by four nucleotides on the promoter of target genes (18). In the absence of ligands (Fig. 1B), LXRs bind to the cognate LXRE in complex with corepressors such as silencing mediator of retinoic acid and thyroid hormone receptor and nuclear receptor corepressor (19, 20). Under these conditions the transcriptional activity of the receptor is suppressed. In the presence of ligands (Fig. 1C), LXR undergoes a conformational change that induces release of corepressors, recruitment of specific coactivators and, subsequently, transcription of target genes (21).
LXR target genes are involved not only in cholesterol, triglyceride, and bile acid metabolism but also in inflammatory response and energy balance. LXRs act as sterol sensors: when the cellular concentration of oxysterols increases, LXR induces the transcription of genes that protect the cell from cholesterol overload.
In the liver, LXR activation promotes cholesterol elimination in two different ways: first, by induction of the expression of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the classical pathway of bile acid biosynthesis (22); second, by promoting the transcription of genes for ATP-binding cassette transporters, ABCG5 and ABCG8 that transport cholesterol from the hepatocytes to the bile canaliculi (23, 24). At the same time, hepatic LXR activation also increases the synthesis of fatty acids and triglycerides by up-regulating sterol regulatory-binding protein 1c, the key regulator of hepatic lipogenesis (12) and other genes in the pathway such as fatty acid synthase, acyl-coenzyme A carboxylase, and stearoyl-coenzyme A desaturase 1 (25, 26). For this reason, treatment with LXR agonists usually increases hepatic and serum levels of triglycerides, an unwanted side effect for this class of pharmaceuticals.
LXR also offers extrahepatic tissues protection from cholesterol accumulation. Through the control of reverse cholesterol transport in macrophages, LXR ligands induce the expression of ABCA1, ABCG1, and ABCG4 transporters that promote the efflux of cholesterol to high-density lipoproteins (27, 28, 29, 30). In addition LXRs promote the transcription of apolipoproteins (ApoE and ApoC in macrophages and ApoD in adipose tissue) that act as cholesterol acceptors (31, 32, 33). Moreover, LXRs positively regulate lipoprotein lipase, cholesterol ester transfer protein, and the phospholipid transfer protein (34, 35), all of which are involved in lipoprotein remodeling.
In addition to cholesterol and triglyceride metabolism, LXR activation also influences glucose homeostasis. In particular, in the liver, LXR agonists decrease glucose output and increase glucose utilization by inducing glucokinase and repressing phosphoenolpyruvate carboxykinase and glucose 6-phosphatase (36), whereas in white adipose tissue, glucose transporter 4, an insulin-dependent glucose transporter, is induced (37).
In addition to their important physiological roles as antiatherogenic and antidiabetogenic receptors, LXRs are also immunomodulatory. The antiinflammatory action of LXRs is evident from the down-regulation of inducible nitric oxide synthase, COX2, IL6, and IL1β that occurs in LXRα−/−β−/− mice. So far no LXRE has been identified on the promoters of these repressed genes, and the mechanism through which LXRs exert these immunomodulatory effects remains to be elucidated (38).
Studies on Mice Lacking the LXRβ Isoform
A few years after the cloning of LXRs, the identification of oxysterols as LXR activators and the isolation of an LXRE in the promoter of CYP7A1 raised the hypothesis of a key regulatory role of these nuclear receptors in cholesterol homeostasis. Recently, in vivo studies on the phenotype of LXRα−/− and LXRβ−/− mice have not only confirmed this theory, but also demonstrated specific and distinct roles of each isoform in controlling lipid and glucose metabolism, inflammation, and proliferation.
Metabolic phenotype
The most studied phenotype involves metabolic alterations seen during dietary challenge in the three different genotypes: LXRα−/−, LXRβ−/−, and LXRα−/−β−/− mice. On a standard rodent diet (Table 1), LXRα−/−mice differ from wild-type (WT) mice, first in the amount of perigonadal fat, which tends to be reduced with decreased adipocyte size (39), and second, in the serum and hepatic lipid profile. Serum total cholesterol and very low-density lipoproprotein (LDL) triglycerides are in the normal range whereas LDL cholesterol is increased in parallel with a reduction in high-density lipoprotein (HDL) cholesterol (40). Glucose tolerance is the same as in WT mice, as is hepatic cholesterol; triglycerides in the liver are slightly reduced (40). Interestingly, when LXRα−/− mice are fed with a diet containing 2% of cholesterol (Table 1), they are unable to regulate cholesterol catabolism in the liver, where, as a consequence, cholesterol esters accumulate as early as 8 d after the start of feeding (22, 41). WT mice have the capacity to metabolize high concentrations of dietary cholesterol, mainly by increasing the activity of CYP7A1, the rate-limiting enzyme in the classical pathway of bile acid biosynthesis. Excess cholesterol is then eliminated by its conversion to bile acids. This mechanism is missing in LXRα−/− mice so that, even on a standard diet, they exhibit a decreased bile acid pool and a low ratio between cholic acid and muricholic acid (22). Furthermore, during cholesterol feeding of these mice, serum total cholesterol and LDL cholesterol are significantly increased whereas HDL cholesterol is reduced; liver transaminases are higher than in WT mice, but after 90 d they decrease, probably an indication of liver failure.
Table 1.
Metabolic modifications in LXRα−/− mice during dietary challenge
| Standard chow diet | 2% Cholesterol | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (months) | Ref. | Sex | Age (months) | Ref. | |||||||||
| Body weight | Normal | MF | 12 | (38 43 ) | Unchanged | MF | 2–3 | (21 ) | ||||||
| Perigonadal fat | Reduced | MF | 12 | (38 43 ) | Reduced | MF | 2–3 | (21 ) | ||||||
| Hepatic triglyceride | Tendency to be reduced | – | 4 | (39 ) | Reduced | MF | 2–3 | (21 ) | ||||||
| Hepatic cholesterol | Normal | MF | 3–4 | (21 39 40 ) | Increased | MF | 3–4 | (21 40 ) | ||||||
| Serum cholesterol | Normal | MF | 4 | (39 40 ) | Increased | MF | 3–4 | (40 ) | ||||||
| LDL | Tendency to be increased | – | 4 | (39 ) | Increased | MF | 3–4 | (40 ) | ||||||
| HDL | Tendency to be reduced | – | 4 | (39 ) | – | – | – | – | ||||||
| Very LDL | Normal | – | 4 | (39 ) | – | – | – | – | ||||||
| LDL/HDL ratio | Increased | – | 4 | (39 ) | – | – | – | – | ||||||
| Serum triglycerides | Normal | – | 3 | (39 ) | Tendency to be reduced | MF | 3–4 | (21 ) | ||||||
| Serum glucose | Normal | F | 6–9 | (38 ) | Tendency to be reduced | MF | 3–4 | (21 ) | ||||||
| ALT | Normal | MF | 3–4 | (40 ) | Increased | MF | 3–4 | (21 40 ) | ||||||
| AST | – | – | – | – | Increased | MF | 2–3 | (21 ) | ||||||
| Albumin | – | – | – | – | Normal | MF | 2–3 | (21 ) | ||||||
| BA excretion | Normal | MF | 3 | (21 ) | Reduced | MF | 2–3 | (21 ) | ||||||
| BA pool | Reduced | MF | 3 | (21 ) | Reduced | MF | 2–3 | (21 ) | ||||||
| Ratio CA/MCA | Reduced | MF | 3 | (21 ) | Reduced | MF | 2–3 | (21 ) | ||||||
| Leptin | Normal | F | 12 | (38 ) | – | – | – | – | ||||||
| Adiponectin | Normal | F | 12 | (38 ) | – | – | – | – | ||||||
| Resistin | Normal | F | 12 | (38 ) | – | – | – | – | ||||||
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BA, bile acid; CA, cholic acid; F, female; MF, male and female; MCA, muricholic acid.
LXRβ−/− mice, when fed with a standard diet (Table 2), differ from WT mice in terms of a reduction in the amount of perigonadal fat (39), a decrease in hepatic and serum levels of triglycerides (40), and a decrease in serum insulin (39). Surprisingly, on a diet containing 2% cholesterol (Table 2), the response of LXRβ−/− mice is similar to WT mice; in particular, they maintain the physiological capacity to eliminate excess cholesterol by increasing bile acid synthesis. Indeed, in these mice, the expression of enzymes involved in bile acid metabolism (CYP7A1, CYP7B, CYP8B1, CYP27) is not affected (39). Interestingly, in LXRβ−/− mice fed a high-fat diet, body weight and amount of the perigonadal fat pad were lower than in WT mice on the same diet (39). These data together represent compelling evidence that LXRα plays a central role in maintaining cholesterol homeostasis in the liver, whereas LXRβ has a different role in fat metabolism, in particular related to a resistance in gaining weight.
Table 2.
Metabolic modifications in LXRβ−/− mice during dietary challenge
| Standard chow diet | 2% Cholesterol | High-fat diet | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (months) | Ref. | Sex | Age (months) | Ref. | Sex | Age (months) | Ref. | |||||||||||||
| Body weight | Normal/reduced | MF | 12 | (38 43 ) | – | – | – | – | Reduced | F | – | (38 ) | |||||||||
| Perigonadal fat | Reduced | MF | 12 | (38 43 ) | – | – | – | – | Reduced | F | – | (38 ) | |||||||||
| Food intake | Normal in males/increased in females | MF | 6–12 | (38 43 ) | – | – | – | – | – | – | – | – | |||||||||
| O2 consumption | Normal | M | 6 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
| Serum insulin | Reduced | MF | 6–9 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
| Hepatic triglyceride | Decreased | F | 4–12 | (38 39 ) | – | – | – | – | – | – | – | – | |||||||||
| Hepatic cholesterol | Normal | – | 4 | (39 ) | Normal | MF | 3–4 | (40 ) | – | – | – | – | |||||||||
| Serum cholesterol | Normal | – | 4 | (39 ) | Normal | MF | 3–4 | (40 ) | – | – | – | – | |||||||||
| Serum triglycerides | Tendency to be reduced | – | 4–12 | (38 39 ) | – | – | – | – | – | – | – | – | |||||||||
| ALT | Normal | MF | 3–4 | (40 ) | Normal | MF | 3–4 | (40 ) | – | – | – | – | |||||||||
| LDL | Normal | – | 4 | (39 ) | – | – | – | – | – | – | – | – | |||||||||
| HDL | Normal | – | 4 | (39 ) | – | – | – | – | – | – | – | – | |||||||||
| VLDL | Tendency to be reduced | – | 4 | (39 ) | – | – | – | – | – | – | – | – | |||||||||
| Leptin | Normal | F | 12 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
| Adiponectin | Normal | F | 12 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
| Resistin | Normal | F | 12 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
ALT, Alanine aminotransferase; F, female; MF, male and female.
The lack of LXRβ is responsible for the lean phenotype observed in LXRα−/−β−/− mice. On a standard diet the body weight of these mice is reduced as is the size of the perigonadal fat pad and adipocytes (39, 42, 43). Triglycerides are reduced both in liver and serum as in LXRβ−/− mice whereas, as in LXRα−/− mice, total cholesterol and LDL cholesterol are increased, HDL cholesterol is decreased in serum, and hepatic cholesterol is increased. Interestingly, LXRα−/−β−/− mice are also resistant to obesity when fed a Western diet containing 0.2% cholesterol, but they gain weight when only fed a high-fat diet (39).
Pancreatic phenotype
We have investigated the mechanism behind the resistance of LXRβ−/− mice to gain weight and found a malabsorption syndrome due to chronic pancreatitis (44) with the following abnormalities: serum levels of α-amylase and lipase and fecal levels of proteases are drastically reduced; pancreatic ducts are invaded by immune infiltrations, and epithelial cells have a high rate of cell death; and intralobular pancreatic ducts are filled with dense secretion with laminar structures typical of cystic fibrosis. Interestingly, both mRNA and protein expression of aquaporin-1 (AQP-1), a water channel transporting water into the pancreatic lumen, are markedly reduced in LXRβ−/− mice. To determine whether LXR is directly involved in the regulation of pancreatic AQP-1 expression, we treated WT mice with the LXR agonist, TO2030. We found a significant increase in AQP-1 in the pancreatic ductal epithelium indicating a very interesting new role of LXRβ in controlling water transport.
Neurological phenotype
Given that LXRβ−/− mice maintain their capacity to metabolize dietary cholesterol in the liver, the function of LXRβ has been studied in other organs in which cholesterol plays a physiological key role, especially the central nervous system.
Male LXRβ−/− mice at 7 months of age develop a motor impairment, measured as a reduced ability, compared with WT littermates, to stay on a rotor rod. Learning ability and muscle strength are not affected. Histopathological analysis of LXRβ−/− spinal cords revealed a loss of large α motor neurons in the lateroventral horn and, in the few motor neurons left, there were cytoplasmic lipid accumulations and protein aggregates which stained positive for TDP-43 and ubiquitin (45). The number of astrocytes was increased, and an axonal atrophy was detectable (46). These features, evident only in male mice, are typical of the chronic motor neuron disease amyotrophic lateral sclerosis (ALS). In LXRβ−/− mice, accumulation of lipids in motor neurons could be due to a decrease in the expression of transporters such as ABCG5, ABCG8, and ABCA1 and could lead to motor neuron degeneration. Interestingly, in the spinal cord of patients affected by ALS, high levels of sphingomyelin, ceramides, and cholesterol esters have been demonstrated (47). Moreover, mice carrying a Cu/Zn superoxide dismutase 1 mutation, considered to be an animal model of ALS, show accumulation of phytosterols in the spinal cord (47). Plant sterols are transported back to the intestinal lumen by ABCG5 and ABCG8 transporters, and mice lacking these transporters accumulate plant sterols in the brain (48).
In LXRα−/−β−/− mice, as expected, there is a down-regulation of ABCG5 and ABCG8 transporters. This is accompanied by lipid overload in the brain particularly in the choroid plexus with the result that the lateral ventricles are obliterated by lipid-laden cells. There is also proliferation of astrocytes, loss of neurons, and disorganized myelin sheaths (49).
Interestingly, in the population of Guam, when the diet was based on Cycas micronesica, a vegetable rich in β-sitosterol, there was a very high incidence of ALS associated with Parkinson′s dementia (PDC). Feeding of monkeys with up to 2 g of cycad flour does not lead to any neurological disease (50). Thus it is thought that the indigenous Guam population has a genetic predisposition that renders them susceptible to the toxic effects of cycad flour. Our studies (45) suggest that LXRβ could be a genetic factor involved in PDC pathogenesis: LXRβ−/− mice fed β-sitosterol for 3 wk develop a phenotype that resembles PDC. In the spinal cord, there is a drastic reduction in the number of motor neurons whereas in the substantia nigra, dopaminergic neurons were shrunken with reduced numbers of projections, and there was an increase in the number of activated microglia in the pars reticulata.
In regard to cerebral phenotype, histopathological data on LXRβ−/− brain are not yet available, but low mRNA levels of ABCG1 and ABCA1 have been demonstrated (51). Interestingly, after experimental ischemic stroke in mice lacking LXRβ, there was a wider ischemic area (52), and administration of an LXR agonist decreased the volume of the infarct in wild type, but not in LXRβ−/−, mice (52).
Embryonic phenotype
Studies from our own laboratory have demonstrated that LXRβ has an important role in the development of the cerebral cortex. At late stage of embryogenesis (embryonic d 18.5) and in neonates (postnatal d 2), brains of LXRβ−/− mice are smaller than those of WT littermates with a reduction in the number of neurons in the superficial cortical layers. During development, neurons migrate from lower layers to superficial layers. After birth (postnatal d 2), in LXRβ−/− mice the number of neurons is higher in lower cortical layers (IV) whereas in WT mice more neurons are in the upper layer (II–III) indicating that in the absence of LXRβ there is a migration defect (53).
Reproductive phenotype
During pregnancy, cholesterol plays an important role in modulating membrane activity and stability of oxytocin receptor (54). When activated by its specific hormone, oxytocin, this receptor induces contractile activity in the myometrium during labor. A balanced cholesterol distribution in uterus has a central role in contractile function: depletion increases abnormal contraction of uterine smooth muscle cells in vitro, whereas cholesterol accumulation in myometrium reduces the amplitude of contractions during labor (55). In LXRβ−/− mice, there is a high accumulation of cholesterol esters in longitudinal and circular layers of myometrium, probably due to a reduction in the expression of transporters, ABCA1 and ABCG1, which regulate cholesterol efflux and are under the control of LXRβ. As a consequence, uteri of LXRβ−/− mice have an impaired contractile function in response to oxytocin or prostaglandin F2α stimulation, and these mice show several signs of fetal resorption in the uterine horns, unexpulsed pups, or death during delivery (56). Interestingly, this role of LXRβ in cholesterol modulation in uterus seems very specific because LXRα−/− mice do not exhibit any uterine abnormalities (56). Moreover, ovarian function is impaired in LXRα−/−β−/− mice; in particular, oocytes lacking both LXRs fail to resume meiosis after FSH stimulation (57).
LXRβ also controls cholesterol balance in the male reproductive tract: in the testis of LXRβ−/− mice, there is an accumulation of cholesterol as early as at 2.5 months of age in Sertoli cells that provide structural and nutritional support to germ cells (58). There is also a lower proliferation rate of germ cells (59). Furthermore, although Leydig cells are not affected by lipid accumulation in LXRβ−/− mice, the secretion rate of testosterone is markedly reduced in LXRα−/−β−/− mice. No structural or functional alterations are detectable in testis from LXRα−/− mice, indicating a dominant role of LXRβ. In addition, the epithelial structure in the epididymis is affected in LXRα−/−β −/− mice but not in single-mutant mice. This defect results in sperm head fragility (60).
All the described alterations, both in the female and male reproductive tract, may together be responsible for the reduced fertility and decreased number of pups per litter, described in LXRβ−/− mice and LXRα−/−β−/− mice (57). Furthermore, LXRα−/−β−/− mice become completely infertile by 4–5 months of age (58, 59), indicating a more severe phenotype in mice lacking both isoforms.
Immunological phenotype
By the age of 5–6 months, in LXRβ−/− mice there is splenomegaly and lymphoadenopathy with expansion of total number of T and B lymphocytes. A prevalence of B lymphocytes was noticed only in lymph nodes. Moreover, LXRβ−/− T lymphocytes respond to T cell receptor stimulation with a higher proliferative rate than T lymphocytes from LXRα−/− and WT mice (61). Conversely, in WT mouse T lymphocytes in cell culture (but not in LXRβ−/− T lymphocytes), proliferation is inhibited when LXR synthetic agonist, RXR agonist or 22(R)-hydroxycholesterol are added to the culture medium (61). In activated LXRβ−/− T lymphocytes in cell culture, the proliferation rate is higher that that of WT mouse T cells with an increased fraction of cells in S and G2, M phase (61).
Cardiovascular phenotype
There are abnormalities in the vascular system in LXRα−/−β−/− mice that are most likely due to a combination of both the increase in LDL-cholesterol and of the down-regulation of genes involved in reverse cholesterol transport (ABCG1, ABCA1). In LXRα−/−β−/− mice there is accumulation of foam cells with a significant increase in lipid infiltration all around the intima-media layer in the aortic root. Accumulation of lipid in the aorta is less pronounced in single-mutant mice (40). Except for a report that there is lack of neutral lipid accumulation in cardiac myocytes (56), there is no published information about the cardiac phenotype of LXRβ−/− mice. Interestingly, LXRβ activation in LXRα−/−ApoE−/− mice reduces plasma cholesterol and atherosclerosis (62).
Moreover, LXRβ binds to the promoter of renin, and LXR agonist treatment leads to an up-regulation of renin mRNA in kidney (63). Thus it is anticipated that LXRβ mice might be hypotensive, but, so far, no studies on blood pressure in these mice have been published.
Dermatological phenotype
Oxysterols, ligands for LXRs, stimulate keratinocyte differentiation by increasing the expression of epidermal differentiation markers such as involucrin, filaggrin, and loricrin, in WT and LXRα−/− mice but not in LXRβ−/−mice (64, 59). The epidermis of LXRβ−/− mice is thinner with a reduced number of proliferating cell nuclear antigen-positive cells in the basal layer and a slightly decreased expression of differentiation markers. However, in these mice, no macroscopic cutaneous abnormalities or functional impairments are detectable, indicating that LXRβ−/− mice use some compensatory pathways to overcome the consequences of the lack of keratinocyte differentiation (64).
Bone phenotype
LXRβ−/− female mice exhibit increased levels of alkaline phosphatase and osteocalcin in serum, probably indicating an increased osteoblast activity. In addition, the volume of osteoclasts in the trabecular compartment is lower that that in WT mice (65). However, no particular alterations have been detected in the cortical or in the trabecular compartment of the femurs of these mice.
LXRβ Genetics in Human Diseases
The role of genetic mutations or gene polymorphisms of LXRs in human diseases corresponding to the phenotypes described in the transgenic animals is almost completely unexplored; at the moment only three such studies have been published.
According to the database of National Center for Biotechnology Information, four single-nucleotide polymorphisms (SNPs) of LXRβ (chromosome 19) have been identified: LXR1 in intron 5, LXR2 in intron 7, LXR3 in the 3′-untranslated region, and LXR4 in intron 2. An association between the risk of developing late-onset (age at onset after 60 yr) Alzheimer disease and LXR2 and LXR4 has been shown in an American population of 931 Alzheimer disease patients (66). Although LXR2 seems to be a silent SNP, LXR4 is likely to be functional, residing in either a coding region or in a splicing junction. Moreover, this association has been confirmed also in a Spanish population of 414 Alzheimer disease patients. In this study there was an increased risk if these SNPs (LXR2, LXR4, LXR1) are associated with a SNP in heme-oxygenase-1 (413 TT) (67).
The third study involves a Swedish population of 559 obese patients. This study revealed that one LXRα (rs2279238) and two LXRβ SNPs (LB44732G>A and rs2695121), in the promoter region and in intron 2, are associated with obesity (68).
More studies are required to confirm that these SNPs have a functional role in the susceptibility of Alzheimer disease and obesity.
Conclusions
Studies on the phenotype of LXRβ−/− mice have revealed important roles for LXRβ in controlling lipid metabolism in the central nervous system, in particular in the spinal cord where its deficiency causes lipid accumulation and death of motor neurons leading to a picture resembling ALS. Studies in humans are strongly encouraged to identify possible genetic mutations that could work in concert with environmental factors, such as high phytosterol intake, in the pathogenesis of ALS.
Our studies have revealed that, in addition to lipid metabolism, LXRβ also controls water transport in pancreatic tissue. LXRβ deficiency leads to a down-regulation of AQP-1 and as a consequence, there is dense pancreatic secretion containing laminar structures typically seen in cystic fibrosis. In this perspective, LXRβ could be considered as a water transport regulator and a promising target in the treatment of disease associated with a dysregulation of the fluid balance such as in pancreatic insufficiency or cystic fibrosis.
Table 3.
Metabolic modifications in LXRα−/−β−/− mice during dietary challenge
| Standard chow diet | Western diet + CH | Western diet no CH | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (m) | Ref | Sex | Age (m) | Ref | Sex | Age (m) | Ref | |||||||||||||
| Body weight | Reduced | MF | 4–5–12 | (41 43 ) | Reduced | M | 4–5 | (42 ) | Unchanged | M | 4–5 | (42 ) | |||||||||
| Perigonadal fat | Reduced | MF | 12–18 | (38 41 43 ) | – | – | – | – | – | – | – | – | |||||||||
| Food intake | normal | MF | 12 | (43 ) | Unchanged | M | 4–5 | (42 ) | Unchanged | M | 4–5 | (42 ) | |||||||||
| Fat absorption | Reduced | M | 4–5 | (42 ) | Unchanged | M | 4–5 | (42 ) | Unchanged | m | 4–5 | (42 ) | |||||||||
| Serum insulin | Reduced | M | 4–5 | (42 ) | Tendency to be reduced | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| Hepatic Triglyceride | Reduced | – | 4 | (39 ) | Reduced | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| Hepatic Cholesterol | Increased | – | 4 | (39 ) | Increased | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| Serum cholesterol | Normal | – | 4 | (39 ) | Tendency to be reduced | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| LDL | Incresed | – | 4 | (39 ) | Increased | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| HDL | tendency to be reduced | – | 4 | (39 ) | – | – | – | – | – | – | – | – | |||||||||
| LDL/HDL ratio | Increased | – | 4 | (39 ) | – | – | – | – | – | – | – | – | |||||||||
| Serum Triglycerides | Reduced | – | 4 | (39 ) | Reduced | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| TSH | Normal | M | 4–5 | (42 ) | – | – | – | – | – | – | – | – | |||||||||
| T3 | Normal | M | 4–5 | (42 ) | – | – | – | – | – | – | – | – | |||||||||
| T4 | Normal | M | 4–5 | (42 ) | – | – | – | – | – | – | – | – | |||||||||
| Leptin | Normal / lower | F | 12 | (3842 ) | Tendency to be reduced | M | 4–5 | (42 ) | – | – | – | – | |||||||||
| Adiponectin | Reduced | F | 12 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
| Resistin | normal | F | 12 | (38 ) | – | – | – | – | – | – | – | – | |||||||||
NURSA Molecule Pages:
Ligands: 22α-Hydroxycholesterol | 24(S) | GW 3965;
Nuclear Receptors: LXRα | LXRβ.
Footnotes
This work was supported by a grant from the Swedish Research Council (70192301, K2008-67X-02819-3).
Disclosure Statement: J.-Å.G. is consultant, shareholder, and grant receiver from KaroBio AB, Stockholm, Sweden. The other authors declare no conflict of interests.
First Published Online December 12, 2008
Abbreviations: ABC, ATP-binding cassette; ALS, amyotrophic lateral sclerosis; Apo, apolipoprotein; AQP-1, aquaporin-1; CYP7A1, cholesterol 7α-hydroxylase; DBD, DNA-binding domain; HDL, high-density lipoprotein; LBD, ligand-binding domain; LDL, low-density lipoprotein; LXR, liver X receptor; LXRE, LXR-responsive element; PDC, Parkinson′s dementia; RXR, retinoid X receptor; SNP, single-nucleotide polymorphism; WT, wild type.
References
- 1.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ2001. Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870 [DOI] [PubMed] [Google Scholar]
- 2.Giguere V, Hollenberg SM, Rosenfeld MG, Evans RM1986. Functional domains of the human glucocorticoid receptor. Cell 46:645–652 [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P1987. Functional domains of the human estrogen receptor. Cell 51:941–951 [DOI] [PubMed] [Google Scholar]
- 4.Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB1991. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 352:497–505 [DOI] [PubMed] [Google Scholar]
- 5.Graupner G, Wills KN, Tzukerman M, Zhang XK, Pfahl M1989. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature 340:653–656 [DOI] [PubMed] [Google Scholar]
- 6.Webster NJ, Green S, Jin JR, Chambon P1988. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell 54:199–207 [DOI] [PubMed] [Google Scholar]
- 7.Kliewer SA, Lehmann JM, Willson TM1999. Orphan nuclear receptors: shifting endocrinology into reverse. Science 284:757–760 [DOI] [PubMed] [Google Scholar]
- 8.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ1999. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc Natl Acad Sci USA 96:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E2007. The nuclear receptor LXR is a glucose sensor. Nature 445:219–223 [DOI] [PubMed] [Google Scholar]
- 10.Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C2008. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest 118:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, Plunket KD, Morgan DG, Beaudet EJ, Whitney KD, Kliewer SA, Willson TM2002. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem 45:1963–1966 [DOI] [PubMed] [Google Scholar]
- 12.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B2000. Role of LXRs in control of lipogenesis. Genes Dev 14:2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song C, Hiipakka RA, Liao S2000. Selective activation of liver X receptor α by 6α-hydroxy bile acids and analogs. Steroids 65:423–427 [DOI] [PubMed] [Google Scholar]
- 14.Molteni V, Li X, Nabakka J, Liang F, Wityak J, Koder A, Vargas L, Romeo R, Mitro N, Mak PA, Seidel HM, Haslam JA, Chow D, Tuntland T, Spalding TA, Brock A, Bradley M, Castrillo A, Tontonoz P, Saez E2007. N-Acylthiadiazolines, a new class of liver X receptor agonists with selectivity for LXRβ. J Med Chem 50:4255–4259 [DOI] [PubMed] [Google Scholar]
- 15.Kainu T, Kononen J, Enmark E, Gustafsson JA, Pelto-Huikko M1996. Localization and ontogeny of the orphan receptor OR-1 in the rat brain. J Mol Neurosci 7:29–39 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Mangelsdorf DJ2002. LuXuRies of lipid homeostasis: the unity of nuclear hormone receptors, transcription regulation, and cholesterol sensing. Mol Interv 2:78–87 [DOI] [PubMed] [Google Scholar]
- 17.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M1994. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol 14:7025–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiebel FF, Gustafsson JA1997. Heterodimeric interaction between retinoid X receptor α and orphan nuclear receptor OR1 reveals dimerization-induced activation as a novel mechanism of nuclear receptor activation. Mol Cell Biol 17:3977–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JD, Evans RM1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- 20.Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, Rosenfeld MG1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- 21.Glass CK, Rosenfeld MG2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- 22.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR α. Cell 93:693–704 [DOI] [PubMed] [Google Scholar]
- 23.Berge KE, von Bergmann K, Lutjohann D, Guerra R, Grundy SM, Hobbs HH, Cohen JC2002. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res 43:486–494 [PubMed] [Google Scholar]
- 24.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ2002. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors α and β. J Biol Chem 277:18793–18800 [DOI] [PubMed] [Google Scholar]
- 25.Horton JD, Goldstein JL, Brown MS2002. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 67:491–498 [DOI] [PubMed] [Google Scholar]
- 26.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P2002. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem 277:11019–11025 [DOI] [PubMed] [Google Scholar]
- 27.Costet P, Luo Y, Wang N, Tall AR2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 275:28240–28245 [DOI] [PubMed] [Google Scholar]
- 28.Engel T, Lorkowski S, Lueken A, Rust S, Schluter B, Berger G, Cullen P, Assmann G2001. The human ABCG4 gene is regulated by oxysterols and retinoids in monocyte-derived macrophages. Biochem Biophys Res Commun 288:483–488 [DOI] [PubMed] [Google Scholar]
- 29.Kennedy MA, Venkateswaran A, Tarr PT, Xenarios I, Kudoh J, Shimizu N, Edwards PA2001. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem 276:39438–39447 [DOI] [PubMed] [Google Scholar]
- 30.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529 [DOI] [PubMed] [Google Scholar]
- 31.Hummasti S, Laffitte BA, Watson MA, Galardi C, Chao LC, Ramamurthy L, Moore JT, Tontonoz P2004. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J Lipid Res 45:616–625 [DOI] [PubMed] [Google Scholar]
- 32.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P2001. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 98:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak PA, Laffitte BA, Desrumaux C, Joseph SB, Curtiss LK, Mangelsdorf DJ, Tontonoz P, Edwards PA2002. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors α and β. J Biol Chem 277:31900–31908 [DOI] [PubMed] [Google Scholar]
- 34.Laffitte BA, Joseph SB, Chen M, Castrillo A, Repa J, Wilpitz D, Mangelsdorf D, Tontonoz P2003. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol Cell Biol 23:2182–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Tall AR2000. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest 105:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P2003. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA 100:5419–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalen KT, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI2003. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor α. J Biol Chem 278:48283–48291 [DOI] [PubMed] [Google Scholar]
- 38.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9:213–219 [DOI] [PubMed] [Google Scholar]
- 39.Gerin I, Dolinsky VW, Shackman JG, Kennedy RT, Chiang SH, Burant CF, Steffensen KR, Gustafsson JA, MacDougald OA2005. LXRβ is required for adipocyte growth, glucose homeostasis, and β cell function. J Biol Chem 280:23024–23031 [DOI] [PubMed] [Google Scholar]
- 40.Schuster GU, Parini P, Wang L, Alberti S, Steffensen KR, Hansson GK, Angelin B, Gustafsson JA2002. Accumulation of foam cells in liver X receptor-deficient mice. Circulation 106:1147–1153 [DOI] [PubMed] [Google Scholar]
- 41.Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Bjorkhem I, Pettersson S, Gustafsson JA2001. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRβ-deficient mice. J Clin Invest 107:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juvet LK, Andresen SM, Schuster GU, Dalen KT, Tobin KA, Hollung K, Haugen F, Jacinto S, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI2003. On the role of liver X receptors in lipid accumulation in adipocytes. Mol Endocrinol 17:172–182 [DOI] [PubMed] [Google Scholar]
- 43.Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ2005. LXRs regulate the balance between fat storage and oxidation. Cell Metab 1:231–244 [DOI] [PubMed] [Google Scholar]
- 44.Gabbi C, Kim HJ, Hultenby K, Bouton D, Toresson G, Warner M, Gustafsson JA2008. Pancreatic exocrine insufficiency in LXRβ−/− mice is associated with a reduction in aquaporin-1 expression. Proc Natl Acad Sci USA 105:15052–15057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, Gustafsson JA2008. Liver X receptor β (LXRβ): a link between β-sitosterol and amyotrophic lateral sclerosis-Parkinson’s dementia. Proc Natl Acad Sci USA 105:2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersson S, Gustafsson N, Warner M, Gustafsson JA2005. Inactivation of liver X receptor β leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA 102:3857–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP2002. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol 52:448–457 [DOI] [PubMed] [Google Scholar]
- 48.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC2004. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J Lipid Res 45:301–307 [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA2002. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci USA 99:13878–13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer PS1987. Guam ALS/Parkinsonism-dementia: a long-latency neurotoxic disorder caused by “slow toxin(s)” in food? Can J Neurol Sci 14:347–357 [DOI] [PubMed] [Google Scholar]
- 51.Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P2007. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver X receptors. Proc Natl Acad Sci USA 104:10601–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA2008. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation 118:1450–1459 [DOI] [PubMed] [Google Scholar]
- 53.Fan X, Kim HJ, Bouton D, Warner M, Gustafsson JA2008. Expression of liver X receptor β is essential for formation of superficial cortical layers and migration of later-born neurons. Proc Natl Acad Sci USA 105:13445–13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gimpl G, Fahrenholz F2001. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683 [DOI] [PubMed] [Google Scholar]
- 55.Smith RD, Babiychuk EB, Noble K, Draeger A, Wray S2005. Increased cholesterol decreases uterine activity: functional effects of cholesterol alteration in pregnant rat myometrium. Am J Physiol Cell Physiol 288:C982–C988 [DOI] [PubMed]
- 56.Mouzat K, Prod'homme M, Volle DH, Sion B, Dechelotte P, Gauthier K, Vanacker JM, Lobaccaro JM2007. Oxysterol nuclear receptor LXRβ regulates cholesterol homeostasis and contractile function in mouse uterus. J Biol Chem 282:4693–4701 [DOI] [PubMed] [Google Scholar]
- 57.Steffensen KR, Robertson K, Gustafsson JA, Andersen CY2006. Reduced fertility and inability of oocytes to resume meiosis in mice deficient of the Lxr genes. Mol Cell Endocrinol 256:9–16 [DOI] [PubMed] [Google Scholar]
- 58.Robertson KM, Schuster GU, Steffensen KR, Hovatta O, Meaney S, Hultenby K, Johansson LC, Svechnikov K, Soder O, Gustafsson JA2005. The liver X receptor-β is essential for maintaining cholesterol homeostasis in the testis. Endocrinology 146:2519–2530 [DOI] [PubMed] [Google Scholar]
- 59.Volle DH, Mouzat K, Duggavathi R, Siddeek B, Dechelotte P, Sion B, Veyssiere G, Benahmed M, Lobaccaro JM2007. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol Endocrinol 21:1014–1027 [DOI] [PubMed] [Google Scholar]
- 60.Frenoux JM, Vernet P, Volle DH, Britan A, Saez F, Kocer A, Henry-Berger J, Mangelsdorf DJ, Lobaccaro JM, Drevet JR2004. Nuclear oxysterol receptors, LXRs, are involved in the maintenance of mouse caput epididymidis structure and functions. J Mol Endocrinol 33:361–375 [DOI] [PubMed] [Google Scholar]
- 61.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P2008. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134:97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P2007. Ligand activation of LXR β reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR α and apoE. J Clin Invest 117:2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morello F, de Boer RA, Steffensen KR, Gnecchi M, Chisholm JW, Boomsma F, Anderson LM, Lawn RM, Gustafsson JA, Lopez-Ilasaca M, Pratt RE, Dzau VJ2005. Liver X receptors α and β regulate renin expression in vivo. J Clin Invest 115:1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komuves LG, Schmuth M, Fowler AJ, Elias PM, Hanley K, Man MQ, Moser AH, Lobaccaro JM, Williams ML, Mangelsdorf DJ, Feingold KR2002. Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-β in murine epidermis. J Invest Dermatol 118:25–34 [DOI] [PubMed] [Google Scholar]
- 65.Robertson KM, Norgard M, Windahl SH, Hultenby K, Ohlsson C, Andersson G, Gustafsson JA2006. Cholesterol-sensing receptors, liver X receptor α and β, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res 21:1276–1287 [DOI] [PubMed] [Google Scholar]
- 66.Adighibe O, Arepalli S, Duckworth J, Hardy J, Wavrant-De Vrieze F2006. Genetic variability at the LXR gene (NR1H2) may contribute to the risk of Alzheimer’s disease. Neurobiol Aging 27:1431–1434 [DOI] [PubMed] [Google Scholar]
- 67.Infante J, Rodriguez-Rodriguez E, Mateo I, Llorca J, Vazquez-Higuera JL, Berciano J, Combarros O, Gene-gene interaction between heme oxygenase-1 and liver X receptor-β and Alzheimer’s disease risk. Neurobiol Aging, in press [DOI] [PubMed]
- 68.Dahlman I, Nilsson M, Jiao H, Hoffstedt J, Lindgren CM, Humphreys K, Kere J, Gustafsson JA, Arner P, Dahlman-Wright K2006. Liver X receptor gene polymorphisms and adipose tissue expression levels in obesity. Pharmacogenet Genomics 16:881–889 [DOI] [PubMed] [Google Scholar]


