Abstract
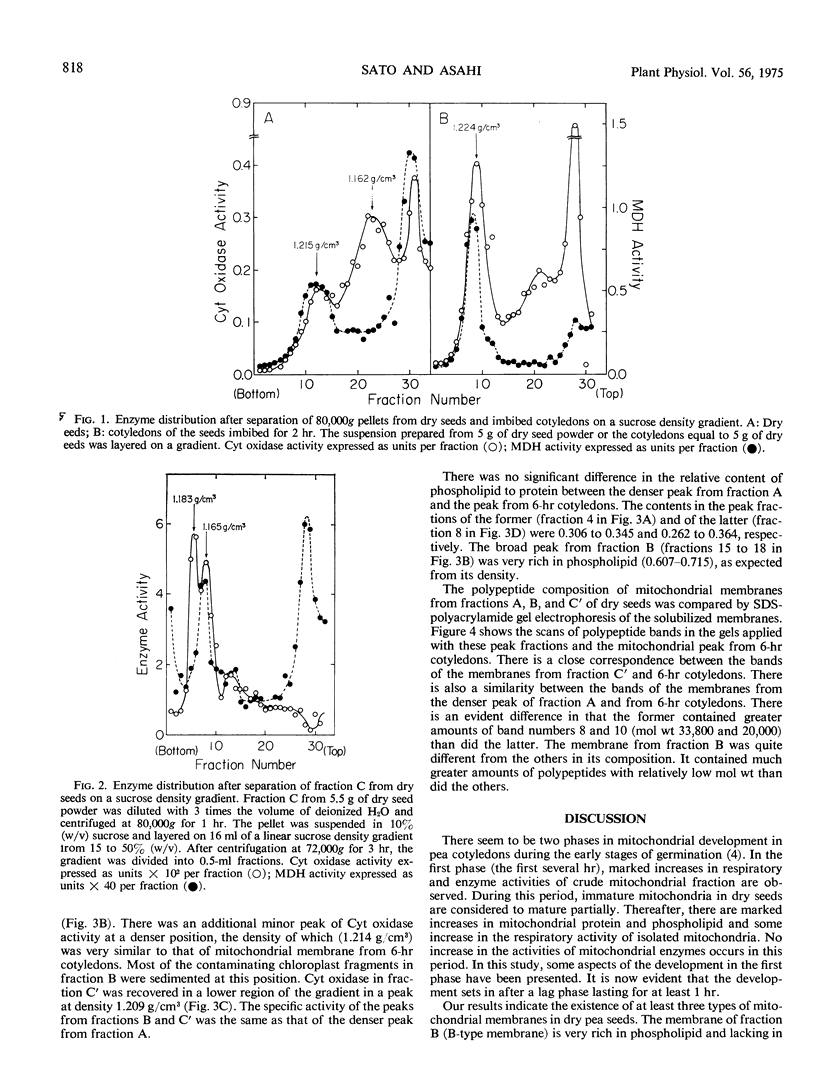
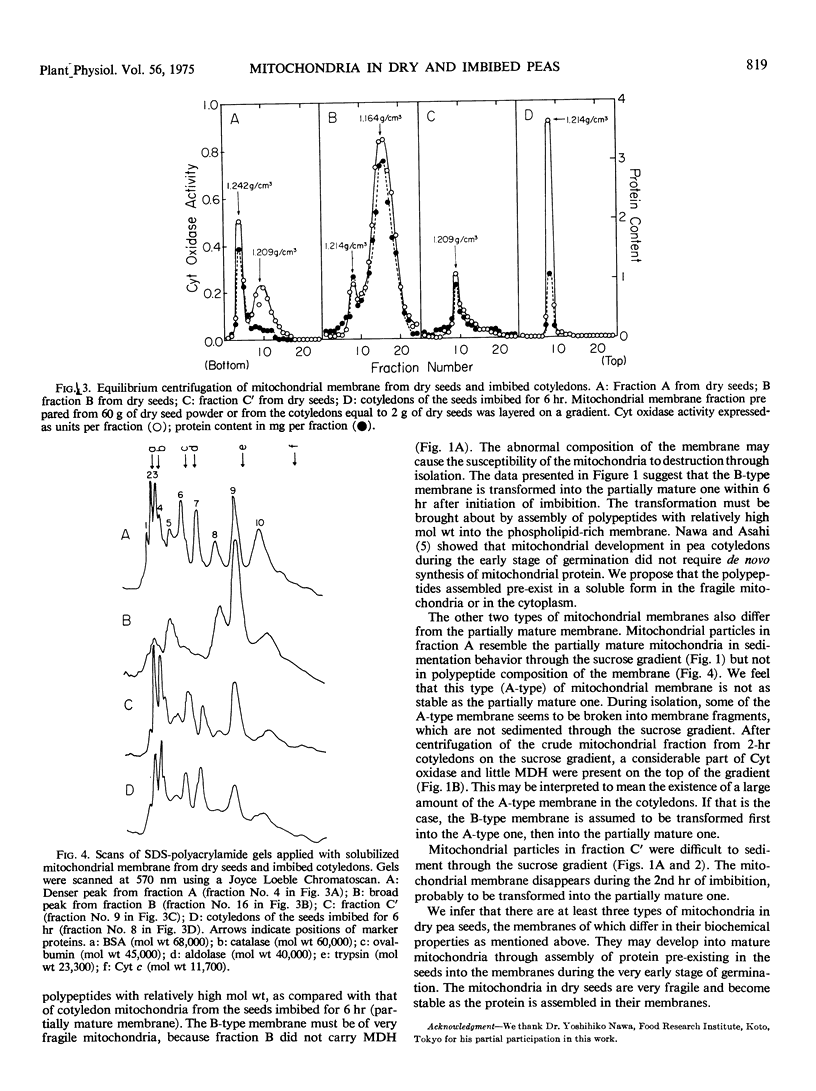
An attempt to isolate intact mitochondria from dry pea seeds (Pisum sativum var. Alaska) ended in failure. Cytochrome oxidase in crude mitochondrial fraction from dry seeds was separated into three fractions by sucrose density gradient centrifugation. Two of the fractions contained malate dehydrogenase, whereas the other did not. Equilibrium centrifugation of mitochondrial membrane on sucrose gradients revealed that the membrane from the fraction without malate dehydrogenase was lighter than that from the others. Differences were observed in relative content of phospholipid to protein and in polypeptide composition analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis among the membranes from three fractions and imbibed cotyledons. Membrane from the fraction without malate dehydrogenase was rich in phospholipid and lacking in polypeptides with relatively high molecular weights as compared with that from others. During imbibition, the fraction without malate dehydrogenase and one of the other two disappeared rapidly after a lag phase lasting for at least 1 hour. Concomitantly, active and stable mitochondria increased in the cotyledons. The results were interpreted to indicate that there were at least three types of mitochondria in dry seeds, the membranes of which differed in their biochemical properties, and that the mitochondria became active and stable through assembly of protein into the membranes during imbibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Biochemical Studies on Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination: Effects of Antibiotics on the Development. Plant Physiol. 1973 May;51(5):833–838. doi: 10.1104/pp.51.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971 Dec;48(6):671–674. doi: 10.1104/pp.48.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Malhotra S. S., Prasad S., Malhotra S. K., Spencer M. Biochemical and structural changes in mitochondria and other cellular components of pea cotyledons during germination. Can J Biochem. 1972 Jul;50(7):725–737. doi: 10.1139/o72-101. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


