Introduction
KEY TEACHING POINTS
|
The study of left atrial (LA) fibrosis with delayed enhancement–magnetic resonance imaging (DE-MRI) is an established technique that provides a noninvasive means of detecting the electroanatomic substrate of atrial fibrillation (AF).1 We present a case of a middle-aged woman with systemic lupus erythematosus (SLE) and persistent AF who had extensive atrial fibrosis as detected by DE-MRI before the ablation procedure.
Case Report
In October 2010, a 46-year-old woman was admitted to our cardiology department for an ablation procedure of symptomatic persistent AF. She had SLE since 1990, which started as articular and then became renal involvement (diffuse proliferative glomerulonephritis, class IV) and was treated with steroid therapy, cyclosporine, and cyclophosphamide bolus. All therapies were discontinued in 2006 because of a complete remission of the disease. No cardiovascular risk factor was present, in particular no family history of cardiomyopathy. She was symptomatic for impaired functional status, and she was refractory to pharmacological treatment with antiarrhythmic drugs of class 1C. The echocardiogram revealed normal dimensions of the left ventricle with overall normal systolic function. The LA was moderately enlarged, and the right atrium was only slightly enlarged. Mild mitral regurgitation was found.
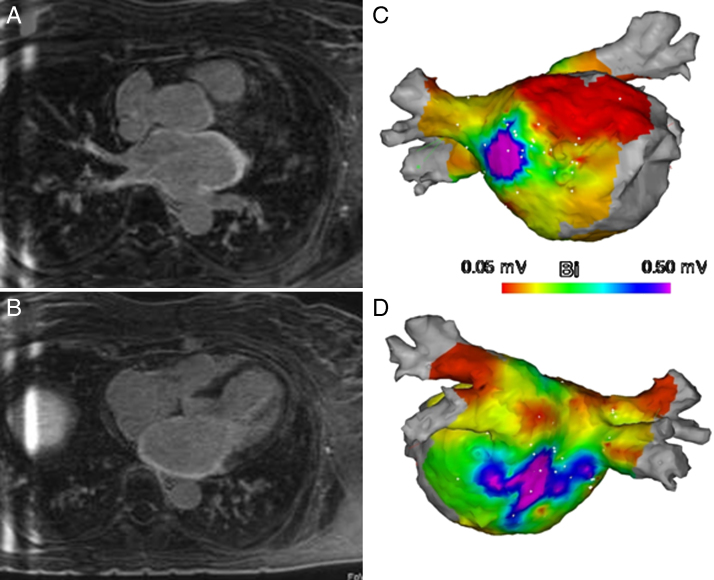
Before the ablation procedure, she underwent MRI with atrial fibrosis detection (DE-MRI), as reported previously.1 DE-MRI was performed with a 1.5-T scanner (MAGNETOM Avanto, Siemens Medical Systems, Erlangen, Germany) 15 minutes after a bolus injection of 0.1 mmol/kg of gadobenate dimeglumine (MultiHance, Bracco Spa, Milan, Italy) using a 3-dimensional (3D) inversion recovery prepared, respiration navigated, ECG-gated, gradient echo pulse sequence with fat saturation (Gradient Echo 3-dimensional Inversion Recover Turbo Flash). The axial DE-MRI images revealed massive delayed enhancement in different regions of the LA (see the Online Video), including anterior wall (Figure 1A), posterior wall, and septum (Figure 1B). The percentage of fibrosis relative to the volume of the LA wall2 was 40%. Furthermore, a significant homogeneous thickening distribution of the atrial wall was present (measured thickness 6.8 mm). The maximum LA volume and LA emptying fraction3 were 38 ml/mq and 47%, respectively. The DE imaging performed using a 2D inversion recovery sequence after contrast injection demonstrated a small area of fibrosis in the inferolateral mid-wall ventricular myocardium.
Figure 1.
Two-dimensional slice from the delayed enhancement–magnetic resonance imaging scan shows massive delayed enhancement in the anterior (A) and posterior (B) walls of the left atrium. An electroanatomic map shows large patches of low bipolar voltage (<0.05 mV) in the anterior wall and septum (C), interspersed with electrically abnormal tissue (0.0 5 mV < bipolar voltage <1 mV) in the posterior wall and roof (D).
During the electrophysiological study (CARTO3, Biosense Webster, Inc., Diamond Bar, CA), the endocardial electroanatomic map of the LA confirmed large areas of low bipolar voltage (<0.05 mV) in the anterior wall and septum (Figure 1C) and an area of electrically abnormal tissue (0.05 mV < bipolar voltage <1 mV) in the posterior wall and roof (Figure 1D). The percentage of fibrosis relative to the LA surface estimated by using the CARTO3 system was 52%.
The ablation procedure was performed without complications; nevertheless, during follow-up, the patient had recurrences of atrial tachycardia.
SLE is an inflammatory autoimmune disease that can virtually affect all organ systems.4 Cardiovascular involvement occurs frequently (58%–77%).5 The most common cardiac manifestations include pericarditis (~25% of all patients with SLE),6, 7 myocarditis,8 coronary artery disease,9 valvular lesion such as valvular thickening and regurgitation, and Libman-Sacks endocarditis (prevalence 11%–74%).10 In the context of cardiac arrhythmias, sinus tachycardia (50% of patients), AF, and atrial ectopic beats are the most frequent.11 All types of atrioventricular blocks, intraventricular conduction disturbances, and sick sinus syndrome are also observed.12, 13
Very little is known about the pathogenesis of cardiac manifestations in SLE.6 Histological features of SLE cardiomyopathy include initially inflammatory cell infiltrates and, as the disease advances, myocardial necrosis, fibrotic replacement, and scarring.14, 15 Cases of intramural and subepicardial ventricular fibrosis detected by DE-MRI have been described in the literature.14 In addition, in other inflammatory autoimmune and vasculitic diseases, DE-MRI most frequently detected a midwall ventricular fibrosis, probably owing to a combination of subclinical inflammatory and immunological processes,16 while a mosaic patchy distribution of myocardial fibrosis is a pathognomonic feature of systemic sclerosis17 and recently all ventricular patterns (subendocardial, subepicardial, intramural, and transmural) are observed in these diseases.18 Nevertheless, to of our knowledge, atrial fibrosis has not been previously reported in SLE or other autoimmune diseases. However, the study of atrial fibrosis using DE-MRI is recent and not widely implemented in the clinical setting of patients with AF.
Herein, we also highlight the following peculiar features of this case:
-
1.
The occasional feedback of a significant thickening of the atrial wall in a patient with SLE and AF, a finding of DE-MRI that is frequently detected in storage disorders, such as amyloidosis, that have a known etiopathogenetic mechanism. DE-MRI helped us make a differential diagnosis between these abnormalities.
-
2.
The extension of atrial fibrosis by at least 40% compared to the volume of the LA wall, as evidenced by DE-MRI. It is known that atrial fibrosis with an increase in extracellular matrix deposition results in intra-atrial conduction disturbances (ie, anisotropy and zigzag conduction), thus creating a substrate for AF.19, 20
These findings are matched to a given reduced atrial functionality that could be interpreted as a predictor of cardiovascular events.3, 21, 22
In conclusion, in a patient with SLE with no other cardiovascular risk factors, the presence of an uncommon thickening of the LA wall associated with an extensive atrial fibrosis could, in our opinion, justify the refractoriness of AF. The important question that needs to be answered is whether SLE leads to atrial fibrosis, and other studies are necessary to confirm a correlation between them.
Footnotes
Supplementary material cited in this article is available online at doi:10.1016/j.hrcr.2015.02.014.
Appendix. Supplementary data
Video. DE-MRI images revealed large amounts of DE in different regions of the LA including anterior wall, posterior wall and septum.
References
- 1.Oakes R.S., Badger T.J., Kholmovski E.G. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravanelli D., dal Piaz E.C., Centonze M., Casagranda G., Marini M., Del Greco M., Karim R., Rhode K., Valentini A. A novel skeleton based quantification and 3-D volumetric visualization of left atrium fibrosis using late gadolinium enhancement magnetic resonance imaging. IEEE Trans Med Imaging. 2014;33:566–576. doi: 10.1109/TMI.2013.2290324. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S., Matulevicius S.A., Ayers C.R., Berry J.D., Patel P.C., Markham D.W., Levine B.D., Chin K.M., de Lemos J.A., Peshock R.M., Drazner M.H. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278–285. doi: 10.1093/eurheartj/ehs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh Y.P., Naidoo P., Ngian G.S. Imaging of systemic lupus erythematosus, Part I: CNS, cardiovascular, and thoracic manifestations. Clin Radiol. 2013;68:181–191. doi: 10.1016/j.crad.2012.06.110. [DOI] [PubMed] [Google Scholar]
- 5.Hejtmancik M.R., Wright J.C., Quint R., Jennings F.L. The cardiovascular manifestations of systemic lupus erythematosus. Am Heart J. 1964;68:119–130. doi: 10.1016/0002-8703(64)90248-0. [DOI] [PubMed] [Google Scholar]
- 6.Miner J.J., Kim A.H.J. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40:51–60. doi: 10.1016/j.rdc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Moder K.G., Miller T.D., Tazelaar H.D. Cardiac involvement in systemic lupus erythematosus. Mayo Clin Proc. 1999;74:275–284. doi: 10.4065/74.3.275. [DOI] [PubMed] [Google Scholar]
- 8.Doherty N., Siegel R. Cardiovascular manifestations of systemic lupus erythematosus. Am Heart J. 1985;110:1257–1265. doi: 10.1016/0002-8703(85)90023-7. [DOI] [PubMed] [Google Scholar]
- 9.Sarzi-Puttini P., Atzeni F., Gerli R., Bartoloni E., Doria A., Barskova T., Matucci-Cerinic M., Sitia S., Tomasoni L., Turiel M. Cardiac involvement in systemic rheumatic disease: an update. Autoimmun Rev. 2010;9:849–852. doi: 10.1016/j.autrev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Moyssakis I., Tektonidou M.G., Vasilliou V.A. Libman-Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med. 2007;120:636–642. doi: 10.1016/j.amjmed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Seferović P.M., Ristić A.D., Maksimović R., Simeunović D.S., Ristić G.G., Radovanović G., Seferović D., Maisch B., Matucci-Cerinic M. Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology (Oxford) 2006;45:39–42. doi: 10.1093/rheumatology/kel315. [DOI] [PubMed] [Google Scholar]
- 12.Doria A., Iaccarino L., Sarzi-Puttini P., Atzeni F., Turriel M., Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus. 2005;14:683–686. doi: 10.1191/0961203305lu2200oa. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira R.A., Borba E.F., Bonfá E., Martinelli Filho M. Arrhythmias in systemic lupus erythematosus. Rev Bras Reumatol. 2010;50:81–89. [PubMed] [Google Scholar]
- 14.Ashrafi R., Garg P., McKay E., Gosney J., Chuah S., Davis G. Aggressive cardiac involvement in systemic lupus erythematosus: a case report and a comprehensive literature review. Cardiol Res Pract. 2011;15:578390. doi: 10.4061/2011/578390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansari A., Larson P.H., Bates H.D. Cardiovascular manifestations of systemic lupus erythematosus: current perspective. Prog Cardiovasc Dis. 1985;27:421–434. doi: 10.1016/0033-0620(85)90003-9. [DOI] [PubMed] [Google Scholar]
- 16.Edwards N.C., Ferro C.J., Townend J.N., Steeds R.P. Myocardial disease in systemic vasculitis and autoimmune disease detected by cardiovascular magnetic resonance. Rheumatology (Oxford) 2007;46:1208–1209. doi: 10.1093/rheumatology/kem077. [DOI] [PubMed] [Google Scholar]
- 17.Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol. 2014;6:993–1005. doi: 10.4330/wjc.v6.i9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavrogeni S., Sfikakis P.P., Karabela G., Stavropoulos E., Spiliotis G., Gialafos E., Panopoulos S., Bournia V., Manolopoulou D., Kolovou G., Kitas G. Cardiovascular magnetic resonance imaging in asymptomatic patients with connective tissue disease and recent onset left bundle branch block. Int J Cardiol. 2014;171:82–87. doi: 10.1016/j.ijcard.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Allessie M., Ausma J., Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 20.Yue L., Xie J., Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welles C.C., Ku I.A., Kwan D.M., Whooley M.A., Schiller N.B., Turakhia M.P. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. 2012;59:673–680. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staszewsky L., Latini R. What is the atrium trying to tell us? Eur Heart J. 2013;34:255–257. doi: 10.1093/eurheartj/ehs327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. DE-MRI images revealed large amounts of DE in different regions of the LA including anterior wall, posterior wall and septum.