Introduction
KEY TEACHING POINTS
|
Short QT syndrome (SQTS) is an inherited, rare, potentially lethal disease characterized by ventricular repolarization alterations, predisposing individuals to atrial fibrillation (AF), syncope, and high incidence of sudden cardiac death (SCD), often during the first years of life.1, 2 SQTS was first described in a family in which some members showed an abnormally short QT interval and 1 of them suffered from AF. An additional patient had a short QT interval, recurrent ventricular arrhythmias, and, ultimately, SCD.3 The diagnosis of SQTS requires a short QT interval on the resting electrocardiogram (ECG), although its importance remains ambiguous. There is debate over what constitutes a short QT interval and the lower limit of normal changes with age.4 Recently the first diagnostic criteria for SQTS have been published,5 although several diagnostic limitations have been discussed.6 Recently a multicenter pediatric cohort of 25 patients with SQTS has been described.7
Genetic publications have reported gain-of-function pathogenic mutations mainly in 3 different potassium channels (KCNQ1, KCNH2, and KCNJ2).8, 9, 10 In 2011, a pathogenic variant in CACNA2D1 was reported to be associated with SQTS.11 In addition, pathogenic mutations in 2 L-type calcium channels (CACNA1C and CACNB2B)12 have also been reported in a specific phenotype of Brugada syndrome with shortened QT interval. At present, robust genotype–phenotype correlation data are not yet available for SQTS, and thus management must rely on clinical findings.13
AF is defined as an erratic activation of the atria, causing an irregular ventricular response.14 AF is the most common distressing cardiac arrhythmia encountered in clinical practice in the adult population.15 In the pediatric population AF is an extremely rare entity.16 Genetic predisposition contributes to the development of AF.17 To date, more than 15 genes have been described.18 AF has been associated with long QT syndrome19, 20, 21, 22 and with SQTS.23, 24, 25, 26
In 2005, a case showing AF in utero with concomitant bradycardia, short QT interval, and a structurally normal heart was described. Genetic analysis revealed a de novo point mutation (p.V141M) in the KCNQ1 gene.27 The KCNQ1 gene codifies KVLQT1 (Kv7.1), a voltage-activated potassium channel alpha-subunit expressed in various cell types, including cardiac myocytes. In the publication by Villafane et al7 3 of those 25 patients had SQTS and AF. Of those, 1 has the previously reported p.V141M_KCNQ1 mutation. Recently, Villafane et al28 have also reported 2 neonates with AF, structurally normal hearts, and a slow ventricular response rate occurring in association with SQTS. In both individuals, the p.V141M mutation in the KCNQ1 gene was identified. Here we report the clinical follow-up of 3 new unrelated babies, all them diagnosed in utero with the same clinical diagnosis of AF with concomitant bradycardia and short QT interval.
Methods
Clinical analysis
Individuals were clinically evaluated in pediatric arrhythmology services of university hospitals following the same diagnostic techniques for fetal ultrasound, pediatric ECG, and echocardiography. All individuals are white.
Genetic analysis
Informed consent for genetic analysis was obtained from all patients or their legal representatives, in accordance with local institutional review board guidelines of Hospital Josep Trueta (Girona, Spain) and Universitat of Girona (Girona, Spain). The study protocol conforms to the Declaration of Helsinki.
Blood samples were obtained from all 3 patients (3 mL) and from their parents (10 mL). Mutational analysis genomic DNA was isolated from peripheral blood leukocytes using a commercial kit (Gentra System; Puregene). Genetic screening was performed by direct sequencing of the exons and exon–intron boundaries. The exons were amplified and analyzed by direct sequencing. Polymerase chain reaction products were purified with a commercial reagent (ExoSAP-IT, USB) and were directly sequenced from both directions using Genetic Analyzer 3130XL (Applied Biosystems, Life Technologies).
Genetic analysis was performed in the 3 main SQTS-related genes published to date. Therefore, we examine the contribution of KCNQ1 (NM000218.2), KCNH2 (NM000238.2), and KCNJ2 (NM000891.2) in all 3 unrelated cases (UCSC Genome bioinformatics/NCBI Mendelian Inheritance in Man). The previously reported variants were named with Human Gene Mutation Database accession codes. Data regarding genetic variants identified were consulted in available online databases such as the 1000 Genomes project (http://browser.1000genomes.org/) and Exome Sequencing Project (http://evs.gs.washington.edu/EVS/) to study their frequency in the general population. Alignment of species was consulted in the UniProt database (http://www.uniprot.org/). The potential pathogenic role of missense genetic variants was consulted in the CONDEL (CONsensus DELeteriousness score of missense SNVs) database (http://bg.upf.edu/condel/home), Mutation Taster (www.mutationtaster.org), and PROVEAN (Protein Variation Effect Analyzer) (http://provean.jcvi.org/index.php).
Results
Clinical findings
Patient 1 presented with asymptomatic fetal arrhythmia at 24 gestational weeks. Cardiac rhythm was assessed using M-mode and ascending aorta–superior vena cava Doppler recordings suggesting AF. The baby was delivered at 37 weeks gestation. After birth, this female newborn was hemodynamically stable, but an irregular rhythm was auscultated. The 12-lead ECG showed AF, narrow QRS response at 80–120 bpm, and short QTc interval between 270 and 310 ms. Chest radiograph, echocardiography, and electrolyte levels were normal. Electric cardioversion was attempted with no success. The patient was put under daily aspirin treatment. She remained asymptomatic until the age of 2.5 years when she complained of dizziness. A 24-hour ECG Holter test showed symptomatic 5-second pauses; therefore, a pacemaker was implanted using an epicardial right ventricle (RV) lead. She did well during the first 12 months after implant, but she progressively developed dilated cardiomyopathy refractory to diuretic treatment (furosemide, spirinolactone) plus digoxin and carvedilol; thus, resynchronization was attempted by implanting an epicardial left ventricle (LV) lead. Unfortunately, 1 week later the RV lead failed and no further surgical interventions were attempted; therefore, she was left under epicardial LV stimulation. Mildly improved LV function was observed so she was kept under diuretic, digoxin, and carvedilol and followed closely by her cardiologist. At age 8, progressive worsening of the LV function was observed and while she was being evaluated for an eventual heart transplant, she died suddenly (no available recordings). Clinical assessment of parents showed no cardiac alterations.
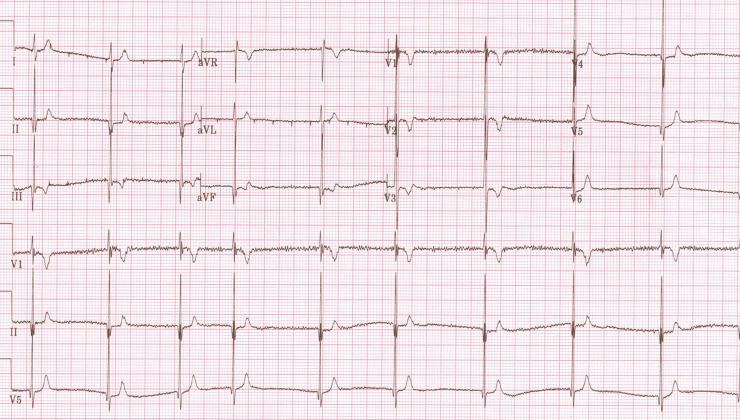
Patient 2 presented with persistent fetal bradycardia at 26.3 gestational weeks. As congenital AV block was suspected, betamethasone was started. Maternal autoimmune screening was negative. At 28.4 weeks the mother was hospitalized for preterm labor and a terbutaline pump was started. The heart rate varied from 70 to 120 bpm. At 35.5 weeks the fetus had a heart rate of 70 bpm, no signs of hydrops, and only a slightly dilated inferior vena cava. At 37 gestational weeks the infant male was delivered by caesarean section. Postdelivery, the ECG showed short a QTc interval (290 ms) and AF (Figure 1). Electric cardioversion was unsuccessful even after quinidine load. Owing to persistent bradycardia, an epicardial DDD device was placed initially; however, because of poor atrial thresholds and the presence of AF, the device was programmed VVIR and has remained so since then. At 8 years of age the child is asymptomatic with mild left ventricular dilatation but normal LV function. He has been on aspirin chronically since birth. Clinical assessment of parents showed no cardiac alterations.
Figure 1.
Electrocardiogram of patient 2 at birth, showing irregular rhythm with no P or F waves and a QT interval of 290 msec.
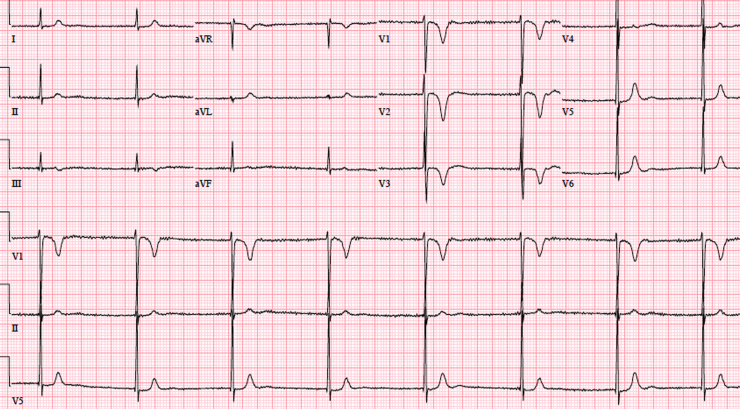
Patient 3 was first seen as a 25-week fetus, for bradycardia. As AV block was suspected, the mother was started on terbutaline. No signs of hydrops were observed throughout the pregnancy. Maternal autoimmune screening was negative. A female baby was born with uncomplicated elective caesarean section, Apgar 7 and 9. After birth the ECG showed AF with junctional scape rhythm around 80 bpm, QTc interval 290 ms. Echocardiography was normal. At day of life 14, an electrophysiology study was performed using an esophageal lead. Neither atrial nor ventricular pace capture was possible, and intravenous adenosine had no effects. Multiple electric cardioversions were attempted, with no success. Finally aspirin and amiodarone were started. Two months later, another electric cardioversion was performed and this successfully restored sinus rhythm. One month later, AF was again noted, amiodarone was discontinued, and flecainide was started. Another electric cardioversion was attempted a month later, with initial success, but eventually AF with rapid ventricular response returned. The patient was weaned off antiarrhythmic drugs. At age 4, the child remained in AF with short QT interval. Echocardiography showed mild LV dilatation (LV dimensions +2 to +2.5 z-score for body surface area). At last follow-up, at age 9, she remains in AF and short QT interval (Figure 2). Echocardiography shows mild LV dilatation (LV dimensions +2 to +2.5 z-score for body surface area) with severe aortic root dilatation (z score +4.02). So far, no pacemaker has been placed in this girl, as she remains asymptomatic, though considering the aortic root dilatation, a pacemaker is being considered. Clinical assessment of parents showed no cardiac alterations.
Figure 2.
Electrocardiogram of patient 3 at 9 years of age, showing atrial fibrillation, narrow QRS complexes, and short QTc interval.
Genetic analysis
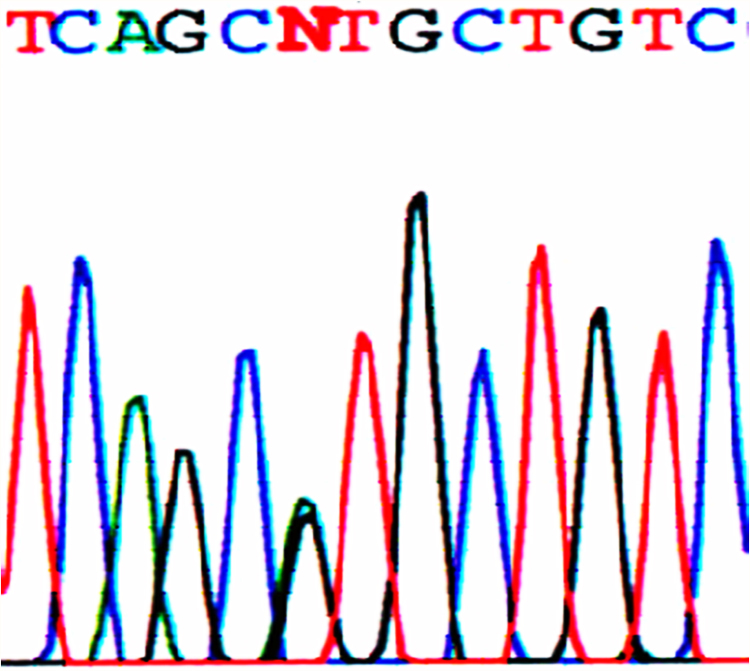
The genetic analysis revealed the same missense p.V141M pathogenic mutation in all 3 samples. This pathogenic mutation, which induces a substitution of G to A at nucleotide 421, c.421G>A (GTG to ATG) and a substitution of valine (Val, V) to methionine (Met, M) at position 141 (Figure 3), was already described.27 Sequence analysis of the biological parents’ DNA of each baby failed to identify the mutation, indicating that p.V141M_KCNQ1 represents a de novo pathogenic mutation in all 3 infants.
Figure 3.
Sequencing analysis displaying a de novo missense pathogenic mutation (p.V141M) in exon 2 of the KCNQ1 gene that codifies the potassium channel KvLQT1. Change of G to A in the KCNQ1 gene nucleotide 421 (c.421G>A), which carries a replacement of GTG valine (V, Val) by ATG, methionine (M, Met), at position 141 (p.V141M, p.Val141Met) of the transmembrane domain S1.
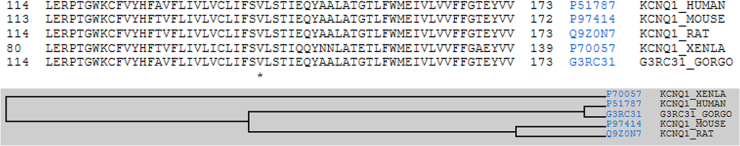
The p.V141M_KCNQ1 genetic variation was described as disease-associated in 2005.27 In order to update genetic data, we consulted the current online databases. No report of this variant in the control population was identified. In addition, new current in silico analyses were used and they all predicted a deleterious effect of the variation. Finally, alignment between species showed a high conserved position (Figure 4).
Figure 4.
Multiple sequence alignment and taxonomy. Conservation of the altered amino acid in the genetic variation p.V141M. The asterisk (*) indicates the position of conserved amino acid between species.
Discussion
In 2005, Hong et al27 identified a missense mutation (p.V141M) in the KCNQ1 gene, which causes AF and shortens the QT interval by altering the gating of IKs channels—to our knowledge, the only pathogenic mutation associated with this rare entity, so far. In 2008, Restier et al29 described that p.V141 causes AF and SQTS, mainly owing to the markedly slow IKs deactivation, such that a proportion of the channels remain in the open state. Recently, Chan et al30 provided AF mechanisms for understanding the effects on channel deactivation underlying the mutation. The constitutive activation is predicted to markedly shorten action potential duration in the atria and ventricles, displaying V141M KCNQ1-KCNE1 heteromultimers and abnormal gating, thereby predisposing to AF and short QT.
The identification of similar clinical findings, previously reported by Hong et al, in 2 more unrelated children who carry the same de novo genetic alteration is an uncommon genetic phenomenon. Thus, from the clinical point of view, this syndrome is responsible for a very specific clinical phenotype. In other clinical-genetic syndromes it has been difficult to link the different mutations to a possible syndrome because of the broad distribution of mutations. It is reasonable to expect that the p.V141M mutation would also shorten atrial action potentials and cause AF in affected children. All children presented in this report showed AF with very poor AV conduction causing severe bradycardia while in utero. When they were born, their heart rates were in the 60–80 bpm range, much lower than the expected average heart rate of 140 bpm.31 In 2007, a knock-in p.S140G_KCNQ1 mouse model was reported showing gain-of-function that causes AV node dysfunction.32 However, how AV conduction can be disrupted by a gain of IKs function is still, at present, speculative.27
Furthermore, from the genetic point of view, the identification of the same de novo mutation in 3 unrelated children suggests a highly probabe mutation-specific disease. The pathogenic role of this variant is supported by in silico predictions and conserved protein region between species. In addition, the mutation is located in codon 141 and from position I132 to A150, 11 more pathogenic mutations have been described (https://portal.biobase-international.com/hgmd/pro/allmut.php), suggesting that the domain S1 appears to be a crucial segment in the function of the protein. The fact that 3 unrelated families showed the same de novo mutation suggests that this protein domain could be more predisposed to suffer alterations, possibly owing to the ineffective DNA mismatch repair system. All these facts suggest this site as a mutational hotspot. However, further mechanistic studies should be performed to clarify this exceptionally unusual genetic phenomenon.
Interestingly, all patients developed LV dilatation in various severities from mild to severe dilated cardiomyopathy. The first pathophysiological suspected mechanism was pacemaker-induced cardiomyopathy. In fact, patient 1 developed dilatation while being paced in the RV, and when she was switched to LV stimulation she had a slight improvement. But, contrary to what happens in pacemaker-induced cardiomyopathy, replacement of the stimulation site did not significantly improve her dilatation. In addition, patient 3 (not paced) presented with moderate dilatation of the LV. Furthermore, patient 2 had a pacemaker and was briefly treated with quinidine. The role of quinidine in LV dilatation is certainly unknown and the sample size is not sufficient to perform statistical analysis. Thus, the role of this channelopathy in causing structural heart disease remains unclear and a matter for future research.
Conclusions
We have described the clinical late follow-up of 3 unrelated patients who presented with AF and bradycardia in utero, associated with SQTS. All 3 carry the same de novo pathogenic mutation, p.V141M_KCNQ1. We support the highly pathogenic role of this mutation, associated with development of ventricular dilatation and heart failure. We recommend genetic analysis in patients with similar clinical findings.
Footnotes
Dr Sarquella-Brugada and Campuzano contributed equally. This work was supported by Fundació “La Caixa.”
References
- 1.Patel U., Pavri B.B. Short qt syndrome: A review. Cardiol Rev. 2009;17:300–303. doi: 10.1097/CRD.0b013e3181c07592. [DOI] [PubMed] [Google Scholar]
- 2.Cross B., Homoud M., Link M., Foote C., Garlitski A.C., Weinstock J., Estes N.A., 3rd The short qt syndrome. J Interv Card Electrophysiol. 2011;31:25–31. doi: 10.1007/s10840-011-9566-0. [DOI] [PubMed] [Google Scholar]
- 3.Gussak I., Brugada P., Brugada J., Wright R.S., Kopecky S.L., Chaitman B.R., Bjerregaard P. Idiopathic short qt interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 4.Anttonen O., Junttila M.J., Rissanen H., Reunanen A., Viitasalo M., Huikuri H.V. Prevalence and prognostic significance of short qt interval in a middle-aged finnish population. Circulation. 2007;116:714–720. doi: 10.1161/CIRCULATIONAHA.106.676551. [DOI] [PubMed] [Google Scholar]
- 5.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–1963. [DOI] [PubMed]
- 6.Veltmann C., Borggrefe M. Arrhythmias: a ׳schwartz score׳ for short qt syndrome. Nature reviews. Cardiology. 2011;8:251–252. doi: 10.1038/nrcardio.2011.51. [DOI] [PubMed] [Google Scholar]
- 7.Villafane J., Atallah J., Gollob M.H. Long-term follow-up of a pediatric cohort with short qt syndrome. J Am Coll Cardiol. 2013;61:1183–1191. doi: 10.1016/j.jacc.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Bellocq C., van Ginneken A.C., Bezzina C.R., Alders M., Escande D., Mannens M.M., Baro I., Wilde A.A. Mutation in the kcnq1 gene leading to the short qt-interval syndrome. Circulation. 2004;109:2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 9.Brugada R., Hong K., Dumaine R. Sudden death associated with short-qt syndrome linked to mutations in herg. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 10.Priori S.G., Pandit S.V., Rivolta I. A novel form of short qt syndrome (sqt3) is caused by a mutation in the kcnj2 gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 11.Templin C., Ghadri J.R., Rougier J.S. Identification of a novel loss-of-function calcium channel gene mutation in short qt syndrome (sqts6) Eur Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antzelevitch C., Pollevick G.D., Cordeiro J.M. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by st-segment elevation, short qt intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brugada J., Gussak I., Brugada P. Short qt syndrome: a predictable story. Cardiology. 2014;128:231–233. doi: 10.1159/000359995. [DOI] [PubMed] [Google Scholar]
- 14.Camm A.J., Kirchhof P., Lip G.Y. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the european society of cardiology (esc) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki S., Shah A.J., Scherr D., Haissaguerre M. Atrial fibrillation: pathophysiology and current therapy. Ann Med. 2011;43:425–436. doi: 10.3109/07853890.2011.554426. [DOI] [PubMed] [Google Scholar]
- 16.Mills L.C., Gow R.M., Myers K., Kantoch M.J., Gross G.J., Fournier A., Sanatani S. Lone atrial fibrillation in the pediatric population. Can J Cardiol. 2013;29:1227–1233. doi: 10.1016/j.cjca.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Brugada R., Tapscott T., Czernuszewicz G.Z., Marian A.J., Iglesias A., Mont L., Brugada J., Girona J., Domingo A., Bachinski L.L., Roberts R. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 18.Campuzano O., Brugada R. Genetics of familial atrial fibrillation. Europace. 2009;11:1267–1271. doi: 10.1093/europace/eup199. [DOI] [PubMed] [Google Scholar]
- 19.Bartos D.C., Duchatelet S., Burgess D.E. R231c mutation in kcnq1 causes long qt syndrome type 1 and familial atrial fibrillation. Heart Rhythm. 2011;8:48–55. doi: 10.1016/j.hrthm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson J.N., Tester D.J., Perry J., Salisbury B.A., Reed C.R., Ackerman M.J. Prevalence of early-onset atrial fibrillation in congenital long qt syndrome. Heart Rhythm. 2008;5:704–709. doi: 10.1016/j.hrthm.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrier K., Czosek R.J., Spar D.S., Anderson J. Long qt genetics manifesting as atrial fibrillation. Heart Rhythm. 2013;10:1351–1353. doi: 10.1016/j.hrthm.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Mandyam M.C., Soliman E.Z., Alonso A. The qt interval and risk of incident atrial fibrillation. Heart Rhythm. 2013;10:1562–1568. doi: 10.1016/j.hrthm.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schimpf R., Wolpert C., Gaita F., Giustetto C., Borggrefe M. Short qt syndrome. Cardiovasc Res. 2005;67:357–366. doi: 10.1016/j.cardiores.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Giustetto C., Di Monte F., Wolpert C., Borggrefe M., Schimpf R., Sbragia P., Leone G., Maury P., Anttonen O., Haissaguerre M., Gaita F. Short qt syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27:2440–2447. doi: 10.1093/eurheartj/ehl185. [DOI] [PubMed] [Google Scholar]
- 25.Deo M., Ruan Y., Pandit S.V., Shah K., Berenfeld O., Blaufox A., Cerrone M., Noujaim S.F., Denegri M., Jalife J., Priori S.G. Kcnj2 mutation in short qt syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci U S A. 2013;110:4291–4296. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benito B., Brugada R., Perich R.M., Lizotte E., Cinca J., Mont L., Berruezo A., Tolosana J.M., Freixa X., Brugada P., Brugada J. A mutation in the sodium channel is responsible for the association of long qt syndrome and familial atrial fibrillation. Heart Rhythm. 2008;5:1434–1440. doi: 10.1016/j.hrthm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Hong K., Piper D.R., Diaz-Valdecantos A. De novo kcnq1 mutation responsible for atrial fibrillation and short qt syndrome in utero. Cardiovasc Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Villafane J., Fischbach P., Gebauer R. Short qt syndrome manifesting with neonatal atrial fibrillation and bradycardia. Cardiology. 2014;128:236–240. doi: 10.1159/000360758. [DOI] [PubMed] [Google Scholar]
- 29.Restier L., Cheng L., Sanguinetti M.C. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol. 2008;586:4179–4191. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan P.J., Osteen J.D., Xiong D., Bohnen M.S., Doshi D., Sampson K.J., Marx S.O., Karlin A., Kass R.S. Characterization of kcnq1 atrial fibrillation mutations reveals distinct dependence on kcne1. J Gen Physiol. 2012;139:135–144. doi: 10.1085/jgp.201110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rijnbeek P.R., Witsenburg M., Schrama E., Hess J., Kors J.A. New normal limits for the paediatric electrocardiogram. Eur Heart J. 2001;22:702–711. doi: 10.1053/euhj.2000.2399. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Liu Y., Dong X. Human kcnq1 s140g mutation is associated with atrioventricular blocks. Heart Rhythm. 2007;4:611–618. doi: 10.1016/j.hrthm.2007.01.029. [DOI] [PubMed] [Google Scholar]