Introduction
KEY TEACHING POINTS
|
Ventricular assist devices (VADs) are playing a large role in the management of end-stage heart failure. Approximately 35% of patients with a VAD have ventricular arrhythmias within the first month of implantation.1 Electrophysiologic ablations in the setting of a VAD are limited to left VADs (LVADs). To our knowledge, we describe the first report of ablation of a hemodynamically significant ventricular tachycardia in a patient with a biventricular assist device (BiVAD).
Case report
A 63-year-old female with a history of nonischemic cardiomyopathy underwent implantation of a HeartWare LVAD (Framingham, MA) in June 2013, but postoperatively developed severe right ventricular (RV) heart failure and underwent HeartWare RVAD placement. In February 2014, the patient presented with recurrent presyncope due to sustained ventricular tachycardia (VT). Despite the use of multiple antiarrhythmic drugs, multiple cardioversions, and changes in RV and left ventricular inlet flows, the patient continued to have recurrent, sustained VT, leading to hemodynamic compromise. The sustained VT caused a decrease in mean arterial blood pressure by roughly 10 mm Hg and decreased BiVAD cannulae flows.
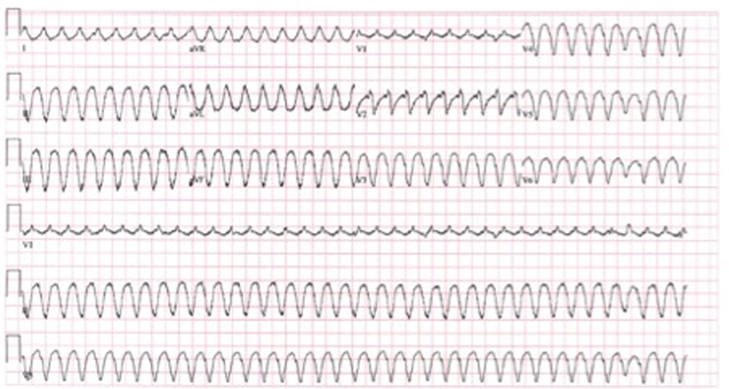
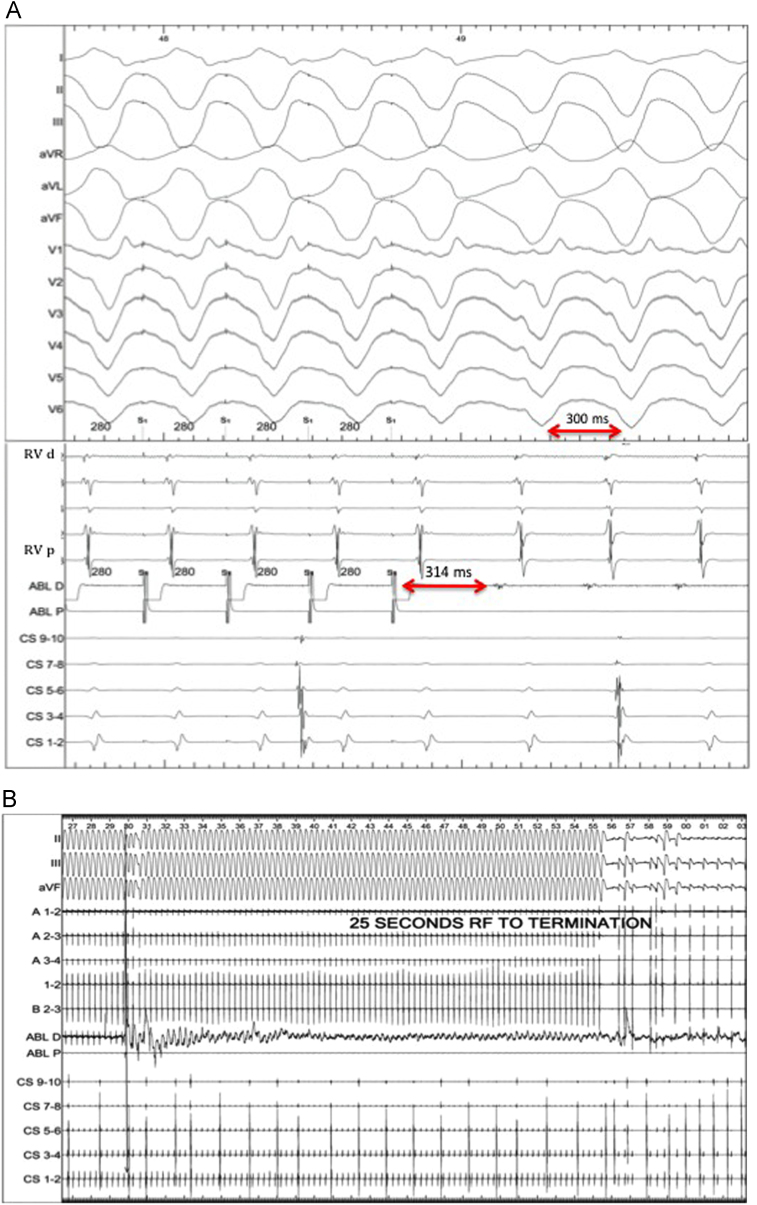
Because of sustained and symptomatic VT despite biventricular support, the patient was taken for electrophysiologic study and VT ablation. The clinical VT had a cycle length of 300 milliseconds, with axis and precordial transition suggestive of an inferoapical exit site (Figure 1). Extensive mapping was performed in the right ventricle. Overdrive pacing in the right ventricle at presystolic sites near the RV inlet cannula led to good pace maps of the VT with a postpacing interval minus tachycardia cycle length of 14 milliseconds (Figure 2A). Neither broad activation mapping to suggest a macroreentrant circuit nor maneuvers to demonstrate progressive fusion were performed, so it is unclear if the VT mechanism was focal or reentrant. Catheter ablation was performed at this site using a ThermoCool SF catheter (Biosense Webster, Diamond Bar, CA) at 35 W with a maximum temperature of 40°C. Twenty-five seconds into ablation, the VT terminated (Figure 2B). Further ablation was performed in this region, toward the RV inlet cannula (Figure 3). VT was noninducible despite triple extrastimuli and changes in RV inlet flow (previously this method had reproducibly induced the clinical VT). Mapping of the left ventricle was not performed, as the arrhythmia was not inducible after termination. Afterward, administration of the antiarrhythmic agents lidocaine, amiodarone, and propranolol was discontinued. At similar pump speeds, the RVAD pump flow increased from 4.4 L/min to 5 L/min and the LVAD pump flow increased from 3.5 L/min to 5 L/min postablation. The patient was symptom free in subsequent follow-up clinic visits, and she underwent successful heart transplantation in June 2014. Direct visualization of the explanted heart showed extensive scarring within the right ventricle in the region of successful ablation.
Figure 1.
A 12-lead electrocardiogram of clinical ventricular tachycardia.
Figure 2.
A: Overdrive pacing with postpacing interval–tachycardia cycle length of 14 milliseconds at the site of presystolic potentials, and B: delayed termination during ablation at that site. Lines A1-2 and B2-3 reflect the right ventricular decapolar catheter. Abl D = distal ablation; Abl P = proximal ablation; CS = coronary sinus; RVd = distal RV recording; RVp = proximal RV recording; RF = radiofrequency ablation.
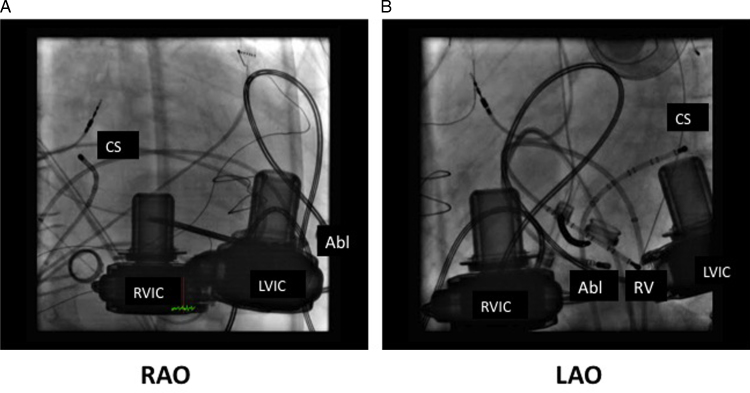
Figure 3.
A: Right anterior oblique and B: left anterior oblique projections of the successful ablation site. The ablation catheter lies in the distal apicoseptal right ventricle. Abl = ablation catheter; CS = coronary sinus catheter; LVIC = left ventricular inlet cannula; RV = right ventricular decapolar catheter; RVIC = right ventricular inlet cannula.
Discussion
Ventricular arrhythmias are known to occur after LVAD implantation.2 Although initial reports suggest that ventricular arrhythmias may be tolerated in patients with LVADs,3 prolonged of RV failure, thrombus formation, or decreased flows via the LVAD may lead to undesired consequences. Further, recent data have suggested that ventricular arrhythmias post–LVAD implantation in this patient population may be associated with increased mortality.4 Given these considerations, catheter ablation for patients with LVAD has been reported.1, 5
Traditionally, with BiVAD support, ventricular arrhythmias are generally tolerated given both RV and left ventricular support. Refaat et al6 reported no difference in survival to transplant in BiVAD patients with an implantable cardioverter-defibrillator. We, however, report a case of symptomatic, hemodynamically significant VT despite BiVAD support. Continuous flow VADs are known to cause “septal push,” which can induce VT from the inlet cannula as it creates a suction effect against the myocardium. In our patient, despite multiple changes in flow rates, VT persisted. The patient’s symptoms potentially were from lack of pulsatile flow in VT, as pulsatility was restored after ablation, with improved BiVAD cannulae flows. Given the patient’s symptoms and that no donor heart was available, the decision was made to pursue catheter ablation.
The successful site of ablation in this patient was near the RV inlet cannula within the right ventricle. Although reentrant VT can occur around an inlet cannula, intrinsic myocardial scar–related VT has been the predominant mechanism reported in LVAD patients.1, 5 Entrainment maneuvers were not performed around the various cannulae to elucidate if this VT was focal or a reentrant one involving the RV, LV, or both inlet cannulae. This patient had an underlying nonischemic cardiomyopathy and electrocardiogram features suggestive of an epicardial exit.7 Further, unipolar RV voltage mapping revealed a large area of low voltage (<5.5 mV) in this region, also suggestive of epicardial scar.8 This finding is potentially why there was a delayed termination during ablation.
In conclusion, despite biventricular VAD support, patients may experience symptomatic ventricular tachycardia, which could be effectively treated with catheter ablation.
References
- 1.Cantillon D.J., Bianco C., Wazni O.M., Kanj M., Smedira N.G., Wilkoff B.L., Starling R.C., Saliba W.I. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm. 2012;9:859–864. doi: 10.1016/j.hrthm.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Ziv O., Dizon J., Thosani A., Naka Y., Magnano A.R., Garan H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005;45:1428–1434. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Oz M.C., Rose E.A., Slater J., Kuiper J.J., Catanese K.A., Levin H.R. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. J Am Coll Cardiol. 1994;24:1688–1691. doi: 10.1016/0735-1097(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 4.Brenyo A., Rao M., Koneru S., Hallinan W., Shah S., Massey H.T., Chen L., Polonsky B., McNitt S., Huang D.T., Goldenberg I., Aktas M. Risk of mortality for ventricular arrhythmia in ambulatory lvad patients. J Cardiovasc Electrophysiol. 2012;23:515–520. doi: 10.1111/j.1540-8167.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 5.Dandamudi G., Ghumman W.S., Das M.K., Miller J.M. Endocardial catheter ablation of ventricular tachycardia in patients with ventricular assist devices. Heart Rhythm. 2007;4:1165–1169. doi: 10.1016/j.hrthm.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Refaat M.M., Tanaka T., Kormos R.L., McNamara D., Teuteberg J., Winowich S., London B., Simon M.A. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J Card Fail. 2012;18:140–145. doi: 10.1016/j.cardfail.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valles E., Bazan V., Marchlinski F.E. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 8.Polin G.M., Haqqani H., Tzou W., Hutchinson M.D., Garcia F.C., Callans D.J., Zado E.S., Marchlinski F.E. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:760–783. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]