KEY TEACHING POINTS
|
Introduction
In corrected transposition of the great arteries (CTGA), the presence of 2 atrioventricular nodes (twin AVNs), their Purkinje systems, and a specific conduction system (Mönckeberg sling) connecting them to each other has been demonstrated using histologic and electrophysiological techniques.1, 2. Twin AVNs can induce supraventricular tachycardia (SVT) as a preoperative, perioperative, or postoperative arrhythmic complication. Therefore, preoperative electrophysiological evaluation is important.3 However, clinical reports describing the electrophysiological properties of the sling are limited.4, 5 In this case report, we confirmed the presence of a sling between twin AVNs and determined that it functioned as a part of the reentrant circuit of SVT associated with the twin AVNs.
Case report
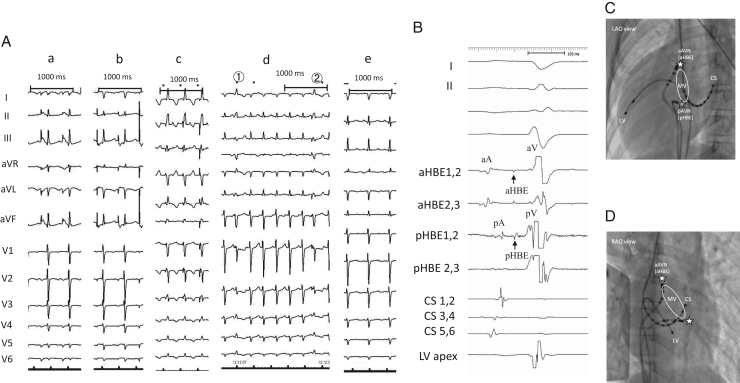
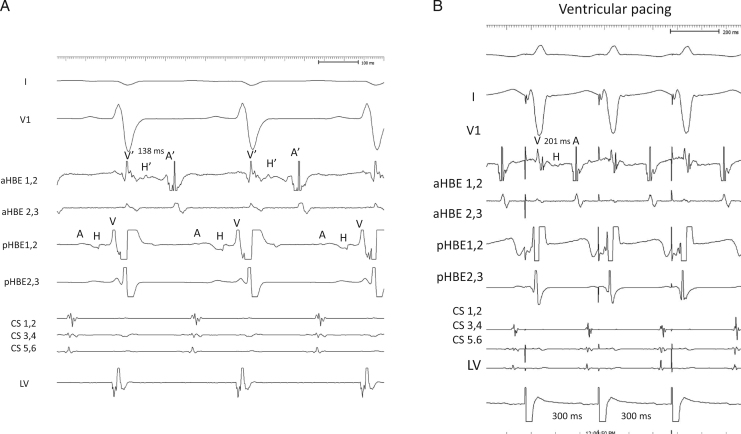
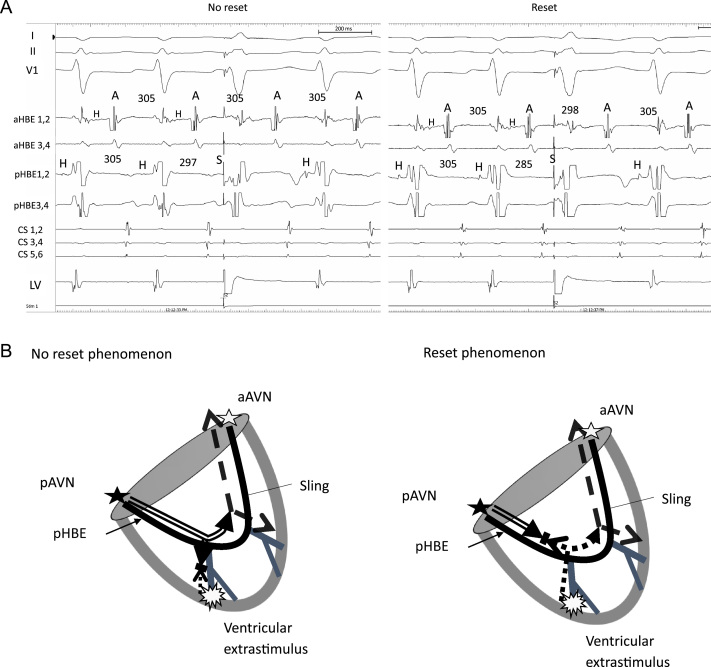
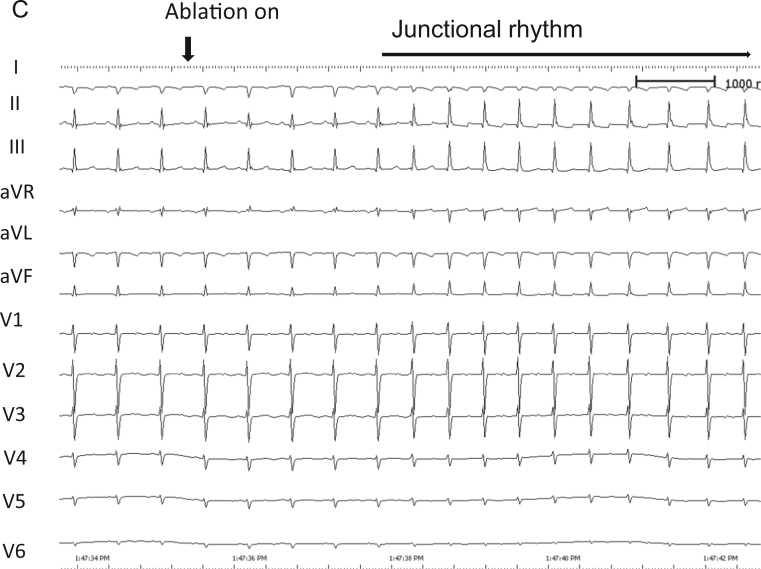
The patient was a 6-year-old girl with CTGA (SLL) with a perimembranous ventricular septal defect, a persistent left superior vena cava, 2 atrioventricular valves, and dextrocardia without native outflow tract stenosis who underwent surgical placement of a pulmonary artery band at 4 months of age. At 6 years of age, she developed SVT during cardiac catheterization conducted for evaluation prior to intracardiac repair; the SVT terminated by intravenous administration of adenosine triphosphate. An electrophysiological study was performed under general anesthesia to preoperatively evaluate the SVT and devise ablation therapy. A decapolar catheter was positioned in the right coronary sinus and the great cardiac vein. A bipolar catheter was positioned in the right side of the morphological left ventricle. Of 2 quadrupolar catheters with a deflectable tip, 1 catheter was positioned in the posterior His bundle (pHB) lesion on the posterior mitral valve annulus, whereas the other catheter was positioned in the anterior His bundle (aHB) lesion on the anterior mitral valve annulus. Baseline 12-lead electrocardiography results showed sinus rhythm without delta waves. High and low right atrial (RA) pacing induced 2 discrete non-preexcited QRS complexes (qrs pattern in lead I and rSr׳ in lead aVR during high RA pacing and low RA pacing; rS pattern in lead I and qR in lead aVR during low RA pacing), indicating the presence of twin AVNs (Figure 1A). Subsequently, His bundle (HB) electrography mapping was performed during sinus rhythm. As shown in Figure 1, Figure 2 and C distinctive HB potentials (HBEs) with different HV times (38 milliseconds at the aHB and 31 milliseconds at the pHB) were identified at the high anterior and low posterior sites of the mitral valvular annulus, indicating that the HB was independently located at both the anterior and posterior aspects. During high RA pacing, the HV time at the aHB was constant, whereas the HV time at the pHB was shortened. In contrast, during low RA pacing, the HV time at the pHB was constant, whereas the HV time at the aHB was shortened. This suggested that the sinus beat coincided with antegrade conduction at the anterior and posterior atrioventricular nodes (aAVN and pAVN, respectively), resulting in a fusion beat. When atrial pacing was performed near 1 of the twin AVNs, the QRS complex morphology indicated pure antegrade conduction at 1 of the twin AVNs and bystander conduction at the other. The antegrade AVN conduction was smooth and decremental, with prolongation of the AH interval without shortening of the HV time. The Wenckebach rate was 280 beats per minute at both the aAVN and pAVN during RA overdrive pacing. The effective refractory period at the AVNs was continuous during atrial pacing (basic cycle length, 500 milliseconds) with an atrial extrastimulus of 190 milliseconds at the aAVN and 240 milliseconds at the pAVN. Neither AVN showed an AH jump, which suggested that dual AVN physiology was not present. On ventricular pacing, the earliest retrograde atrial activation was observed at the aHB. The QRS morphology of clinical SVT (cycle length, 315 milliseconds) induced by programmed ventricular stimulation (Figure 2A) was identical to the QRS morphology during low RA pacing (Figure 1A), indicating that the anterograde AV conduction during SVT and low RA pacing was through the pAVN. Intracardiac electrography tracings during SVT showed an A-H-V sequence at the pHB and a V-H-A sequence at the anterior aHB, with the earliest atrial excitation occurring at the aHB (Figure 2A). These findings suggested that the SVT involved the pAVN as an anterograde limb and the aAVN as a retrograde limb. Ventricular overdrive pacing during the SVT reset the tachycardia without changing the retrograde atrial activation sequence or the His-atrial interval of 49 milliseconds at the aHB. The His-atrial interval during ventricular pacing was the same as that during the SVT. However, the earliest ventricular-to-aHB interval was prolonged from 89 milliseconds to 152 milliseconds (Figure 2B). A premature ventricular extrastimulus before the posterior HB potential (pHBE) with an H-S interval of 297 milliseconds did not reset the AA interval (Figure 3A, left panel), and the stimulus before the pHBE refractory period with a short H-S interval of 285 milliseconds advanced the retrograde atrial conduction at the aHB (Figure 3A, right panel). The QRS morphology of the H-S intervals with the durations of 297 milliseconds and 285 milliseconds was relatively narrow, resembling that during ventricular overdrive pacing (Figure 1A). These findings suggested that there was a connecting sling between the pAVN and aAVN (Figure 3B). Because the pAVN was predominantly involved during sinus rhythm and the aAVN was a retrograde conducting limb for reentry, we attempted catheter ablation of the aAVN during sinus rhythm. A junctional rhythm was observed at the start of the ablation (Figure 3C), and the QRS morphology after the successful ablation was the same as that during SVT (Figure 1A).
Figure 1.
A: QRS morphologies in the 12-lead electrocardiogram. a: Preablation QRS morphology of high atrial pacing. b: Preablation QRS morphology of low atrial pacing. c: Preablation QRS morphology of ventricular pacing during sinus rhythm. d: Preablation QRS morphology upon delivering a ventricular extrastimulus during tachycardia. 1: H-S interval of 297 milliseconds; 2: H-S interval of 285 milliseconds. e: Postablation QRS morphology with pure posterior atrioventricular node conduction. B: Two simultaneously recorded His bundle potentials with different HV intervals during sinus rhythm. aHBE-V time, 38 milliseconds; pHBE-V time, 31 milliseconds. C: Left anterior oblique (LAO) fluoroscopic view illustrating locations on the anterior and posterior His bundles where potentials were recorded. D: Right anterior oblique (RAO) fluoroscopic view illustrating locations on the anterior and posterior His bundles where potentials were recorded. aHBE = anterior His bundle potential; aAVN = anterior atrioventricular node; CS = coronary sinus; LV = anatomical left ventricle; MV = mitral valve; pAVN = posterior atrioventricular node; pHBE = posterior His bundle potential.
Figure 2.
A: Intracardiac recording during supraventricular tachycardia before ventricular overdrive pacing. B: Intracardiac recording of ventricular overdrive pacing during tachycardia. Although the HA interval at the aHB was unchanged before and after ventricular overdrive pacing (49 milliseconds), the earliest V-to-aHBE interval increased from 89 to 152 milliseconds. As a result, the VA interval increased. A = atrial potential; aHB = anterior His bundle; CS = coronary sinus; HBE = His bundle potential; LV =anatomical left ventricle; pHBE = posterior His bundle potential; V = ventricular potential.
Figure 3.
A: Intracardiac recording of a ventricular extrastimulus prior to the possible antegrade conduction via the posterior atrioventricular His bundle during the supraventricular tachycardia. Left panel: During the tachycardia, a ventricular extrastimulus with an H-S interval of 297 milliseconds was delivered between 2 His bundle potentials (interval, 305 milliseconds). However, this did not affect the AA interval in the aAVN (interval, 305 milliseconds), indicating no advanced atrial activation. The tachycardia cycle length decreased from 315 to 306 milliseconds after isoproterenol infusion. Right panel: During the tachycardia, a ventricular extrastimulus with an H-S interval of 285 milliseconds was delivered between 2 His bundle potentials (interval, 305 milliseconds). As a result, the AA interval in the aAVN decreased from 305 milliseconds to 298 milliseconds. B: Schematics of advanced atrial activation induced by a ventricular extrastimulus prior to posterior His bundle potential during the tachycardia. Left panel: A ventricular extrastimulus with a long H-S interval resulted in no resetting. Right panel: The tachycardia was reset by a ventricular extrastimulus with a short H-S interval. C: The 12-lead electrocardiogram recorded during catheter ablation of the aAVN. Junctional rhythms were observed at the start of the ablation of the aAVN, whereas after the successful ablation, the QRS morphology was indicative of antegrade conduction through the posterior atrioventricular node. A = atrial potential; aAVN, anterior atrioventricular node; aHBE = anterior His bundle potential; CS, coronary sinus; H = His bundle potential; H-S = posterior His bundle potential to ventricular extrastimulus; LV = anatomical left ventricle; pAVN = posterior atrioventricular node; pHBE = posterior His bundle potential; S = ventricular extrastimulus; V = ventricular potential.
The VA interval at the aHB during ventricular pacing after ablation was prolonged, whereas the earliest atrial activation occurred at the pHB. These findings suggested that the aAVN bidirectional conduction was successfully eliminated. SVT did not recur, and no complications were observed after the ablation.
Discussion
In CTGA and other heterotaxy syndromes including atrial isomerism or ventricular inversion, the presence of twin AVNs and a sling may cause an unusual form of reentry SVT.1, 2, 3, 4, 5 In our patient, 12-lead electrocardiography results showed 2 types of QRS morphology not accompanied by preexcitation, whereas intracardiac electrography mapping revealed 2 different HBEs, at the anterior and posterior aspects of the AV annulus. The QRS morphology of the SVT was the same as that observed during pAVN conduction. Based on the electrophysiological findings during the SVT, we made the diagnosis of atrioventricular reentrant tachycardia with the pAVN and aAVN serving as anterograde and retrograde limbs, respectively.
The presence of a conduction system (sling) connecting twin AVNs has been histologically demonstrated.1, 2 In reciprocating tachycardia involving twin AVNs, it is thought that a sling or ventricular cardiac muscles without a sling form a part of the reentry circuit.5 Although cases of reentrant tachycardia involving twin AVNs have been described, the electrophysiological properties of this unusual SVT and the specialized conduction sling connecting the twin AVNs have not been fully characterized. In our patient, the absence of advanced atrial activation in the aAVN by a ventricular extrastimulus prior to the pHB activation, with the H-S interval of 297 milliseconds showing antegrade conduction during SVT, suggested the involvement of a sling in the tachycardia circuit. The ventricular extrastimulus with the coupling interval of 297 milliseconds did not reset the cycle, and that with the coupling interval of 285 milliseconds successfully reset it (Figure 3A). The 2 corresponding QRS complexes looked similar (Figure 1A), indicating the presence of a sling, as illustrated in Figure 3B. Because the ventricular extrastimulus was applied near the fascicle, the excitation induced by ventricular pacing propagated retrogradely (Figure 3B). The paced ventricular wavefront with a longer coupling interval collided with the wavefront of the atrioventricular reentrant tachycardia outside of the circuit, and the wavefront with a shorter coupling interval collided inside the circuit, resulting in the resetting of the tachycardia cycle. The absence of a sling would result in a greater difference in the QRS morphologies corresponding to late premature ventricular extrastimulus and the early extrastimulus. Furthermore, the difference between the QRS morphologies when conducting through pAVN and through aAVN would also be greater than that in Figure 1A.
Bae et al4 reported that HBEs of antegrade and retrograde conduction during tachycardia were observed earlier than the ventricular potentials, suggesting the involvement of a sling in SVT involving twin AVNs, but they did not present detailed electrophysiological data obtained using programmed electrical stimulation. In our patient, the ventricular potential was observed between the HBE in the pAVN, showing antegrade conduction, and that in the aAVN showed retrograde conduction. This activation sequence differed from that reported by Bae et al. When the sling conduction time during tachycardia is relatively long and the activation time of the ventricular bundle branched from the sling is relatively short, the activation sequence observed in our patient may occur.
In addition, the V-H time during the tachycardia was relatively long in this case. If the sling connection entered the Purkinje system, this would be similar to short V-H antidromic atrioventricular reentrant tachycardia associated with the atriofascicular accessory pathway with a retrograde right bundle branch block.6 The atrial extrastimulus from the high or low atrium showed smooth decremental dominant atrioventricular conduction without a short HV interval. This finding supported the hypothesis that the SVT was associated with the twin AVNs and ruled out the possibility of an unusual accessory pathway. When the first ventricular bundle branching from the sling is near the site of the AVN working as an antegrade conductor, the activation time of the ventricular bundle that branches from the sling is relatively short, and the sling conduction time during tachycardia is relatively long, as described above. This can explain the relatively long V-H interval during the tachycardia.
When ablation of 1 of the 2 twin AVNs is required for the treatment of SVT, the AVN with more robust conduction, as indicated by a shorter HV conduction time in atrial rhythm, should be preserved. In patients with CTGA, this is usually the pAVN. Ablation of the aAVN showing retrograde conduction during the tachycardia and no predominant antegrade conduction during sinus rhythm was successfully performed in our case. In a situation in which it is impossible to determine which AVN should be ablated or the superfluous node cannot be accessed, a reversible technique such as cryoablation may be used as an alternative approach.
We believe that the absence of a resetting phenomenon is electrophysiological evidence of the sling operating as a part of the SVT circuit involving twin AVNs. Therefore, such tachycardia can be referred to as “twin AVN reentrant tachycardia via sling connection.”
Footnotes
The first two authors contributed equally to this work. Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Dickinson D.F., Wilkinson J.L., Anderson K.R., Smith A., Ho S.Y., Anderson R.H. The cardiac conduction system in situs ambiguus. Circulation. 1979;59:879–885. doi: 10.1161/01.cir.59.5.879. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R.H., Becker A.E., Arnold R., Wilkinson J.L. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911–923. doi: 10.1161/01.cir.50.5.911. [DOI] [PubMed] [Google Scholar]
- 3.Wu M.H., Wang J.K., Lin J.L., Lin M.T., Chiu S.N., Chen C.A. Long-term outcome of twin atrioventricular node and supraventricular tachycardia in patients with right isomerism of the atrial appendage. Heart Rhythm. 2008;5:224–229. doi: 10.1016/j.hrthm.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Bae E.J., Noh C.I., Choi J.Y., Yun Y.S., Kim W.H., Lee J.R., Kim Y.J. Twin AV node and induced supraventricular tachycardia in Fontan palliation patients. Pacing Clin Electrophysiol. 2005;28:126–134. doi: 10.1111/j.1540-8159.2005.09450.x. [DOI] [PubMed] [Google Scholar]
- 5.Epstein M.R., Saul J.P., Weindling S.N., Triedman J.K., Walsh E.P. Atrioventricular reciprocating tachycardia involving twin atrioventricular nodes in patients with complex congenital heart disease. J Cardiovasc Electrophysiol. 2001;12:671–679. doi: 10.1046/j.1540-8167.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 6.Gandhavadi M., Sternick E.B., Jackman W.M., Wellens H.J., Josephson M.E. Characterization of the distal insertion of atriofascicular accessory pathways and mechanisms of QRS patterns in atriofascicular antidromic tachycardia. Heart Rhythm. 2013;10:1385–1392. doi: 10.1016/j.hrthm.2013.07.009. [DOI] [PubMed] [Google Scholar]