Abstract
This article highlights the most significant scientific achievements of June 2007 to June 2008 in nuclear receptors (NRs) and coregulators. These molecules are the subjects of nine studies in three key areas of endocrinology: molecular endocrinology, endocrine metabolism, and endocrine pathology. In each case, the relevant NR or coregulator was found to play an integral role in the study, whether in elucidating a formerly unknown pathway or in initiating or facilitating a disease process. As more NRs and coregulators are researched, more therapeutic approaches to human disease can potentially be developed.
AS PART OF The Year in Basic Science session, a selection was made of the top research papers in Nuclear Receptors and Coregulators from June 2007 through June 2008. Narrowing the list down to manageable size was not easy because of the sheer volume of outstanding work done during this past year. Worldwide, there are more than 100,000 scientists working in nuclear receptors and generally related fields. Using submitted nominations from more than 20 U.S. scientists, top-scoring research papers were selected for three subject areas: molecular endocrinology, endocrine metabolism, and endocrine pathology.
Briefly, the pathway relevant to all of these nine papers involves nuclear receptors (NRs). NRs are activated when a hormone (ligand) binds and incites them to dimerize. Thus activated, the receptors locate their target genes in the nucleus and recruit coregulators to activate or corepressors to repress genes. For gene activation, the coactivators then take over, accumulating RNA polymerase, general transcription and splicing factors, and increasing synthesis of mRNA, all of which result in the production of new proteins that regulate endocrine target cell function.
MOLECULAR ENDOCRINOLOGY
Peroxisome proliferator-activated receptor-γ (PPARγ), androgen receptor (AR), estrogen receptor (ER), and steroid receptor coactivator 3 (SRC-3) are the NRs and coregulators subjected to the following studies in molecular endocrinology.
Crystal Structure of a Full-Length NR (1)
In molecular endocrinology, the number 1-ranked scientific advance of the year was the crystallization of a full-length NR pair: PPARγ and retinoid X receptor-α (RXRα). In 1991, crystallization of the DNA-binding domain was accomplished for glucocorticoid receptor; then, in 1995, the ligand-binding domain was completed for thyroid hormone receptor and RXR. Now, as of this year, crystallization of a full-length liganded receptor has been achieved for the first time (1). PPARγ and RXRα reside in an arrangement dictated by the 5′-extension of the DR1, which also dictates binding to the target gene DNA (Fig. 1). Significantly, the PPAR ligand-binding domain dominates the entire RXR molecule in the dimeric structure and seems to play the larger role of the two. The PPAR ligand-binding domain also comprises the center of the organized DNA binding complex, contacting all ordered domains from both proteins. It contacts the three distinct heterodimerization interfaces that exist between PPARγ and RXRα and is important in all phases of dimeric interaction. Moreover, the PPAR domain essentially directs the DNA-binding domains of both receptor molecules, assisting them in their binding to the target response element DNA. Also surprising is that switching among three different ligands did not result in major domain rearrangements within the molecule.
Fig. 1.

Crystal Structure of a Full-Length NR (See Ref. 1 )
ER and AR: Location of Target Genes (2)
The next published study in molecular endocrinology sought to determine how ER molecules and AR molecules locate their target genes to regulate them. It long has been established that DNA-binding sequences are critical for this process and, most would probably agree, that other proteins in the enhancer region and chromatin epigenetic signals also factor into the location. A new study elucidates the relationship of these three factors in determining that receptor binding occurs at specific sites in the genome. What the researchers found, for a limited number of chromosomes analyzed (nos. 8, 11, and 12) is that about half of the ER binding sites in MCF7 (breast cancer) cells and about half of the androgen-receptor binding sites in LNCaP (prostate cancer) cells are associated with, or directly overlap with, the forkhead box transcription factor A1 (FOXA1). The authors refer to FOXA1 as a “pioneer factor” that aids in the location of the AR or ER. It turns out that the proximity of FOXA1 and the steroid-receptor binding sites is deliberate; a specific arrangement occurs that dictates function at these sites.
There is yet another step in determining what happens next at the ER and AR sites, however (Fig. 2). In an unknown manner, the cells create an important chromatin epigenetic signal at the site, one that will dictate the later receptor binding at the site. This developmental chromosomal epigenetic modification, which in the case of breast and prostate cancer cells is on histone 3 and is a lysine 4 methylation (H3K4me1/me2), then recruits FOXA1 to the region. This particular combination of factors provides the mechanism by which ERs and ARs locate the genomic target site. Once there, the receptors promote (or inhibit) transcription of nearby genes, perhaps even genes up to 200,000 bp away in the DNA.
Fig. 2.
How ER and AR Locate Their Target Genes in Chromatin (2 )
DHT, Dihydrotestosterone; E2, 17β-estradiol.
Regulation of SRC-3 Ubiquitin-Dependent Clock (3)
The third paper in the section on molecular endocrinology deals with the origin of genomic clocks, clocks that operate while a gene is being transcribed and control the functional lifetime of certain coactivators such as SRC-3. The SRC-3 clock constitutes the fastest running mammalian clock described to date. Whereas it was formerly believed that adding a monoubiquitin to SRC-3 initiated the process of degradation, a recent publication showed that, conversely, it superactivates SRC-3, and this monoubiquination comprises the point at which the clock starts. With estrogen, a kinase-induced phosphorylation signal occurs on the SRC-3 molecule, inducing the assembly of the active coregulator complex (Fig. 3). Joining the complex is the ubiquitin ligase, Fbw7a, which adds a monoubiquitin on SRC-3. Thus monoubiquinated, SRC-3 becomes superactivated, and it develops specificity for ERs and ARs, and now displays decreased affinity for the progesterone and glucocorticoid receptors. With each round of transcription, another ubiquitin is added to SRC-3, and it gradually becomes less efficient. With the accumulation of five ubiquitins, SRC-3 becomes inactive and the 19S regulatory cap then arrives at the gene site to transport the polyubiquitinated SRC-3 to the proteasome where it is degraded. The SRC-3 clock then stops. Ironically, in the transcription process, activating coactivators inherently dooms them to death, turning the genome back to its natural state, which is “off.”
Fig. 3.
ER-Mediated Transcription Occurs via a SRC-3-Dependent Ubiquitin Clock (3 )
HSP, Heat shock protein; Ub, ubiquitin.
Calculations indicate that one tick of the clock is equal to one RNA polymerase chain initiation, taking about 6 sec. Five ticks equals five ubiquitins added to SRC-3 before it’s destroyed, giving it a 30-sec functional halftime; thus it is a very fast clock and one that is critical for efficient expression of our target genes. Regulating the level of these powerful coactivator molecules is crucial for normal gene function.
ENDOCRINE METABOLISM
The next subject area discussed in The Year in Nuclear Receptors and Coregulators is that of endocrinology and metabolism. Here, Rev-erbα, ER-related receptor-α (ERRα), PPARα, and PPAR coactivator (PGC)-1α dominate the stage.
A Heme-Regulated Circadian Clock (4, 5)
Simultaneous studies from two laboratories examine another clock, our daily 24-h (circadian rhythm) clock, and in the course describe a new ligand for a NR. BMAL1 is an essential transcription factor component of the circadian clock that exists in the hypothalamic superchiasmatic nucleus of our brain. New research shows that BMAL1 is regulated by a heme-binding protein, Rev-erbα. In the presence of heme, Rev-erbα will bind NR corepressor and recruit histone deacetylase 3 to the gene, in effect, shutting down transcription of BMAL1 (Fig. 4). Because heme is a true ligand for Rev-erbα, and heme is a lipophilic metabolite, the BMAL1-suppressing activity of Rev-erbα in turn suppresses metabolism. In terms of the circadian clock, the heme-activated Rev-erbα represents the up phase of the circadian cycle, whereas the repression of BMAL1 represents the down phase. How this relates to general body metabolism is beyond the scope of this article but, basically, heme activates this cycle, shuts it down, and then allows reactivation in a 24-h cycle. Rev-erbα suppresses gluconeogenesis, via aminolevulinate synthase, the enzyme that actually makes heme. Heme also is required for oxidative metabolism. Being thus central to metabolism and a specific receptor ligand, heme is, in effect, a new hormone that regulates NR-dependent corepressor recruitment to achieve physiological results.
Fig. 4.
A New Hormone, Heme, Regulates the 24-h Circadian Clock (4 5 )
HDAC, Histone deacetylase; N-CoR, nuclear receptor corepressor.
PGC-1 and ERRα Collaborate for Vascular Endothelial Growth Factor (VEGF) Production (6)
The next study in the area of endocrine metabolism reveals the collaboration between the transcriptional coactivator, PGC-1, and ERRα, for production of VEGF during exercise. ERRα plus PGC-1 induce a major stimulation of transcription of the VEGF gene. Increased levels of VEGF result in greatly increased capillary proliferation. Exercise signals agents, such as Ca2+ and cAMP, to activate PGC-1α. The PGC-1α coactivator then drives ERRα to produce VEGF, as well as mitochondrial biogenesis. PGC-1α also activates MEF2 for switching muscle fibers to the endurance type of fibers that are optimum for exercise (Fig. 5). Thus, exercise (and hypoxia) induces a change in physiology that is coordinated by pathways that function via a single coactivator, PGC-1α, and the required combination of increased blood flow and metabolism ensues.
Fig. 5.
PGC-1α Enhances ERRα Induction of VEGF Gene Expression
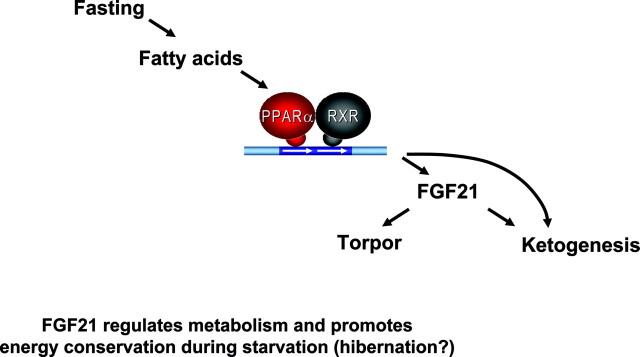
Endocrine Basis of Fasting and Energy Conservation (7)
Last in endocrine metabolism is an examination of the key molecule of a process called torpor: a hibernation-like state of regulated hypothermia. Fibroblast growth factor 21 (FGF21) is significant because it induces ketogenesis by increasing both enzymes (CPT1α and HMGCS2) that produce ketone bodies, and also, by increasing lipolysis to produce fatty acid substrates for ketones (Fig. 6). In transgenic mice that overproduce FGF21, a dramatic drop in body temperature occurs, compared with that in wild-type mice. This decrease in temperature is similar to that induced in animals and people subjected to prolonged fasting, cold, or darkness. Mammals also use this state of torpor for energy conservation.
Fig. 6.
PGC-1 and ERRα Collaborate to Regulate VEGF Production (6 )
The pathway begins with fasting or starvation eliciting the production of fatty acids, which, in turn, activate the heterodimer PPARα and RXR (similar to the key heterodimer, PPARγ and RXRα from PAPER 1, incidentally). This heterodimer then activates the FGF21 gene, which induces ketogenesis and torpor. This condition of induced hypothermia is actually a protective state, decreasing metabolism and conserving energy. This process conserves energy in the brain and, at the same time, feeds it by ensuring continued production of ketones, which are essential energy sources for the brain. Elucidation of this pathway for torpor allows an understanding of hibernating animals in light of the metabolism-regulating, energy-conserving FGF21 gene.
ENDOCRINE PATHOLOGY
Specific advances in Endocrine Pathology involve chicken ovalbumin upstream promoter-transcription factor II (COUP-TF II), ERα, and NR4A1 and NR4A3.
Orphan NR-Controlled Molecular Reproduction (8)
A breakthrough this year in Endocrine Pathology involved elucidation of the pathway of steroid hormones in facilitating decidualization of the uterus and subsequent embryo attachment. Although it has been accepted that estrogen and progesterone somehow control uterine decidualization and embryo attachment, and that those two processes must occur in conjunction for pregnancy, a recent study shows that COUP-TF II is actually integral to the process as well. Estrogen is fundamental to preparing the uterus for an eventual pregnancy; however, constant estrogen or ER activity prevents embryo implantation without the advent of progesterone. The orphan receptor COUP-TF II is involved in two key ways with this function of the progesterone receptor (PR).
First, progesterone activates PR, which increases Indian hedgehog (Ihh) signaling in the epithelium. Ihh then signals to the two transcription factors, Patched and Smoothened (Ptc/Smo), in the stroma. Ptc/Smo next activate COUP-TFII, which, in turn, launches production of bone morphogenetic protein 2, and uterine decidualization ensues.
The second PR signaling event also emanates from COUP-TF II when it simultaneously activates PR to inhibit estrogen activity, removing the block on blastocyst implantation. Figure 7 shows successful blastocyst implantation and attachment and uterine decidualization in wild-type mice contrasted with the failure of those three processes in mice with a deletion of the COUP-TF II gene. Although more work remains to be done to fully understand the complete cycle, it is now clear that COUP-TF II is a key to pregnancy and fertility.
Fig. 7.
An Endocrine Basis of Fasting and Energy Conservation (7 )
Osteoclast-Induced Postmenopausal Osteoporosis (9)
The next study in endocrine pathology seeks to solve the problem of why the decrease in ovarian estrogen in menopause leads to dramatically reduced bone mass and whether the ER in question is either ERα or ERβ. Before menopause, there is an equal amount of bone formation and resorption. Osteoblasts lay down new bone whereas osteoclasts destroy old bone, resulting in an equilibrium cycle that keeps bone strong. After menopause, however, the ratio of formation and resorption tips in favor of resorption. According to a recent study, this shift occurs because of decreased osteoclast apoptosis, leading to more osteoclasts and more activity.
In wild-type mice, estrogen is shown to protect bone, whereas in mice with the ERα deleted, bone maintenance is not in evidence. In the wild-type animals, osteoclasts are seen to be undergoing continuous apoptosis. In the knock-out mice, however, apoptotic activity is missing, and the increased levels of osteoclasts act to destroy bone (Fig. 8). Thus, the relationship between ERα and osteoclast apoptosis appears to promote osteoporosis.
Fig. 8.
A New Molecular Mechanism for Orphan Receptor-Controlled Reproduction (8 )
E2, 17β-Estradiol; TRAP, thyroid hormone receptor-associated protein; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling.
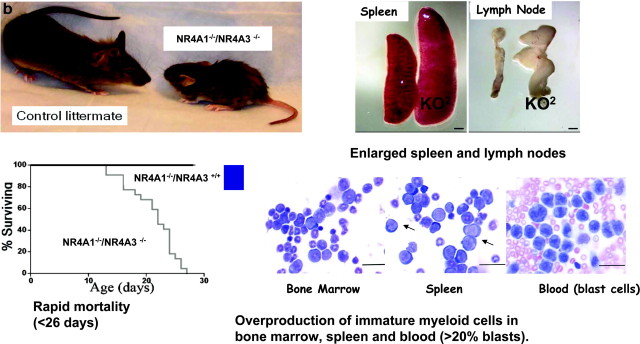
Orphan NRs and Cancer (10)
Two orphan receptors are at the center of this final study in the area of Endocrine Pathology. Specifically, the disruption of the activities of receptors NR4A1 (Nurr77) and NR4A3 (Nor-1) appears to be implicated in the development of florid acute myeloid leukemia (AML). Mice that have both NR4A genes knocked out were less than half the size of wild-type mice, and did not survive past 26 d. In addition, they revealed grossly enlarged spleens and lymph nodes and overproduction of myeloid cells in bone marrow, the spleen, and the blood (Fig. 9).
Fig. 9.
A Mechanism for Osteoclast-Induced Postmenopausal Osteoporosis
Significantly, the study goes on to show that NR4A1 and NR4A3 gene activities are virtually silenced in human patients with AML as well. Consequently, NR4A1 receptor levels are dramatically lower in AML patients, and NR4A3 receptor levels are barely detectable. This study naturally invokes the intriguing possibility of reactivating the NR4A receptor genes as a potential therapeutic approach to treating AML.
CONCLUSION
In a summary of the relationships of coregulators to human diseases displayed in Table 1, the pathologic potency of coactivators is underscored (11). In the past decade, approximately 300 coactivators and corepressors have been identified. Already, 165 of these molecules have been directly associated with human diseases. Multiple cancers and certain diseases of the central nervous system and endocrine system have been shown to be attributable, at least in part, to these coregulators. This finding has major implications for human physiology and pathology. Far from playing only a role in transcription at the gene site, coregulators are responsible for misexpression, translocation, polymorphism, and other types of cancer- and disease-dependent processes. Perhaps in the near future, we will gain a capacity to manipulate the functions of these coregulator molecules to prevent, diagnose, and ameliorate human disease.
Table 1.
NR Coregulators and Human Disease (11 )
| Coregulators and Diseases | |
|---|---|
| Totals (165 of 300) | |
| Cancers | 123 |
| Aging; Alzheimer; Parkinson | 11 |
| Schizophrenia; bipolar | 9 |
| Metabolism: diabetes; obesity | 10 |
| Mechanisms | |
| Over/under expression | 100 |
| Translocations | 25 |
| Polymorphisms; mutations | 38 |
| CAN (cancer gene database) | 9 |
NURSA Molecule Pages:
Coregulators: PGC-1 | SRC-1;
Nuclear Receptors: AR | COUP-TFII | ERα | ERRα | NGFIB | NOR1 | PPARα | PPARγ | PR | REV-ERBα.
Footnotes
This commentary is based on a talk presented on Sunday, June 15, 2008, at ENDO 2008, San Francisco, CA. Draft prepared by Kelly Horvath.
Disclosure Statement: The author has nothing to disclose.
First Published Online October 9, 2008
Abbreviations: AML, Acute myeloid leukemia; AR, androgen receptor; COUP-TF, chicken ovalbumin upstream promoter-transcription factor; ER, estrogen receptor; ERR, ER-related receptor-α; FGF, fibroblast growth factor; FOXA1, forkhead box transcription factor A1; NR, nuclear receptor; PGC, PPAR coactivator; PPAR, peroxisome proliferator-activated receptor; PR, progesterone receptor; RXR, retinoid X receptor; SRC, steroid receptor coactivator; VEGF, vascular endothelial growth factor.
References
- 1.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F, 29 October 2008. Structure of the intact PPAR-γ-RXR-α nuclear receptor complex on DNA. Nature, Epub ahead of print [DOI] [PMC free article] [PubMed]
- 2.Lupien M, Eeckhoute J, Meyer CA, Wang O, Zhang Y, Li W, Carroll JS, Liu XS, Brown M 2008. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132:958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu RC, Feng Q, Lonard DM, O'Malley BW 2007. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129:1125–1140 [DOI] [PubMed] [Google Scholar]
- 4.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA 2007. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318:1730–1731 [DOI] [PubMed] [Google Scholar]
- 5.Baghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F 2007. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REF-ERBβ. Nat Struct Mol Biol 12:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Gimun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman B 2008. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451:1008–1012 [DOI] [PubMed] [Google Scholar]
- 7.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Klewer SA 2007. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 6:415–425 [DOI] [PubMed] [Google Scholar]
- 8.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY 2007. COUP-TFll mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 6:e102 [DOI] [PMC free article] [PubMed]
- 9.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007. Estrogen prevents bone loss via estrogen receptor-α and reduction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- 10.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM 2007. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med 6:730–735 [DOI] [PubMed] [Google Scholar]
- 11.Lonard DM, Lanz RB, O'Malley BW 2007. Nuclear receptor coregulators and human disease. Endocr Rev 28:575–587 [DOI] [PubMed] [Google Scholar]