Abstract
Pleiotropic actions of cocaine- and amphetamine-regulated transcript (CART) are well described in the central nervous system and periphery, but the intracellular mechanisms mediating biological actions of CART are poorly understood. Although CART is not expressed in mouse ovaries, we have previously established CART as a novel intracellular regulator of estradiol production in bovine granulosa cells. We demonstrated that inhibitory actions of CART on estradiol production are mediated through inhibition of FSH-induced cAMP accumulation, Ca2+ influx, and aromatase mRNA expression via a Go/i-dependent pathway. We also reported that FSH-induced estradiol production is dependent on Erk1/2 and Akt signaling, and CART may regulate other signaling proteins downstream of cAMP essential for estradiol production. Here, we demonstrate that CART is a potent inhibitor of FSH-stimulated Erk1/2 and Akt signaling and the mechanisms involved. Transient CART stimulation of bovine granulosa cells shortens the duration of FSH-induced Erk1/2 and Akt signaling whereas a prolonged (24 h) CART treatment blocks Erk1/2 and Akt activation in response to FSH. This CART-induced accelerated termination of Erk1/2 and Akt signaling is mediated both by induced expression and impaired ubiquitin-mediated proteasome degradation of dual specific phosphatase 5 (DUSP5) and protein phosphatase 2A. Results also support existence of a negative feedback loop in which CART via a Go/i-MAPK kinase dependent pathway activates Erk1/2, and the latter induces DUSP5 expression. Moreover, small interfering RNA mediated ablation of DUSP5 and/or protein phosphatase 2A prevents the CART-induced early termination of Erk1/2 and Akt signaling. Results provide novel insight into the intracellular mechanism of action of CART in regulation of FSH-induced MAPK signaling.
PRODUCTION OF ESTRADIOL (E) is critical both within and outside the reproductive system and is essential for reproductive success and women’s health. Whereas the key role of pituitary gonadotropins such as FSH in regulation of E production is well established, the local regulatory molecules that influence gonadotropin action and their cognate signaling pathways are not completely understood, especially the inhibitory factors regulating E production. We have recently published (1, 2) studies establishing a novel intraovarian role for the cocaine and amphetamine-regulated transcript (CART) peptide in negative regulation of E production in bovine granulosa cells (GCs). CART peptides are well-established neuromodulators, with wide expression (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12) and varied pleiotropic actions (8, 13, 14, 15, 16) in different tissues, suggesting diverse physiological roles for CART. Despite numerous responses to CART, the intracellular mechanisms that mediate the biological actions of CART are poorly understood. Also, the CART receptor is yet to be identified although binding studies using the AtT20 cell line (17), PC12 cells (18), and primary neural cell cultures (19) provide direct evidence for existence of a putative CART receptor.
Our laboratory has established a direct link between CART-induced signaling pathways and FSH-stimulated E production in bovine granulosa cells (1, 2). We have demonstrated that CART negatively regulates E production by GCs through inhibition of FSH-induced cAMP accumulation, Ca2+ influx, and aromatase mRNA expression via a Go/i-dependent pathway (2). Furthermore, bovine GC CART expression is negatively associated with follicle health status and E-producing capacity (1). Production of E is critical for follicle growth, to trigger the preovulatory gonadotropin surge and promote resumption of meiosis and ovulation (20, 21, 22, 23, 24). Thus, CART inhibition of E production may have a pivotal role in regulation of numbers of follicles that grow and ovulate during reproductive cycles for single- vs. multiple-ovulating species such as cattle and perhaps human beings and may explain why studies in our laboratory (our unpublished data) as well as by Murphy et al. (25) failed to detect CART expression in mouse ovaries and CART mutant mice are fertile (26). Taken together, these species-specific observations enhance the relevance and utility of the bovine vs. mouse model for studies of CART signaling pathways that regulate FSH signaling and E production.
Whereas above results support prominent effects of CART on E production linked to inhibition of cAMP production, evidence also supports potential regulation at the level of Erk1/2 and Akt. We have also reported that FSH-induced E production is dependent on Erk1/2 and Akt signaling, and pharmacological stimulation of GCs by 8-bromo-cAMP fails to block the inhibitory actions of CART on E production (2). This suggests that reduced cAMP is not the sole factor mediating the negative effects of CART on FSH-stimulated E production and that CART may regulate other signaling proteins downstream of cAMP essential for E production. Both Erk1/2 and Akt are important downstream convergence points for signaling pathways regulating expression of genes necessary for follicular development, selection, cell proliferation and differentiation, cell survival, and steroidogenesis (27, 28, 29, 30, 31, 32, 33, 34).
The objectives of the present study were to determine the effects of CART on FSH-induced Erk1/2 and Akt activation in bovine GCs and the underling mechanisms involved. Results suggest that CART treatment accelerates the dephosphorylation of phosphorylated Erk1/2 (pErk1/2) and Akt (pAkt), and this early termination of the FSH-induced Erk1/2 and Akt signaling pathways by CART is mediated by inducing the expression and decreasing the degradation of two MAPK phosphatases (MKP), DUSP5 (dual specific phosphatase 5) and PP2A (protein phosphatase 2A). Furthermore, in this study we report the existence of a negative feedback loop in which CART treatment stimulates expression [via a Go/i-, protein kinase C (PKC)-, MAPK kinase (MEK)-, and Erk1/2-dependent pathway] of DUSP5 that, in turn, terminates Erk1/2 signaling. Results provide novel insights into the mechanism of action of CART that are relevant to the reproductive system and beyond.
RESULTS
Evidence of CART-Induced Early Termination of Erk1/2 and Akt Activation
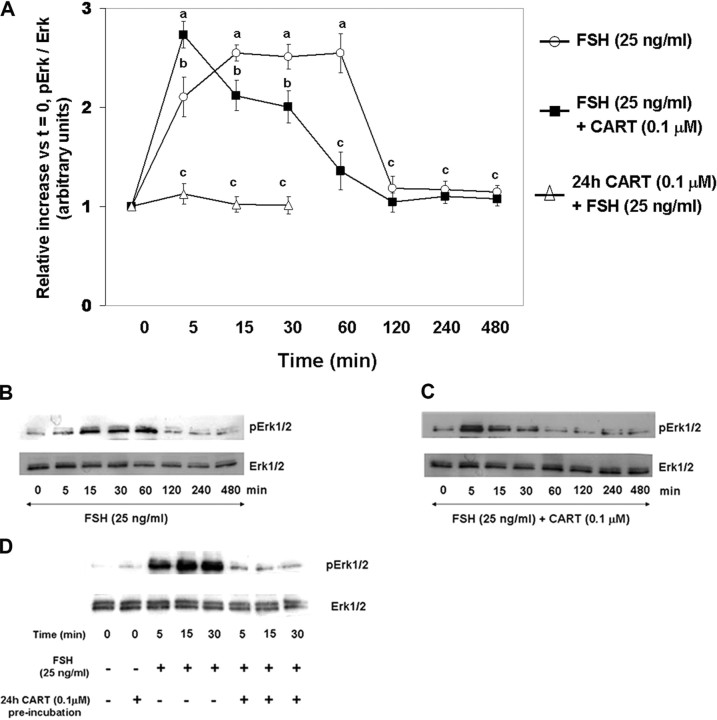
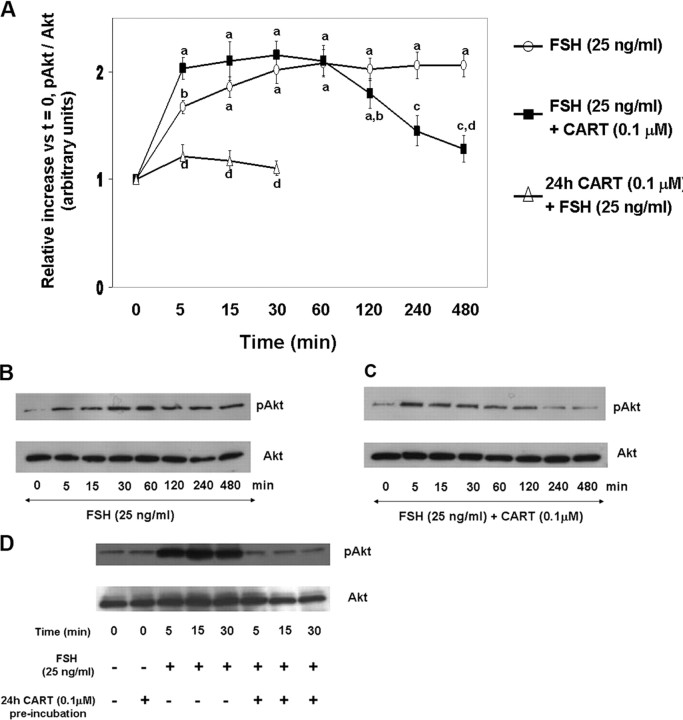
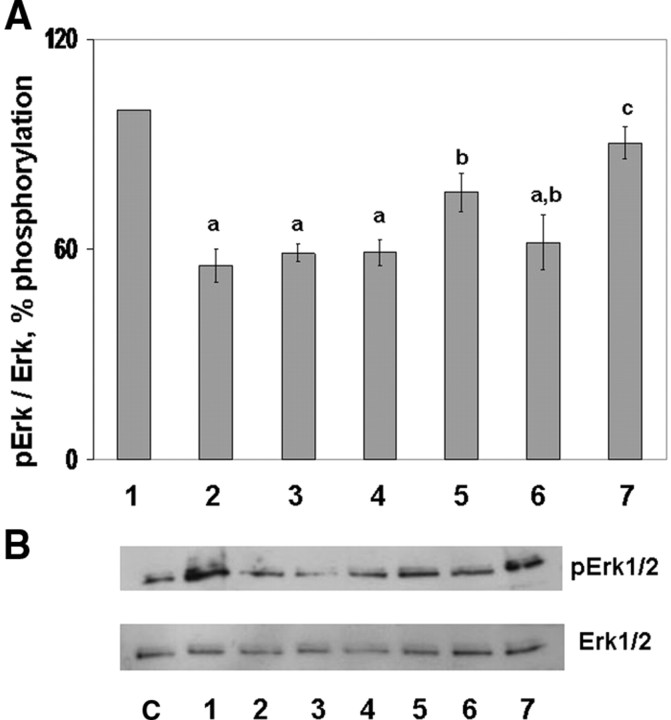
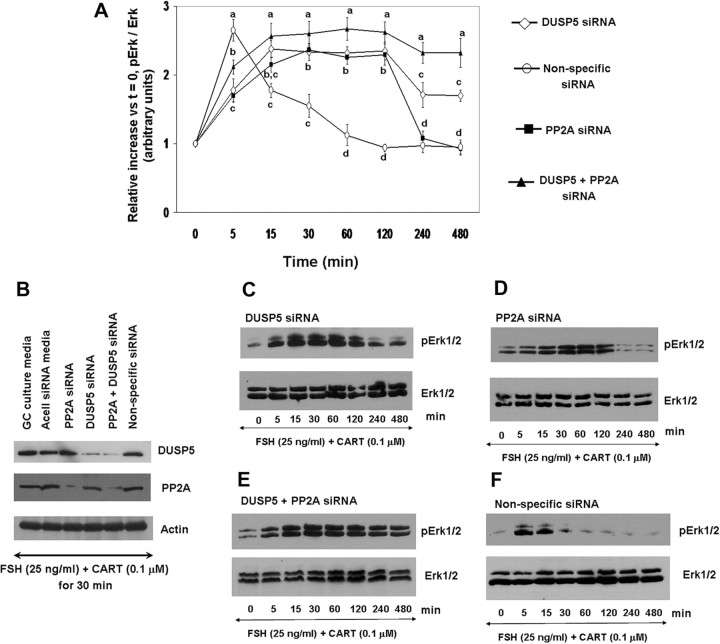
To investigate the effect of exogenous CART on FSH-induced Erk1/2 (Fig. 1) and Akt (Fig. 2) activation, GCs were stimulated with FSH (25 ng/ml) or FSH + CART (0.1 μm; vehicle was water) for 0, 5, 15, 30, 60, 120, 240, and 480 min. Also, the effect of long-term CART treatment on FSH-induced Erk1/2 and Akt activation was studied by preincubating GCs with/without CART (0.1 μm) for 24 h before FSH stimulation for 0, 5, 15, and 30 min. Thereafter, phosphorylated and total Erk1/2 and Akt levels were determined using Western blot analysis. Stimulation of GCs with FSH alone (Fig. 1A) resulted in an increase in Erk1/2 activation within 15 min (P < 0.01) that was maintained through 60 min of stimulation and returned to basal levels by 120 min. In contrast, when GCs were costimulated with FSH + CART (Fig. 1A), Erk1/2 activation was accelerated to maximal levels within 5 min (P < 0.01) but linearly decreased to basal values (P < 0.01) by 60 min of stimulation, thus demonstrating an earlier termination of Erk1/2 signaling. In contrast, pretreatment of GCs with CART for 24 h completely blocked FSH-induced Erk1/2 activation (P < 0.01; Fig. 1A). Panels B and C of Fig. 1, are representative Western blots demonstrating the pattern of Erk1/2 activation in GCs stimulated with FSH alone (Fig. 1B) and FSH + CART (Fig. 1C) whereas Fig. 1D demonstrates the pattern of Erk1/2 activation in GCs preincubated with/without CART (0.1 μm) for 24 h before FSH stimulation. Activation of Akt peaked by 30 min (P < 0.01) and was maintained through 480 min of FSH stimulation (Fig. 2, A and B) whereas FSH + CART treatment (Fig. 2, A and C) resulted in a decline (P < 0.01) in the activated levels of Akt within 240 min of stimulation. Pretreatment (24 h) with CART blocked FSH-induced Akt activation (Fig. 2, A and D). To demonstrate specificity of the CART-induced accelerated termination of FSH-stimulated Erk1/2 and Akt activation, GCs were costimulated with FSH and an inactive CART peptide 55–76 (0.1 μm). The inactive CART peptide did not have any effect on FSH-induced Erk1/2 (supplemental Fig. 1A published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojornals.org) and Akt (supplemental Fig. 1B) activation. Hence we conclude that CART treatment results in early termination of FSH-induced Erk1/2 and Akt activation in bovine GCs.
Fig. 1.
CART Regulation of FSH-Induced Erk1/2 Signaling
Time course of the relative increase in Erk1/2 activation (pErk/total Erk) vs. time zero observed for bovine GCs collected after stimulation with 25 ng/ml FSH, 25 ng/ml FSH preceded by 24 h preincubation in the presence of 0.1 μm CART, or after costimulation with FSH + CART (A). Representative Western blots demonstrating the pattern of Erk1/2 activation in GCs stimulated with FSH alone (B), FSH + CART; vehicle was water (C) or subjected to 24 h CART pretreatment followed by FSH stimulation (D). The data depicted in panel A are represented as the mean ± sem from three replicate experiments and different superscripts denote significant differences across and within treatments and time points (P < 0.05).
Fig. 2.
CART Regulation of FSH-Stimulated Akt Activation
Time course of the relative increase in Akt activation (pAkt-Ser473/total Akt) vs. time zero observed for bovine GCs collected after stimulation with 25 ng/ml FSH, 25 ng/ml FSH preceded by 24 preincubation in the presence of 0.1 μm CART, or after costimulation with FSH + CART (A). Representative Western blots demonstrating the pattern of Akt activation in GCs stimulated with FSH alone (B), FSH + CART; vehicle was water (C) or subjected to 24 h CART pretreatment followed by FSH stimulation (D). The data depicted in panel A are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences across and within treatments and time points (P < 0.05).
Acute CART Treatment Stimulates Erk1/2 But Not Akt Activation
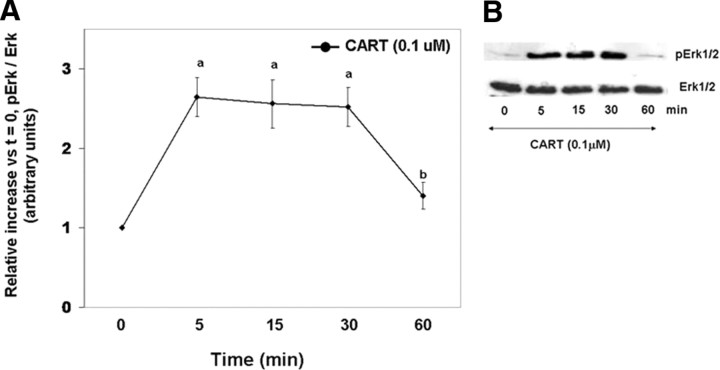
The increase in Erk1/2 activation to maximal levels within 5 min of FSH + CART costimulation (Fig. 1, A and C) followed by a linear decline to basal levels suggests that CART treatment may activate Erk1/2, leading to desensitization of GCs to subsequent FSH-induced Erk1/2 activation. Thus, to test the effect of acute CART treatment on Erk1/2 and Akt activation, GCs were stimulated with CART (0.1 μm) alone for 0, 5, 15, 30, and 60 min, and phosphorylated and total Erk1/2 and Akt levels were determined using Western blot analysis. Figure 3A is the relative increase in phosphorylated/total ERK1/2 vs. time zero whereas Fig. 3B is a representative Western blot of CART-induced Erk1/2 activation over time. A transient Erk1/2 activation was observed in GCs within 5–15 min of treatment with CART alone (P < 0.01), and Erk1/2 activation returned to basal levels within 60 min (Fig. 3). In contrast, treatment with CART alone did not stimulate Akt activation (data not shown). It is well established that cAMP activates Erk1/2 and Akt signaling in GCs (35, 36, 37, 38) as well as in other cell types (39, 40), and previously we have reported that long-term CART treatment inhibits the FSH-induced rise in intracellular cAMP levels (2). Thus, to confirm that the actions of CART on Erk1/2 and Akt are cAMP independent, cAMP levels were measured after acute CART stimulation. No effects of acute CART treatment on intracellular cAMP levels were observed (supplemental Fig. 2). Collectively, results indicate that CART treatment stimulates bovine GC Erk1/2, but not Akt activation.
Fig. 3.
CART-Induced Erk1/2 Activation
Time course of the relative increase in Erk1/2 activation (pErk/total Erk) vs. time zero observed for bovine GCs collected after stimulation with 0.1 μm CART (A). Representative Western blots demonstrating the pattern of Erk1/2 activation in GCs stimulated with CART (B). The data depicted in panel A are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences in Erk1/2 activation between time points (P < 0.05).
CART Induces Erk1/2 Activation via a Go/i-, PKC-, and MEK-Dependent Pathway
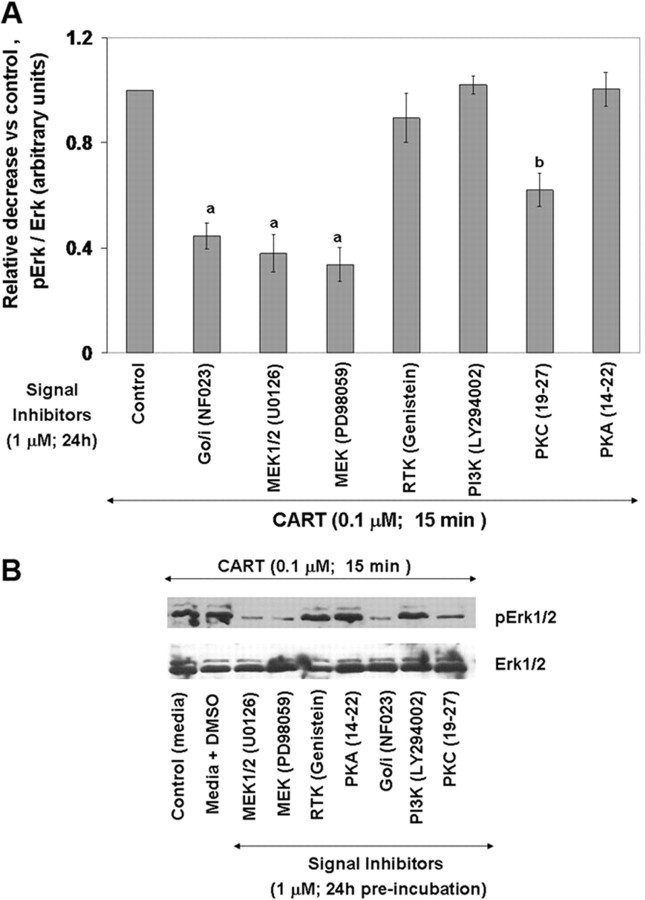
To elucidate the signaling pathways involved in CART-induced Erk1/2 activation, GCs were preincubated with media alone, with media containing 1 μm of different signaling protein inhibitors [protein kinase C (PKC), protein kinase A (PKA), phospho inositol-3 kinase (PI3K), Go/i, MEK, MEK1/2, and receptor tyrosine kinase (RTK)] or with media containing equivalent volume of dimethylsulfoxide (DMSO) (DMSO was vehicle used for PI3K inhibitor-LY294002, genistein, and MEK inhibitors U0126 and PD98059; water was vehicle used for PKA inhibitor peptide 14–22, PKC inhibitor peptide 19–27, and Go/i inhibitor NF023) for 24 h followed by 15 min of CART stimulation. Dose of inhibitors used was based on results of our previous studies (2) in which similar results were obtained when two concentrations (1 and 10 μm) of each inhibitor were used, and a similar effect was observed for both concentrations as well as based on previous results in the literature referenced below. Figure 4A demonstrates the relative decrease in phosphorylated/total Erk1/2 (15 min after CART treatment) in response to preincubation with various signaling protein inhibitors relative to preincubation control (media alone), and Fig. 4B is a representative Western blot showing effects of treatments. Inhibition of Go/i (NF023) and MEK (PD98059 and U0126) significantly decreased (P < 0.01) CART-induced Erk1/2 activation whereas the PKC inhibitor peptide 19–27 only partially blocked (P < 0.01) CART actions (Fig. 4A). None of the other signaling protein inhibitors or DMSO (vehicle treatment) altered CART-stimulated Erk1/2 activation. Results suggest that CART causes Erk1/2 activation via a Go/i-, PKC-, and MEK-dependent pathway. To demonstrate the effectiveness and specificity of the protein inhibitors used in this study with bovine GCs and further confirm lack of nonspecific effects of corresponding vehicles for each inhibitor (described above), GCs were pretreated (24 h) with each inhibitor (1 μm), with equivalent volume of vehicle (DMSO control and water control), or with media alone followed by FSH (25 ng/ml) stimulation for 30 min. Activation of Erk1/2 and Akt was then determined by Western blot analysis (supplemental Fig. 3, A and B). Consistent with previous reports, the PI3K inhibitor LY294002 (31, 34) blocked FSH-induced Akt activation whereas the MEK inhibitors U0126 and PD98059 (32), genistein (32), and PKA inhibitor peptide 14–22 (myristoylated) (32) blocked FSH-induced Erk1/2 activation. The PKC inhibitor peptide 19–27 (myristoylated) significantly reduced, but did not totally block, FSH-induced Erk1/2 activation, and no effect of respective vehicle treatments was observed (supplemental Fig. 3). We have also shown previously (2) that the MEK and PI3K (LY294002) inhibitors used here inhibit FSH-induced E production, thus demonstrating that these inhibitors indeed block recognized physiological responses in bovine GCs. Moreover, the Go/i inhibitor NF023, which is a suramin analog (41, 42) and suppresses GTPγS binding to the α-subunit of the Go/i group and inhibits the formation of agonist-specific ternary complex (agonist/receptor/G-protein) did not have any effect on FSH-induced Akt and Erk1/2 activation. The Go/i inhibitor NF023 also did not have any effect on FSH-induced cAMP, calcium, or E levels in bovine GCs but specifically blocked the inhibitory effects of CART on FSH signaling (2).
Fig. 4.
Signaling Pathways Involved in CART-Induced Erk1/2 Activation
Relative decrease in Erk1/2 activation (pErk/total Erk) vs. media alone control observed for bovine GCs preincubated in the presence of 1 μm of different signaling protein inhibitors (PKC, PKA, PI3K, Go/i, MEK, MEK1/2, and RTK) for 24 h followed by 15 min CART (0.1 μm) stimulation (A). Representative Western blots demonstrating the pattern of Erk1/2 activation in GCs treated with media containing equivalent volume of DMSO (vehicle) or with above signaling protein inhibitors for 24 h before CART stimulation (B). The data depicted in panel A are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences between treatments (P < 0.05).
CART-Induced Regulation of Phosphatase Activity Is Dependent on de Novo Protein Synthesis
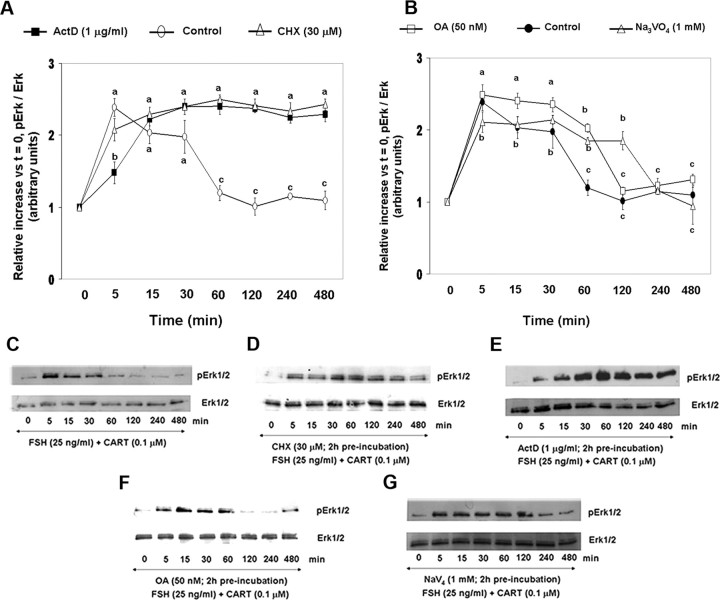
To investigate potential involvement of increased phosphatase activity in CART-induced early termination of Erk1/2 and Akt signaling, an in vitro phosphatase assay was performed as described in Materials and Methods. Phosphatase activity was assessed by Western blot analysis of phosphorylated and total Erk1/2 after coincubation of protein from cell lysates containing high levels of pErk1/2 (activated Erk standard pool; cells treated with FSH for 30 min) with protein from lysates of cells (source of phosphatase activity measured) stimulated with FSH (30, 60, and 120 min), FSH + CART (30 and 120 min) or CART alone (60 min). Figure 5A illustrates changes in phosphatase activity (denoted as percent of Erk phosphorylation) whereas Fig. 5B is a corresponding representative Western blot showing the decrease in Erk phosphorylation in response to coincubation with lysates from cells exposed to the various treatments mentioned above. Lane 1 (Fig. 5) depicts the percent of pErk1/2 in cells treated with FSH alone for 30 min (standard pool of activated Erk1/2). A maximum decrease (P < 0.01) in levels of phosphorylated Erk1/2 (Fig. 5A) was detected when lysates harvested within 30 and 120 min of FSH + CART stimulation (lanes 2 and 3), as well as when lysates harvested within 60 min of treatment with CART alone (lane 4), were coincubated with those stimulated with FSH alone (standard pool of activated Erk1/2). In contrast, a linear decrease (P < 0.01) in percent of pErk1/2 was observed after coincubation of lysates from GCs stimulated for 30, 60, and 120 min with FSH alone (lanes 1, 5, and 6, respectively; Fig. 5A). To demonstrate the specificity of the observed phosphatase activity, GCs were preincubated for 120 min with phosphatase inhibitors [sodium orthovanadate (1 mm), a tyrosine phosphatase inhibitor; and okadaic acid (50 nm; OA), a serine/threonine phosphatase inhibitor] followed by stimulation with FSH + CART for 120 min. Protein isolated from these cells was subjected thereafter to phosphatase assay as described above. Combined pretreatment of GCs with both the serine/threonine (OA) and tyrosine (Na3VO4) phosphatase inhibitors almost completely blocked the dephosphorylation of Erk1/2 by lysates from FSH + CART-stimulated cells (lane 7; Fig. 5, A and B), suggesting that cotreatment of GCs with FSH + CART induces maximal phosphatase activity at an earlier time point than in GCs treated with FSH alone.
Fig. 5.
CART-Induced Phosphatase Activity
Decrease in percentage of Erk1/2 phosphorylation (phosphatase activity) observed after coincubation of 100 μg protein collected from bovine GCs containing high levels of phosphorylated Erk1/2 (activated Erk standard pool; GCs treated with 25 ng/ml FSH for 30 min; lane 1) with 100 μg of protein from lysates of GCs stimulated with FSH (25 ng/ml) + CART (0.1 μm) for 30 (lane 2) and 120 min (lane 3), CART (0.1 μm) for 60 min (lane 4), FSH (25 ng/ml) for 60 (lane 5) and 120 min (lane 6) and from GCs preincubated for 120 min with phosphatase inhibitors [sodium orthovanadate (1 mm); a tyrosine phosphatase inhibitor and OA (50 nm); a serine/threonine phosphatase inhibitor] and then stimulated with FSH + CART for 120 min (lane 7) (A). Representative Western blots demonstrating decrease in phosphorylated Erk1/2/total Erk1/2 induced by coincubation of activated Erk lysate (standard pool) with the above-mentioned samples (lanes 1–7 denoted in panel A, source of phosphatase activity being quantified). Lysates of GCs treated with media alone were also used as a control (lane C) (B). The data depicted in panel A are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences between treatments (P < 0.05).
To examine whether the CART-induced increase in phosphatase activity is transcription and (or) translation dependent, GCs were preincubated in the presence of the protein synthesis inhibitor cycloheximide (CHX; 30 μm), the transcription inhibitor, actinomycin D (ActD; 1 μg/ml), or media alone for 120 min followed by FSH + CART stimulation. Furthermore, to identify the class of phosphatases involved, GCs were also preincubated with the above described serine/threonine or tyrosine phosphatase inhibitors [Na3VO4 (1 mm) or OA (50 nm)] for 120 min followed by FSH + CART stimulation. The effective concentrations of CHX, ActD, Na3VO4, and OA used in this study have been previously established (43, 44, 45, 46). Figure 6 depicts the observed increase in phosphorylated/total ERK1/2 relative to time zero (Fig. 6, A and B) and respective representative Western blots (Fig. 6, C–G) for GCs treated with media alone (Fig. 6, A and C), CHX (Fig. 6, A and D), ActD (Fig. 6, B and E), OA (Fig. 6, B and F), and Na3VO4 (Fig. 6, B and G). As demonstrated above, in GCs pretreated with media alone followed by FSH + CART stimulation, levels of pErk1/2 peaked (P < 0.01) within 5 min of stimulation, began to decline (P < 0.01) within 15 min, and reached basal levels by 60 min (Fig. 6F). In contrast, blocking transcription or translation by pretreatment with ActD or CHX, respectively, resulted in maintenance (P < 0.01) of Erk1/2 activation through 480 min of stimulation (Fig. 6F). Pretreatment with OA and Na3VO4 also delayed (P < 0.01) the dephosphorylation of Erk1/2 for up to 60 min and 120 min, respectively, after FSH + CART treatment (Fig. 6G). Levels of pErk1/2 ultimately declined to basal values, suggesting the involvement of both classes of phosphatases in regulating Erk1/2 signaling.
Fig. 6.
Effect of Transcription, Translation, and Serine/Threonine and Tyrosine Phosphatase Inhibitors on CART-Induced Phosphatase Activity
Relative increase in Erk1/2 activation (pErk/total Erk) vs. time zero observed after preincubation of bovine GCs in media alone, CHX (30 μm), and ActD (1 μg/ml) for 120 min followed by FSH (25 ng/ml) + CART (0.1 μm) stimulation (A). Relative increase in Erk1/2 activation vs. time zero after preincubation of GCs in media alone, OA (50 nm) and Na3VO4 (1 mm) for 120 min followed by FSH + CART stimulation (B). Representative Western blots demonstrating the pattern of Erk1/2 activation in GCs preincubated with media alone (C), CHX (D), ActD (E), OA (F), or Na3VO4 (G) for 120 min followed by FSH + CART stimulation. The data depicted in panels A and B are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences across and within treatments and time points (P < 0.05).
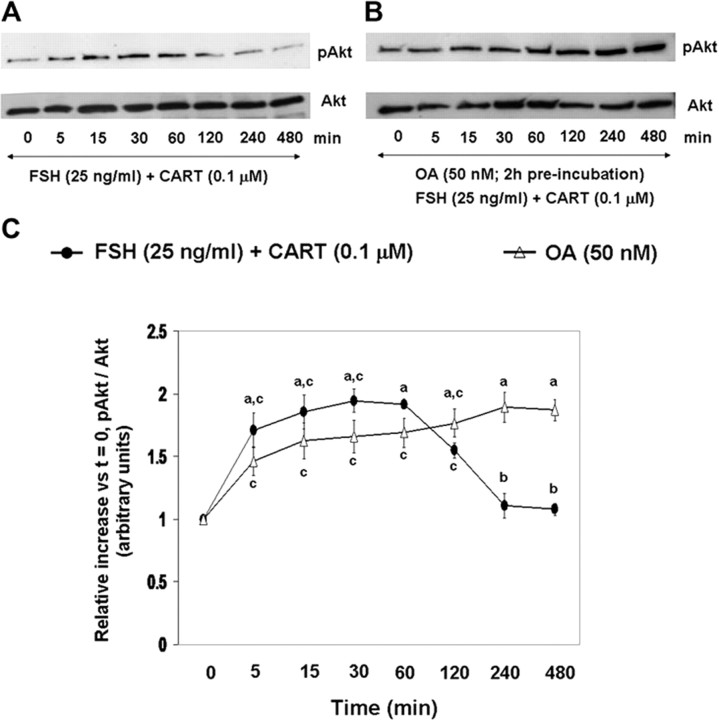
Because Akt is activated by phosphorylation at serine/threonine residues (47), the effects of OA only (serine/threonine-specific phosphatase inhibitor) on Akt activation in GCs stimulated with FSH + CART were investigated. Panels A and B of Fig. 7 are representative Western blots demonstrating differences in the pattern of changes in phosphorylated and total Akt in GCs treated with/without OA followed by FSH + CART treatment, respectively. Figure 7C depicts a comparison of the FSH + CART-induced changes in Akt activation over time (relative to time zero) for bovine GCs pretreated with OA vs. controls pretreated with media alone. Pretreatment with OA resulted in maintenance of Akt activation (Fig. 7C) through 480 min after FSH + CART stimulation (P < 0.01). However, because OA alone enhanced basal Akt phosphorylation (Fig. 7, A and B), the effects of OA specifically on CART-induced early termination of FSH-induced Akt activation were difficult to interpret. Taken together, results suggest that CART-induced acceleration of dephosphorylation of pErk1/2 is mediated, at least in part, by increased tyrosine phosphatase and/or serine/threonine phosphatase activity that is transcription and translation dependent, and CART-induced acceleration of termination of Akt signaling is potentially serine/threonine phosphatase mediated.
Fig. 7.
Serine/Threonine Phosphatase Inhibitor Effects on Akt Activation in Response to FSH + CART Treatment
Representative Western blots demonstrating the pattern of phosphorylated (Ser473) and total Akt in bovine GCs preincubated with media alone (A) or with 50 nm OA (B) for 120 min followed by FSH (25 ng/ml) + CART (0.1 μm) stimulation. Relative increase in Akt activation (pAkt-Ser473/total Akt) vs. time zero observed for GCs subjected to above treatments (C). The data depicted in panel C are represented as the mean ± sem from three replicate experiments. Different superscripts denote significant differences across and within treatments and time points (P < 0.05).
CART-Induced Expression and Regulation of Specific Phosphatase Isoforms
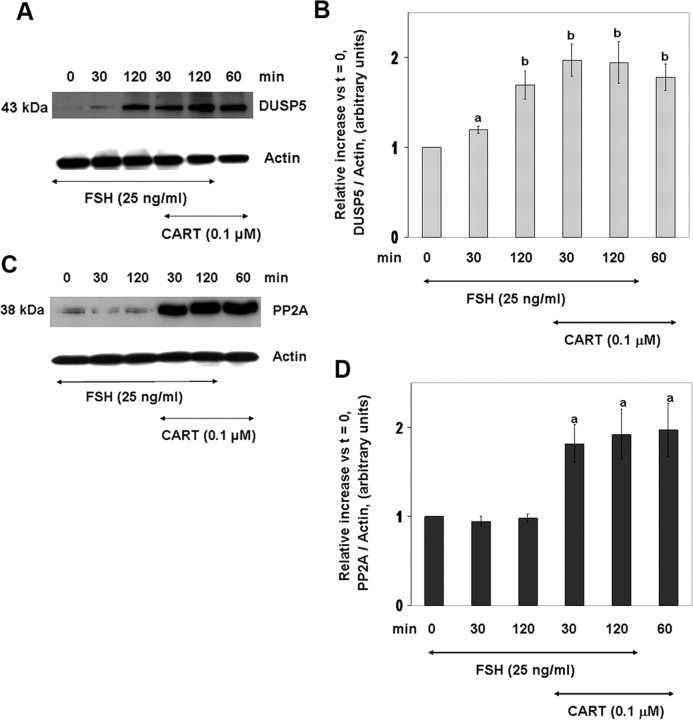
CART regulation of specific phosphatase isoforms was investigated by Western blot analysis. Based on the pattern of Erk1/2 activation (Fig. 1) and results of phosphatase activity assay (Fig. 5), 30 and 120 min were selected as the time points to investigate the expression and role of specific phosphatase isoforms in the termination of Erk1/2 signaling. Results demonstrate that abundance of DUSP5 (Fig. 8, A and B) and PP2A (Fig. 8, C and D) is elevated in response to FSH and/or CART treatment. The antibody used against PP2A (PP2A-C) is specific for the catalytic subunit of PP2A and, unless otherwise mentioned, PP2A-C has been referred to as PP2A throughout the study. Panels A and C of Fig. 8 depict representative Western blots for DUSP5 (Fig. 8A) and PP2A (Fig. 8C), and panels B and D are graphic depictions of the relative increase in DUSP5 and PP2A abundance vs. time zero, respectively, in GC-stimulated with FSH, FSH + CART, or CART alone. FSH alone induced a time-dependent linear increase (P < 0.05) in DUSP5 expression with highest levels of DUSP5 observed after only 120 min of stimulation whereas in GCs treated with FSH + CART, expression of DUSP5 reached maximum (P < 0.01) levels within 30 min of stimulation (Fig. 8B). Moreover, maximum (P < 0.01) levels of DUSP5 expression were also achieved after 60 min of CART stimulation. In contrast, expression of PP2A was not increased in response to FSH treatment and was observed to be solely regulated by CART, reaching maximal (P < 0.01) levels within 30 min of stimulation (Fig. 8D). In addition, expression of MKP-1 and -3 in GCs was also observed. Increased levels of MKP-1 were observed after 120 min of stimulation by FSH and FSH + CART, whereas elevated but lower levels were also observed in response to 60 min CART treatment (P < 0.01; supplemental Fig. 4, A and B). MKP-3 was not regulated in response to FSH and CART treatment (data not shown). Expression of MKP-2 was not detected in bovine GCs. These results indicate that the CART-induced increase in DUSP5 and PP2A proteins may promote accelerated termination of Erk1/2 and Akt signaling.
Fig. 8.
CART-Induced Expression of DUSP5 and PP2A
Representative Western blots demonstrating levels of DUSP5 in bovine GCs stimulated with FSH (25 ng/ml) for 0, 30, and 120 min, CART (0.1 μm) for 60 min, or FSH + CART for 30 and 120 min (A). Relative increase in expression of DUSP5 vs. time zero observed in response to above treatments (B). Representative Western blots demonstrating levels of PP2A-catalytic subunit (PP2A) in GCs stimulated with FSH (25 ng/ml) for 0, 30, and 120 min, CART (0.1 μm) for 60 min, or FSH + CART for 30 and 120 min (C). Relative increase in expression of PP2A vs. time zero observed in response to above treatments (D). The data in panels B and D are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences between treatments (P < 0.05).
Next, we investigated the signaling pathways and the underlying mechanisms mediating the CART-induced increase in DUSP5 and PP2A. GCs were preincubated in the presence or absence of inhibitors (1 μm) of MEK and PKC for 24 h (d 6) and then subjected to FSH (0 and 30 min), CART (60 min), and FSH + CART (30 min) stimulation on d 7 of culture. Figure 9A depicts representative Western blots illustrating the effects of preincubation with respective signaling protein inhibitors on changes in DUSP5 and PP2A protein abundance in response to above treatments, and panels B and C of Fig. 9 graphically depict the effect of pretreatment with respective inhibitors on the relative increase (vs. time zero) in DUSP5 (Fig. 9B) and PP2A (Fig. 9C) in GCs stimulated with FSH, FSH + CART, or CART alone. Stimulation of GCs with FSH + CART or CART alone for 30 and 60 min, respectively, induced DUSP5 expression to maximum levels (P < 0.01) as compared with FSH treatment alone (Fig. 9B). Inhibition of MEK by U0126 completely blocked (P < 0.01) the FSH + CART and CART-stimulated increase in DUSP5 protein levels, whereas the PKC inhibitor was ineffective in blocking the increase in DUSP5 (Fig. 9B). In contrast, inhibition of both MEK and PKC did not have any effect on PP2A expression (Fig. 9C).
Fig. 9.
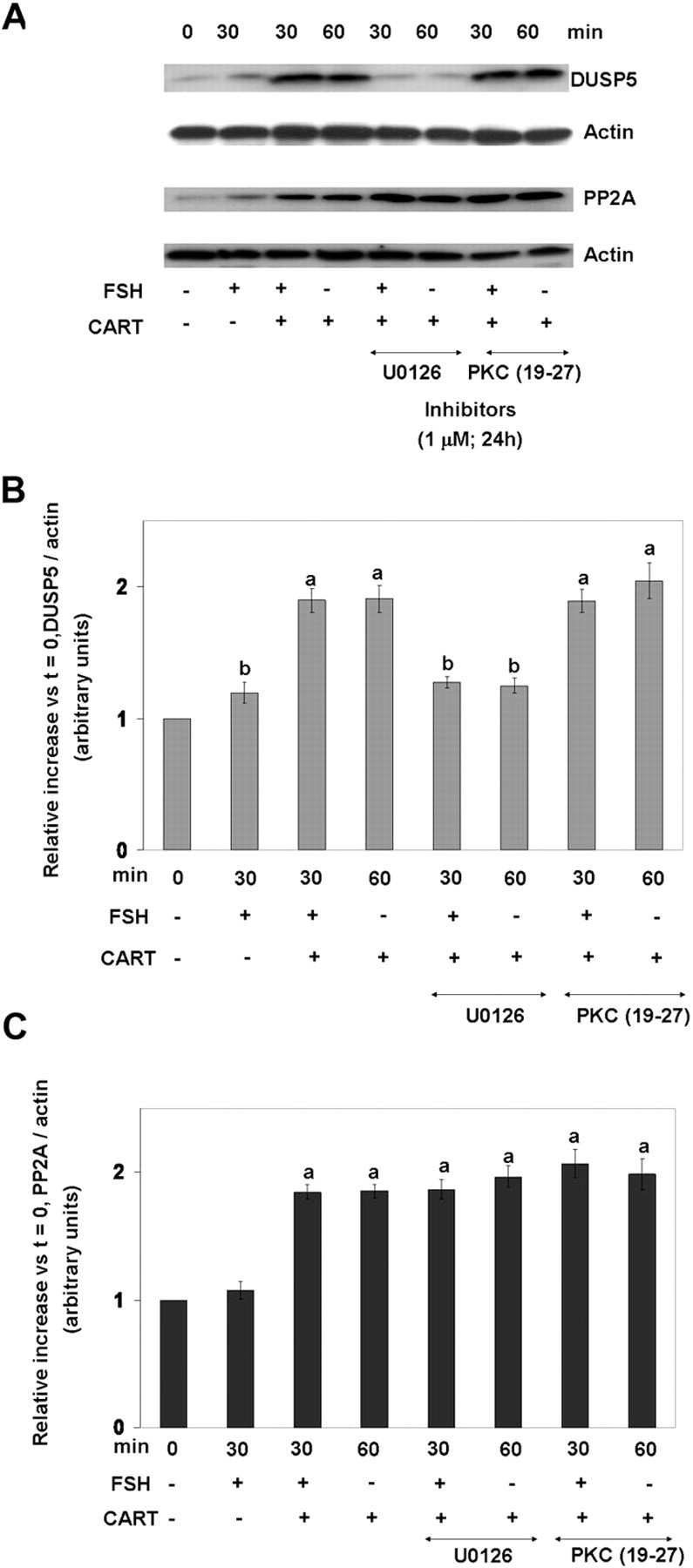
Signaling Pathways Involved in CART-Induced Expression of DUSP5 and PP2A
Representative Western blots demonstrating levels of DUSP5 and PP2A-catalytic subunit (PP2A) in bovine GCs preincubated with media alone or with 1 μm MEK1/2 inhibitor (U0126) or 1 μm PKC peptide inhibitor 19–27 for 24 h before stimulation with FSH (25 ng/ml) for 0 and 30 min, CART (0.1 μm) for 60 min, or FSH + CART for 30 min (A). Relative increase in GC expression of DUSP5 (B) and PP2A (C) vs. time zero observed in response to above treatments. The data depicted in panels B and C are represented as the mean ± sem from three replicate experiments. Different superscripts denote significant differences between treatments (P < 0.05).
To further demonstrate that the increase in DUSP5 and PP2A in response to CART stimulation is regulated at the transcription level, GCs were preincubated (2 h) with the transcription inhibitor ActD (1 μg/ml), and DUSP5 and PP2A levels were visualized by Western blot analysis (Fig. 10A) and quantified using computer-aided densitometry (Fig. 10, B and C). Inhibition of transcription in GCs stimulated with FSH + CART (30 and 120 min) and CART (60 min) alone, completely blocked the increase in DUSP5 (Fig. 10B; P < 0.01). In contrast, inhibition of transcription only partially suppressed (P < 0.01) the increase in PP2A (Fig. 10C), suggesting a contribution of a transcription-independent mechanism to PP2A regulation by CART. Collectively, results demonstrate the existence of a negative feedback loop whereby CART treatment stimulates Erk1/2 activation that induces the expression of DUSP5. The latter, in turn, causes deactivation of Erk1/2 signaling. We also conclude that CART treatment induces an increase in PP2A via transcription-dependent and -independent mechanisms.
Fig. 10.
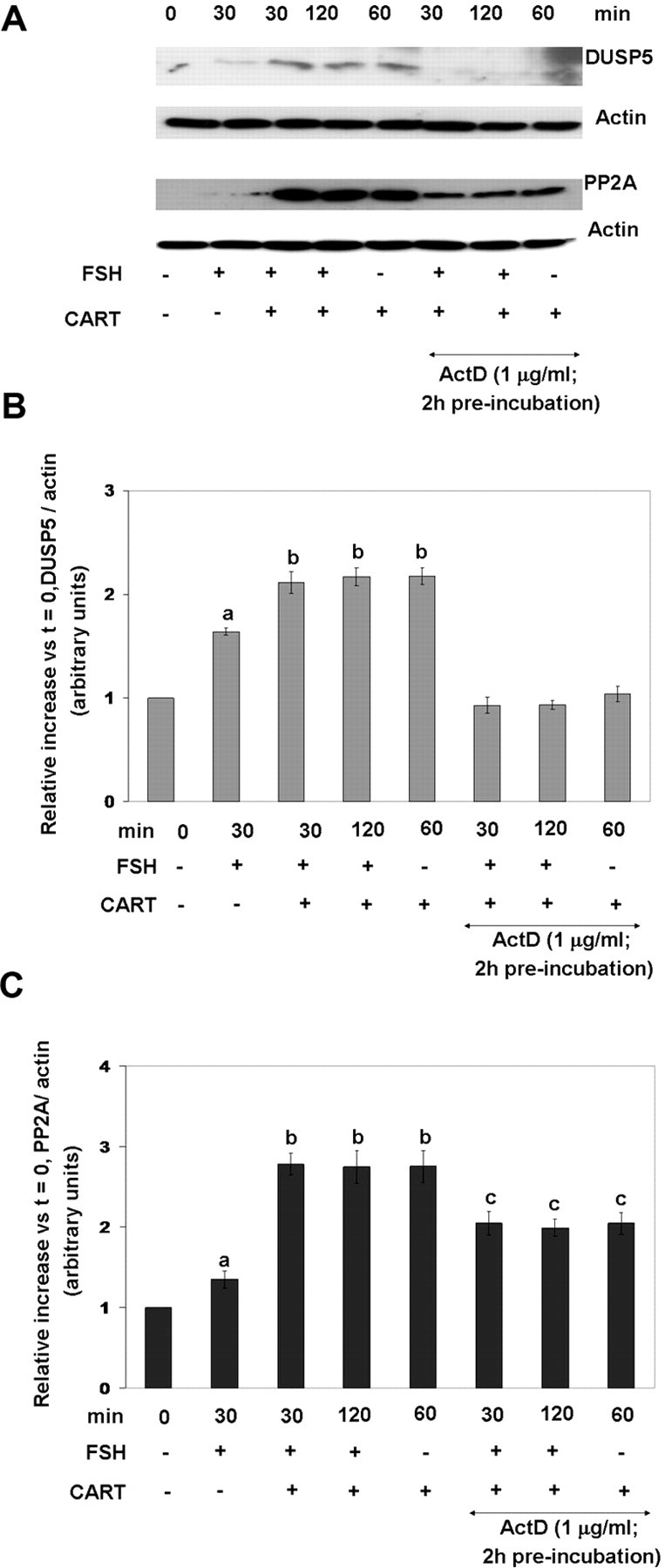
Transcriptional Dependence of CART-Induced Regulation of DUSP5 and PP2A
Representative Western blots demonstrating levels of DUSP5 and PP2A-catalytic subunit (PP2A) in bovine GCs preincubated with media alone or with ActD (1 μg/ml) for 120 min before stimulation with FSH (25 ng/ml) for 0 and 30 min, CART (0.1 μm) for 60 min, or FSH + CART for 30 min (A). Relative increase in GC expression of DUSP5 (B) and PP2A (C) vs. time zero observed in response to above treatments. The data depicted in panels B and C are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences between treatments (P < 0.05).
Small Interfering RNA (siRNA)-Mediated Ablation of DUSP5 and PP2A in Bovine GCs Prevents CART-Induced Early Termination of Erk1/2 and Akt Activation
To demonstrate a direct relationship between CART-induced expression of DUSP5 and PP2A and observed early termination of Erk1/2 and Akt activation, GCs were treated with siRNA delivery media alone or with delivery media containing siRNAs targeting DUSP5, PP2A, DUSP5 siRNA + PP2A siRNA, or with a nonspecific siRNA control. Approximately 96 h after siRNA delivery, GCs were stimulated with FSH + CART, and cells were harvested at 0, 5, 15, 30, 60, 120, 240, and 480 min after stimulation. Effects of treatments on DUSP5 and PP2A expression levels, as well as phosphorylated and total Erk1/2, were determined using Western blot analysis. Figure 11A depicts quantification of effects of treatment with respective siRNAs on the time course of Erk1/2 activation in response to FSH + CART treatment. Figure 11B depicts a representative Western blot illustrating the effects of the above described siRNA treatments on abundance of DUSP5 and PP2A proteins whereas panels C–F of Fig. 11 depict Western blots demonstrating the pattern of Erk1/2 activation in GCs treated with the various combinations of DUSP5, PP2A, and nonspecific siRNAs. As shown in Fig. 11B, treatment with siRNA for DUSP5 resulted in a dramatic decrease in DUSP5 protein abundance in bovine GCs, with no effect on PP2A abundance. Likewise, treatment with PP2A siRNA also resulted in a significant decrease in PP2A protein abundance with no effect on DUSP5 protein abundance, and coincubation with both siRNAs was effective in reducing abundance of both DUSP5 and PP2A. Effects of the nonspecific siRNA or the siRNA delivery media alone on DUSP5 and PP2A protein abundance were not observed (Fig. 11B). Furthermore, off-target effects of the DUSP5 and PP2A siRNAs on MKP1 protein abundance in bovine GCs were not observed (data not shown). Ablation of DUSP5 resulted in sustained Erk1/2 activation for up to 120 min after FSH + CART treatment, after which activated Erk1/2 levels declined slightly but were still significantly higher (P < 0.01) than basal levels (Fig. 11, A and C). In GCs in which PP2A was knocked down, Erk1/2 activation was prolonged through 120 min of FSH + CART treatment, and basal levels were reached by 240 min after stimulation (Fig. 11, A and D). In contrast, ablation of both DUSP5 and PP2A completely blocked the CART-induced early termination of Erk1/2 activation for the full 8-h sampling period after FSH + CART treatment (Fig. 11, A and E). The pattern of Erk1/2 activation in GCs treated with a nonspecific siRNA (Fig. 11, A and F) was used as a control and was similar to GCs stimulated with FSH + CART alone.
Fig. 11.
Effect of siRNA-Mediated Knockdown of DUSP5 and PP2A on CART-Induced Early Termination of Erk1/2 Activation
Time course of the relative increase in Erk1/2 activation (pErk/total Erk) vs. time zero observed in response to stimulation with 25 ng/ml FSH and 0.1 μm CART for bovine GCs pretreated with siRNAs targeting DUSP5, PP2A-catalytic subunit (PP2A), DUSP5 siRNA + PP2A siRNA, or Accell nontargeting pool (nonspecific negative control) siRNAs (A). Representative Western blots illustrating effects of above described siRNA treatments on GC abundance of DUSP5 and PP2A proteins (B) and effects of treatment with DUSP5 siRNA (C), PP2A siRNA (D), DUSP5 siRNA + PP2A siRNA (E), or Accell nontargeting pool (nonspecific negative control) siRNA (F) on the pattern of Erk1/2 activation in GCs stimulated with FSH+CART. The data depicted in panel A are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences between treatments (P < 0.05).
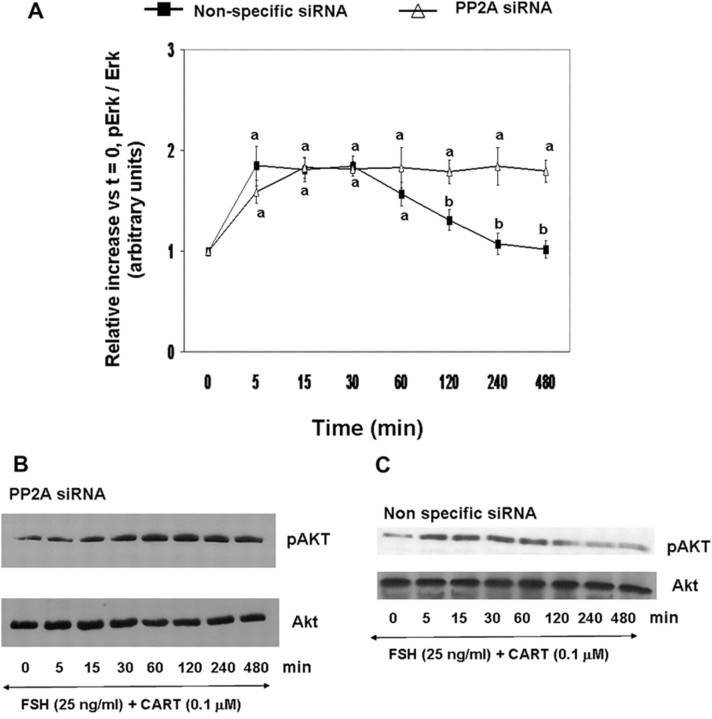
In contrast to the observed effect of PP2A knockdown on Erk1/2 activation, PP2A siRNA treatment completely abolished (P < 0.01) the early termination of Akt activation in FSH + CART-stimulated GCs (Fig. 12A). Panels B and C of Fig. 12 are representative Western blots demonstrating the pattern of Akt activation in GCs treated with PP2A and nonspecific siRNA before FSH + CART stimulation as described above. Collectively, results provide functional evidence that CART-induced expression of both DUSP5 and PP2A is required for observed early termination of Erk1/2 signaling in bovine GCs in response to FSH + CART treatment, whereas PP2A is involved in CART-induced termination of FSH-induced Akt activation.
Fig. 12.
Effect of siRNA-Mediated Knockdown of PP2A on CART Regulation of FSH-Induced Akt Signaling
Time course of the relative increase in Akt activation (pAkt-Ser473/total Akt) vs. time zero observed in response to stimulation with 25 ng/ml FSH and 0.1 μm CART for bovine GCs pretreated with siRNAs targeting PP2A-catalytic subunit (PP2A) or Accell nontargeting pool (nonspecific negative control) siRNAs (A). Representative Western blots illustrating effects of treatment with PP2A siRNA (B) or Accell nontargeting pool (nonspecific negative control) siRNA (C) on the time course of Akt activation in GCs stimulated with FSH + CART. The data depicted in panel A are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences across treatments (P < 0.05).
CART Inhibits Proteasome Degradation of Phosphatase Isoforms
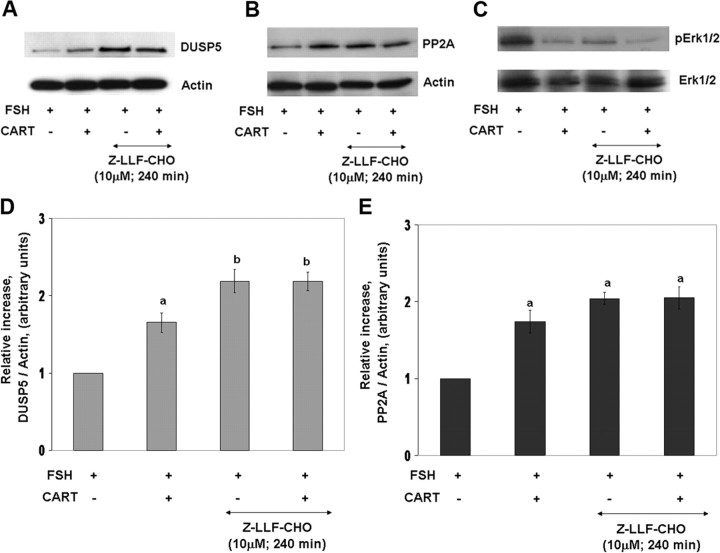
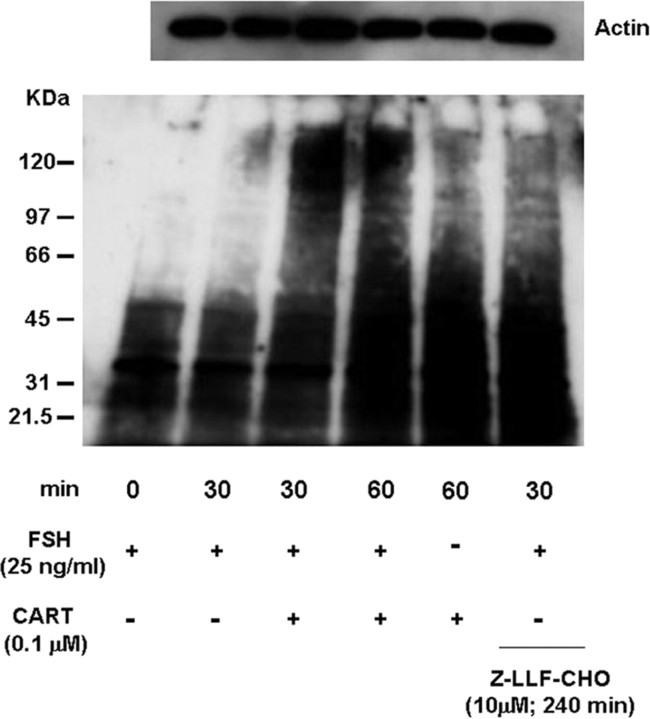
Above results also support a transcription-independent mechanism for CART regulation of PP2A (Fig. 10C). Previous studies have shown that proteasomal inhibition by benzyloxycarbonyl-leu-leu-phenylalaninal (Z-LLF-CHO) alone induces increased protein phosphatase levels in unstimulated cells, resulting in deactivation of MAPK signaling (48). Thus, we investigated whether regulation of proteasome degradation contributes to CART regulation of DUSP5 and/or PP2A and thus Erk1/2 and Akt signaling. Granulosa cells were preincubated (240 min) in the presence or absence of the proteasome inhibitor, Z-LLF-CHO (10 μm), and then stimulated with FSH alone or FSH + CART for 30 min (Fig. 13). Panels A–C of Fig. 13 depict representative Western blots demonstrating the effect of proteasome inhibition on FSH and FSH + CART-induced DUSP5 and PP2A expression and Erk1/2 activation, respectively. Panels D and E of Fig. 13 depict quantification of effects of treatments on DUSP5 (Fig. 13D) and PP2A (Fig. 13E) protein abundance. As demonstrated above, FSH + CART treatment significantly increased (P < 0.01) DUSP5 and PP2A expression in comparison with FSH alone. In contrast, treatment with Z-LLF-CHO mimicked CART actions. Inhibition of proteasome activity increased (P < 0.01) levels of both DUSP5 (Fig. 13D) and PP2A (Fig. 13E) relative to GCs stimulated with FSH alone. Moreover, Z-LLF-CHO pretreatment before FSH + CART stimulation further increased (P < 0.05) DUSP5 but not PP2A expression above that observed in GCs stimulated with FSH + CART alone. The increase in DUSP5 and PP2A in response to proteasome inhibition is accompanied by decreased pErk1/2 levels in response to the same treatments (Fig. 13C). Furthermore, to establish that proposed CART-induced impairment of ubiquitin-mediated proteasome degradation is linked to increased accumulation of DUSP5 and PP2A, we measured the amount of polyubiquitinated proteins in GCs stimulated with FSH (0 and 30 min), CART (60 min), FSH + CART (30 and 60 min), or GCs preincubated with Z-LLF-CHO (240 min) followed by FSH (30 min) treatment (Fig. 14). CART treatment increased the accumulation of polyubiquitinated GC proteins, which appear as a smear (Fig. 14), as compared with FSH treatment alone for 0 and 30 min. The CART-induced increase in accumulation of polyubiquitinated proteins was mimicked in GCs pretreated with Z-LLF-CHO (240 min) followed by FSH (30 min) stimulation (Fig. 14). Results suggest that CART-induced up-regulation of DUSP5 and PP2A, leading to termination of Erk1/2 and Akt signaling, is mediated, at least in part, by an impairment in the proteasome-mediated degradation process for these specific phosphatase isoforms.
Fig. 13.
CART Regulation of Proteasomal Degradation of DUSP5 and PP2A
Representative Western blots demonstrating the effect of treatment of bovine GCs with the proteasome inhibitor Z-LLF-CHO (10 μm) on FSH (25 ng/ml) and FSH + CART (0.1 μm)-induced protein expression for DUSP5 (A) and PP2A-catalytic subunit (PP2A) (B) and on Erk1/2 activation (pErk/total Erk) (C). Relative increase in GC expression of DUSP5 (D) and PP2A (E) proteins vs. time zero observed in response to above treatments. The data depicted in panels D and E are represented as the mean ± sem from three replicate experiments, and different superscripts denote significant differences across treatments (P < 0.05).
Fig. 14.
CART Regulation of Accumulation of Polyubiquitinated Proteins
Representative Western blot for polyubiquitinated proteins in cell lysates from bovine GCs stimulated with FSH (25 ng/ml) for 0 and 30 min, CART (0.1 μm) for 60 min, FSH + CART for 30 and 60 min, or preincubated with the proteasome inhibitor, Z-LLF-CHO (10 μm) for 240 min followed by stimulation with FSH for 30 min. Polyubiquitinated proteins detected appear as a smear. Corresponding Western blot for actin was used as a loading control.
DISCUSSION
Results of the present studies (in bovine GCs) demonstrate for the first time that CART shortens the duration of FSH-induced Erk1/2 and Akt activation. Based on these results, we propose a model (Fig. 15) to describe the cross talk between CART and FSH signaling in bovine GCs. We show that the inhibitory action of CART on FSH-induced Erk1/2 and Akt signaling is mediated through increased phosphatase activity attributed to up-regulation of specific MAPK phosphatase isoforms (DUSP5, PP2A) through transcription-dependent mechanisms and via inhibition of their degradation via the proteasome. Erk1/2 and Akt are important downstream signaling proteins involved in a wide variety of cellular functions (49, 50, 51, 52, 53, 54, 55, 56), and duration of Erk1/2 and Akt activity plays an important role in regulating the specificity of cellular outcomes in response to multiple external stimuli in different cell types (54, 57, 58, 59). Both Erk1/2 and Akt are involved in FSH signaling (37, 60, 61), and our previous studies demonstrated that FSH-stimulated E production in GCs is Erk1/2 and Akt dependent whereas CART inhibits FSH-stimulated E production (2). In an in vivo context, during follicular development, there are marked differences in Erk1/2 and Akt signaling among dominant vs. subordinate follicles, which suggest that both these signaling proteins play an important role in regulating the fate of GCs during follicular development (27, 28). Thus, this study not only sheds novel insight into the intracellular mechanism of CART action but also provides a greater understanding of regulation of Erk1/2 and Akt signaling during follicular development.
Fig. 15.
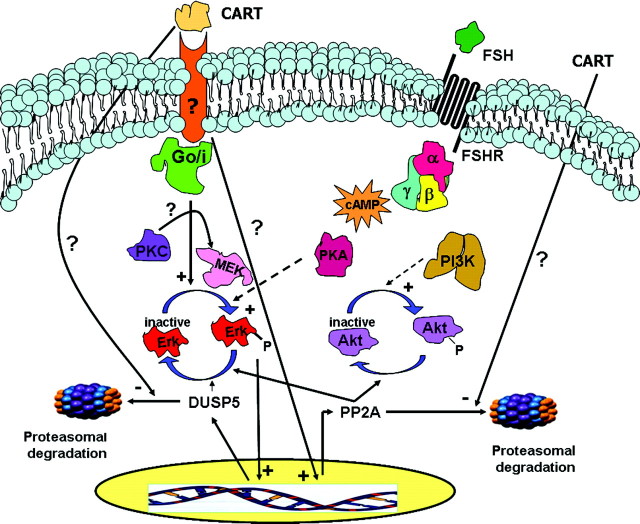
Proposed Model for the Intracellular Mechanism of CART Action in Bovine GCs
FSH stimulates Erk1/2 and Akt activation whereas CART treatment accelerates the termination of FSH-induced Erk1/2 and Akt signaling. CART stimulation alone also induces Erk1/2 activation in bovine GCs via a Go/i-PKC-MEK-dependent pathway. Whether the CART-induced Go/i protein directly phosphorylates PKC, which in turn activates MEK and thus Erk1/2, or whether Erk1/2 is also activated in a PKC-independent manner is not clear. However, the CART-stimulated Erk1/2 activation is independent of PKA, PI3K, and RTK. Expression of DUSP5 is dependent on Erk1/2, thereby exhibiting a negative feedback loop in which activated Erk1/2 induces DUSP5 expression, which in turn terminates Erk1/2 activation. CART-induced DUSP5 expression is functionally required for CART-induced termination of Erk1/2 signaling and is mediated by Erk1/2- and transcription-dependent mechanisms. CART increases PP2A expression, which is also functionally required for CART-induced termination of Erk1/2 and Akt activation, and CART regulation of PP2A expression is mediated by transcription-dependent and -independent mechanisms. CART treatment also impairs the proteasomal degradation of both DUSP5 and PP2A, resulting in their accumulation. FSHR, FSH receptor.
We propose that CART may function as a gatekeeper of the ovulatory quota in monoovulatory species. In the bovine ovary, CART mRNA and peptide are present in granulosa and cumulus cells, and the oocyte and GC CART mRNA and follicular fluid concentrations of CART peptide are higher in estrogen inactive atretic subordinate vs. estrogen active healthy dominant follicles (1). In cattle and humans, growth of antral follicles occurs in a characteristic wave-like pattern, and a single follicle gets selected from the original cohort that achieves dominance (62, 63, 64, 65, 66). Enhanced capacity of the dominant follicle to produce E is critical to sustain dominant follicle growth and trigger the preovulatory gonadotropin surge, resumption of meiosis, and ovulation (21, 22) whereas in subordinate follicles, E producing capacity is lost much before morphological signs of atresia (64, 67). Given the higher expression of CART in GCs of subordinate vs. dominant follicles and the negative effects of CART on FSH signaling and E production, we speculate that CART plays an important role in negative regulation of FSH action in monoovulatory species, such as the cow and perhaps the human, and is critical to dominant follicle selection. In contrast, we hypothesize that the mouse, being a polyovulatory species, requires a less stringent selection mechanism to ovulate multiple follicles, as a result of which CART is not expressed in the ovary and CART mutant mice are fertile. However, further studies are required to substantiate this hypothesis. Moreover, what regulates differential expression of CART between subordinate vs. dominant follicles is still unknown.
A transient activation of Erk1/2, but not Akt, in response to acute CART treatment was observed in the described studies. Similar to published studies using the AtT20 cell line (68) and primary cortical neurons (69), we too observed that acute CART-induced Erk1/2 activation in bovine GCs occurs via a Go/i-MEK-dependent pathway. However, partial inhibition of CART-stimulated Erk1/2 activation in response to pretreatment with a PKC inhibitor suggests a potential role for PKC in CART signaling. In human T cells PLC/PKC and Erk1/2 signaling pathways are activated via a Go/i-coupled receptor (70, 71), and a substantial amount of literature also indicates existence of PKC-dependent and -independent pathways of Erk1/2 activation (39). Thus, as represented in the model (Fig. 15), it is possible that CART via a Go/i-dependent pathway activates MEK-Erk1/2 in a PKC-dependent and -independent fashion. More importantly, our results clearly show that PKA, PI3K, and RTK are not involved in CART-induced Erk1/2 activation.
It is well established that FSH induces an increase in cAMP levels (60), and cAMP activates both Erk1/2 and Akt signaling pathways in GCs (35, 36, 37, 38) as well as in other cell types (39, 40). Therefore, it was conceivable that the CART-induced early termination of FSH-stimulated Erk1/2 and Akt activation may be due to a negative effect of CART on FSH-induced cAMP accumulation. However, acute CART treatment did not have any effect on intracellular cAMP levels, suggesting that the early transient deactivation of Erk1/2 and Akt by CART is not due to a decrease in cAMP levels (termination of positive signal) but is mediated by a different mechanism. In contrast, we have previously reported that CART pretreatment (24 h) decreases FSH-induced intracellular cAMP accumulation (2), which may be one of the causes for the complete inhibition of FSH-induced Erk1/2 and Akt activation by long-term (24 h) CART treatment observed in this study. It is also possible that prolonged CART treatment induces FSH receptor internalization, resulting in the desensitization of GCs to subsequent FSH-induced Erk1/2 and Akt activation. However, further studies are required to prove this hypothesis. Thus, in bovine GCs the effect of CART on FSH-induced Erk1/2 and Akt signaling and the underlying mechanism involved varies based on the duration of CART treatment.
Regulation of Erk1/2 and/or Akt activity involves different intracellular mechanisms (39, 49, 72, 73) and MKPs play a significant role in regulating the duration of Erk1/2 and Akt signaling (74, 75). We observed that whereas inhibition of tyrosine or serine/threonine phosphatases individually extended the duration of Erk1/2 activation, effects were only partial, implying that both classes of phosphatases are involved in CART-induced deactivation of Erk1/2 signaling. More convincing evidence was obtained from results of the phosphatase activity assay in which a more pronounced inhibition of CART-induced Erk1/2 deactivation was observed in GCs cotreated with both types of phosphatase inhibitors (OA and Na3VO4). In contrast, because Akt activation involves phosphorylation of serine/threonine residues (47), inhibition of the serine/threonine phosphatases by OA was potent enough to completely maintain Akt activation over time.
Results show that the pattern of DUSP5 and PP2A expression is inversely related to activated Erk1/2 levels and correlates with phosphatase activity observed in GCs stimulated with FSH, FSH + CART, or CART alone. Here we propose that CART-induced rapid induction of DUSP5 and PP2A expression accounts for the early termination of FSH-induced Erk1/2 and Akt signaling in bovine GCs (Fig. 15). Rapid (within 30 min) ligand-induced MKP expression is a common mechanism of MAPK regulation and has also been reported in breast cancer cells (76), cardiac myocytes (77), and endothelial cells (78). We also observed that both FSH and CART regulate the expression of MKP-1, but given the fact that MKP-1 expression increased only after 120 min of FSH and/or CART stimulation, we believe that MKP-1 is not involved in the CART-induced early termination of Erk1/2 and Akt signaling.
The increase in the duration of Erk1/2 activation when DUSP5 or PP2A were knocked down demonstrates a direct functional relationship between the CART-induced increase in DUSP5 and PP2A expression and early termination of Erk1/2 and Akt activation. However, when DUSP5 and PP2A were knocked down separately, the effect was only partial, suggesting a cooperative role for the two phosphatases. In contrast, ablation of both DUSP5 and PP2A completely blocked the CART-induced early termination of Erk1/2 activation. Similar results were also observed when OA and/or Na3VO4 treatments were used as described above. Thus, taken together, these experiments establish that both DUSP5 and PP2A are the major protein phosphatases involved in CART regulation of Erk1/2 and Akt signaling in bovine GCs.
Although the structure and substrate specificity of DUSP5 and PP2A are well established, understanding of the mechanisms regulating their expression is limited. Based on the cell type, different factors probably regulate the expression of DUSP5 in an Erk1/2-dependent manner. For example, in T cells, IL-2 activates DUSP5 (79), whereas in human kidney-2 cells DUSP5 expression is regulated by epidermal growth factor (80). Woods and Johnson (43) reported that TGFα regulation of DUSP5 expression in hen GCs is Erk1/2 dependent. As depicted in Fig. 15, results of this study establish the existence of a CART-induced negative feedback loop in bovine GCs in which Erk1/2 activation induces the expression of DUSP5 that, in turn, helps terminate FSH-induced Erk1/2 signaling. In contrast, whereas substrate specificity and posttranslational regulation (81, 82, 83) of PP2A has been extensively investigated, very little is known about the regulation of PP2A gene expression. In our study, CART stimulation of PP2A accumulation was regulated partially at the transcription level and independent of PKC and Erk1/2, thereby suggesting the involvement of a possible prominent secondary mechanism mediating the CART-induced increase in PP2A levels.
Regulation of phosphatase degradation is another well-established intracellular mechanism controlling the duration of Erk1/2 and Akt activation (84, 85). Based on results of the present study, we propose (Fig. 15) that, in addition to inducing DUSP5 and PP2A expression, CART also inhibits the ubiquitin-mediated proteasome degradation of such phosphatases, resulting in observed accumulation of DUSP5 and PP2A in response to CART treatment. A link between regulation of proteasome degradation, MKP accumulation, and MAPK signaling is well established, and studies indicate that the underlying mechanism of regulation of MKPs differs among cell types and MKP isoforms (43, 85, 86, 87, 88, 89, 90, 91, 92). Our data suggest that in bovine GCs there may be different underlying mechanisms involved in CART regulation of DUSP5 and PP2A. The observed complete inhibition of CART-induced DUSP5 expression by ActD pretreatment, along with the observed increase in DUSP5 accumulation in GCs pretreated with Z-LLF-CHO before FSH + CART stimulation, suggests that CART regulation of DUSP5 is primarily at the transcription level. In contrast, the partial inhibitory effects of ActD on CART-induced PP2A expression and maximal accumulation of PP2A within 30 min of FSH + CART stimulation with no further effect in response to Z-LLF-CHO pretreatment suggests that inhibition of proteasome degradation may be the predominant mechanism of CART-induced regulation of PP2A. Whereas our results clearly demonstrate a CART-induced increase in accumulation of polyubiquitinated proteins, the underlying mechanism(s) involved in CART regulation of the ubiquitin-proteasome pathway are unclear. Interferon-γ and TGFβ regulate proteasome degradation by altering proteasome subunit composition (93) whereas another way of blocking the ubiquitin-proteasome pathway is by inhibiting the ubiquitination process (94). However, because we observed an accumulation of polyubiquitinated proteins in response to CART treatment in this study, it is likely that CART impairs proteasome activity and the degradation process rather than the ubiquitination pathway. Further studies are needed to understand the mechanism of how CART inhibits ubiquitin-mediated proteasome degradation. In summary, we suggest that FSH or CART stimulation alone results in Erk1/2 activation and DUSP5 expression but at a latter time point (120 and 60 min), costimulation of GCs with FSH + CART results in accelerated Erk1/2 activation to maximal levels within 5 min of stimulation, which in turn induces a rapid expression of DUSP5 that reaches peak level within 30 min of stimulation. CART also induces PP2A expression by transcription-dependent and -independent mechanisms; the underlying mechanism of transcription-independent regulation is unknown, but most probably involves impairment of the proteasome degradation process. This rapid expression and accumulation of DUSP5 and PP2A by CART, in turn, cause an early termination of FSH-induced Erk1/2 and Akt signaling (Fig. 15). Results of the present studies provide extensive novel insight into the cross talk between CART and FSH signaling and establish CART as an important regulator of the duration of Erk1/2 and Akt signaling. More importantly, our results not only demonstrate CART as a novel inducer of DUSP5 and PP2A expression, but also implicate CART for the first time as an inhibitor of the proteasome degradation process. Given the importance of FSH signaling to E production and wide expression and varied functions of CART across species and cell/tissue types, results of described studies form a foundation for future studies of CART signaling pathways critical to relevant physiological processes within and beyond the reproductive system.
MATERIALS AND METHODS
Long-Term Bovine GC Culture
Serum-free long-term bovine GC culture was performed as described previously (2) before initiation of indicated treatments.
Treatment Administration
For studies of CART regulation of FSH-induced Erk1/2 activation, GCs were stimulated on d 7 of culture with 25 ng/ml of FSH (NIDDK-oFSH-20, Harbor-University of California Los Angeles Research and Education Institute, Los Angeles, CA) + water (vehicle for CART), CART peptide 55–102 (0.1 μm; American Peptide Co., Sunnyvale, CA), FSH + CART or FSH + inactive CART peptide 55–76 (American Peptide), or preincubated with/without (vehicle only) CART (0.1 μm) for 24 h (d 6) before FSH (25 ng/ml) stimulation. Cells were then harvested at 0, 5, 15, 30, 60, 120, 240, and 480 min after stimulation. GCs were also stimulated with CART (0.1 μm) alone for 0, 5, 15, 30, and 60 min. For studies of signaling pathways and mechanisms involved in CART-induced Erk1/2 activation, GCs were preincubated with media containing equivalent volume of vehicle or with media containing 1 μm of signaling protein inhibitors [PI3K inhibitor LY294002, PKA inhibitor peptide 14–22 (myristoylated), PKC inhibitor peptide 19–27 (myristoylated), Go/i inhibitor NF023, MEK1/2 inhibitor U0126, and MEK inhibitor PD98059; Calbiochem, San Diego, CA] for 24 h (d 6), whereas in experiments with the transcription (1 μg/ ml ActD; Sigma Chemical Co., St. Louis, MO), translation (30 μm CHX; Sigma), or phosphatase inhibitors (50 nm OA; and/or 1 mm sodium orthovanadate; Sigma) GCs were pretreated for only 120 min before CART (0.1 μm), FSH (25 ng/ml), or FSH + CART stimulation for different time points on d 7 of culture. GCs were also preincubated (d 7) with the proteasome inhibitor, Z-LLF-CHO (10 μm; Calbiochem) for 240 min followed by FSH (25 ng/ml) or FSH + CART (0.1 μm) stimulation for 0, 30, or 60 min. After the stipulated duration of treatments, cells were harvested and pooled from four wells for each treatment/time point of stimulation and stored at −80 C until Western blot analysis.
Western Blot Analysis
Western blot analysis was performed as described previously (95, 96, 97, 98). Briefly, protein was isolated from GCs by sonication and protein concentration was determined spectrophotometrically (at 280 nm). After Western blot analysis of increasing protein concentrations from cell lysates, the optimal protein amount to quantify Erk1/2, pErk1/2, Akt, and pAkt was determined to be 10 μg/lane whereas 25 μg/lane of total protein was used to detect the MAPK phosphatase isoforms. The following primary antibodies were used in this study at dilutions indicated: mouse anti-actin monoclonal antibody [1:5000 (vol/vol), Calbiochem], rabbit anti-Erk2 (C-14) polyclonal antibody, mouse anti-pErk (E-4) monoclonal antibody, rabbit anti-PP2A-C (FL-309) polyclonal antibody (detects catalytic subunit), rabbit anti-MKP-1(V-15) polyclonal antibody, rabbit anti-MKP-2 (S-18) polyclonal antibody, rabbit anti-MKP-3 (H-130) polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) rabbit anti-Akt polyclonal antibody, rabbit anti-pAkt (Ser473) antibody (Cell Signaling Technology, Inc., Danvers, MA), rabbit anti-DUSP5 polyclonal antibody [1:1000 (vol/vol), Abcam, Inc., Cambridge, MA], and Ub (P4D1) mouse monoclonal antibody [1:250 (vol/vol), Santa Cruz Biotechnology]. After detection of pErk1/2 or pAkt, membranes were stripped by treatment with restore Western blot stripping buffer and reprobed for total Erk1/2 or Akt to confirm equal loading. Validation of the Western blot analysis and stripping conditions has been determined previously (95, 96, 97, 98). Phosphorylated and total Erk1/2 and Akt levels were determined using computer-aided densitometry, and Erk1/2 and Akt activation was expressed as relative increase in phosphorylated/total Erk1/2 and Akt vs. time 0, respectively. Expression of MAPK phosphatase isoforms was normalized to corresponding actin levels.
In Vitro Phosphatase Assay
An in vitro phosphatase assay was performed as described by Woods and Johnson (43) with slight alterations. Briefly, GCs were stimulated with 1) FSH (25 ng/ml) + CART (0.1 μm) for 30 and 120 min, 2) CART (0.1 μm) for 60 min, and 3) FSH (25 ng/ml) for 30, 60, and 120 min. Thereafter, media were removed, cells were washed with PBS, and cell lysates were prepared by sonication in phosphatase buffer [10 mm EDTA, 10 mm EGTA, 50 mm HEPES (pH 7.6), 1 mm phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin, 10 mg/ml aprotinin]. Phosphatase activity in each sample was assessed by coincubating (60 min at 30 C) 100 μg of protein from cell lysates containing high levels of pErk1/2 (activated Erk standard pool; cells treated with FSH for 30 min) with 100 μg of protein from lysates of cells subjected to the above mentioned treatments. Thereafter, Western blot analysis was used to determine decrease in pErk/Erk induced by coincubation of activated Erk lysate (standard pool) with the above-mentioned samples (source of phosphatase activity being quantified). Lysates of GCs treated with media alone (no pErk1/2) were used as a control. Based on a preliminary study involving different coincubation times (0–60 min), 60 min was established as the optimal incubation time to detect maximal phosphatase activity (data not shown). In a separate experiment, cells were preincubated for 120 min with phosphatase inhibitors [sodium orthovanadate (1 mm); a tyrosine phosphatase inhibitor and okadaic acid (50 nm; OA); a serine/threonine phosphatase inhibitor] followed by stimulation with FSH + CART for 120 min. Thereafter, media was removed, cells were washed with PBS, and cell lysates were prepared by sonication in phosphatase buffer before coincubation of lysates with the standard pool of activated Erk1/2 (as described above). Relative levels of phosphorylated Erk1/2/total Erk1/2 were determined by Western blot analysis. The decrease in percent of Erk phosphorylation (pErk/Erk) in response to coincubation with lysates from cells exposed to various treatments was used as an indicator of phosphatase activity.
RNA Interference in Bovine GCs in Vitro
Granulosa cells were cultured for 4 d in the long-term bovine GC culture system (2) with media changed every 48 h. Thereafter, the cells were cultured in 1) Accell siRNA delivery media alone or in Accell siRNA delivery media containing 2) DUSP5 siRNA, 3) PP2A siRNA, 4) DUSP5 + PP2A siRNA, or 5) Accell nontargeting pool (negative control) siRNA for an additional 3 d. Based on a preliminary dose-response experiment for DUSP5 and PP2A siRNAs (data not shown), a 10 μm concentration of siRNA was selected for use in all experiments. All siRNAs were designed by custom Accell SMARTpool (Dharmacon, Inc. Chicago, IL) against the bovine sequences for DUSP5 (GenBank accession no. XM_583612) and PP2A (GenBank accession no. NM_181031). The Accell nontargeting pool siRNA (Dharmacon) that was used as negative control is a pool of four siRNAs designed not to target any genes in human, mouse, or rat genomes and is microarray tested. The Accell siRNA delivery media (Dharmacon) was supplemented with HEPES (20 mm), antibiotics (100 IU/ml penicillin and 0.1 mg/ml streptomycin), fungizone-amphotericin B (250 μg/ml), long R3-IGF-I (Sigma; 1 ng/ml), and androstenedione (Sigma; 10−7 m). After 72 h of siRNA delivery (d 7 of culture), the Accell siRNA delivery media were removed, and fresh long-term GC culture media were added. On d 8 (96 h after siRNA delivery) GCs were stimulated with FSH (25 ng/ml) + CART (0.1 μm), and cells were harvested at 0, 5, 15, 30, 60, 120, 240, and 480 min after stimulation. Cell viability was determined by the trypan blue exclusion method. Approximately 75% of cells were viable on d 8 of culture, and there was no effect of treatments on cell viability (data not shown). Thereafter, cells from four wells were pooled per treatment for each time point of stimulation and phosphorylated, and total Erk1/2 levels were determined using Western blot analysis as described above. Specificity and efficacy of above siRNA treatments (treatments 1–5 described above) in reducing abundance of DUSP5 and PP2A protein were determined by Western blot analysis of cell lysates harvested 30 min after treatment with FSH + CART.
Statistical Analysis
Generalized mixed linear models were fit to the data using the MIXED procedure of the statistical software SAS (version 9.1; SAS Institute Inc., Cary, NC). In all cases, model assumptions were evaluated and considered to be appropriately met. Multiple comparisons between treatments were performed using Tukey-Kramer adjustments. P < 0.05 was considered significant. All experiments were replicated at least three times.
Acknowledgments
We thank Janet Ireland and Fermin-Jimenez Krassel for their help with follicle dissection; Dr. Stephen R Hammes of University of Texas Southwestern Medical Center (Dallas, TX) for allowing Aritro Sen to perform the siRNA experiments in his laboratory; and Liliana Carbajal and Maria Beckford for assistance with ovary collections.
Footnotes
This work was supported by the Michigan Agricultural Experiment Station, a seed grant from the Michigan State University Reproductive and Developmental Sciences Program, and by National Research Initiative Competitive Grant 2005-35203-16011 from the US Department of Agriculture Cooperative State Research, Education, and Extension Service.
Disclosure Statement: The authors of this manuscript have nothing to declare.
First Published Online September 25, 2008
Abbreviations: ActD, Actinomycin D; CART, cocaine- and amphetamine-regulated transcript; CHX, cycloheximide; DUSP, dual specific phosphatase; E, estradiol; GCs, granulosa cells; MKP, MAPK phosphatase; OA, okadaic acid; PP2A, protein phosphatase 2A; PPT, protein phosphatase; PI3K, phospho inositol-3 Kinase; PKA, protein kinase A; PKC, protein kinase C; RTK, receptor tyrosine kinase; Z-LLF-CHO, benzyloxycarbonyl-leu-leu-phenylalaninal.
References
- 1.Kobayashi Y, Jimenez Krassel F, Li Q, Yao J, Huang R, Ireland JJ, Coussens PM, Smith GW 2004. Evidence that cocaine- and amphetamine-regulated transcript is a novel intraovarian regulator of follicular atresia. Endocrinology 145:5373–5383 [DOI] [PubMed] [Google Scholar]
- 2.Sen A, Bettegowda A, Jimenez-Krassel F, Ireland JJ, Smith GW 2007. Cocaine- and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells. Endocrinology 148:4400–4410 [DOI] [PubMed] [Google Scholar]
- 3.Thim L, Kristensen P, Nielsen PF, Wulff BS, Clausen JT 1999. Tissue-specific processing of cocaine- and amphetamine-regulated transcript peptides in the rat. Proc Natl Acad Sci USA 96:2722–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurd YL, Fagergren P 2000. Human cocaine- and amphetamine-regulated transcript (CART) mRNA is highly expressed in limbic- and sensory-related brain regions. J Comp Neurol 425:583–598 [DOI] [PubMed] [Google Scholar]
- 5.Smith Y, Koylu EO, Couceyro P, Kuhar MJ 1997. Ultrastructural localization of CART (cocaine- and amphetamine-regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse 27:90–94 [DOI] [PubMed] [Google Scholar]
- 6.Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ 1997. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol 9:823–833 [DOI] [PubMed] [Google Scholar]
- 7.Douglass J, McKinzie AA, Couceyro P 1995. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci 15:2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuriyama G, Takekoshi S, Tojo K, Nakai Y, Kuhar MJ, Osamura RY 2004. Cocaine- and amphetamine-regulated transcript peptide in the rat anterior pituitary gland is localized in gonadotrophs and suppresses prolactin secretion. Endocrinology 145:2542–2550 [DOI] [PubMed] [Google Scholar]
- 9.Jensen PB, Kristensen P, Clausen JT, Judge ME, Hastrup S, Thim L, Wulff BS, Foged C, Jensen J, Holst JJ, Madsen OD 1999. The hypothalamic satiety peptide CART is expressed in anorectic and non-anorectic pancreatic islet tumors and in the normal islet of Langerhans. FEBS Lett 447:139–143 [DOI] [PubMed] [Google Scholar]
- 10.Wierup N, Kuhar M, Nilsson BO, Mulder H, Ekblad E, Sundler F 2004. Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J Histochem Cytochem 52:169–177 [DOI] [PubMed] [Google Scholar]
- 11.Ekblad E, Kuhar M, Wierup N, Sundler F 2003. Cocaine- and amphetamine-regulated transcript: distribution and function in rat gastrointestinal tract. Neurogastroenterol Motil 15:545–557 [DOI] [PubMed] [Google Scholar]
- 12.Wierup N, Gunnarsdottir A, Ekblad E, Sundler F 2007. Characterisation of CART-containing neurons and cells in the porcine pancreas, gastro-intestinal tract, adrenal and thyroid glands. BMC Neurosci 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrang N, Larsen PJ, Kristensen P, Tang Christensen M 2000. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 141:794–801 [DOI] [PubMed] [Google Scholar]
- 14.Raptis S, Fekete C, Sarkar S, Rand WM, Emerson CH, Nagy GM, Lechan RM 2004. Cocaine- and amphetamine-regulated transcript co-contained in thyrotropin-releasing hormone (TRH) neurons of the hypothalamic paraventricular nucleus modulates TRH-induced prolactin secretion. Endocrinology 145:1695–1699 [DOI] [PubMed] [Google Scholar]
- 15.Baranowska B, Wolinska Witort E, Chmielowska M, Martynska L, Baranowska Bik A 2003. Direct effects of cocaine-amphetamine-regulated transcript (CART) on pituitary hormone release in pituitary cell culture. Neuroendocrinol Lett 24:224–226 [PubMed] [Google Scholar]
- 16.Smith SM, Vaughan JM, Donaldson CJ, Rivier J, Li C, Chen A, Vale WW 2004. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology 145:5202–5209 [DOI] [PubMed] [Google Scholar]
- 17.Vicentic A, Lakatos A, Kuhar MJ 2005. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: Specific binding in AtT20 cells. Eur J Pharmacol 528:188–189 [DOI] [PubMed] [Google Scholar]
- 18.Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Sloncova E, Elbert T, Slaninova J, Zelezna B 2007. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol 559:109–114 [DOI] [PubMed] [Google Scholar]
- 19.Douglas C, Jones MJK 2008. CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse 62:122–127 [DOI] [PubMed] [Google Scholar]
- 20.Fortune JE 1994. Ovarian follicular growth and development in mammals. Biol Reprod 50:225–232 [DOI] [PubMed] [Google Scholar]
- 21.Greenwald GS, Roy SK 1994. Follicular development and its control. In: Knobil E, Neill JD, eds. The physiology of reproduction, 2nd ed. New York: Raven Press; 629–724
- 22.Vander AJ, Sherman JH, Luciano DS 1994. Reproduction. In: Prancan KM, Bradley JW, eds. Human physiology: the mechanisms of body function. New York: McGraw-Hill; 661–692
- 23.Fair T 2003. Follicular oocyte growth and acquisition of developmental competence. Anim Reprod Sci 78:203–216 [DOI] [PubMed] [Google Scholar]
- 24.Johnson AL 2003. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim Reprod Sci 78:185–201 [DOI] [PubMed] [Google Scholar]
- 25.Murphy KG, Abbott CR, Mahmoudi M, Hunter R, Gardiner JV, Rossi M, Stanley SA, Ghatei MA, Kuhar MJ, Bloom SR 2000. Quantification and synthesis of cocaine- and amphetamine-regulated transcript peptide (79–102)-like immunoreactivity and mRNA in rat tissues. J Endocrinol 166:659–668 [DOI] [PubMed] [Google Scholar]
- 26.Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Koster A 2001. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 142:4394–4400 [DOI] [PubMed] [Google Scholar]
- 27.Ryan KE, Casey SM, Canty MJ, Crowe MA, Martin F, Evans ACO 2007. Akt and Erk signal transduction pathways are early markers of differentiation in dominant and subordinate ovarian follicles in cattle. Reproduction 133:617–626 [DOI] [PubMed] [Google Scholar]
- 28.Evans ACO, Martin F 2000. Kinase pathways in dominant and subordinate ovarian follicles during the first wave of follicular development in sheep. Anim Reprod Sci 64:221–231 [DOI] [PubMed] [Google Scholar]
- 29.Woods DC, Haugen MJ, Johnson AL 2007. Actions of epidermal growth factor receptor/mitogen-activated protein kinase and protein kinase C signaling in granulosa cells from gallus gallus are dependent upon stage of differentiation. Biol Reprod 77:61–70 [DOI] [PubMed] [Google Scholar]
- 30.Babu PS, Krishnamurthy H, Chedrese PJ, Sairam MR 2000. Activation of extracellular-regulated kinase pathways in ovarian granulosa cells by the novel growth factor type 1 follicle-stimulating hormone receptor. Role in hormone signaling and cell proliferation. J Biol Chem 275:27615–27626 [DOI] [PubMed] [Google Scholar]
- 31.Hu C-L, Cowan RG, Harman RM, Quirk SM 2004. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Mol Endocrinol 18:326–338 [DOI] [PubMed] [Google Scholar]
- 32.Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M 2003. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem 278:7167–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M 2004. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 279:19431–19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS 2000. Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14:1283–1300 [DOI] [PubMed] [Google Scholar]
- 35.Makarevich A, Sirotkin A, Chrenek P, Bulla J, Hetenyi L 2000. The role of IGF-I, cAMP/protein kinase A and MAP-kinase in the control of steroid secretion, cyclic nucleotide production, granulosa cell proliferation and preimplantation embryo development in rabbits. J Steroid Biochem Mol Biol 73:123–133 [DOI] [PubMed] [Google Scholar]
- 36.Woods DC, Johnson AL 2007. Protein kinase C activity mediates LH-induced ErbB/Erk signaling in differentiated hen granulosa cells. Reproduction 133:733–741 [DOI] [PubMed] [Google Scholar]
- 37.Richards JS 2001. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- 38.Donadeu FX, Ascoli M 2005. The differential effects of the gonadotropin receptors on aromatase expression in primary cultures of immature rat granulosa cells are highly dependent on the density of receptors expressed and the activation of the inositol phosphate cascade. Endocrinology 146:3907–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enrique R 2007. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213:589–602 [DOI] [PubMed] [Google Scholar]
- 40.Monje PV, Bunge MB, Woods PM 2006. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia 53:649–659 [DOI] [PubMed] [Google Scholar]
- 41.Beindl W, Mitterauer T, Hohenegger M, Ijzerman AP, Nanoff C, Freissmuth M 1996. Inhibition of receptor/G protein coupling by suramin analogues. Mol Pharmacol 50:415–423 [PubMed] [Google Scholar]
- 42.Freissmuth M, Boehm S, Beindl W, Nickel P, Ijzerman AP, Hohenegger M, Nanoff C 1996. Suramin analogues as subtype-selective G protein inhibitors. Mol Pharmacol 49:602–611 [PubMed] [Google Scholar]
- 43.Woods DC, Johnson AL 2006. Phosphatase activation by epidermal growth factor family ligands regulates extracellular regulated kinase signaling in undifferentiated hen granulosa cells. Endocrinology 147:4931–4940 [DOI] [PubMed] [Google Scholar]
- 44.Johnson AL, Bridgham JT 2000. Caspase-3 and -6 expression and enzyme activity in hen granulosa cells. Biol Reprod 62:589–598 [DOI] [PubMed] [Google Scholar]
- 45.Johnson AL, Solovieva EV, Bridgham JT 2002. Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. Biol Reprod 67:1313–1320 [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez Reyes J, Santana P, Gonzalez Robaina I, Cabrera Oliva J, Estevez F, Hernandez I, Lopez Blanco F, Quintana Aguiar J, Fanjul LF, Ruiz de Galarreta CM 1997. Effect of the protein phosphatase inhibitor okadaic acid on FSH-induced granulosa cell steroidogenesis. J Endocrinol 152:131–139 [DOI] [PubMed] [Google Scholar]
- 47.Scheid MP, Woodgett JR 2003. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett 546:108–112 [DOI] [PubMed] [Google Scholar]
- 48.Orlowski RZ, Small GW, Shi YY 2002. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J Biol Chem 277:27864–27871 [DOI] [PubMed] [Google Scholar]
- 49.Ebisuya M, Kondoh K, Nishida E 2005. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci 118:2997–3002 [DOI] [PubMed] [Google Scholar]
- 50.Datta SR, Brunet A, Greenberg ME 1999. Cellular survival: a play in three Akts. Genes Dev 13:2905–2927 [DOI] [PubMed] [Google Scholar]
- 51.Tunquist BJ, Maller JL 2003. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev 17:683–710 [DOI] [PubMed] [Google Scholar]
- 52.Mirza AM, Gysin S, Malek N, Nakayama K, Roberts JM, McMahon M 2004. Cooperative regulation of the cell division cycle by the protein kinases RAF and AKT. Mol Cell Biol 24:10868–10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C, Jacobson K, Schaller MD 2004. MAP kinases and cell migration. J Cell Sci 117:4619–4628 [DOI] [PubMed] [Google Scholar]
- 54.Murphy LO, Smith S, Chen R-H, Fingar DC, Blenis J 2002. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol 4:556–564 [DOI] [PubMed] [Google Scholar]
- 55.Murphy LO, MacKeigan JP, Blenis J 2004. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol Cell Biol 24:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balmanno K, Cook S 1999. Sustained MAPK activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085–3097 [DOI] [PubMed] [Google Scholar]
- 57.Marshall CJ 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179–185 [DOI] [PubMed] [Google Scholar]
- 58.Longo PG, Laurenti L, Gobessi S, Petlickovski A, Pelosi M, Chiusolo P, Sica S, Leone G, Efremov DG 2006. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia 21:110–120 [DOI] [PubMed] [Google Scholar]
- 59.Pouyssegur J, Lenormand P 2003. Fidelity and spatio-temporal control in MAP kinase (ERKs) signaling. Eur J Biochem 270:3291–3299 [DOI] [PubMed] [Google Scholar]
- 60.Hunzicker-Dunn M, Maizels ET 2006. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wayne CM, Fan H-Y, Cheng X, Richards JS 2007. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957 [DOI] [PubMed] [Google Scholar]
- 62.Ginther OJ, Beg MA, Bergfelt DR, Donadeu FX, Kot K 2001. Follicle selection in monovular species. Biol Reprod 65:638–647 [DOI] [PubMed] [Google Scholar]
- 63.Baerwald AR, Adams GP, Pierson RA 2003. Characterization of ovarian follicular wave dynamics in women. Biol Reprod 69:1023–1031 [DOI] [PubMed] [Google Scholar]
- 64.Sunderland SJ, Crowe MA, Boland MP, Roche JF, Ireland JJ 1994. Selection, dominance and atresia of follicles during the oestrous cycle of heifers. J Reprod Fertil 101:547–555 [DOI] [PubMed] [Google Scholar]
- 65.Mihm M, Good TE, Ireland JL, Ireland JJ, Knight PG, Roche JF 1997. Decline in serum follicle-stimulating hormone concentrations alters key intrafollicular growth factors involved in selection of the dominant follicle in heifers. Biol Reprod 57:1328–1337 [DOI] [PubMed] [Google Scholar]
- 66.Richards J, Imagawa W, Balakrishnan A, Edery M, Nandi S 1988. The lack of effect of phenol red or estradiol on the growth response of human, rat, and mouse mammary cells in primary culture. Endocrinology 123:1335–1340 [DOI] [PubMed] [Google Scholar]
- 67.Austin EJ, Mihm M, Evans AC, Knight PG, Ireland JL, Ireland JJ, Roche JF 2001. Alterations in intrafollicular regulatory factors and apoptosis during selection of follicles in the first follicular wave of the bovine estrous cycle. Biol Reprod 64:839–848 [DOI] [PubMed] [Google Scholar]
- 68.Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ 2005. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett 384:198–202 [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, Hurn PD, Martinez-Murillo F, Alkayed NJ 2006. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci USA 103:14489–14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE 1998. The α-chemokine, stromal cell-derived factor-1 α, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 273:23169–23175 [DOI] [PubMed] [Google Scholar]
- 71.Wang J-F, Park I-W, Groopman JE 2000. Stromal cell-derived factor-1 α stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood 95:2505–2513 [PubMed] [Google Scholar]
- 72.Gavi S, Shumay E, Wang HY, Malbon CC 2006. G-protein-coupled receptors and tyrosine kinases: crossroads in cell signaling and regulation. Trends Endocrinol Metab 17:48–54 [DOI] [PubMed] [Google Scholar]
- 73.Du K, Tsichlis PN 2005. Regulation of the Akt kinase by interacting proteins. Oncogene 24:7401–7409 [DOI] [PubMed] [Google Scholar]
- 74.Keyse SM 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr Opin Cell Biol 12:186–192 [DOI] [PubMed] [Google Scholar]
- 75.Dickinson RJ, Keyse SM 2006. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci 119:4607–4615 [DOI] [PubMed] [Google Scholar]
- 76.Wu W, Pew T, Zou M, Pang D, Conzen SD 2005. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem 280:4117–4124 [DOI] [PubMed] [Google Scholar]
- 77.Lim HW, New L, Han J, Molkentin JD 2001. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem 276:15913–15919 [DOI] [PubMed] [Google Scholar]
- 78.Kiemer AK, Weber NC, Furst R, Bildner N, Kulhanek-Heinze S, Vollmar AM 2002. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-α-induced actin polymerization and endothelial permeability. Circ Res 90:874–881 [DOI] [PubMed] [Google Scholar]
- 79.Kovanen PE, Rosenwald A, Fu J, Hurt EM, Lam LT, Giltnane JM, Wright G, Staudt LM, Leonard WJ 2003. Analysis of γ c-family cytokine target genes. Identification of DUSP5 as a regulator of MAPK activity in interleukin-2 signaling. J Biol Chem 278:5205–5213 [DOI] [PubMed] [Google Scholar]
- 80.Sarközi R, Miller B, Pollack V, Feifel E, Mayer G, Sorokin A, Schramek H 2007. ERK1/2-driven and MKP-mediated inhibition of EGF-induced ERK5 signaling in human proximal tubular cells. J Cell Physiol 211:88–100 [DOI] [PubMed] [Google Scholar]
- 81.Janssens V, Goris J 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J 353:417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldberg Y 1999. Protein phosphatase 2A: who shall regulate the regulator? Biochem Pharmacol 57:321–328 [DOI] [PubMed] [Google Scholar]
- 83.Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S 2005. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem 280:36029–36036 [DOI] [PubMed] [Google Scholar]
- 84.Lin YW, Chuang SM, Yang JL 2003. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem 2778:21534–21541 [DOI] [PubMed] [Google Scholar]
- 85.Steinmetz R, Wagoner HA, Zeng P, Hammond JR, Hannon TS, Meyers JL, Pescovitz OH 2004. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol Endocrinol 18:2570–2582 [DOI] [PubMed] [Google Scholar]
- 86.Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassan M, Cato A 2001. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J 20:7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei Q, Xia Y 2006. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem 281:21652–21659 [DOI] [PubMed] [Google Scholar]
- 88.Rosini P, De Chiara G, Bonini P, Lucibello M, Marcocci ME, Garaci E, Cozzolino F, Torcia M 2004. Nerve growth factor-dependent survival of CESS B cell line is mediated by increased expression and decreased degradation of MAPK phosphatase 1. J Biol Chem 279:14016–14023 [DOI] [PubMed] [Google Scholar]
- 89.Brondello JM, Pouyssegur J, McKenzie FR 1999. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286:2514–2517 [DOI] [PubMed] [Google Scholar]
- 90.Lin YW, Yang JL 2006. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem 281:915–926 [DOI] [PubMed] [Google Scholar]
- 91.Lin Y-W, Chuang S-M, Yang J-L 2003. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem 278:21534–21541 [DOI] [PubMed] [Google Scholar]
- 92.Marchetti S, Gimond C, Chambard J-C, Touboul T, Roux D, Pouyssegur J, Pages G 2005. Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol Cell Biol 25:854–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akiyama K, Yokota K, Kagawa S, Shimbara N, Tamura T, Akioka H, Nothwang H, Noda C, Tanaka K, Ichihara A 1994. cDNA cloning and interferon γ down-regulation of proteasomal subunits X and Y. Science 265:123–134 [DOI] [PubMed] [Google Scholar]
- 94.Berleth ES, Kasperek EM, Grill SP, Braunscheidel JA, Graziani LA, Pickart CM 1992. Inhibition of ubiquitin-protein ligase (E3) by mono- and bifunctional phenylarsenoxides. Evidence for essential vicinal thiols and a proximal nucleophile. J Biol Chem 267:16403–16411 [PubMed] [Google Scholar]
- 95.Sen A, Browning J, Inskeep EK, Lewis P, Flores JA 2004. Expression and activation of protein kinase C isozymes by prostaglandin F(2α) in the early- and mid-luteal phase bovine corpus luteum. Biol Reprod 70:379–384 [DOI] [PubMed] [Google Scholar]
- 96.Sen A, Choudhary E, Inskeep EK, Flores JA 2005. Effects of selective protein kinase C isozymes in prostaglandin 2α-induced Ca2+ signaling and luteinizing hormone-induced progesterone accumulation in the mid-phase bovine corpus luteum. Biol Reprod 72:976–984 [DOI] [PubMed] [Google Scholar]
- 97.Sen A, Wright M, Inskeep EK, Flores JA 2005. Participation of specific PKC isozymes in the inhibitory effect of ET-1 on progesterone accumulation in cells isolated from early- and mid-phase corpora lutea. Domest Anim Endocrinol 31:284–299 [DOI] [PubMed] [Google Scholar]
- 98.Li Q, Bakke LJ, Pursley JR, Smith GW 2004. Localization and temporal regulation of tissue inhibitors of metalloproteinases 3 and 4 in bovine preovulatory follicles. Reproduction 128:555–564 [DOI] [PubMed] [Google Scholar]