Abstract
Myostatin is a secreted TGF-β family member that controls skeletal muscle growth. Humans, cattle, and dogs carrying natural loss-of-function mutations in the myostatin gene and myostatin knockout mice exhibit significant increases in skeletal muscle mass. Treatment of adult mice with antimyostatin antibodies also resulted in significant muscle mass increases. However, myostatin-knockout mice that were treated with a soluble form of the activin type II receptor (ActRII) B increased their muscle mass by an additional 15–25%, indicating that there is at least one additional ligand, in addition to myostatin, that functions to limit muscle growth. Here, both soluble ActRII and -IIB fragment-crystallizable proteins were used to affinity purify their native ligands from human and mouse sera. Using mass spectrometry-based proteomics and in vitro binding assays we have identified and confirmed that a number of TGF-β family members, including myostatin, activins-A, -B, and -AB, bone morphogenetic proteins (BMPs) -9, -10, and -11, bind to both ActRIIs. Many of these factors, such as BMPs-11, -9, and -10 were discovered in systemic circulation for the first time, indicating that these ligands may also act in an endocrine fashion. Using a promoter-specific gene reporter assay, we demonstrated that soluble ActRIIB fragment-crystallizable proteins can inhibit the canonical signaling induced by these ligands. In addition, like myostatin, these factors were able to block the differentiation of myoblast cells into myotubes. However, in addition to myostatin, only BMP-11, and activins-A, -B, and -AB could be blocked from inhibiting the myoblast-to-myotube differentiation with both soluble ActRIIs, thus implicating them as potential novel regulators of muscle growth.
MYOSTATIN, ALSO KNOWN as growth differentiation factor 8 (GDF-8), is a TGF-β superfamily member that is known for its role in regulating muscle mass. Myostatin knockout mice exhibit significant increases in skeletal muscle mass (1), and loss-of-function myostatin gene mutations in cattle, dogs, and humans result in similar muscular phenotypes (2, 3, 4, 5). Conversely, overexpression of myostatin in mice induced cachexia (6). Homozygous myostatin knockout mice also displayed improved muscle healing through enhanced muscle regeneration and reduced fibrosis (7). Moreover, blockade of myostatin resulted in functional improvement of dystrophic muscle in the mdx mouse model of Duchenne Muscular Dystrophy (DMD) (8, 9). Thus, myostatin is an attractive therapeutic target for treating muscle-wasting diseases such as DMD, cachexia, and sarcopenia.
Multiple myostatin binding proteins have been shown to inhibit myostatin activity in vivo (8, 9, 10, 11, 12, 13, 14, 15, 16). These proteins, which include a monoclonal antibody, JA16, raised against myostatin (8, 15) and a mutant form of the myostatin propeptide (16), have been shown to be capable of increasing skeletal muscle mass by about 25% when administered to mice. Both JA16 and the myostatin propeptide have been shown to specifically bind myostatin circulating in the blood (11, 12), and thus, in both cases, the observed muscle increase was primarily due to the reduction of myostatin in circulation.
Myostatin most likely signals through both activin type II receptors (ActRII and ActRIIB) in vivo, because it has been shown to bind to both receptors in transfected COS cells (13, 17). In addition, mice carrying mutations in either ActRII or ActRIIB genes displayed significant increases in muscle mass, yet these increases were somewhat attenuated when compared with those of the myostatin knockout animals, suggesting a redundant role for the ActRIIs in respect to myostatin signaling (18). Not surprisingly, mice overexpressing a truncated form of ActRIIB, which resulted in loss of signal transduction, were also found to have marked skeletal muscle increases (13). Interestingly, Lee et al. (18) also demonstrated that wild-type mice treated with a soluble form of ActRIIB [ActRIIB-fragment crystallizable (Fc), the extracellular ligand-binding domain of ActRIIB fused to the human Fc] gained more muscle mass than mice treated with JA16 or the myostatin propeptide. Furthermore, treatment of myostatin knockout mice with ActRIIB-Fc, but not JA16, produced a further 15–25% increase in muscle mass in addition to the muscle gain that resulted from the deletion of myostatin (18). This clearly indicated the presence of at least one other ligand, in addition to myostatin, that could regulate muscle mass homeostasis.
Although, in vitro, ActRIIs have been shown to bind other TGF-β family members in addition to myostatin (19), it is not known whether these factors exist in systemic circulation and thus could potentially be neutralized by the administered ActRIIB-Fc leading to muscle mass increases. To find other natural factors that could act similarly to myostatin in controlling muscle growth, here, we combined affinity purification and mass spectrometry to identify natural ligands of ActRIIs circulating in mouse and human sera and investigated their potential roles in controlling the differentiation of C2C12 cells along the myogenic pathway.
RESULTS
ActRII-Fc and ActRIIB-Fc Pull Down Additional TGF-β Superfamily Members, in Addition to Myostatin, from Human and Mouse Sera
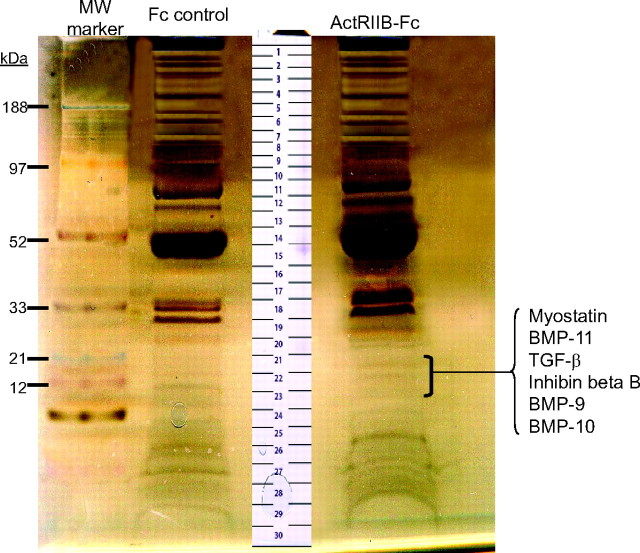
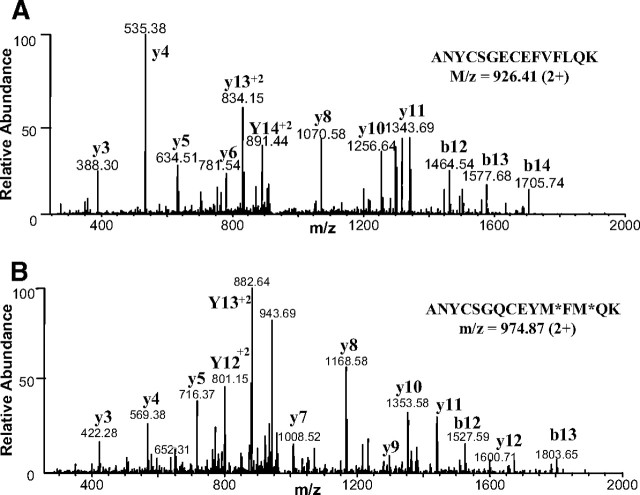
To identify candidate factors that regulate muscle growth in addition to myostatin, we used ActRIIB-Fc precoupled to agarose beads to capture its native ligands circulating in vivo in human and mouse sera. Fc-alone coupled to agarose beads was used as a control for nonspecific protein interactions. Captured proteins were eluted off the beads using sodium dodecyl sulfate (SDS) sample buffer and then separated by SDS-PAGE. A reducing silver-stained gel of proteins affinity-purified using ActRIIB-Fc or Fc-alone from mouse serum is shown in Fig. 1. Both control and experimental lanes were subjected to whole-gel proteomic analysis as described in Materials and Methods. Comparison between the experimental and the control samples allowed us to identify a number of proteins that bound to the ActRIIB-Fc coupled beads but not to the corresponding Fc-bead controls. Among the potential ActRIIB-Fc-binding proteins identified in the mouse serum, we found a number of members from the TGF-β family including myostatin, bone morphogenetic protein (BMP)-11, TGF-β, inhibin βB, BMP-9, and BMP-10 (Table 1). Peptides for these TGF-β family members were all identified from bands 21, 22, and 23 (Fig. 1) in the molecular mass range of ′12–15 kDa. This matched the theoretical molecular masses of the reduced mature chains of the identified ligands. Whereas protein bands within this gel region were only very lightly stained due to their low abundance, the same bands were not visible in the corresponding region of the Fc-control lane. For each of these TGF-β family members, multiple unique peptides were identified with high confidence, and none of these peptides were identified in the Fc-control sample. Two representative tandem mass spectrometry (MS/MS) spectra that uniquely identify the highly homologous proteins, myostatin and BMP-11, are shown in Fig. 2, A and B, respectively.
Fig. 1.
Affinity Purification of ActRIIB-Fc-Interacting Proteins from BALB/c Mouse Serum
A silver-stained reducing gel of proteins pulled down with ActRIIB-Fc (right lane) and with Fc-control protein (left lane) is shown. Both the experimental and control lanes were excised entirely into approximately 30-gel slices for subsequent comparative proteomics analysis. Gel slices containing factors from the TGF-β family are indicated. MW, Molecular weight.
Table 1.
TGF-β Family Members Identified in ActRIIB-Fc Pulldowns from Mouse and Human Sera
| GI No.1 | Protein Name | % Sequence Coverage2 | Sequence | z3 | Xcorr4 |
|---|---|---|---|---|---|
| Mouse serum | |||||
| 111494000 | Myostatin | 45 | (R)DFGLDCDEHSTESR | 2+ | 4.52 |
| (K)ANYCSGECEFVFLQK | 2+ | 4.49 | |||
| (K)M* SPINM* LYFNGK5 | 2+ | 3.37 | |||
| (K)GSAGPCCTPTK(M) | 2+ | 2.77 | |||
| (K)IPAM* VVDR | 2+ | 2.48 | |||
| 4160597 | BMP-11 | 67 | (R)NLGLDCDEHSSESR | 2+ | 4.72 |
| (K)ANYCSGQCEYM* FM* QK | 2+ | 4.58 | |||
| (K)M* SPINM* LYFNDK | 2+ | 3.54 | |||
| (K)YPHTHLVQQANPR | 3+ | 3.51 | |||
| (R)GSAGPCCTPTK | 2+ | 3.14 | |||
| (K)IPGMVVDR | 2+ | 2.18 | |||
| (K)IPGM* VVDR | 2+ | 2.11 | |||
| 50148 | Inhibin βB | 38 | (K)LSSM* SM* LYFDDEYNIVKR | 3+ | 2.94 |
| (R)GLNPGPVNSCCIPTK | 2+ | 2.90 | |||
| (R)GLNPGPVNSCC#IPTK6 | 2+ | 2.87 | |||
| (R)DVPNM* IVEECGCA | 2+ | 3.00 | |||
| 74184431 | TGF-β1 | 21 | (−)ALDTNYCFSSTEK | 2+ | 3.84 |
| (K)VEQLSNMIVR | 2+ | 3.43 | |||
| (K)VEQLSNM* IVR | 2+ | 3.07 | |||
| 5932440 | BMP-9 | 28 | (R)VNFEDIGWDSWIIAPK | 3+ | 4.09 |
| (K)GGCFFPLADDVTPTK | 2+ | 3.53 | |||
| 3873289 | BMP-10 | 29 | (K)LDPISILYLDK | 2+ | 2.78 |
| (K)YEGM* AVSECGCR | 2+ | 2.36 | |||
| (K)TPLYIDFK | 2+ | 2.35 | |||
| Human serum | |||||
| 4885259 | Myostatin | 39 | (K)ANYCSGECEFVFLQK | 2+ | 4.40 |
| DFGLDCDEHSTESR | 3+ | 2.60 | |||
| M* SPINMLYFNGK | 2+ | 2.70 | |||
| GSAGPCCTPTK | 2+ | 2.40 | |||
| 6649914 | BMP-11 | 35 | (K)M* SPINM* LYFNDK | 2+ | 2.40 |
| (R)GSAGPCCTPTK | 2+ | 2.40 | |||
| (R)RNLGLDCDEHSSESR | 3+ | 2.60 | |||
| 186417 | Inhibin βB | 39 | (R)GLNPGTVNSCCIPTK | 2+ | 3.30 |
| (R)DVPNM* IVEECGCA | 2+ | 2.20 | |||
| (K)LSTM* SM* LYFDDEYNIVK | 2+ | 4.70 | |||
| 755740 | Inhibin βA | 24 | (K)DIQNM* IVEECGCS | 2+ | 3.84 |
| (K)QFFVSFK | 2+ | 2.30 | |||
| (K)KQFFVSFK | 2+ | 3.10 | |||
| (K)SCCVPTK | 2+ | 2.04 | |||
| 22761274 | Inhibin βE | 38 | (K)ANNPWPASTSCCVPTAR | 2+ | 3.50 |
| (K)TDVPDM* VVEACGCS | 2+ | 2.80 | |||
| (R)TPTCEPATPLCCR | 2+ | 3.60 | |||
| 5031795 | Inhibin βC | 61 | (R)AGGQCPACGGPTLELESQR | 2+ | 5.20 |
| (K)ANTAAGTTGGGSCCVPTAR | 2+ | 5.00 | |||
| (R)GIDCQGGSR | 2+ | 2.90 | |||
| (R)RPLSLLYYDRDSNIVK | 3+ | 3.10 | |||
| (R)QEFFVDFR | 2+ | 2.10 | |||
| 67463993 | BMP-97 | 14 | (K)GGCFFPLADDVTPTK | 2+ | 4.90 |
| 75516754 | BMP-10 | 35 | (K)ACCVPTKLEPISILYLDK | 3+ | 4.15 |
| (K)LEPISILYLDK | 2+ | 2.84 | |||
| (R)TPLYIDFK | 2+ | 2.66 | |||
| (K)YEGM* AVSECGCR | 2+ | 3.30 |
GI No., GenInfo Identifier, used to access protein sequence records in NCBI.
Percent sequence coverage represents the percent of the protein amino acid sequence that can be mapped by the identified peptides from MS.
z is the charged state of the parent peptide ion.
Xcorr is the Sequest search cross-correlation value. It increases with peptide length and charge.
M*, Methionine oxidation.
C#, Iodoacetamide modification of cysteine.
Annotated fragment ion spectrum of this peptide is shown in Supplemental Fig. 1a.
Fig. 2.
Representative Fragment Ion Spectra of Identified Peptides from Myostatin and BMP-11
MS/MS spectra of similar tryptic peptides unique to myostatin (panel A) and BMP-11 (panel B) were obtained from the ActRIIB-Fc pulldown from mouse serum. m/z, Mass to charge ratio.
To test whether ActRIIB-Fc could also pull down ligands from human peripheral blood, we performed a similar study using pooled normal human serum as the source of native proteins. Our initial attempt using 15 ml of human serum identified only one myostatin peptide and one BMP-11 peptide with very low Xcorr values (data not shown). However, when we incubated ActRIIB-Fc-coupled beads with 160 ml of pooled human serum, all of the previously identified TGF-β family members from the mouse serum, except TGF-β, were positively identified in the ActRIIB-Fc pull-down sample but not in the Fc-control sample (Table 1). In addition to these ligands, we also identified three other inhibin β family members, βA, βC, and βE. Thus, from both human and mouse sera, we were able to uncover native interactions between ActRIIB and several members of the TGF-β family including myostatin, and for the first time showed that BMP-9, BMP-10, and BMP-11 exist in the peripheral circulation.
Because myostatin can bind to both ActRIIB and ActRII in transfected COS cells (13, 17), and both the ActRIIB and the ActRII knockout mice result in significant skeletal muscle increases (18), we performed a similar pull-down study from human and mouse sera using ActRII-Fc as the bait. The TGF-β family members identified from mouse serum are summarized in Table 2. In comparison with the ActRIIB-Fc study, myostatin, BMP-11, and inhibin β isomers were also positively identified, but TGF-β, BMP-9, and BMP-10 were not. Interestingly, inhibin α as well as the myostatin propeptide were identified in the ActRII-Fc pulldown but were not present in the previous ActRIIB-Fc pulldowns. On the other hand, from the human serum, we were able to pull down BMP-11, BMP-10, and the inhibins βC and βE but not myostatin or BMP-9 using ActRII-Fc (Table 2). This indicates that ActRIIB and ActRII may have different binding specificities or affinities toward these natural ligands and also suggests that the abundance of these ligands is very low in the human serum. Thus, we were able to successfully pull down and identify several members of the TGF-β family from human and mouse sera using ActRII- and ActRIIB-Fc.
Table 2.
TGF-β Family Members Identified in ActRII-Fc Pulldowns from Mouse and Human Sera
| GI No.1 | Protein Name | % Sequence Coverage2 | Sequence | z3 | Xcorr4 |
|---|---|---|---|---|---|
| Mouse serum | |||||
| 111494000 | Myostatin (mature) | 49 | (R)YPLTVDFEAFGWDWIIAPK | 2+ | 4.83 |
| (K)ANYCSGECEFVFLQK | 2+ | 4.55 | |||
| (K)MSPINMLYFNGK | 2+ | 3.61 | |||
| (K)IPAMVVDR | 2+ | 2.47 | |||
| (K)GSAGPCCTPTK | 2+ | 2.30 | |||
| 111494000 | Myostatin (propeptide) | 24 | (K)ALDENGHDLAVTFPGPGED-GLNPFLEVK | 3+ | 4.57 |
| (K)QPESNLGIEIK | 2+ | 2.31 | |||
| (K)TPTTVFVQILR | 2+ | 3.29 | |||
| (K)TVLQNWLK | 2+ | 2.54 | |||
| 296838 | Inhibin α | 40 | (R)LLQRPPEEPAAHAFCHR | 3+ | 3.90 |
| (K)PCCAALPGSMR | 2+ | 3.30 | |||
| (R)STPSVPWPWSPAALR | 2+ | 2.60 | |||
| (R)TTSDGGYSFK | 2+ | 2.60 | |||
| 4160597 | BMP-11 | 28 | (R)YPLTVDFEAFGWDWIIAPK | 2+ | 4.83 |
| (K)GSAGPCCTPTK | 2+ | 2.30 | |||
| 50148 | Inhibin βB | 23 | (R)GLNPGPVNSCCIPTK | 2+ | 3.27 |
| (R)DVPNM* IVEECGCA5 | 2+ | 2.93 | |||
| 14714539 | Inhibin βE | 54 | (R)RPLSLLYLDHNGNVVK | 3+ | 5.31 |
| (K)ANNPWPAGSSCCVPTAR | 2+ | 3.36 | |||
| (R)RTPTCEPETPLCCR | 3+ | 3.34 | |||
| (K)TDVPDM* VVEACGCS | 2+ | 3.30 | |||
| (R)TPTCEPETPLCCR | 2+ | 3.29 | |||
| 74203474 | Inhibin βC | 28 | (R)GSCCVPTSR | 2+ | 2.68 |
| (R)GIDCQGASR | 2+ | 2.63 | |||
| (K)TDIPDM* VVEACGCS | 2+ | 2.53 | |||
| Human serum | |||||
| 6649914 | BMP-116 | 14 | (R)RNLGLDCDEHSSESR | 3+ | 2.58 |
| 75516754 | BMP-106 | 7 | (R)TPLYIDFK | 2+ | 2.68 |
| 5031795 | Inhibin βC | 24 | (R)AGGQCPACGGPTLELESQR | 2+ | 4.63 |
| (R)GIDCQGGSR | 2+ | 2.34 | |||
| 22761274 | Inhibin βE | 36 | (K)ANNPWPASTSCCVPTAR | 2+ | 3.47 |
| (R)DHYVDFQELGWR | 2+ | 3.60 | |||
| (R)TPTCEPATPLCCR | 2+ | 3.61 |
GI No., GenInfo Identifier used to access protein sequence records in NCBI.
Percent sequence coverage represents the percent of the protein amino acid sequence that can be mapped by the identified peptides from MS.
z is the charged state of the parent peptide ion.
Xcorris the Sequest search cross-correlation value. It increases with peptide length and charge.
M*, Methionine oxidation.
Annotated fragment ion spectra of these peptides are shown in Supplemental Fig. 1b (BMP-10) and 1c (BMP-11).
Specific Binding between Identified Ligands and ActRII-Fc Proteins in Vitro
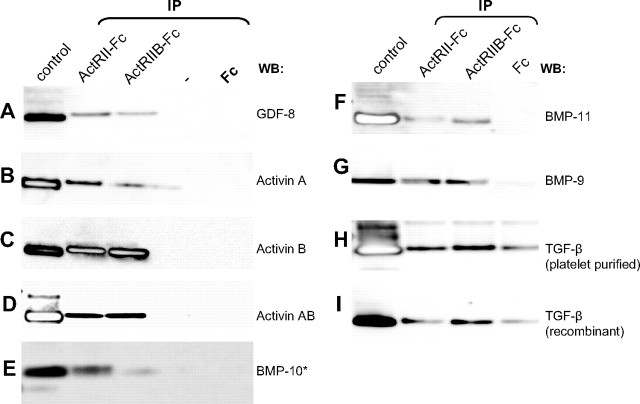
After successfully identifying a number of native TGF-β family members that were bound to the activin receptor-Fc proteins from both mouse and human sera, we performed a series of in vitro affinity purification studies followed by Western blot (WB) to demonstrate that the ligand-receptor binding was due to direct and specific interactions. Figure 3 clearly shows that purified myostatin, BMP-9, -10, and -11 all bind to both ActRIIB-Fc and ActRII-Fc but not to the Fc-control proteins, indicating that the binding is specific for both receptors. In addition to these growth factors, several inhibin β-chains were identified in our pulldowns from serum. Inhibin β-chains are known to exist as either homo- or heterodimers (activins) in vivo (20); therefore, we tested the binding between the receptors and the activin ligands that have been shown to be biologically active: activin A, activin B, and activin AB (21). Again, all activins bound both ActRIIB-Fc and ActRII-Fc but not the Fc-control protein (Fig. 3). On the other hand, inhibins are heterodimers consisting of an inhibin α- and an inhibin β-chain (20), and both the α- and β-chains were detected in the pulldowns with ActRII-Fc. Unfortunately, we did not test for specific binding between the activin receptors and inhibin because we could not obtain the recombinant protein from any commercial source. Finally, two versions of the TGF-β protein (recombinant or platelet purified) bound to both the activin receptor-Fc molecules and the Fc-control protein. To ensure that the TGF-β proteins we used in our assays were properly folded and active, we treated serum-starved C2C12 cells with the two versions of TGF-β protein for 1 h and assayed for Sma- and Mad-related protein (SMAD)2 phosphorylation using WB (22). For both versions of TGF-β protein, the treatment resulted in SMAD2 phosphorylation (data not shown), revealing that these proteins were active and properly folded. This implied that the binding between TGF-β and the activin receptor-Fc molecules could be nonspecific. Such a notion is also consistent with the fact that TGF-β was only found once in the ActRIIB-Fc pulldown from mouse serum but not in any other samples. Thus, with in vitro binding assays we confirmed that there was specific binding between ActRII- or ActRIIB-Fc’s and the ligands: myostatin, BMP-9, -10, and -11, and activins A, AB, and B.
Fig. 3.
Confirmation of Specific and Direct Binding between ActRII-Fc Receptors and the Identified Ligands
Each recombinant growth factor (250 ng) was spiked into 1% BSA in PBS and pulled down with ActRII-Fc, ActRIIB-Fc, or Fc-control proteins precoupled to agarose beads. Eluted proteins were run on a reducing SDS-PAGE gel and immunoblotted with factor-specific antibodies (except for BMP-10, which was detected by silver stain). A–G, Recombinant myostatin, activins A, B, and AB, and BMP-9, -10, and -11 were all specifically purified by both ActRII and -IIB-Fc’s, whereas (H–I) TGF-β nonspecifically interacted with the Fc-control protein as well as the receptor-Fc’s. Control lanes contain 250 ng of each recombinant factor. IP, Immunoprecipitation.
ActRIIB-Fc Can Neutralize the Signaling Activity of the Ligands in A204 and C2C12 Cells
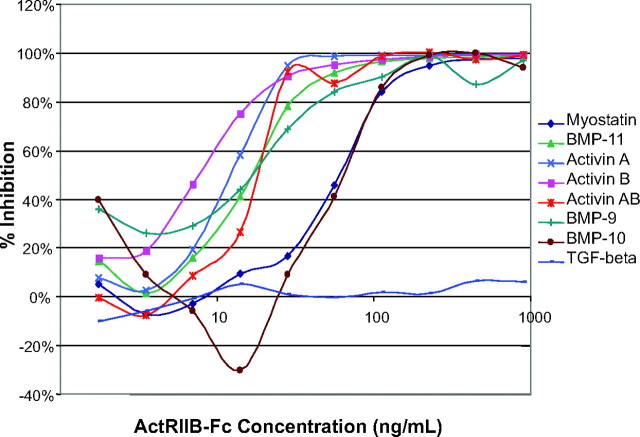
It is known that myostatin can activate the Smad-dependent signaling in A204 and myoblast C2C12 cells and that this activation can be inhibited by multiple myostatin-binding proteins (14). We used these assays to test whether ActRIIB-Fc can neutralize the functional activity of these ligands by inhibiting their signaling. To this end, we performed reporter gene assays using the (CAGA)12 and the (BRE)2 reporter constructs (23, 24), which get turned on in response to Smad2,3 and Smad1,5,8 activation, respectively. As expected, the identified ligands all activated Smad signaling in a dose-dependent manner (data not shown), and all of the ligands, with the exception of TGF-β, could be effectively inhibited from signaling by ActRIIB-Fc (Fig. 4). Furthermore, this inhibition was specific for ActRIIB-Fc and was not observed when the Fc-control protein was used (data not shown). Thus, this result demonstrated that ActRIIB-Fc could functionally block the activity of myostatin and the novel candidates for regulating muscle mass homeostasis, which include BMP-9, -10, and -11, activins A, AB, and B, but not TGF-β.
Fig. 4.
ActRIIB-Fc Can Neutralize Smad Signaling Activity of All Ligands except TGF-β
Using reporter gene constructs that monitor either Smad2,3 [pGL3-(CAGA)12] or Smad1,5,8 (pBRE-Luc9) signaling, ActRIIB-Fc was able to inhibit ligand-mediated signaling in a dose-dependent manner for all ligands with the exception of TGF-β. Combined inhibition curves demonstrate ActRIIB-Fc’s potential to neutralize Smad signaling. To activate the reporters, the ligands were added at their EC50 concentrations: GDF-8, 30 ng/ml; GDF-11, 11 ng/ml; TGFβ, 0.35 ng/ml; activin A, 11 ng/ml; activin B, 5 ng/ml; activin AB, 11 ng/ml; BMP-9, 1 ng/ml; BMP-10, 30 ng/ml.
Myotube Differentiation of C2C12 Cells Can Be Inhibited by the Identified Ligands and ActRII-Fc’s Neutralize this Effect
C2C12 cells are mesenchymal stem cells (myoblasts) that can terminally differentiate into mature myotubes when cultured in DM (2% horse serum) for 3–4 d (Fig. 5A), and such differentiation can be inhibited by myostatin (Fig. 5B, top left panel) (25, 26, 27, 28). To determine whether any of the other identified ligands had similar effects on muscle cell differentiation, we cultured C2C12 cells in DM for 4 d in the presence of the identified factors and examined the cell morphology using phase-contrast microscopy (Fig. 5B, left panel). Similarly to myostatin, all of the factors could inhibit the differentiation of C2C12 cells into myotubes at both 8 μg/ml (data not shown) and 4 μg/ml doses. Additionally, we observed that the cells treated with BMP-9 and -10 underwent a similar morphological change (Fig. 5B, left panel) that was distinct from the cells treated with the other ligands. Because the osteogenic activity of BMP-9 has been well documented (29, 30, 31), we compared the effects of BMP-9 and -10 to that of BMP-2, another TGF-β family member well known for its osteogenic properties and reported to inhibit the terminal differentiation of myogenic cells (32, 33, 34). As expected, BMP-2 also blocked the differentiation of C2C12 cells (Fig. 5B, left panel). Moreover, the cells treated with BMP-2 underwent a very similar morphological change as observed upon treatment with BMP-9 and -10.
Fig. 5.
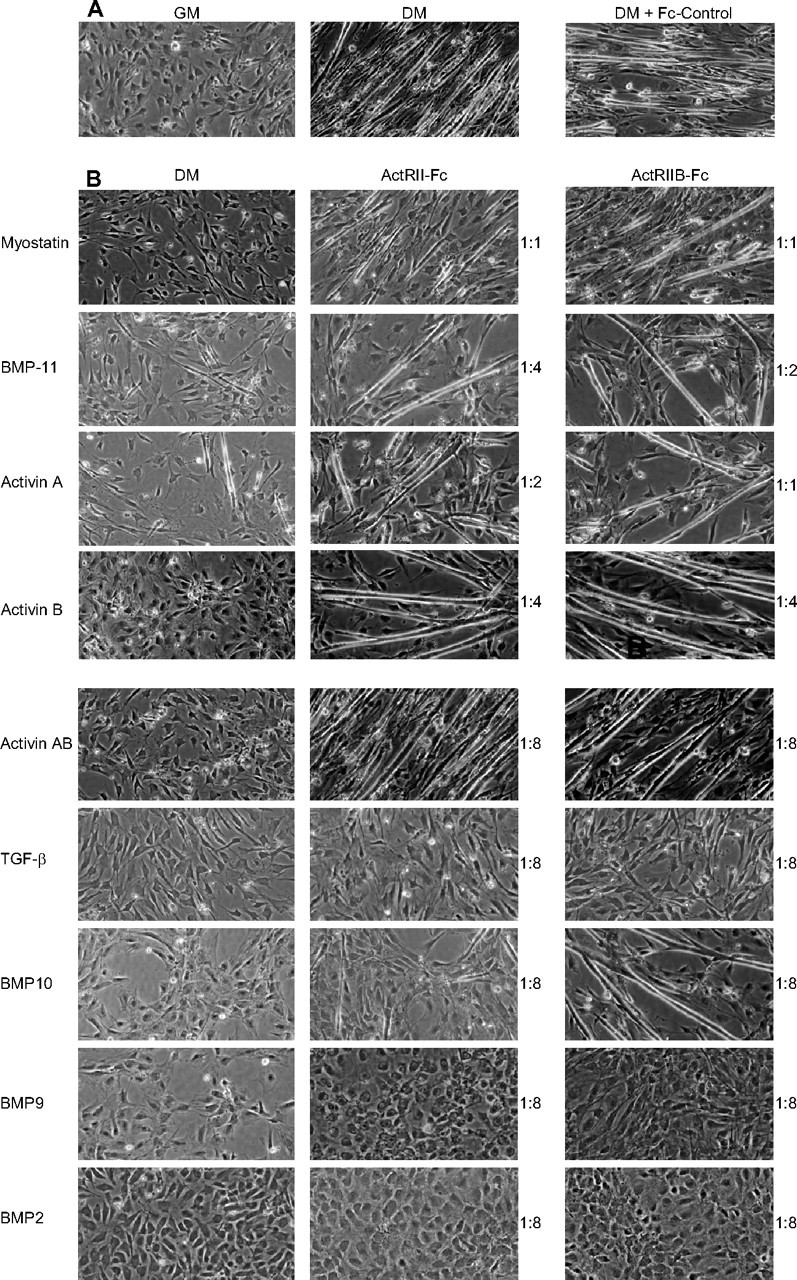
Identified Ligands Inhibit C2C12 Cell Differentiation, and this Inhibition Is Neutralized by ActRII/IIB-Fc’s
A, C2C12 cells differentiate into long myotubes after culture in DM for 96 h, and such a process was not affected by the addition of the Fc-control protein (8:1 molar ratio). However, (B) all identified ligands, including BMP-2, added at 4 μg/ml, could inhibit the differentiation of C2C12 cells into myotubes (left panel). Adding either ActRIIB-Fc (right panel) or ActRII-Fc (middle panel) before ligand treatment could effectively neutralize the inhibition effect of myostatin, BMP-11, activin A, activin B, activin AB, but not of TGF-β, BMP-9, and BMP-2. Inhibition by BMP-10 could be attenuated by ActRIIB-Fc but only partially by ActRII-Fc at the highest concentration tested (molar ratio of 8:1). Minimum molar ratios of ligand-receptor-Fc proteins necessary for the inhibition are shown to the right of the panels.
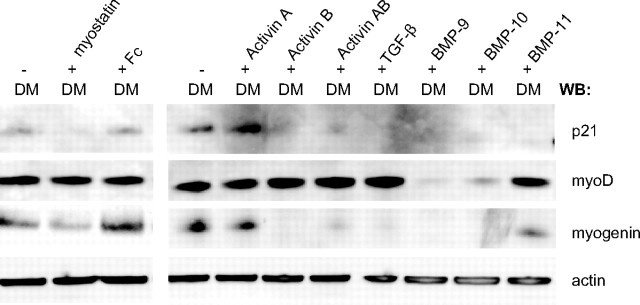
To determine whether the ligands inhibit myogenesis by a mechanism similar to myostatin, we looked at the expression levels of key proteins necessary for myotube differentiation. Myogenic differentiation (MyoD) and myogenin are myogenic regulatory factors that are required for differentiation of myoblasts into mature myotubes (35, 36, 37). MyoD is an early marker that determines the myogenic lineage, whereas myogenin is necessary for the terminal differentiation into myotubes and is activated upon differentiation (38). In addition, cyclin kinase inhibitor p21 is up-regulated to promote differentiation by removing cells from the cell cycle (hold proliferation) (39). Thus, during terminal differentiation of myoblasts, myogenin and p21 protein levels increase, and this increase can be blocked by myostatin (26). As shown in Fig. 6, levels of both myogenin and p21 were significantly lower after treatment with all the ligands except for activin A, which only slightly down-regulated both myogenin and p21. MyoD expression level was not affected by myostatin, BMP-11, TGF-β, and the activins but was highly down-regulated in response to BMP-9 and -10 treatments, indicating that BMP-9 and -10 have driven the C2C12 cells away from the myogenic lineage. This action of BMP-9 and -10 is, again, very similar to that of BMP-2, which also inhibits myogenic differentiation by suppressing both MyoD and myogenin protein expression (34).
Fig. 6.
Expression of MyoD, Myogenin, and p21 in Response to Ligand Treatment
C2C12 cells were cultured in DM (2% horse serum) for 96 h in the presence (+) or absence (−) of indicated ligands, added at 8 μg/ml. Cell lysates were run on a reducing SDS-PAGE gel and immunoblotted for p21, myoD, myogenin, and β-actin proteins. Both myogenin and p21 were down-regulated by the treatment of all ligands, with the exception of activin A. In contrast, the levels of MyoD were down-regulated only when treated with BMP-9 and -10.
Because ActRIIB-Fc treatment of myostatin knockout mice resulted in additional muscle mass increase, it was important to determine whether ActRII-Fc’s could neutralize the blocking effect of these ligands on myoblast differentiation. Although all ligands could inhibit the differentiation of C2C12 cells into myotubes, only the effects of myostatin, BMP-11, and activins A, B, and AB could be neutralized by ActRII-Fc proteins (Fig. 5B, middle and right panels). At a higher receptor-ligand ratio (8:1), inhibition by BMP-10 could be neutralized with ActRIIB-Fc but only partially with ActRII-Fc; and inhibition by TGF-β, BMP-9, or BMP-2 could not be neutralized with either ActRIIB-Fc or ActRII-Fc. This again suggested that the interactions between ActRII-Fc’s and TGF-β were not specific and that BMP-9 and -10 function similarly to BMP-2 (40).
BMP-9 and -10 Elevate Alkaline Phosphatase (AP) Activity in C2C12 Cells
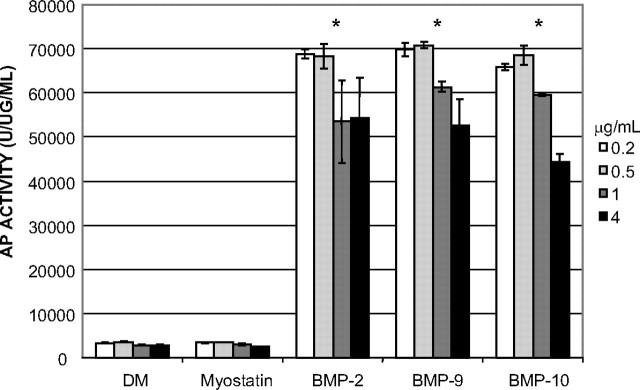
To further confirm whether BMP-9 and -10 act similarly to BMP-2 and drive the C2C12 cells away from the myogenic lineage and toward the osteogenic pathway, we assayed for AP activity, an early marker of osteogenic differentiation (reviewed in Ref. 41). Because it is known that BMP-2 can induce osteoblast differentiation and AP activity in C2C12 cells (33), we cultured C2C12 cells in DM for 4 d in the presence of myostatin, BMP-9, -10, or -2 and examined levels of AP activity in the cell lysates (Fig. 7). As expected, no increase in AP activity was observed after myostatin treatment. However, treatment with BMP-9, -10, or -2 resulted in significant increase in AP activity even at low doses (0.2 and 0.5 μg/ml). This further demonstrated that, like BMP-2, BMP-9 and BMP-10 can divert C2C12 cells away from the myogenic pathway and into the osteogenic lineage.
Fig. 7.
BMP-9 and -10 Induce AP Activity during C2C12 Cell Differentiation
C2C12 cells were cultured in DM (2% horse serum) for 96 h alone or in the presence of myostatin, BMP-2, -9, or -10 at the indicated doses (white bars , 0.2 μg/ml; light gray bars, 0.5 μg/ml; dark gray bars, 1 μg/ml; black bars, 4 μg/ml). AP activity was measured as units of fluorescence released divided by the protein concentration in each well. Error bars indicate sd in the three replicates. Treatment with either BMP-9 (P < 0.001, Student’s t test) or BMP-10 (P = 0.002, Student’s t test), but not with myostatin, resulted in a significant increase of AP activity, which resembled that of cells treated with BMP-2 (P < 0.001, Student’s t test).
DISCUSSION
Using affinity purification and mass spectrometry, we have successfully identified a number of natural TGF-β family ligands, in addition to myostatin, that bound to both ActRIIs in human and mouse sera. These ligands include BMP-11 and activins A, B, and AB, which have all been previously shown to signal through both ActRIIs (19), two BMP family members BMP-9 and BMP-10, as well as TGF-β1 and inhibin. Not all the ligands were identified in each pull-down sample. These discrepancies could be attributed to the biological differences between ligands in the human and mouse species or the different binding affinities of ActRIIB and ActRII. On the other hand, because the concentration of these ligands in human serum is in the single digit ng/ml level (11, 12, 42, 43), the stochastic nature of the MS/MS data acquisition may miss the detection of a given peptide that is low in abundance in a complex mixture.
Because we had to use 10 times more human serum than mouse serum to capture enough of each ligand to enable mass spectrometric identification, this indicates that the concentration of these ligands in human serum is much lower than in mouse serum. It is not known whether these concentration differences represent mechanistic changes between mouse and human species in regulating the signaling of these factors. However, because some of these ligands including myostatin are potentially useful therapeutic targets for treating muscular disorders, this between-species concentration difference will certainly need to be considered when designing proper dosing regimens for humans.
Several of the ligands, including BMP-9, -10, and -11, were not previously known to exist in systemic circulation. Thus our results suggest that these proteins may also act in an endocrine fashion, even though many of these ligands are only expressed in very selective tissues; for example, BMP-10 was shown to be expressed exclusively in the heart (44). To test whether any of these ligands were enriched in muscle tissue, we also performed an affinity purification study using ActRIIB-Fc as the bait using 20 g of BALB/c mouse muscle extracts and subjected the eluted samples to the same kind of mass spectrometry-based proteomic analysis. We were able to find only a trace signal of one myostatin-derived peptide (data not shown). Although this lack of detection could have been due to the increased complexity of the muscle tissue sample, it could also imply that these ligands, including myostatin, may not be enriched in the muscle tissue, which is contrary to what one might originally expect based on the fact that myostatin is almost exclusively expressed in muscle (1).
Mice administered with ActRIIB-Fc gained nearly twice as much muscle mass as those treated with antimyostatin monoclonal antibodies (18). Previously, we have used an antimyostatin monoclonal antibody (JA16) to immunoprecipitate its binding targets, and only myostatin and several of its binding proteins were found (11, 12). Thus, in theory, all the other ligands identified here in addition to myostatin could contribute to the extra muscle mass observed in the mice treated with ActRIIB-Fc. Although TGF-β was positively identified in the ActRIIB-Fc pulldown from mouse serum, it was not found in the pulldown from human serum or in the ActRII-Fc pulldown from mouse serum. In addition, in vitro pull-down studies demonstrated that TGF-β1 could bind to both the receptors-Fc’s and the Fc-control protein without any specificity. This indicates that the identification of TGF-β in the affinity mass spectrometry experiment was a result of nonspecific binding between mouse TGF-β and ActRIIB-Fc. This hypothesis was further substantiated by the fact that at the highest concentration of receptor-Fc’s tested, neither ActRIIB-Fc nor ActRII-Fc could block TGF-β1 signaling or the TGF-β1-induced inhibition of C2C12 cell differentiation. Therefore, although TGF-β1 is a well-known inhibitor of myocyte terminal differentiation in vitro (45, 46, 47), it is unlikely that the additional muscle mass increase was due to the sequestration of TGF-β1 by ActRIIB-Fc.
Both BMP-9 and -10 were shown to bind specifically to ActRIIB and ActRII and to drive C2C12 cells away from the myogenic pathway into the osteoblast lineage. The depletion of circulating BMP-9 and -10 by ActRIIB-Fc may indirectly contribute to the increase in muscle mass by maintaining a higher number of myogenic stem cells (satellite cells) in the skeletal muscle. However, at high concentrations (receptor-ligand molar ratio of 8:1), ActRIIB-Fc or ActRII-Fc could barely block the effects of BMP-9 and -10 on C2C12 cells in vitro. This is likely due to the signaling of BMP-9 and -10 through their high-affinity type II receptor, the BMP type II receptor, rather than that of ActRII (48, 49), similar to BMP-2. Thus, BMP-9 and BMP-10 are not likely responsible for the muscle phenotype resulting from ActRIIB-Fc treatment.
The remaining four identified ligands, BMP-11 and activins A, B, and AB, all specifically bound to both ActRIIs and exhibited inhibitory activities on myocyte differentiation in vitro very similar to myostatin. More importantly, their inhibitory effect on C2C12 cell differentiation as well as their activation of canonical smad signaling [in the (CAGA)12 gene reporter assay] were all effectively neutralized by ActRIIB-Fc and ActRII-Fc. Noncanonical signaling has also been implicated in the mechanism of myostatin action. The pathways involved are still being elucidated and thus were not tested here (reviewed in Ref. 50). Further work will certainly be necessary to determine whether these potential muscle regulators also use similar noncanonical signaling mechanisms to potentiate their effects.
BMP-11 is the most closely related member to myostatin in the TGF-β family (only eight residues are different in their mature regions). In addition, BMP-11 is known to act as an inhibitory factor in the neurogenesis of the olfactory epithelium (51) and in the nerve growth factor-induced differentiation of PC12 cells (52), which is reminiscent of myostatin’s role in regulating muscle growth. BMP-11 has also been shown to be a negative regulator of myogenesis in the developing chick limb (53). Discovering that native BMP-11 is circulating in the blood and could bind to the administered ActRIIB-Fc specifically provides further evidence that BMP-11 may contribute to the control of normal muscle growth together with myostatin. Similarly, activin A has also been shown to inhibit the formation of skeletal muscle during chick development (54) and the development of muscle in primary cell culture (55). Very recently, Lee (56) reported that myostatin knockout mice carrying a follistatin transgene had about 4 times the muscle mass of wild-type mice, again indicating the existence of other regulators of muscle mass with similar activity to myostatin. Follistatin is well known for binding activins, thus regulating their activities (57, 58, 59). In vitro, follistatin has also been shown to bind to both myostatin and BMP-11 with high affinities and to antagonize their activities in vivo (6, 60, 61). Thus, combining our findings together with the existing data in the literature, we hypothesize that BMP-11 and activins A, B, and AB may all work together with myostatin to limit the muscle growth in vivo in a higher capacity than myostatin does alone. Therefore, neutralizing these ligands with ActRIIB-Fc or follistatin resulted in a greater muscle mass increase than inhibiting myostatin alone.
Our study provides a short list of candidate proteins that could be novel regulators of skeletal muscle mass in addition to myostatin. However, further work is necessary to elucidate which, if any, of these ActRII ligands could negatively regulate muscle mass in vivo. Future experiments, such as in vivo pharmacology studies, with monoclonal antibodies specific for each ligand combined with conditional knockout animals of these ligands are currently underway. This information will help guide the design of better therapeutic agents that can simultaneously target multiple muscle growth regulators to achieve the highest therapeutic benefit for patients with muscle disorders such as DMD, cachexia, and sarcopenia.
MATERIALS AND METHODS
Recombinant Proteins and Antibodies
The recombinant ActRIIB-Fc was expressed and purified using previously described methods (18). Recombinant human myostatin was purified from Chinese hamster ovary cell conditioned media as described previously (14). ActRII-Fc and all other recombinant TGF-β family cytokines used here were purchased from R&D Systems (Minneapolis, MN). JA16 mouse monoclonal antibody (mAb) was generated as described elsewhere (11) and was used to detect myostatin and BMP-11 on WB. Anti-BMP9 mouse mAb was generated in house. Anti-TGF-β mAb [56E4] was purchased from Cell Signaling Technology, Inc. (Danvers, MA). Activin A was detected with mouse anti-inhibin βA [E4] mAb purchased from Abcam, Inc. (Cambridge, MA). Activin B and activin AB were detected with mouse antihuman activin B [146807] mAb purchased from R&D Systems. Antiactin [sc-1616] and anti-myogenin [sc-576] antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-MyoD and anti-p21 antibodies were purchased from BD Biosciences (Palo Alto, CA).
Affinity Purification
ActRIIB-Fc, ActRII-Fc, or the Fc-control protein (1 mg) was covalently coupled to 100 μl of agarose beads (Sigma H-8635; Sigma Chemical Co., St. Louis, MO) as described previously (11). These preloaded beads were incubated with 15 ml of pooled wild-type BALB/c mouse serum (The Jackson Laboratory, Bar Harbor, ME) or 160 ml of pooled human serum (ICN Biomedicals, Inc., Aurora, OH), separately, overnight at 4 C. The beads were washed twice with cold 1% Triton in PBS and then twice with cold PBS only. The bound proteins were then stripped off the beads using 80 μl of 2× Tris-glycine SDS buffer (Invitrogen, Carlsbad, CA) followed by another 80 μl of 1× SDS buffer. Both fractions were combined for subsequent analysis.
Mass Spectrometry
The eluted protein samples were reduced by 10 mm dithiothreitol for 30 min at 90 C and alkylated with iodoacetamide (at 2.2 times the molar ratio of dithiothreitol used for reduction) for 30 min at room temperature in the dark. The proteins were separated immediately on 12% Tris-glycine five-well gels (Invitrogen) and silver stained using a glutaraldehyde-free system (62). To preserve the resolution of the gel separation, each entire sample lane was excised into approximately 30 gel slices of similar size such that the high-intensity bands were isolated in their own slices and were not present in slices containing the low-intensity bands or the blank areas. Each gel slice was further cut into 1 × 1-mm2 pieces and subjected to robotic in-gel digestion by trypsin as previously described (11). The resultant peptides were loaded onto a PicoFrit column (New Objective) packed with reverse-phase Magic C18 beads (5 μm, 200 Å, 75 μm × 10 cm) and coupled with a linear ion trap mass spectrometer [(LTQ, Thermo Finnigan, San Jose, CA)]. Peptides were separated at a flow rate of 0.2 μl/min using a 90-min linear gradient from 2%–55% solvent B (solvent A, 2% acetonitrile/0.1% formic acid; solvent B, 90% acetonitrile/0.1% formic acid). The MS/MS data were acquired using an MS data-dependent mode in which each full scan (375–1200 mass-to-charge ratio) was followed by four MS/MS scans of the top four most abundant ions detected in the immediate prior MS scan. The peak lists were generated using ExtractMSN version 3 using the following parameters: number of ions, 40 or greater; total ion current, 20 or greater, MH+ range from 750-3600 daltons; no grouping. Proteins were identified by searching the MS/MS spectra using Sequest version 27 (rev.13) from Thermo Finnigan against an National Center for Biotechnology Information nonredundant protein database appended with its corresponding decoy database consisting of fully inverted protein sequences. For samples from human serum a database containing only human proteins was used (updated on October 25, 2006, consisting of 153,068 proteins); for samples from mouse serum a database containing human, mouse, and rat proteins was used (updated on October 25, 2006, consisting of 299,095 proteins). The search parameters used were: enzyme, trypsin (KR/P); precursor ion mass tolerance, 2.5 Da; fragment ion mass tolerance, 1.0 Da; static modification, carboxymethylation (+57.02) for Cys; differential modifications, methionine oxidation (+16) and acrylamide modification for Cys (+14); allowed enzyme missed cleavages, 2. If peptides matched to multiple members of a protein family, the protein chosen had to contain the highest number of matching peptides; otherwise, if peptide numbers were equal, then the protein selected had the highest accession number. After applying charge-dependent Xcorr filtering criteria, the false discovery rate (FDR) was calculated using the formula: FDR = 2 × Ndecoy/ Ntotal, where Ndecoy is the number of hits against the decoy database, and Ntotal is the number of hits against the entire database (original plus its decoy database). The Xcorr cutoff value for each charge state was adjusted so that the FDR for each charge state was similar to the overall FDR. Identified peptides with less than 5.1% FDR were included in the final analysis. Fragment ion spectra of peptides for proteins identified with less than three unique peptides were also manually validated, and annotated fragment ion spectra of peptides for single-peptide-based protein identifications are shown in supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org. Finally, all sample analyses have been repeated at least once.
In Vitro Binding Assay
Agarose beads were coupled with ActRII-Fc, ActRIIB-Fc, or Fc-control proteins as described above with the exception that 100 μg protein/100 μl of resin was used. The coupled beads were incubated with 250 ng of exogenous protein ligands in 1 ml 1% BSA in PBS for 4 h at 4 C. The beads were washed, and the bound proteins were eluted as described above. After reduction with NuPage 10X reducing agent (Invitrogen), the samples were run on a SDS-PAGE gel (Invitrogen), transferred to a polyvinylidine difluoride membrane (Invitrogen), and immunoblotted with their corresponding primary antibodies: JA-16 (1:200), anti-BMP9 (1:200), anti-inhibin βA (1:200), antihuman activin B (1:500), anti-TGF-β (1:1000) and detected by chemiluminescence (Pierce Chemical Co., Rockford, IL). BMP-10 was visualized on SDS-PAGE gel using silver stain because no suitable antibody against BMP-10 was available.
Myoblast Differentiation Assay
C2C12 myoblasts (American Type Culture Collection, Manassas, VA) were cultured in growth medium consisting of DMEM, modified to contain 4.5 g/liter glucose, 1.5 g/liter sodium bicarbonate, 10% fetal bovine serum (Invitrogen), 1 mm sodium pyruvate (Invitrogen), 100 U/ml penicillin (Sigma) and 100 mg/liter streptomycin (Sigma), and grown at 37 C and in 5% CO2 humidified atmosphere. At 85% confluence, cells were placed into differentiation media (DM) containing 2% horse serum instead of the fetal calf serum in the medium described above. Once in DM, cells were treated with TGF-β family ligands at 4–8 μg/ml and grown for 72–96 h. For competition studies ActRII-Fc, -IIB-Fc, or Fc alone was added at 1–8 times the molar ratio of the ligand. After completion of the experiment, cell lysates were made using RIPA buffer (Cell Signaling Technology) containing protease inhibitors (Sigma) according to manufacturer’s protocol and stored at −80 C. Cell extracts were denatured and reduced with 4× LDS Sample Buffer (Invitrogen) and 10× reducing agent (Invitrogen) and boiled at 90 C for 10 min. Extracts were then separated on a 4–12% gradient NuPage gel (Invitrogen), transferred to a polyvinylidine difluoride membrane (Invitrogen), and immunoblotted with the appropriate primary antibodies: anti-p21 (1:200), anti-MyoD (1:300), antimyogenin (1:200), and anti-β-actin (1:5000). Proteins were visualized using chemiluminescence (Pierce).
Reporter Gene Assay
An A204 cell line (American Type Culture Collection) stably transfected with the luciferase reporter construct, pGL3-(CAGA)12 (23), was used to monitor Smad2,3 activity. Cells were incubated with an appropriate concentration (EC50 value) of each ligand for 6 h at 37 C and assayed as described previously (6, 14). The inhibition of each identified ligand by ActRIIB-Fc or Fc-control protein was evaluated over a broad dose range. TGF-β activity was monitored in a similar fashion using C2C12 cells stably transfected with the pGL3-(CAGA)12 reporter. To monitor Smad1,5,8 signaling, C2C12 cells were transiently transfected with a pBRE-Luc9 reporter (24). Transfections were normalized using the Renilla reporter pPRL-TK (Promega Corp., Madison, WI), which was cotransfected into the cells.
Alkaline Phosphatase Assay
C2C12 myoblasts were cultured in growth medium in 96-well plates. At 85% confluence, the medium was changed to DM, and the cells were treated or left untreated with TGF-β family ligands at 0.2–4 μg/ml for 96 h. Cells were washed twice with calcium/magnesium free PBS and incubated at 37 C for 15 min with 50 μl of 4-methylumbelliferyl phosphate (Sigma), an AP substrate that releases fluorescence when dephosphorylated. Fluorescence was measured using a Victor luminometer (excitation at 355 nm and emission at 460 nm) for 1 sec per well. After the activity reading, 50 μl of 2× lysis buffer (200 mm Tris-HCl, pH 9.8; 0.4% Triton X-100) was added to each well, and protein concentration was determined using a BCA protein assay kit (Pierce) according to the manufacturer’s protocol. AP activity was represented as units of fluorescence released divided by the protein concentration in each well. Each assay was done in triplicate.
Footnotes
Disclosure Statement: T.A.S. is employed by Wyeth. Y.G., J.Z., and P.J.Y. are employed by Wyeth and have equity interest in Wyeth. X.C. and Y.Q. were previously employed by Wyeth. J.J.H. has nothing to disclose.
First Published Online October 16, 2008
Abbreviations: ActRII, Activin type II receptor; AP, alkaline phosphatase; BMP, bone morphogenetic protein; DM, differentiation medium; DMD, Duchenne muscular dystrophy; Fc, fragment crystallizable; FDR, false discovery rate; GDF, growth differentiation factor; GM, growth medium; mAb, monoclonal antibody; MS, mass spectrometry; MS/MS, tandem mass spectrometry; MyoD, myogenic differentiation 1; SDS, sodium dodecyl sulfate; SMAD, Sma- and Mad-related protein; WB, Western blot.
References
- 1.McPherron AC, Lawler AM, Lee SJ 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90 [DOI] [PubMed] [Google Scholar]
- 2.McPherron AC, Lee SJ 1997. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ 2004. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688 [DOI] [PubMed] [Google Scholar]
- 4.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA 2007. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3:e79 [DOI] [PMC free article] [PubMed]
- 5.Shelton GD, Engvall E 2007. Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul Disord 17:721–722 [DOI] [PubMed] [Google Scholar]
- 6.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ 2002. Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488 [DOI] [PubMed] [Google Scholar]
- 7.McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R 2005. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 118:3531–3541 [DOI] [PubMed] [Google Scholar]
- 8.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS 2002. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420:418–421 [DOI] [PubMed] [Google Scholar]
- 9.Bogdanovich S, McNally EM, Khurana TS 2008. Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve 37:308–316 [DOI] [PubMed] [Google Scholar]
- 10.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS 2005. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19:543–549 [DOI] [PubMed] [Google Scholar]
- 11.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y 2002. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277:40735–40741 [DOI] [PubMed] [Google Scholar]
- 12.Hill JJ, Qiu Y, Hewick RM, Wolfman NM 2003. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol 17:1144–1154 [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, McPherron AC 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thies RS, Chen T, Davies MV, Tomkinson KN, Pearson AA, Shakey QA, Wolfman NM 2001. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors 18:251–259 [DOI] [PubMed] [Google Scholar]
- 15.Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM 2003. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300:965–971 [DOI] [PubMed] [Google Scholar]
- 16.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ 2003. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 100:15842–15846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L 2003. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol 23:7230–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM 2005. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 102:18117–18122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Caestecker M 2004. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev 15:1–11 [DOI] [PubMed] [Google Scholar]
- 20.Pangas SA, Woodruff TK 2000. Activin signal transduction pathways. Trends Endocrinol Metab 11:309–314 [DOI] [PubMed] [Google Scholar]
- 21.Phillips DJ 2000. Regulation of activin’s access to the cell: why is mother nature such a control freak? Bioessays 22:689–696 [DOI] [PubMed] [Google Scholar]
- 22.Kretzschmar M, Massague J 1998. SMADs: mediators and regulators of TGF-β signaling. Curr Opin Genet Dev 8:103–111 [DOI] [PubMed] [Google Scholar]
- 23.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM 1998. Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17:3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korchynskyi O, ten Dijke P 2002. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 277:4883–4891 [DOI] [PubMed] [Google Scholar]
- 25.Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G 2003. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286:263–275 [DOI] [PubMed] [Google Scholar]
- 26.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R 2002. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277:49831–49840 [DOI] [PubMed] [Google Scholar]
- 27.Rios R, Carneiro I, Arce VM, Devesa J 2002. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282:C993–C999 [DOI] [PubMed]
- 28.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R 2000. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243 [DOI] [PubMed] [Google Scholar]
- 29.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC 2004. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther 11:1312–1320 [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC 2003. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am [Erratum (2004) 86-A:141] 85-A:1544–1552 [DOI] [PubMed]
- 31.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC 2007. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 25:665–677 [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S 1991. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol 113:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T 1994. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127:1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katagiri T, Akiyama S, Namiki M, Komaki M, Yamaguchi A, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T 1997. Bone morphogenetic protein-2 inhibits terminal differentiation of myogenic cells by suppressing the transcriptional activity of MyoD and myogenin. Exp Cell Res 230:342–351 [DOI] [PubMed] [Google Scholar]
- 35.Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH 1993. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364:501–506 [DOI] [PubMed] [Google Scholar]
- 36.Weintraub H 1993. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75:1241–1244 [DOI] [PubMed] [Google Scholar]
- 37.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S 1991. The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251:761–766 [DOI] [PubMed] [Google Scholar]
- 38.Megeney LA, Rudnicki MA 1995. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol 73:723–732 [DOI] [PubMed] [Google Scholar]
- 39.Yang W, Zhang Y, Li Y, Wu Z, Zhu D, McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R, Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A, Zhao B, Wall RJ, Yang J, Guttridge DC 2007. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 β pathway and is antagonized by insulin-like growth factor 1. J Biol Chem 282:3799–3808 [DOI] [PubMed] [Google Scholar]
- 40.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA 1988. Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534 [DOI] [PubMed] [Google Scholar]
- 41.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC 2008. Regulation of osteogenic differentiation during skeletal development. Front Biosci 13:2001–2021 [DOI] [PubMed] [Google Scholar]
- 42.Grainger DJ, Kemp PR, Metcalfe JC, Liu AC, Lawn RM, Williams NR, Grace AA, Schofield PM, Chauhan A 1995. The serum concentration of active transforming growth factor-β is severely depressed in advanced atherosclerosis. Nat Med 1:74–79 [DOI] [PubMed] [Google Scholar]
- 43.Muttukrishna S, Knight PG, Groome NP, Redman CW, Ledger WL 1997. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet 349:1285–1288 [DOI] [PubMed] [Google Scholar]
- 44.Neuhaus H, Rosen V, Thies RS 1999. Heart specific expression of mouse BMP-10 a novel member of the TGF-β superfamily. Mech Dev 80:181–184 [DOI] [PubMed] [Google Scholar]
- 45.Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C 1986. Regulation of myogenic differentiation by type β transforming growth factor. J Cell Biol 103:1799–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massague J, Cheifetz S, Endo T, Nadal-Ginard B 1986. Type β transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci USA 83:8206–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J 2004. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164:1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P 2007. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci 120:964–972 [DOI] [PubMed] [Google Scholar]
- 49.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S 2007. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109:1953–1961 [DOI] [PubMed] [Google Scholar]
- 50.Kollias HD, McDermott JC 2008. Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol 104:579–587 [DOI] [PubMed] [Google Scholar]
- 51.Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL 2003. Autoregulation of neurogenesis by GDF11. Neuron 37:197–207 [DOI] [PubMed] [Google Scholar]
- 52.Ge G, Hopkins DR, Ho WB, Greenspan DS 2005. GDF11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of PC12 cells. Mol Cell Biol 25:5846–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gamer LW, Cox KA, Small C, Rosen V 2001. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol 229:407–420 [DOI] [PubMed] [Google Scholar]
- 54.He L, Vichev K, Macharia R, Huang R, Christ B, Patel K, Amthor H 2005. Activin A inhibits formation of skeletal muscle during chick development. Anat Embryol (Berl) 209:401–407 [DOI] [PubMed] [Google Scholar]
- 55.Link BA, Nishi R 1997. Opposing effects of activin A and follistatin on developing skeletal muscle cells. Exp Cell Res 233:350–362 [DOI] [PubMed] [Google Scholar]
- 56.Lee SJ 2007. Quadrupling muscle mass in mice by targeting TGF-β signaling pathways. PLoS ONE 2:e789 [DOI] [PMC free article] [PubMed]
- 57.Shimonaka M, Inouye S, Shimasaki S, Ling N 1991. Follistatin binds to both activin and inhibin through the common subunit. Endocrinology 128:3313–3315 [DOI] [PubMed] [Google Scholar]
- 58.Krummen LA, Woodruff TK, DeGuzman G, Cox ET, Baly DL, Mann E, Garg S, Wong WL, Cossum P, Mather JP 1993. Identification and characterization of binding proteins for inhibin and activin in human serum and follicular fluids. Endocrinology 132:431–443 [DOI] [PubMed] [Google Scholar]
- 59.Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW 2004. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol 225:29–36 [DOI] [PubMed] [Google Scholar]
- 60.Nomura T, Ueyama T, Ashihara E, Tateishi K, Asada S, Nakajima N, Isodono K, Takahashi T, Matsubara H, Oh H 2008. Skeletal muscle-derived progenitors capable of differentiating into cardiomyocytes proliferate through myostatin-independent TGF-β family signaling. Biochem Biophys Res Commun 365:863–869 [DOI] [PubMed] [Google Scholar]
- 61.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K 2004. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30 [DOI] [PubMed] [Google Scholar]
- 62.Shevchenko A, Wilm M, Vorm O, Mann M 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68:850–858 [DOI] [PubMed] [Google Scholar]