Abstract
Physiological and pharmacological processes mediated by glucocorticoids involve tissue- and context-specific regulation of glucocorticoid-responsive gene expression via glucocorticoid receptor (GR). However, the molecular mechanisms underlying such highly coordinated regulation of glucocorticoid actions remain to be studied. We here addressed this issue using atp1a1 and scnn1a, both of which are up-regulated in response to corticosteroids in human embryonic kidney-derived 293 cells, but resistant in liver-derived HepG2 cells. Hexamethylene bisacetamide-inducible protein 1 (HEXIM1) represses gene expression via, at least, two distinct mechanisms, i.e. positive transcription elongation factor b sequestration and direct interaction with GR, and is relatively high in HepG2 cells compared with 293 cells. Given this, we focused on the role of HEXIM1 in transcriptional regulation of these GR target genes. In HepG2 cells, hormone resistance of atp1a1 and scnn1a was diminished by either knockdown of HEXIM1 or overexpression of GR. Such a positive effect of exogenous expression of GR was counteracted by concomitant overexpression of HEXIM1, indicating the balance between GR and HEXIM1 modulates hormonal sensitivity of these genes. In support of this, the hormone-dependent recruitment of RNA polymerase II onto atp1a1 promoter was in parallel with that of GR. Moreover, we revealed that not positive transcription elongation factor b-suppressing activity but direct interaction with GR of HEXIM1 plays a major role in suppression of promoter recruitment of the receptor and subsequent atp1a1 and scnn1a gene activation. Collectively, we may conclude that HEXIM1 may participate in tissue-selective determination of glucocorticoid sensitivity via direct interaction with GR at least in certain gene sets including atp1a1 and scnn1a.
GLUCOCORTICOIDS ARE secreted from the adrenal glands under the strict control of the hypothalamic-pituitary-adrenal axis and maintain homeostasis through the regulation of electrolyte balance, glucose homeostasis, lipid and protein metabolism, and modulation of the immune, cardiovascular, and central nervous system (1, 2, 3). On the other hand, glucocorticoids have been widely and successfully used in treating a number of pathological states, e.g. inflammation and autoimmune disorders (4). It has been demonstrated that such physiological and pharmacological processes mediated by glucocorticoids involve tissue-specific regulation of glucocorticoid-responsive gene expression (3). Moreover, glucocorticoid sensitivity of every single gene has been shown to differ among cells, tissues, and individuals, and even fluctuates not only in pathological states, but also during normal physiological processes, including development and the cell cycle (4, 5). Albeit its importance, the molecular mechanisms underlying highly coordinated tissue- and context-dependent regulation of expression of glucocorticoid-target genes remain to be studied.
atp1a1 is expressed in all mammalian cells, and its product Na+, K+-ATPase α1 plays essential roles in regulating ionic intracellular milieu, the process that is needed for the regulation of metabolism, proliferation, differentiation, and cell volume (6). In addition, in kidney, Na+, K+-ATPase α1 also plays a central role in the fine control of systemic electrolyte balance through hormone-regulated sodium reabsorption, in functional cooperation with amiloride-sensitive Na+ channel, which is encoded by scnn1a (7, 8, 9). atp1a1, as well as scnn1a, is up-regulated in response to corticosteroids in kidney (7). On the other hand, particularly in liver, expression of either atp1a1 or scnn1a is not influenced by glucocorticoids, indicating that these genes are resistant to glucocorticoids, not in kidney but in liver. Interestingly, their hormone resistance seems to be corticosteroid signal selective, because other extracellular stimuli, such as thyroid hormones (10) and low external potassium ion (11), were shown to modulate mRNA expression of atp1a1 even in liver.
Glucocorticoids elicit their hormone actions via a signal pathway involving ubiquitously expressed glucocorticoid receptor (GR), a prototypic member of the nuclear receptor superfamily, which acts as a ligand-dependent transcription factor (12). It is generally believed that, upon binding glucocorticoids, GR translocates into the nucleus and binds the glucocorticoid response element (GRE) on the target gene promoters. Binding of liganded receptors with target DNA is followed by recruitment of mediators and coactivators to the proximity of the target DNA, resulting in RNA polymerase II (RNAPII) recruitment and activation of transcription (4, 13, 14, 15, 16, 17, 18, 19). The recent advent of DNA microarray technology has revealed that there are only modest overlaps in glucocorticoid-regulated gene sets among different cell types. In fact, considerable numbers of genes are responsive to glucocorticoids in certain tissues but resistant in others (20, 21, 22, 23). Already several mechanisms have been postulated for tissue-specific regulation of glucocorticoid actions including different metabolisms of ligands (24), tissue-specific cofactor availability (25), and GR subtype distribution (26).
Hexamethylene bisacetamide-inducible protein 1 (HEXIM1) was originally identified as a nuclear protein, expression of which was induced when human vascular smooth muscle cells were treated with hexamethylene bisacetamide, an inhibitor of cell proliferation (27). HEXIM1 has been shown to regulate mRNA expression via, at least, two distinct mechanisms, i.e. positive transcription elongation factor b (P-TEFb)-dependent (28, 29) and P-TEFb-independent mechanisms (30). P-TEFb, typically composed of cyclin-dependent kinase 9 (CDK9) and its regulatory partner cyclin T1 (CycT1), phosphorylates the C-terminal domain of RNAPII, thereby stimulating transcription elongation (31, 32, 33). P-TEFb recruitment has been reported in diverse class II promoters in association with a certain class of transcription factors, including HIV-1 Tat (34), nuclear factor-κB (35), signal transducer and activator of transcription 3 (STAT3) (36), heat shock factor (HSF) (37), and arylhydrocarbon receptor (AhR) (38). HEXIM1 exerts its inhibitory effect on P-TEFb in vivo and in vitro in a 7SK small nuclear RNA (snRNA)-dependent fashion (28, 29). Upon binding with HEXIM1 and 7SK snRNA, P-TEFb looses its kinase activity, resulting in suppression of transcription elongation (39, 40). On the other hand, several reports described P-TEFb-independent mechanisms of gene regulation by HEXIM1 (30, 41, 42, 43, 44, 45, 46, 47). Among others, we demonstrated that HEXIM1 directly interacts with GR and modulates glucocorticoid-responsive gene expression (30). Moreover, we recently showed that GR, via its hinge region, interacts with central basic amino acid-rich region of HEXIM1 (48). At this moment, however, it remains unknown how genes can differentially use these distinct functions of HEXIM1: inhibitory effects on P-TEFb-dependent elongation and GR-mediated transactivation.
In the present study, we showed that mRNA expression of atp1a1 and scnn1a was up-regulated by treatment with glucocorticoids in human embryonic kidney-derived 293 cells, but not in human liver cancer-derived HepG2 cells. Knockdown of endogenous HEXIM1 in HepG2 cells canceled glucocorticoid resistance of atp1a1 and scnn1a mRNA expression. By creating a system that enables differential analysis of the above-mentioned distinct HEXIM1 functions, we revealed that not P-TEFb-suppressing activity but direct interaction with GR plays a major role in suppression of atp1a1 activation by attenuating promoter recruitment of the receptor and RNAPII. We may conclude, therefore, that HEXIM1 may participate in tissue- and gene-selective determination of glucocorticoid sensitivity via direct interaction with GR, at least in a certain gene set that includes atp1a1 and scnn1a.
RESULTS
Dexamethasone (DEX)-Resistance of atp1a1 and scnn1a Not in 293 Cells but in HepG2 Cells
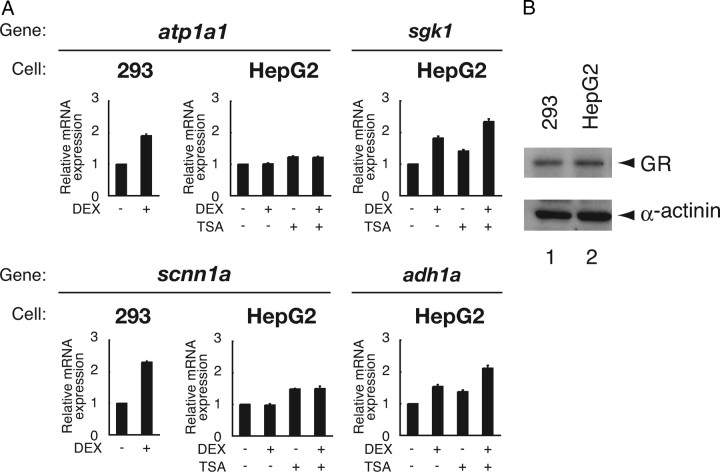
Expression of atp1a1 and scnn1a is shown to be up-regulated by glucocorticoids, as well as aldosterone, in kidney and kidney-derived 293 cells. On the other hand, in liver, their mRNA expression is induced by various intra- or extracellular stimuli except for glucocorticoids (see “Introduction”). That is, atp1a1 and scnn1a appear to be resistant rather selectively against glucocorticoid-GR system particularly in liver. To address the molecular mechanism of such tissue-dependent hormone resistance in gene regulation, we studied HepG2 cells as a model in comparison with 293 cells. When we analyzed their mRNA expression levels using quantitative real-time RT-PCR (qRT-PCR), 6 h treatment of 293 cells with 100 nm DEX induced atp1a1 and scnn1a mRNA expression by 1.9-fold and 2.3-fold, respectively (Fig. 1A). In contrast, mRNA expression of neither atp1a1 nor scnn1a was induced in the presence of 100 nm DEX in HepG2 cells (Fig. 1A). Our previous DNA microarray analysis also supported this notion (data not shown). Note that GR is comparably expressed in HepG2 cells and 293 cells (Fig. 1B), and that mRNA expression of sgk1 and adh1a, both of which are known to be glucocorticoid target genes as well (20, 21, 22, 23), are induced by 1.8-fold and 1.6-fold, respectively, after DEX treatment in HepG2 cells (Fig. 1A). It is indicated, therefore, that, despite the presence of functional GR, mRNA expression of a particular set of genes, i.e. atp1a1 and scnn1a, shows a cell- or tissue-specific resistance to glucocorticoids. Treatment with a histone deacetylase inhibitor trichostatin A (TSA) increased both basal and induced mRNA levels of sgk1 and adh1a, but, in the case of atp1a1 and scnn1a, only basal mRNA levels were increased without restoration of responsiveness to DEX (Fig. 1A). Glucocorticoid insensitivity of atp1a1 and scnn1a in HepG2 cells, thus, does not appear to be related to histone acetylation-dependent mechanisms.
Fig. 1.
DEX Resistance of atp1a1 and scnn1a Not in 293 Cells but in HepG2 Cells
A, 293 cells and HepG2 cells were cultured in phenol red-free Opti-MEM I for 24 h and treated with or without 100 nm DEX for 6 h in the presence or absence of 100 nm TSA, as indicated. Total RNA was prepared and endogenous mRNA for Na+, K+-ATPase α1 (atp1a1), amiloride-sensitive Na+ channel 1α (scnn1a), serum and glucocorticoid-regulated kinase 1 (sgk1), alcohol dehydrogenase 1A (adh1a), and glyceraldehyde-3-phosphate dehydrogenase (gapdh) was measured with qRT-PCR. Samples were normalized to gapdh mRNA levels, and relative expression levels to vehicle-treated samples are presented as relative mRNA expression. Error bars represent sd values of at least three independent experiments. B, Cell lysates from 293 cells and HepG2 cells were subjected to Western blot analysis using indicated antibodies.
Cell Type Difference in Hormone-Dependent GR Recruitment onto GRE in atp1a1 Promoter
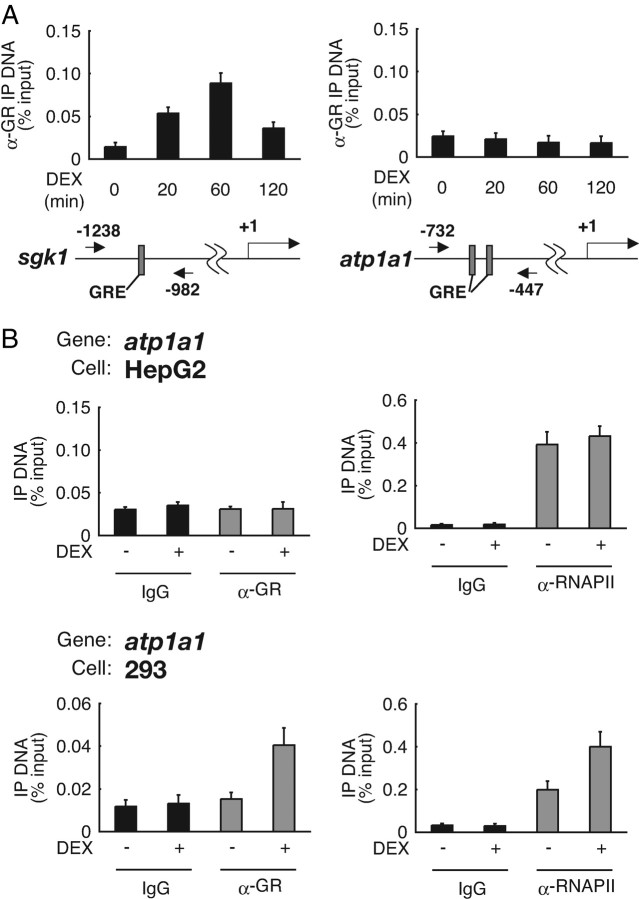
In GR-dependent transactivation, binding of liganded receptors onto target DNA is generally believed to be an essential trigger for transcription initiation and elongation (12, 13, 14, 15). We, therefore, tested in vivo occupancy of atp1a1 promoter by endogenous GR in comparison with sgk1 promoter using chromatin immunoprecipitation (ChIP) assay, because both promoters are known to contain GRE (Fig. 2A) (49, 50). In HepG2 cells, when sgk1 promoter was tested, GR was recruited onto the promoter in a time- and hormone-dependent manner. In contrast, atp1a1 promoter did not recruit GR even in the presence of DEX (Fig. 2A). Next, to examine the relationship between GR recruitment and ongoing transcription, we compared GR binding and RNAPII binding on atp1a1 promoter after DEX treatment. RNAPII binding, as well as that of GR, was not increased after DEX treatment in HepG2 cells. In clear contrast, RNAPII binding was enhanced after DEX treatment in concert with increase of GR binding in 293 cells (Fig. 2B). These results indicate that glucocorticoid resistance of atp1a1 promoter may be due to cell type-dependent deficiency of GR recruitment despite the presence of the receptor.
Fig. 2.
Cell Type Difference in Hormone-Dependent GR Recruitment onto atp1a1 Promoter GRE
A, HepG2 cells were cultured in phenol red-free Opti-MEM I for 24 h and treated with 1 μm DEX for the indicated time periods. ChIP assays were performed with anti-GR polyclonal antibodies, and recovered GRE-containing DNA fragments were measured with qRT-PCR. GREs in sgk1 and atp1a1 5′-flanking regions are indicated as gray boxes. The positions of the primers are shown as numbered arrows. Values are expressed as percentage of immunoprecipitated DNA to input. Error bars represent sd values of at least three independent experiments. B, HepG2 cells and 293 cells were cultured as described in panel A and treated with 1 μm DEX for 60 min. ChIP assays were performed with the indicated antibodies and primer sets as described in Materials and Methods. IP, Immunoprecipitation.
Endogenous HEXIM1 Negatively Modulates Glucocorticoid-Mediated Transcriptional Activation
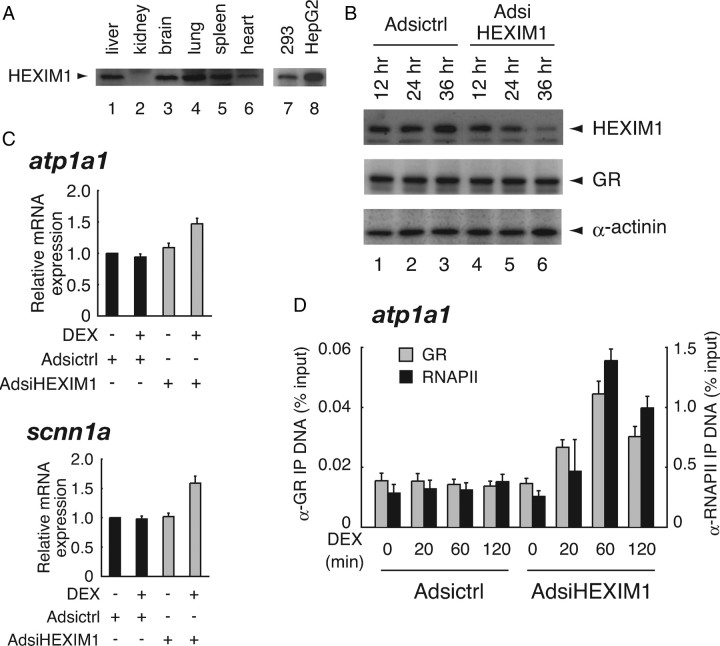
It was previously described that HEXIM1 mRNA is ubiquitously expressed but its expression levels are variable among human tissues (27). Protein expression levels of endogenous HEXIM1 also show a great diversity among tissues in mice; HEXIM1 expression levels appeared to be low in kidney compared with those in liver, brain, lung, spleen, and heart (Fig. 3A). These tissue-dependent differences of HEXIM1 expression levels were also observed in human tissue-derived cell lines, i.e. HepG2 cells abundantly express HEXIM1 compared with 293 cells (Fig. 3A). Together with the fact that certain GR target genes, e.g. atp1a1 and scnn1a, are resistant to hormone treatment in HEXIM1-rich HepG2 cells, we hypothesized that HEXIM1 may participate in cell type-dependent hormone resistance at the level of transcriptional regulation of these genes.
Fig. 3.
Endogenous HEXIM1 Negatively Modulates Glucocorticoid-Mediated Transcriptional Activation
A, Cell lysates from various tissues from adult mice were subjected to Western blot analysis using indicated antibodies. B, HepG2 cells were infected with Adsictrl or AdsiHEXIM1 in phenol red-free Opti-MEM I at MOI of 100 for indicated time periods. Whole-cell extracts were prepared, and protein expression levels of endogenous HEXIM1, GR, and α-actinin were assessed in Western blotting. C, HepG2 cells were infected with the recombinant adenoviruses for 36 h as described in panel B and stimulated with 100 nm DEX for 6 h. Total RNA was prepared and mRNA for atp1a1, scnn1a, and gapdh was measured with qRT-PCR. Samples were normalized to gapdh, and relative expression levels to vehicle-treated samples are presented as relative mRNA expression. Error bars represent sd values of at least three independent experiments. D, HepG2 cells were infected with the recombinant adenoviruses for 36 h as described in panel B and stimulated with 1 μm DEX for indicated time periods. ChIP assays were performed with the indicated antibodies as described in Materials and Methods. IP, Immunoprecipitation.
Given this, we then addressed whether endogenous HEXIM1 contributes to glucocorticoid insensitivity of atp1a1 and scnn1a in HepG2 cells. For that purpose, we constructed the recombinant adenoviruses expressing small interfering RNA (siRNA) against HEXIM1 named Adsi-HEXIM1, and unrelated siRNA named Adsictrl, as described in Materials and Methods. Figure 3B shows that infection of AdsiHEXIM1 diminished endogenous HEXIM1 protein expression down to less than 10% of the control without significant alteration of GR protein expression level. Again, mRNA expression of atp1a1 and scnn1a was not significantly induced in the presence of 100 nm DEX in Adsictrl-infected HepG2 cells (Fig. 3C). Infection of AdsiHEXIM1 had little effect on basal levels of but significant effect on DEX-inducibility of atp1a1 and scnn1a (Fig. 3C). Using atp1a1 as a model, we then studied the influence of knockdown of endogenous HEXIM1 on hormone-dependent GR recruitment onto atp1a1 promoter in ChIP analysis. As shown in Fig. 3D, GR was recruited in a time-dependent manner onto the promoter after DEX treatment in AdsiHEXIM1-infected cells. Moreover, RNAPII was also incorporated to the promoter in parallel with GR recruitment (Fig. 3D). Similar results were obtained when scnn1a promoter was used for ChIP assay (data not shown). These findings strongly support the notion that protein levels of endogenous HEXIM1 might determine GR recruitment onto these promoters and subsequent glucocorticoid-responsive transcription of atp1a1 and scnn1a in HepG2 cells.
GR and HEXIM1 Stochastically Contribute to Hormone-Dependent Transcriptional Regulation of GR-Target Genes
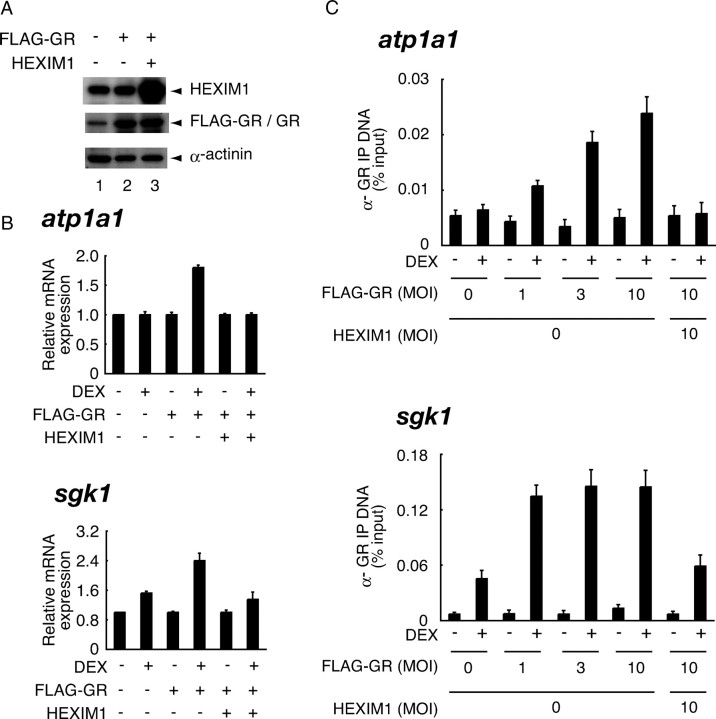
To test relative contribution of GR and HEXIM1 in the expression of atp1a1, we overexpressed FLAG-GR alone or in combination with HEXIM1 in HepG2 cells using recombinant adenoviruses. Western blots showed that the protein levels of expressed FLAG-GR and HEXIM1 were approximately 5-fold and 8-fold compared with those of endogenous GR and HEXIM1, respectively (Fig. 4A). In HepG2 cells, overexpression of FLAG-GR restored glucocorticoid responsiveness and resulted in DEX-dependent induction of atp1a1 mRNA by 1.8-fold, which was again canceled by coexpression of HEXIM1 (Fig. 4B). We may propose, therefore, that high-level expression of HEXIM1 relative to GR confers tissue-specific glucocorticoid resistance of atp1a1 in HepG2 cells. This is also the case in scnn1a mRNA expression (data not shown). Interestingly, mRNA expression of sgk1 was also negatively affected by exogenous expression of HEXIM1 (Fig. 4B). Our ChIP assay revealed that GR overexpression restored hormone-dependent recruitment of GR to atp1a1 promoter in a dose-dependent manner, which was again canceled by overexpression of HEXIM1 (Fig. 4C). In the case of sgk1 promoter as well, overexpression of GR further increased hormone-dependent GR recruitment, which was antagonized by exogenous HEXIM1 (Fig. 4C). These findings strongly support the notion that GR and HEXIM1 stochastically contribute to hormone-dependent transcriptional regulation of both atp1a1 and sgk1, and that atp1a1 promoter is more susceptible to HEXIM1.
Fig. 4.
GR and HEXIM1 Stochastically Contribute to Hormone-Dependent Transcriptional Regulation of GR-Target Genes
A, HepG2 cells were infected with the recombinant adenoviruses expressing FLAG-GR and HEXIM1 in phenol red-free Opti-MEM I at MOI of 5 for 24 h as indicated. Cells were lysed and subjected to Western blot analysis using the indicated antibodies. B, HepG2 cells were infected with the recombinant adenoviruses as described in panel A and treated with or without 100 nm DEX for 6 h. Total RNA was prepared, and mRNA for atp1a1, sgk1, and gapdh was measured with qRT-PCR. Samples were normalized to gapdh, and relative expression levels to vehicle-treated samples are presented as relative mRNA expression. Error bars represent sd values of at least three independent experiments. C, HepG2 cells were infected with the recombinant adenoviruses in phenol red-free Opti-MEM I for 24 h as indicated and treated with or without 1 μm DEX for 20 min. ChIP assays were performed with anti-GR antibodies as described in Materials and Methods. IP, Immunoprecipitation.
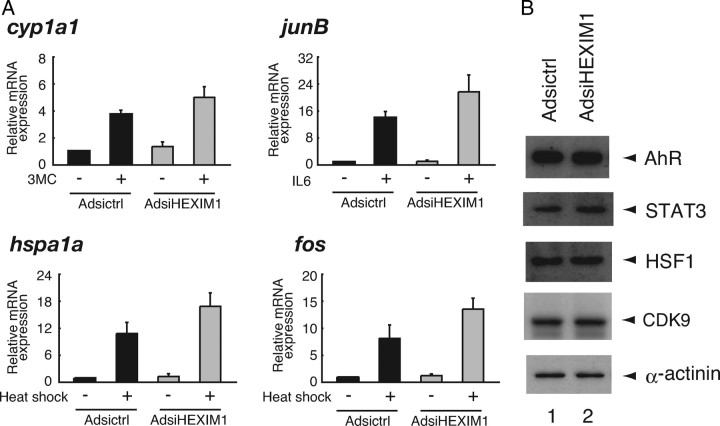
Knockdown of HEXIM1 Enhanced P-TEFb-Dependent Gene Expression
In addition to suppressing GR recruitment onto the target DNA, HEXIM1 is originally reported to inactivate kinase activity of P-TEFb, thereby suppressing transcription elongation (see Introduction). To investigate whether knockdown of HEXIM1 in our system affects P-TEFb-dependent mRNA expression as previously reported (51), we analyzed mRNA expression of several genes, expression of which has been reported to be critically regulated by P-TEFb at the step of transcription elongation.
mRNA level of cyp1a1 was increased after stimulation with 1 h treatment of 10 nm 3-methylcholantrene (3MC) by 3.7-fold, probably via activation of AhR and subsequent recruitment of P-TEFb onto cyp1a1 promoter (38), and AdsiHEXIM1 further enhanced this 3MC effect by 5.0-fold (Fig. 5A). IL-6-mediated expression of junB mRNA (14-fold), which is mediated by STAT3 (52), was also enhanced by AdsiHEXIM1 (22-fold). Finally, heat shock-mediated amplification of mRNA expression of hspa1a and fos (11-fold and 8.1-fold, respectively), which is mediated by HSF1 (53, 54), was further enhanced by AdsiHEXIM1 (17-fold and 14-fold, respectively). Note that protein expression levels of AhR, STAT3, HSF1, and CDK9 were not significantly influenced by infection of AdsiHEXIM1 (Fig. 5B). We may conclude, therefore, that P-TEFb activity was enhanced in HEXIM1-knocked down cells and that the P-TEFb-dependent elongation process was up-regulated in certain genes.
Fig. 5.
Knockdown of HEXIM1 Enhanced P-TEFb-Dependent Gene Expression
A, HepG2 cells were infected with Adsictrl or AdsiHEXIM1 as described in Fig. 3B and stimulated with 10 nm 3MC, 100 ng/ml IL-6, or culture at 42 C (heat shock) for 1 h as indicated. Endogenous mRNA for cytochrome P450, family 1, subfamily A, polypeptide 1 (cyp1a1), JunB (junB), heat shock 70-kDa protein 1A (hspa1a), Fos (fos), and gapdh was measured with qRT-PCR. Samples were normalized to gapdh, and relative expression levels to the Adsictrl-infected and unstimulated samples are presented as relative mRNA expression. Error bars represent sd values of at least three independent experiments. B, HepG2 cells were infected with the recombinant adenoviruses and stimulated as described in panel A. Nuclear extracts were prepared, and protein expression levels of endogenous AhR, STAT3, HSF1, CDK9, and α-actinin were assessed by Western blotting.
P-TEFb Binding and GR Binding Are Separable for HEXIM1
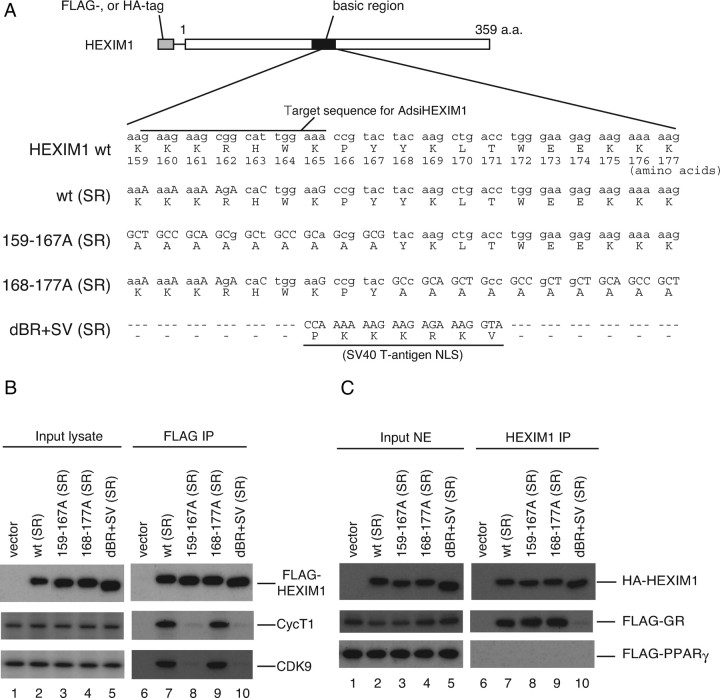
To highlight the GR target gene-selective role of HEXIM1, we established an experimental system that enables us to clarify which function of HEXIM1 is important in regulation of GR-target gene expression, P-TEFb suppression or GR sequestration. In short, endogenous HEXIM1 was knocked down by infection of AdsiHEXIM1, and mutant HEXIM1, which lacks either P-TEFb-suppressing activity or direct interaction with GR, or both, was exogenously complemented. To obtain such a mutant HEXIM1, we focused on basic region (BR) of HEXIM1 and made alanine substitution and domain swap mutants, as schematically depicted in Fig. 6A, because we and others previously showed that BR is essential for nuclear localization, interaction with GR, and P-TEFb-inhibition (28, 30, 41, 55). Because siRNA against HEXIM1 in AdsiHEXIM1 was designed to target the region corresponding to amino acids 159–165 (Fig. 6A), the expression plasmids for siRNA-resistant wild-type (SR) and 168–177A (SR) were created with several nucleotide substitutions in HEXIM1 cDNA without affecting original amino acid sequence (Fig. 6A). In indirect immunofluorescence analysis, every mutant HEXIM1 protein was expressed in the nucleus in transfected cells (data not shown).
Fig. 6.
P-TEFb-Binding and GR Binding Are Separable for HEXIM1
A, Schematic illustration of wild-type (wt) and mutant HEXIM1 used in this study. BR encompassing 150–177 amino acids are depicted as a solid box. Numbers depict positions of amino acids. Nucleotide and amino acid sequences in the BR are shown. Substitutions of nucleotides are shown in uppercase letters. wt (SR) and 168–177A (SR) have nucleotide substitutions in the target nucleotide sequence for AdsiHEXIM1 without affecting original amino acid sequence. 159–167A (SR) and dBR+SV (SR) are resistant to AdsiHEXIM1 by nature. B, HeLa cells were cotransfected with empty vector or expression plasmids for indicated FLAG-tagged mutant HEXIM1. Whole-cell lysates were prepared and subjected to FLAG-affinity purification as described in Materials and Methods. Western blot analysis of input lysates (lanes 1–5) and affinity-purified fractions (lanes 6–10) were performed using anti-FLAG peptide, anti-CycT1, and anti-CDK9 antibodies. C, COS7 cells were cotransfected with empty vector or expression plasmids for indicated HA-tagged mutant HEXIM1 along with either FLAG-tagged GR (middle panel) or FLAG-tagged PPAR γ (lower panel) expression plasmid. Cells were treated with 100 nm DEX (middle panel) or 100 nm TGZ for 2 h. Nuclear extracts were prepared and immunoprecipitated with anti-HEXIM1 antibodies. Western blot analysis of input extracts (lanes 1–5) and immunoprecipitated fractions (lanes 6–10) were performed using anti-HA peptide and anti-FLAG peptide antibodies. a.a., Amino acids; IP, immunoprecipitation; NE, nuclear extract; NLS, nuclear localization signal.
To verify the presence or absence of the interaction between P-TEFb and these mutant FLAG-tagged HEXIM1, we, after transfection of their expression plasmids into HeLa cells, immunoprecipitated cell lysate with anti-FLAG monoclonal antibody, and blots were probed with the antibodies against major P-TEFb subunits CycT1 and CDK9. As expected, substitution of BR to the nuclear localization signal from simian virus (SV) 40 large T antigen, resulting in dBR+SV (SR), completely abolished binding of CycT1 and CDK9 (Fig. 6B). Alanine substitution of amino acids 159–167, which was shown to disrupt the interaction with 7SK snRNA (55), diminished consecutive recruitment of CycT1 and CDK9 (Fig. 6B), as seen in dBR+SV (SR). On the other hand, alteration of amino acids 168–177 to alanines did not affect binding of CycT1 or CDK9 (Fig. 6B).
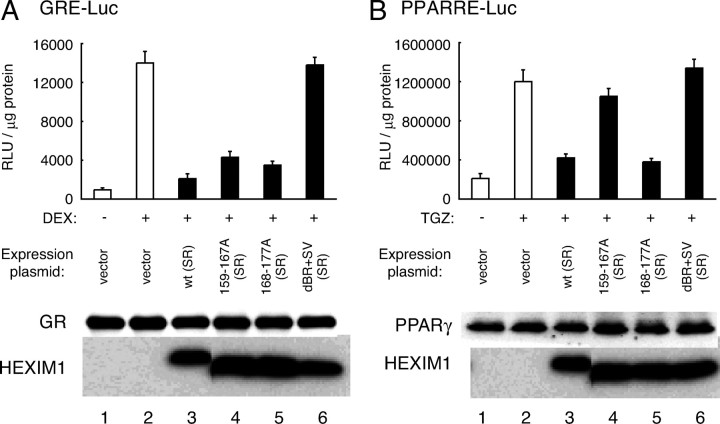
Using these HEXIM1 mutants, we also studied the physical interaction between HEXIM1 and GR. For this purpose, hemagglutinin (HA)-tagged wild-type (SR) and mutant HEXIM1 were expressed in COS7 cells along with either FLAG-tagged GR or FLAG-tagged peroxisome proliferator-activated receptor γ (PPARγ) as a control and immunoprecipitated with anti-HEXIM1 antibodies. As shown in Fig. 6C, GR bound not only wild-type (SR) but also HEXIM1 mutants with alanine substitution, but the swap mutant dBR+SV (SR) did not bind GR. These results may suggest that amino acids 159–177 of HEXIM1 are not critical for binding GR, but protein configuration of BR and its proximity is important for GR recognition. In contrast, PPARγ did not bind wild-type (SR) or any mutant HEXIM1 (Fig. 6C). To further confirm that these HEXIM1 mutants, especially 159–167A (SR), retain not P-TEFb-inhibition but GR suppression, we tested their functions in a GRE-luciferase reporter gene assay (Fig. 7A). PPARγ-dependent reporter gene assay served as a control (Fig. 7B), because neither wild-type (SR) nor any mutant HEXIM1 was capable of binding PPARγ (Fig. 6C). HEXIM1 dBR+SV (SR), which lacks binding activity to either P-TEFb or GR, did not significantly affect either reporter gene activity, as expected (Fig. 7, A and B, top). With respect to GR-driven reporter gene expression, any alanine-substituted HEXIM1 mutant suppressed ligand-dependent activation of the reporter gene as well as wild type (SR), indicating its functional interaction with GR (Fig. 7A, top). In clear contrast, PPARγ-mediated activation of the reporter gene was repressed solely by wild-type (SR) and 168–177A (SR) (Fig. 7B, top). In these experimental settings, protein expression of FLAG-tagged GR or FLAG-tagged PPARγ was not significantly affected by HEXIM1 mutants (Fig. 7, A and B, bottom). It is concluded, therefore, that these HEXIM1 mutants can serve an efficient tool for delineating mechanism of suppressing expression of particular genes by HEXIM1, i.e. P-TEFb suppression or GR binding.
Fig. 7.
Differential Functions of HEXIM1 on P-TEFb- and GR-Dependent Gene Expression
A, COS7 cells were cotransfected with empty vector or expression plasmids for the indicated HA-tagged mutant HEXIM1 along with GR expression plasmid and GRE reporter plasmid. Four hours later, media were replaced, further cultured for 20 h, and treated with vehicle or 100 nm DEX for 18 h as indicated. Cells were lysed and subjected to luciferase assay. Results are presented as relative light units (RLU) per microgram of protein in the lysates. Error bars represent sd values of at least three independent experiments. Protein expression levels of GR and HA-HEXIM1 were assessed in Western blotting. B, COS7 cells were cotransfected with empty vector or expression plasmids for indicated HA-tagged mutant HEXIM1 along with PPARγ expression plasmid and PPARRE reporter plasmid. Media were replaced 4 h later, further cultured for 20 h, and treated with vehicle or 100 nm TGZ for 18 h as indicated. Cells were lysed and subjected to luciferase assay. Results are presented as RLU per microgram of protein in the lysates. Error bars represent sd values of at least three independent experiments. Protein expression levels of PPARγ and HA-HEXIM1 were assessed by Western blotting.
P-TEFb Is Not Involved in HEXIM1-Mediated Suppression of Glucocorticoid Responsiveness
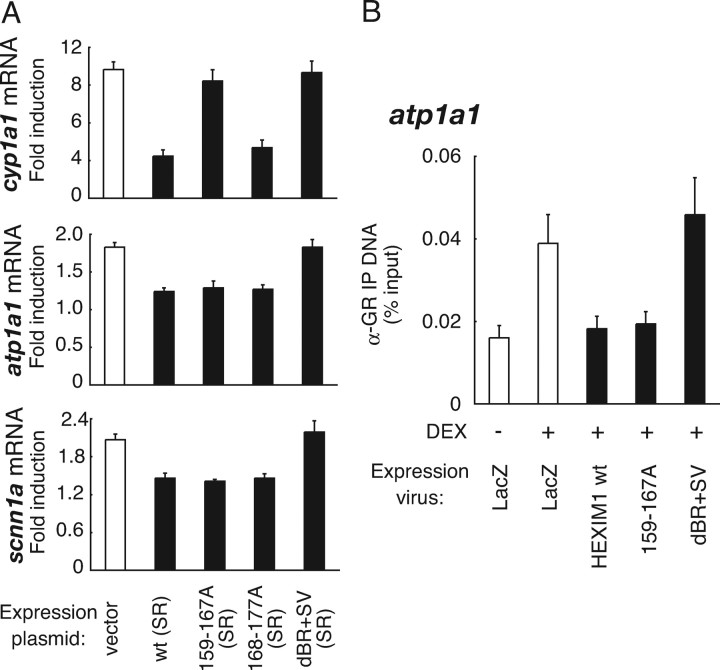
Finally, we differentially evaluated the importance of P-TEFb-suppressing and GR-binding activities of HEXIM1 in regulating glucocorticoid sensitivity of glucocorticoid-inducible mRNA expression of atp1a1 and scnn1a. HeLa cells were transfected with the expression plasmids for HEXIM1 (SR) mutants, infected with AdsiHEXIM1, and treated with the cognate ligands, after which RNA was isolated for qRT-PCR analyses. In HEXIM1 knocked-down cells, mRNA expression of cyp1a1, which is known to be P-TEFb dependent (38), was stimulated by 10-fold in response to 6 h treatment with 10 nm 3MC (Fig. 8A). Adding back of wild-type (SR) HEXIM1 repressed induction of mRNA expression of cyp1a1, to 3.3-fold, suggesting that ectopically expressed HEXIM1 (SR) functionally suppressed P-TEFb activity (Fig. 8A). However, neither 159–167A (SR) nor dBR+SV (SR) repressed cyp1a1 expression, confirming that the suppression of P-TEFb activity may be critical for the repression (Fig. 8A). In support of this, 168–177A (SR), which binds P-TEFb (Figs. 6B and 7B), suppressed AhR-mediated transcription as well as wild type (SR) (Fig. 8A). These effects of wild-type (SR) and mutant HEXIM1 were also observed in the other P-TEFb-regulated genes depicted in Fig. 5A (data not shown). With respect to GR target genes, mRNA expression of atp1a1 and scnn1a was stimulated by 1.8-fold and 2.1-fold, respectively, in response to 6 h treatment with 100 nm DEX in HEXIM1 knocked-down cells (Fig. 8A). Complementation of wild-type (SR) HEXIM1 or 168–177A (SR) significantly repressed induction of mRNA expression of atp1a1 and scnn1a (Fig. 8A). In contrast to cyp1a1, 159–167A (SR) suppressed glucocorticoid-induced enhancement of mRNA expression of atp1a1 and scnn1a comparable to that of wild type (SR) (Fig. 8A), indicating that P-TEFb-binding activity of HEXIM1 is dispensable but GR-binding activity is important for the suppression. Consistently, dBR+SV (SR), which does not bind GR, did not affect mRNA induction of atp1a1 and scnn1a (Fig. 8A). The importance of GR binding of HEXIM1 was also confirmed in ChIP assay. Recombinant adenovirus-mediated expression of 159–167A in HepG2 cells suppressed DEX-dependent recruitment of FLAG-GR onto atp1a1 promoter, whereas dBR+SV did not (Fig. 8B). Using scnn1a, we obtained identical results (data not shown). Taken together, we may conclude that direct interaction between GR and HEXIM1 is critical for HEXIM1-mediated glucocorticoid resistance of atp1a1 and scnn1a in HepG2 cells.
Fig. 8.
P-TEFb Is Not Involved in HEXIM1-Mediated Suppression of Glucocorticoid Responsiveness
A, HeLa cells were transfected with 3 μg of empty vector or expression plasmids for the indicated HA-tagged mutant HEXIM1. Cells were infected 4 h later with AdsiHEXIM1 in phenol red-free Opti-MEM I at MOI of 100 for 36 h, and treated with vehicle or 10 nm 3MC (top panel), or 100 nm DEX (middle and bottom panels) for 6 h. Endogenous mRNA levels for cyp1a1, atp1a1, scnn1a, and gapdh were measured with qRT-PCR. Samples were normalized to gapdh mRNA, and mRNA induction levels by cognate ligands are shown in fold induction. Error bars represent sd values of at least three independent experiments. B, HepG2 cells were infected with FLAG-GR-expressing adenovirus (MOI of 50) along with LacZ- or mutant HEXIM1-expressing adenoviruses (MOI of 40) in phenol red-free Opti-MEM I for 24 h, and the cells were treated with 1 μm DEX for 20 min. ChIP assays were performed with polyclonal anti-GR antibodies as described in Materials and Methods. Error bars represent sd values of at least three independent experiments. IP, Immunoprecipitation.
DISCUSSION
As described in the introductory section, HEXIM1 is currently considered to be a multifunctional protein, acting at a specific stage of gene expression. In the present study, we intended to characterize endogenous HEXIM1 function for modulation of GR-mediated transcriptional regulation. For that purpose, we focused on atp1a1 and scnn1a, because expression of these genes is resistant in HEXIM1-rich HepG2 cells to treatment with DEX (Fig. 1A). Treatment with histone deacetylase inhibitor did not result in liberation of these genes in HepG2 cells (Fig. 1A), suggesting that the observed DEX resistance is not due to irreversible alteration in higher order chromatin structure or histone acetylation-related chromatin packaging. In support of this, these genes retain responsiveness to other extracellular stimuli in liver and HepG2 cells (Refs. 10 and 11 and data not shown). We showed that, in HepG2 cells, knockdown of HEXIM1 by siRNA not only canceled the DEX resistance but also rather enhanced DEX-responsive mRNA expression of these genes (Fig. 3C). Moreover, our ChIP assay clearly demonstrated that siRNA-mediated knockdown of HEXIM1 restored hormone-dependent GR recruitment onto the promoters of those genes in parallel with corresponding increase in RNAPII binding (Fig. 3D). Such effect of reduction in endogenous HEXIM1 level was mimicked by exogenous overexpression of GR (Fig. 4, B and C), indicating that GR-HEXIM1 ratio could be a determinant of glucocorticoid resistance/sensitivity of those genes. As anticipated, overexpression of HEXIM1 turned those promoters more or less resistant to DEX (Fig. 4B).
Endogenous HEXIM1 seems to negatively modulate all GR target genes but not completely diminish DEX responsiveness of all of them in HepG2 cells (Fig. 4), indicating that efficiency of the suppression by HEXIM1 is dependent on gene context. Indeed, our previous DNA microarray analyses showed that the extent of reducing DEX responsiveness by overexpressed HEXIM1 was variable among different genes in HepG2 cells (30). It is also reported that GRE occupancy with GR in alveolar epithelial A549 cells is generally restricted to such genes that are actually regulated by glucocorticoids in those cells (21). This observation strongly supports the idea that gene-specific determination of GR recruitment to GRE is important in tissue-specific regulation of glucocorticoid-responsive gene expression at the level preceding transcription initiation. We recently demonstrated that HEXIM1 directly binds GR and that GR or other oxosteroid receptors are preferential partners of HEXIM1 (48). In this line, we might speculate that HEXIM1 squelches GR in the nucleus and inhibits its access to target gene promoter, and such negative effect of HEXIM1 is, more or less, shared by many genes. Some GR-target genes, including atp1a1 and scnn1a, therefore, might be particularly susceptible to HEXIM1 and resistant to glucocorticoids in HEXIM1-rich cells, i.e. HepG2 cells. Certain promoters, e.g. sgk1 promoter, allow hormone-dependent GR binding in HepG2 cells, strongly supporting the notion that promoter recruitment of GR is determined in a gene context-dependent manner as previously predicted in other GR-regulated genes (56).
We also revealed that P-TEFb-suppressing activity of HEXIM1 is not prerequisite for glucocorticoid resistance of these genes (Fig. 8A). Furthermore, the fact that 159–167A binds GR and suppresses GR recruitment to the target gene (Figs. 6C and 8B) again emphasizes the importance of the suppression of GR recruitment through direct GR-HEXIM1 interaction in the mechanisms of glucocorticoid resistance by HEXIM1. These results highlighted the role of HEXIM1 in P-TEFb-independent and gene-selective suppression of mRNA expression. The bimodal roles of HEXIM1 may differentially contribute to suppressing mRNA expression in a gene context-dependent manner. In this line, it should be noted that other transcription factors, such as estrogen receptor (45) and CCAAT/enhancer binding protein α (44), which were shown to directly interact with HEXIM1, may also be controlled by HEXIM1 through a P-TEFb-independent mechanism. Moreover, the interaction of HEXIM1 with these transcription factors has been shown to be a molecular basis for various physiological or pathological actions of HEXIM1 (44, 45).
Our previous observation revealed that HEXIM1/GR complexes reside in a distinct subnuclear area (30). Given this, HEXIM1 might prevent intranuclear GR from accessing to the promoter and decrease the amount of available GR for transcription. Since we revealed that the central and C-terminal regions of HEXIM1 are indispensable for its proper nuclear localization and GR repression (Refs. 41 and 48 , and data not shown), HEXIM1 might anchor at as yet unknown but saturable subnuclear structure via these regions. Increasing evidence indicates that the C-terminal region of HEXIM1 possesses various functions, e.g. P-TEFb-binding (57, 58), self-oligomerization (59, 60, 61), and interaction with transcription factors (44, 51). Recently, nucleophosmin was shown to bind HEXIM1 via BR and promote its degradation (62). Taken together, it may be indicated that subnuclear localization and function of HEXIM1 might be tightly controlled via multimodal interactions among distinct HEXIM1 domains and various nuclear machineries to elicit fine tuning of transcriptional control of gene expression. In any case, an important question to be solved is how multiple functions of HEXIM1 are rationally regulated in a gene- or tissue-dependent manner.
Expression levels of HEXIM1 vary in different tissues and are modulated during differentiation and development as well as in response to extracellular stimuli (see “Introduction”). Disturbances of tissue-specific glucocorticoid responses have been implicated in pathophysiology of rheumatoid arthritis, osteoarthritis, Crohn’s disease, ulcerative colitis, asthma, AIDS, osteoporosis, and metabolic syndromes (63). Numbers of proteins have been shown to affect GR activity at different steps of GR signaling pathway and indicated to be potentially involved in the pathogenesis of such diseases that have relations to disturbed glucocorticoid responses in particular tissues (63). HEXIM1-mediated repression of GR might be one of such mechanisms and play pathological roles in certain diseases. On the other hand, glucocorticoids are still indispensable in treatment for a numerous diseases (4, 64). However, the desired therapeutic effects are often accompanied by severe side effects. Pharmacological alteration of the expression levels of HEXIM1, if possible, might indirectly modulate glucocorticoid effects in a tissue-specific manner and enable selective expression of pharmacological actions of glucocorticoids in given tissues. Along with development of selective GR modulators (26), HEXIM1 might also be considered as a drug target for tissue-specific modulation of GR actions.
MATERIALS AND METHODS
Reagents and Antibodies
DEX, troglitazone (TGZ), 3MC, and TSA were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human IL-6 was from Peprotech (London, UK). Other reagents were from Nacalai Tesque (Kyoto, Japan) unless otherwise specified. Polyclonal antibodies against CDK9, STAT3, HSF1, CycT1, PPARγ, GR, and HA-peptide were from Santa Cruz Biotechnology, Inc. (sc-484, sc-7179, sc-9144, sc-8127, sc-7196, sc-8992, and sc-805, respectively; Santa Cruz, CA). Polyclonal anti-AhR antibodies were from Biomol (SA-210; Plymouth Meeting, PA). Polyclonal anti-FLAG-peptide antibodies and monoclonal anti-α-actinin antibody were from Sigma-Aldrich (F7425 and A5044, respectively). Monoclonal anti-GR antibody was from BD Biosciences (San Jose, CA). Monoclonal anti-RNAPII antibody was from Covance Laboratories, Inc. (MMS-126R; Princeton, NJ). Rabbit antihuman HEXIM1 antiserum and rabbit antimouse HEXIM1 antiserum were generated against a peptide corresponding from 39–53 amino acids of human HEXIM1 (RVPEEDSRWQSRAFP) and 55–69 amino acids of mouse HEXIM1 (SGSRPGQEGEGGLKH), respectively. Polyclonal anti-HEXIM1 affinity-purified antibodies were obtained from antihuman HEXIM1 antiserum with immunogen-immobilized affinity matrix (Kitayama Labes, Ina, Japan).
Cell Culture and Transfection
HepG2, 293, COS7, and HeLa cells were from RIKEN cell bank (Tsukuba, Japan) and maintained in DMEM supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA) and antibiotics in a humidified atmosphere at 37 C with 5% CO2. Before transfection, cells were washed twice with PBS, and media were replaced with phenol red-free Opti-MEM I (Invitrogen). Transient transfection was performed using TransIt-LT1 transfection reagent (Panvera, Madison, WI) as described previously (65). Total amounts of plasmids to transfect were kept constant by adding empty vector.
Western Blot Analysis
Whole-cell extracts or nuclear extracts were prepared as described previously (30), resolved in sodium dodecyl sulfate (SDS)-polyacrylamide gels, and blotted to polyvinylidene fluoride membranes. The membranes were incubated with Blocking One (Nacalai Tesque) at room temperature for 1 h, incubated with specific antibodies diluted in Blocking One (1:500 dilution for HA-peptide or 1:2000 for the others) at 4 C for 18 h, and then, washed three times with TBS-T (25 mm Tris-HCl, pH 8.0; 125 mm NaCl; 0.1% Tween 20), incubated with secondary antibodies conjugated to horseradish peroxidase (GE Healthcare, Buckinghamshire, UK) at room temperature for 30 min, washed three times with TBS-T, and detected with Chemi-Lumi One L (Nacalai Tesque) according to manufacturer’s instruction.
Recombinant DNA and Adenoviruses
Expression plasmids for FLAG-tagged HEXIM1 (wild-type and dBR+SV) were described previously (30). pFLAG-CMV2-derived mammalian expression plasmids for mutant FLAG-HEXIM1 (159–167A and 168–177A) were generous gifts from Dr. Q. Zhou (University of California, Berkeley, CA). cDNA fragments for wild-type (SR), 159–167A (SR), 168–177A (SR), and dBR+SV (SR) HEXIM1 were generated by a standard PCR protocol using custom-designed primers and subcloned into pCMV-HA (TaKaRa, Otsu, Japan) or pFLAG-CMV2 (Sigma- Aldrich) expression plasmid using blunt-ended EcoRI and XhoI sites. The expression plasmid for human PPARγ, pCMX-6His-PPARγ, was generated by cloning appropriate PCR fragments into pCMX-6His vector (65). The PPAR response element (PPARRE)-driven reporter plasmid p3xPPARRE-LUC was a kind gift from Dr. E. A. Jansson (Karolinska Institutet, Stockholm, Sweden). All plasmids constructed above were verified by DNA sequencing. Recombinant adenoviruses encoding double-stranded hairpin RNAs for siRNA against HEXIM1, AdsiHEXIM1, or control siRNA, Adsictrl, were constructed by subcloning expression cassettes from pSilencer3.1-H1 neo-derived expression plasmids (30) into adenoviral genome using Adenovirus Expression Vector Kit (TaKaRa) according to the manufacturer’s instruction. Recombinant adenoviruses prepared from 293 cells were purified with Virakit AdenoMini-24 (Virapur, San Diego, CA) and titrated using Adeno-X Rapid Titer Kit (TaKaRa).
qRT-PCR
Total RNA was prepared with Sepasol-RNA I super (Nacalai Tesque), reverse-transcribed with oligo-dT primer using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). qRT-PCR was performed with the LightCycler TaqMan Master, Universal ProbeLibrary Set, Human, and LightCycler ST300 systems (Roche, Indianapolis, IN) according to manufacturer’s instructions. Expression levels of mRNA were calculated on the basis of standard curves generated for each gene. mRNA for gapdh was used as an internal control. Sequences of primers used in this study are shown below:
atp1a1: 5′-ccctggctgctttccttt-3′ and 5′-ggcacagaaccaccaggta-3′
scnn1a: 5′-aaccaggtctcctgcaacc-3′ and 5′-gaaagtatagcagtttccatacatcg-3′
sgk1: 5′-cctgagcttatgaatgccaac-3′ and 5′-gccaaggttgatttgctgag-3′
adh1a: 5′-aaggcccatgaagttcgtatt-3′ and 5′-ccacgtggtcatctgtgc-3′
cyp1a1: 5′-cccagctcagctcagtacct-3′ and 5′-ggagattgggaaaagcatga-3′
junb: 5′-atacacagctacgggatacgg-3′ and 5′-gctcggtttcaggagtttgt-3′
hspa1a: 5′-ggagtcctacgccttcaaca-3′ and 5′-ccagcaccttcttcttgtcg-3′
fos: 5′-ctaccactcacccgcagact-3′ and 5′-aggtccgtgcagaagtcct-3′
gapdh: 5′-agccacatcgctcagaca-3′ and 5′-gcccaatacgaccaaatcc-3′
ChIP
ChIP assay was performed with ChIP Assay Kit (Upstate Biotechnology Inc., Lake Placid, NY) according to the manufacturer’s instructions with minor modification. First, HepG2 cells were cultured in phenol red-free Opti-MEM I for 24 h for hormone depletion. Then, the cells were treated with 1 μm DEX or 0.1% ethanol (vehicle) for the indicated time periods. After treatment, the cells were cross-linked in 1% formaldehyde for 10 min at 37 C. Cross-linking was stopped with addition of glycine to medium to a final 125 mm for 5 min at 37 C, after which the cells were rinsed with ice-cold PBS twice and harvested. Cell pellets were collected and resuspended in SDS-lysis buffer (50 mm Tris, pH 8.0; 1% SDS; 10 mm EDTA; 1 μm 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride; 800 nm aprotinin; 15 μm E-64; 20 μm leupeptin-hemisulfate; 50 μm bestatin; and 10 μm pepstatin A) for 10 min at 4 C. Chromatin was sheared to an average size of 500 bp by sonication of the lysate using a Bioruptor Ultrasonicator (Cosmo-Bio, Tokyo, Japan). Lysates corresponding to 2 × 106 cells were diluted 10-fold in ChIP dilution buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mm EDTA; 16.7 mm Tris, pH 8.1; and 167 mm NaCl) and precleared with Salmon Sperm DNA/Protein A Agarose beads (Upstate Biotechnology) at 4 C for 30 min. Supernatants were then collected and incubated with 5 μg of anti-GR polyclonal antibodies or anti-RNAPII monoclonal antibody at 4 C overnight. To collect immune complex, Salmon Sperm DNA/Protein A Agarose beads were added and further incubated at 4 C for 1 h. The beads were then washed twice each with Low-Salt Immune Complex Wash Buffer (0.1% SDS; 1% Triton X-100; 2 mm EDTA; 20 mm Tris, pH 8.1; and 150 mm NaCl), High-Salt Immune Complex Wash Buffer (0.1% SDS; 1% Triton X-100; 2 mm EDTA; 20 mm Tris, pH 8.1; and 500 mm NaCl), LiCl Immune Complex Wash Buffer (0.25 m LiCl; 1% Nonidet P-40; 1% deoxycholate; 1 mm EDTA; and 10 mm Tris, pH 8.1), and Tris-EDTA buffer. Protein-chromatin complex was eluted with elution buffer (10 mm dithiothreitol, 1% SDS, and 0.1 m NaHCO3), and reversal of cross-link of eluates was performed in 200 mm NaCl at 65 C for 6 h, after which proteins were digested with proteinase K at 45 C for 1 h. Precipitated DNA fragments were recovered by QIAquick DNA purification kit (QIAGEN, Chatsworth, CA) and quantified with qRT-PCR using appropriate primer sets. Sequences of primers used in this study are shown below:
sgk1 −1238: 5′-acctcctcacgtgttcttgg-3′ and sgk1 −982: 5′-caagcaaggctgaaaaatcc-3′ for GR
sgk1 −173: 5′-cctctcaatggggacagaac-3′ and sgk1 +85: 5′-ccttagcagcctcagttttca-3′ for RNAPII
atp1a1 −732: 5′-cgcccttcagattctcattt-3′ and atp1a1 −447: 5′-ggactcagggatgctgga-3′ for GR
atp1a1+156: 5′-ccctagctccctccacttg-3′ and atp1a1 +239: 5′-tcgctggagaatcagagagaa-3′ for RNAPII
FLAG-Affinity Purification
HeLa cells (2.5 × 106) were transfected with 4 μg of pFLAG-CMV2-derived expression plasmids. After 4 h, media were replaced with DMEM supplemented with 10% fetal calf serum. After 32 h, cells were lysed in lysis buffer [50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% (wt/vol) Nonidet P-40, 1 μm dithiothreitol, 0.5 μm phenylmethylsulfonyl fluoride], centrifuged at 20,000 × g for 20 min. Supernatant was diluted in FAR buffer [16.7 mm Tris-HCl (pH 8.0), 50 mm NaCl, 0.33% (wt/vol) Nonidet P-40, 0.33 μm dithiothreitol, 0.17 μm phenylmethylsulfonyl fluoride], applied to anti-FLAG M2-agarose beads (Sigma-Aldrich), incubated for 2 h at room temperature. The beads were washed three times with FAR buffer. Bound proteins were eluted with SDS-sample loading buffer and subjected to Western blot analysis using anti-FLAG peptide, anti-CycT1, and anti-CDK9 antibodies.
Luciferase Assay
COS7 cells (1 × 106) were transfected with 2 μg of reporter plasmids (p2xGRE-LUC or p3xPPARRE-LUC), 2.5 ng of expression plasmids for the receptors (pCMX-6His-GR or pCMX-6His-PPARγ), and pCMV-HA-derived HEXIM1 expression plasmids. After 4 h, media were replaced with fresh phenol red-free Opti-MEM I, and infected with recombinant adenoviruses at multiplicity of infection (MOI) of 100. After 20 h, cells were treated with 100 nm DEX, 100 nm TGZ or vehicle (0.1% ethanol), and further cultured for 18 h. Cells were lysed in Cell Culture Lysis Reagent (Promega Corp., Madison, WI), and cellular luciferase activity was measured by using Luciferase Assay System (Promega). Relative light units were normalized to the protein amounts determined with BCA Protein Assay Reagent (Pierce Chemical Co., Rockford, IL).
Acknowledgments
We thank Drs. Q. Zhou and E. A. Jansson for material transfer; Drs. H. Iba, S. Tokudome, and members of the Morimoto laboratory for help; Ms. Y. Nagafuji and Ms. J. Suzuki for clerical work; and Ms. T. Maruyama for diligent technical assistance.
NURSA Molecule Pages:
Coregulators: P-TEFb;
Ligands: Dexamethasone;
Nuclear Receptors: GR.
Footnotes
This work was supported in part by Grants-in-Aid for Science Research and Creative Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, the grant for Research on Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan, and Nakatomi Foundation (to H.T.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 18, 2008
N.S. and N.Y. contributed equally to this work, and both should be considered as first authors.
Abbreviations: AhR, Arylhydrocarbon receptor; BR, basic region; CDK9, cyclin-dependent kinase 9; ChIP, chromatin immunoprecipitation; CycT1, cyclin T1; DEX, dexamethasone; GR, glucocorticoid receptor; GRE, glucocorticoid response element; HA, hemagglutinin; HSF, heat shock factor; 3MC, 3-methylcholantrene; MOI, multiplicity of infection; PPAR, peroxisome proliferator-activated receptor; PPARRE, PPAR response element; qRT-PCR, quantitative real-time RT-PCR; P-TEFb, positive transcription elongation factor b; RNAPII, RNA polymerase II; siRNA, small interfering RNA; SDS, sodium dodecyl sulfate; snRNA, small nuclear RNA; SR, siRNA-resistant; STAT3, signal transducer and activator of transcription 3; SV, simian virus; TGZ, troglitazone; TSA, trichostatin A.
References
- 1.Duma D, Jewell CM, Cidlowski JA 2006. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol 102:11–21 [DOI] [PubMed] [Google Scholar]
- 2.Beato M 1989. Gene regulation by steroid hormones. Cell 56:335–344 [DOI] [PubMed] [Google Scholar]
- 3.Sapolsky RM, Romero LM, Munck AU 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- 4.Rhen T, Cidlowski JA 2005. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med 353:1711–1723 [DOI] [PubMed] [Google Scholar]
- 5.Lu NZ, Cidlowski JA 2004. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann NY Acad Sci 1024:102–123 [DOI] [PubMed] [Google Scholar]
- 6.Lingrel JB, Orlowski J, Shull MM, Price EM 1990. Molecular genetics of Na,K-ATPase. Prog Nucleic Acid Res Mol Biol 38:37–89 [DOI] [PubMed] [Google Scholar]
- 7.Bens M, Chassin C, Vandewalle A 2006. Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells. Pflugers Arch 453:133–146 [DOI] [PubMed] [Google Scholar]
- 8.Garty H, Palmer LG 1997. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77:359–396 [DOI] [PubMed] [Google Scholar]
- 9.Bonny O, Hummler E 2000. Dysfunction of epithelial sodium transport: from human to mouse. Kidney Int 57:1313–1318 [DOI] [PubMed] [Google Scholar]
- 10.Gick GG, Ismail-Beigi F, Edelman IS 1988. Thyroidal regulation of rat renal and hepatic Na,K-ATPase gene expression. J Biol Chem 263:16610–16618 [PubMed] [Google Scholar]
- 11.Ismail-Beigi F, Pressley TA, Haber RS, Gick GG, Loeb JN, Edelman IS 1988. Kinetic analysis of Na,K-activated adenosine triphosphatase induced by low external K+ in a rat liver cell line. J Biol Chem 263:8162–8167 [PubMed] [Google Scholar]
- 12.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995. The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beato M, Herrlich P, Schutz G 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- 14.Belandia B, Parker MG 2003. Nuclear receptors: a rendezvous for chromatin remodeling factors. Cell 114:277–280 [DOI] [PubMed] [Google Scholar]
- 15.Lonard DM, O'Malley B W 2007. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Thompson EB 2005. Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol 94:383–394 [DOI] [PubMed] [Google Scholar]
- 17.Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA 2006. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev 58:782–797 [DOI] [PubMed] [Google Scholar]
- 18.Woychik NA, Hampsey M 2002. The RNA polymerase II machinery: structure illuminates function. Cell 108:453–463 [DOI] [PubMed] [Google Scholar]
- 19.Kadonaga JT 2004. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell 116:247–257 [DOI] [PubMed] [Google Scholar]
- 20.Phuc Le P, Friedman JR, Schug J, Brestelli JE, Parker JB, Bochkis IM, Kaestner KH 2005. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet 1:e16 [DOI] [PMC free article] [PubMed]
- 21.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR 2007. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet 3:e94 [DOI] [PMC free article] [PubMed]
- 22.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD 2004. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res 64:1757–1764 [DOI] [PubMed] [Google Scholar]
- 23.Rogatsky I, Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Ha CM, Darimont BD, Garabedian MJ, Yamamoto KR 2003. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci USA 100:13845–13850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM 2004. 11β-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 25:831–866 [DOI] [PubMed] [Google Scholar]
- 25.Lonard DM, O'Malley BW 2005. Expanding functional diversity of the coactivators. Trends Biochem Sci 30:126–132 [DOI] [PubMed] [Google Scholar]
- 26.Lu NZ, Cidlowski JA 2006. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol 16:301–307 [DOI] [PubMed] [Google Scholar]
- 27.Kusuhara M, Nagasaki K, Kimura K, Maass N, Manabe T, Ishikawa S, Aikawa M, Miyazaki K, Yamaguchi K 1999. Cloning of hexamethylene-bis-acetamide-inducible transcript, HEXIM1, in human vascular smooth muscle cells. Biomed Res 20:273–279 [Google Scholar]
- 28.Michels AA, Nguyen VT, Fraldi A, Labas V, Edwards M, Bonnet F, Lania L, Bensaude O 2003. MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol Cell Biol 23:4859–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q 2003. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell 12:971–982 [DOI] [PubMed] [Google Scholar]
- 30.Shimizu N, Ouchida R, Yoshikawa N, Hisada T, Watanabe H, Okamoto K, Kusuhara M, Handa H, Morimoto C, Tanaka H 2005. HEXIM1 forms a transcriptionally abortive complex with glucocorticoid receptor without involving 7SK RNA and positive transcription elongation factor b. Proc Natl Acad Sci USA 102:8555–8560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sims RJ, 3rd, Belotserkovskaya R, Reinberg D 2004. Elongation by RNA polymerase II: the short and long of it. Genes Dev 18:2437–2468 [DOI] [PubMed] [Google Scholar]
- 32.Malik S, Roeder RG 2005. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem Sci 30:256–263 [DOI] [PubMed] [Google Scholar]
- 33.Peterlin BM, Price DH 2006. Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23:297–305 [DOI] [PubMed] [Google Scholar]
- 34.Karn J 1999. Tackling Tat. J Mol Biol 293:235–254 [DOI] [PubMed] [Google Scholar]
- 35.Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM 2001. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol Cell 8:327–337 [DOI] [PubMed] [Google Scholar]
- 36.Giraud S, Hurlstone A, Avril S, Coqueret O 2004. Implication of BRG1 and cdk9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene 23:7391–7398 [DOI] [PubMed] [Google Scholar]
- 37.Boehm AK, Saunders A, Werner J, Lis JT 2003. Transcription factor and polymerase recruitment, modification, and movement on dhsp70 in vivo in the minutes following heat shock. Mol Cell Biol 23:7628–7637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Y, Ke S, Chen M, Sheng T 2003. Interactions between the aryl hydrocarbon receptor and P-TEFb. Sequential recruitment of transcription factors and differential phosphorylation of C-terminal domain of RNA polymerase II at cyp1a1 promoter. J Biol Chem 278:44041–44048 [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q, Yik JH 2006. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev 70:646–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dey A, Chao SH, Lane DP 2007. HEXIM1 and the control of transcription elongation: from cancer and inflammation to AIDS and cardiac hypertrophy. Cell Cycle 6:1856–1863 [DOI] [PubMed] [Google Scholar]
- 41.Ouchida R, Kusuhara M, Shimizu N, Hisada T, Makino Y, Morimoto C, Handa H, Ohsuzu F, Tanaka H 2003. Suppression of NF-κB-dependent gene expression by a hexamethylene bisacetamide-inducible protein HEXIM1 in human vascular smooth muscle cells. Genes Cells 8:95–107 [DOI] [PubMed] [Google Scholar]
- 42.Wittmann BM, Wang N, Montano MM 2003. Identification of a novel inhibitor of breast cell growth that is down-regulated by estrogens and decreased in breast tumors. Cancer Res 63:5151–5158 [PubMed] [Google Scholar]
- 43.Haaland RE, Herrmann CH, Rice AP 2005. siRNA depletion of 7SK snRNA induces apoptosis but does not affect expression of the HIV-1 LTR or P-TEFb-dependent cellular genes. J Cell Physiol 205:463–470 [DOI] [PubMed] [Google Scholar]
- 44.Montano MM, Doughman YQ, Deng H, Chaplin L, Yang J, Wang N, Zhou Q, Ward NL, Watanabe M 2008. Mutation of the HEXIM1 gene results in defects during heart and vascular development partly through downregulation of vascular endothelial growth factor. Circ Res 102:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wittmann BM, Fujinaga K, Deng H, Ogba N, Montano MM 2005. The breast cell growth inhibitor, estrogen down regulated gene 1, modulates a novel functional interaction between estrogen receptor alpha and transcriptional elongation factor cyclin T1. Oncogene 24:5576–5588 [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Cooper JJ, Altwerger GH, Feldkamp MD, Shea MA, Price DH 2007. HEXIM1 is a promiscuous double-stranded RNA-binding protein and interacts with RNAs in addition to 7SK in cultured cells. Nucleic Acids Res 35:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang F, Wagner M, Siddiqui MA 2004. Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death. Mech Dev 121:559–572 [DOI] [PubMed] [Google Scholar]
- 48.Yoshikawa N, Shimizu N, Sano M, Ohnuma K, Iwata S, Hosono O, Fukuda K, Morimoto C, Tanaka H 2008. Role of the hinge region of glucocorticoid receptor for HEXIM1-mediated transcriptional repression. Biochem Biophys Res Commun 371:44–49 [DOI] [PubMed] [Google Scholar]
- 49.Shull MM, Pugh DG, Lingrel JB 1990. The human Na, K-ATPase α 1 gene: characterization of the 5′-flanking region and identification of a restriction fragment length polymorphism. Genomics 6:451–460 [DOI] [PubMed] [Google Scholar]
- 50.Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP 2002. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5′-flanking region. Am J Physiol Endocrinol Metab 283:E971–E979 [DOI] [PubMed]
- 51.Kohoutek J, Blazek D, Peterlin BM 2006. Hexim1 sequesters positive transcription elongation factor b from the class II transactivator on MHC class II promoters. Proc Natl Acad Sci USA 103:17349–17354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aida M, Chen Y, Nakajima K, Yamaguchi Y, Wada T, Handa H 2006. Transcriptional pausing caused by NELF plays a dual role in regulating immediate-early expression of the junB gene. Mol Cell Biol 26:6094–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishikawa T, Igarashi T, Hata K, Fujita T 1999. c-fos induction by heat, arsenite, and cadmium is mediated by a heat shock element in its promoter. Biochem Biophys Res Commun 254:566–571 [DOI] [PubMed] [Google Scholar]
- 54.Ni Z, Schwartz BE, Werner J, Suarez JR, Lis JT 2004. Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol Cell 13:55–65 [DOI] [PubMed] [Google Scholar]
- 55.Yik JH, Chen R, Pezda AC, Samford CS, Zhou Q 2004. A human immunodeficiency virus type 1 Tat-like arginine-rich RNA-binding domain is essential for HEXIM1 to inhibit RNA polymerase II transcription through 7SK snRNA-mediated inactivation of P-TEFb. Mol Cell Biol 24:5094–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starr DB, Matsui W, Thomas JR, Yamamoto KR 1996. Intracellular receptors use a common mechanism to interpret signaling information at response elements. Genes Dev 10:1271–1283 [DOI] [PubMed] [Google Scholar]
- 57.Schulte A, Czudnochowski N, Barboric M, Schonichen A, Blazek D, Peterlin BM, Geyer M 2005. Identification of a cyclin T-binding domain in Hexim1 and biochemical analysis of its binding competition with HIV-1 Tat. J Biol Chem 280:24968–24977 [DOI] [PubMed] [Google Scholar]
- 58.Dames SA, Schonichen A, Schulte A, Barboric M, Peterlin BM, Grzesiek S, Geyer M 2007. Structure of the Cyclin T binding domain of Hexim1 and molecular basis for its recognition of P-TEFb. Proc Natl Acad Sci USA 104:14312–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dulac C, Michels AA, Fraldi A, Bonnet F, Nguyen VT, Napolitano G, Lania L, Bensaude O 2005. Transcription-dependent association of multiple positive transcription elongation factor units to a HEXIM multimer. J Biol Chem 280:30619–30629 [DOI] [PubMed] [Google Scholar]
- 60.Barboric M, Kohoutek J, Price JP, Blazek D, Price DH, Peterlin BM 2005. Interplay between 7SK snRNA and oppositely charged regions in HEXIM1 direct the inhibition of P-TEFb. EMBO J 24:4291–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blazek D, Barboric M, Kohoutek J, Oven I, Peterlin BM 2005. Oligomerization of HEXIM1 via 7SK snRNA and coiled-coil region directs the inhibition of P-TEFb. Nucleic Acids Res 33:7000–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gurumurthy M, Tan CH, Ng R, Zeiger L, Lau J, Lee J, Dey A, Philp R, Li Q, Lim TM, Price DH, Lane DP, Chao SH 2008. Nucleophosmin interacts with HEXIM1 and regulates RNA polymerase II transcription. J Mol Biol 378:302–317 [DOI] [PubMed] [Google Scholar]
- 63.Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP 2003. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol 85:457–467 [DOI] [PubMed] [Google Scholar]
- 64.Tanaka H, Yoshikawa N, Shimizu N, Morimoto C 2004. Selective modulation of glucocorticoid receptor function toward development of novel anti-inflammation: lessons from a phenyl-pyrazolo-steroid cortivazol. Mod Rheumatol 14:347–355 [DOI] [PubMed] [Google Scholar]
- 65.Yoshikawa N, Yamamoto K, Shimizu N, Yamada S, Morimoto C, Tanaka H 2005. The distinct agonistic properties of the phenylpyrazolosteroid cortivazol reveal interdomain communication within the glucocorticoid receptor. Mol Endocrinol 19:1110–1124 [DOI] [PubMed] [Google Scholar]