KEY TEACHING POINTS
|
Introduction
Brugada syndrome (BrS) is an inherited cardiac arrhythmia syndrome associated with electrocardiography (ECG) patterns characterized by incomplete right bundle branch block and ST segment elevation in the anterior precordial leads, correlated to an increased risk of sudden cardiac death. Genetic substrate, ECG manifestation, and risk of arrhythmia in patients with BrS vary considerably. A new strategy for the treatment of BrS patients with recurrent episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) is based on the catheter ablation of the arrhythmogenic substrate in the right ventricular outflow tract (RVOT), identified by invasive cardiac mapping.1, 2 A promising alternative to catheter-based cardiac mapping is a noninvasive electrocardiographic imaging (ECGI).3, 4 The current case study demonstrates the use of ECGI for diagnosis of a patient with BrS.
Case Report
We evaluated members of a family with asymptomatic BrS. Consistent spontaneous coved-type ST segment elevation up to 2 mV as well as T-wave inversion in V1 and V2 leads were diagnosed in a 5-year-old male proband during ECG screening. His 40-year-old mother had isolated syncopes during childhood and slight stress-induced dizziness during the last year. She had no documented heart rhythm disorders or cardiac arrest history. No significant changes were registered in 12-lead ECG as well as during 24-hour Holter monitoring. Cascade familial genetic screening revealed that the proband’s mother and 3 siblings carry mutation c.1233del in SCN5A gene.
An ajmaline challenge was performed for the proband’s mother with intravenous administration of 1 mg per kg bodyweight in 10 minutes. Starting at a dosage of 0.6 mg per kg bodyweight, the patient gradually developed a typical diagnostic ECG pattern of coved-type ST elevation up to 6 mV above baseline with inverted T-waves in leads V1 and V2 (Figure, a). During electrophysiology (EP) study polymorphic VT was induced using standard pacing protocol with double extrastimuli (550/280/220 ms) from the right ventricular apex.
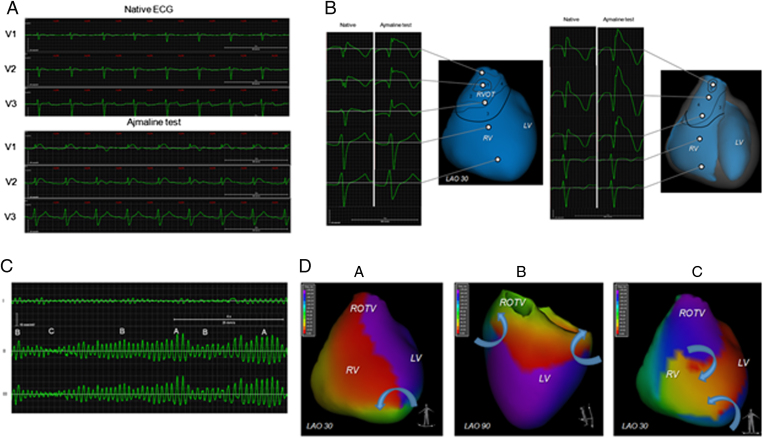
Figure.
A: Electrocardiography (ECG) in V1–V3 leads in the patient with Brugada syndrome before and at sixth minute of ajmaline challenge. B: Reconstructed epicardial and endocardial electrogram before and at sixth minute of ajmaline challenge. C: A, B, and C ECG patterns of induced polymorphic ventricular tachycardia. D: Phase maps of ventricular activation matching the A, B, and C ECG patterns.
Recording of local unipolar electrograms (EG) was performed using the AMYCARD 01C system (EP Solutions SA, Yverdon-les-Bains, Switzerland). This procedure included the following steps: (1) 224 body surface mapping electrodes were applied to the torso of the patient; (2) the patient underwent computed tomography (CT) imaging of the heart and torso (Somatom Definition Flash, Siemens AG, Germany); (3) body surface ECG was recorded at rest, during ajmaline challenge, in the EP laboratory; and finally (4) a CT-based 3D heart model was reconstructed and epicardial/endocardial local unipolar EGs were calculated at rest, during ajmaline challenge, and during EP study. In addition, phase maps of induced polymorphic VT were analyzed.
Despite a normal ECG pattern with standard 12-lead ECG at rest, we observed fractionated EG with ST segment elevation (>2 mV) in the epicardium and ST elevation without fractionation in the endocardium of the RVOT. After ajmaline was administered intravenously, EG changes were augmented, reflecting an expansion of the area with abnormal EG and also the appearance of fragmentation in the right ventricular endocardium, which were not present under resting conditions (Figure, b). The area of EG with the elevated ST segment expanded from 2.7 cm2 to 22.5 cm2 and from 1.8 cm2 to 17.4 cm2 on the epicardium and endocardium, respectively. The area of fractioned EG expanded from 6.7 cm2 to 16.3 cm2 on the epicardium and was 12.5 cm2 on the endocardium.
Induced VT lasted 17 seconds and was terminated by electrical cardioversion. The phase mapping showed the following results. The primary vortex movement of the phase front emerged in the anterior aspect of the RVOT. Immediately after the initiation of tachycardia the core of the first rotor rotating counterclockwise (CCW) was located stationary at the RVOT for 1 cycle. The core of the second rotor, rotating clockwise (CW), drifted toward the posterior part of the left ventricle (LV). Then the CCW rotor moved to the apex and the CW rotor took a position at the basal posteroseptal zone of the LV, and rotation of the phase front around the axis of the heart was observed. The phase singularity points were not identified at the endocardium because the filament connecting phase singularity points was located in the ventricular septum. These sequences were maintained during the 4 cycles of tachycardia (pattern A) (see supplementary material, Video 1). After that, the core of the CCW rotor drifted in the direction of the RVOT and the CW rotor drifted to the lateral wall of the LV. One to 3 secondary vortex waves appeared and the collision of wave fronts was observed (pattern C) (see supplementary material, Video 2). On the eighth second the process became more organized again. The cores of the CCW and CW rotors occupied stable positions at the lateral wall of the RV and the LV, and the rotation axis became perpendicular to the interventricular septum. The points of the phase singularity at the epicardium corresponded to phase singularity points at the endocardium (pattern B) (see supplementary material, Video 3). This pattern was maintained during the 14 cycles of tachycardia. After that, until the VT termination, the multi-wavelet process (pattern C) was observed.
Pattern A corresponded to high-amplitude periodic activity on ECG and cycle length was 176 ms; pattern B corresponded to medium-amplitude periodic activity and cycle length was 168 ms; and pattern C matched low-amplitude, irregular ECG activity (Figure, c and d).
A single-chamber implantable cardioverter-defibrillator (Teligen VR; Boston Scientific, Marlborough, MA) was implanted in accordance with clinical guidelines (IIb class).5
Discussion
Recent studies provided evidence that cellular electrophysiological changes leading to ECG abnormalities and ventricular arrhythmias are located in the RVOT. A number of studies including epicardial and endocardial invasive mapping of the RVOT were performed to identify the electrophysiological substrate of BrS patients.1, 2, 6, 7, 8, 9 Epicardial ablation of those abnormal EG zones led to 12-lead ECG normalization and reduced VF recurrences.1, 8, 9 Yakul and colleagues2 found abnormal late activation zones at the RVOT based on noncontact endocardial mapping. Endocardial catheter ablation of these zones normalized 12-lead ECG and suppressed VT storm.
In the presented case we found significantly modified EG in the RVOT for a patient with normal surface ECG. The noninvasively revealed EG abnormalities at the RVOT corresponded with data described in the above publications. Additionally, EG changes, although less pronounced, were present not only on the epicardium but also in the endocardium. It is concluded that ECGI can visualize the EP substrate of BrS even in patients with normal surface ECG and would be helpful to recognize the potential target for catheter ablation as well.
Two hypotheses were discussed for the underlying mechanism of polymorphic VT and VF: the “mother rotor” and the multi-wavelet hypotheses.10, 11, 12 This observation suggests that there is evidence for both mechanisms of polymorphic VT and VF in BrS patients and these mechanisms are not mutually exclusive. ECGI provides several tools to study cardiac arrhythmias, including analysis of reconstructed local unipolar EG at the cardiac surface and activation mapping of the ventricles. In addition, ECGI supports phase mapping of the reentrant arrhythmias. Rendered phases of EG represent the phases of the electrical activation-recovery cycle of cardiac tissue and can be used for identification of reentry. Moreover, phase mapping allows identification and tracking of the phase singularity point, which corresponds to the center of rotation of the vortex waves (“rotors”).13
In general, the observed patterns of polymorphic VT looked similar to those described in human studies,10, 11, 12 but the observed reentry process was more organized. It is noteworthy that the vortex wave first appeared in the center of the abnormal EG zone of the RVOT. But the rotors’ cores stayed in the RVOT for not more than 20% of the polymorphic VT event duration. From this observation we may draw the conclusion that in patients with BrS the RVOT arrhythmogenic substrate plays an important role for the induction of triggered arrhythmia, but after the ventricular arrhythmia becomes a self-sustaining process that does not depend on the RVOT substrate.
Limitations
ECGI methodology was extensively validated in a series of animal and human studies.3, 4, 14 The combination of optical mapping and phase analysis of optical action potentials has thus become a well-established technique for this kind of experimental investigation of reentrant arrhythmias.13 However, phase mapping based on unipolar EG is a relatively new approach that requires additional validation. It should also be noted that there was no direct comparison between ECGI and intracardiac mapping during pharmacologic testing and VT or VF.
Conclusions
The application of ECGI in addition to conventional diagnostic technique allows the assessment of pathologic EG abnormalities in the endocardium and epicardium of the RVOT in patients with BrS, which are not acquirable in standard 12-lead ECG. Furthermore, this method allows for panoramic phase mapping of VT and VF.
Footnotes
Maria Chaykovskaya and Alexey Tsyganov are consultants to EP Solutions SA.
Supplementary material cited in this article is available online at doi:10.1016/j.hrcr.2015.04.009.
Appendix. Supplementary materials
References
- 1.Nademanee K., Veerakul G., Chandanamattha P., Chaothawee L., Ariyachaipanich A., Jirasirirojanakorn K., Likittanasombat K., Bhuripanyo K., Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123(12):1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 2.Yakul B.S., Yao Y., Thamaree S. Endocardial mapping and catheter ablation for ventricular fibrillation prevention in Brugada syndrome. J Cardiovasc Electrophysiol. 2012;23:S10–S16. doi: 10.1111/j.1540-8167.2012.02433.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghanem R.N., Jia P., Ramanathan C., Ryu K., Markowitz A., Rudy Y. Non-invasive electrocardiographic imaging (ECGI): comparison to intraoperative mapping in patients. Heart Rhythm. 2005;2(4):339–354. doi: 10.1016/j.hrthm.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Cuculich P.S., Zhang J., Desouza K.A., Vijayakumar R., Chen J., Faddis M.N., Lindsay B.D., Smith T.W., Rudy Y. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci Transl Med. 2011;3(98) doi: 10.1126/scitranslmed.3002152. 98ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Nagase S., Kusano K.F., Morita H., Fujimoto Y., Kakishita M., Nakamura K., Emori T., Matsubara H., Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome using the epicardial lead. J Am Coll Cardiol. 2002;39(12):1992–1995. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]; Nagase S., Kusano K.F., Morita H., Nishii N., Banba K., Watanabe A., Hiramatsu S., Nakamura K., Sakuragi S., Ohe T. Longer repolarization in the epicardium at the right ventricular outflow tract causes type 1 electrocardiogram in patients with Brugada syndrome. J Am Coll Cardiol. 2008;51(12):1154–1161. doi: 10.1016/j.jacc.2007.10.059. #. [DOI] [PubMed] [Google Scholar]
- 7.Postema P.G., van Dessel P.F., de Bakker J.M., Dekker L.R., Linnenbank A.C., Hoogendijk M.G., Coronel R., Tijssen J.G., Wilde A.A., Tan H.L. Slow and discontinuous conduction conspire in Brugada syndrome: a right ventricular mapping and stimulation study. Circ Arrhythmia Electrophysiol. 2008;1:379–386. doi: 10.1161/CIRCEP.108.790543. [DOI] [PubMed] [Google Scholar]
- 8.Sacher F., Jesel L, Jais P, Haissaguerre M. Insight into the mechanism of Brugada syndrome: epicardial substrate and modification during ajmaline testing. Heart Rhythm. 2014;11:732–734. doi: 10.1016/j.hrthm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Cortez-Dias N., Placido R., Marta L., Bernardes A., Sobral S., Carpinteiro L., de Sousa J. Epicardial ablation for prevention of ventricular fibrillation in a patient with Brugada Syndrome. Rev Port Cardiol. 2014;33(5):305.e1–305.e7. doi: 10.1016/j.repc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Masse S., Downar E., Chauhan V. Ventricular fibrillation in myopathic human hearts: mechanistic insights from in vivo global endocardial and epicardial mapping. Am J Physiol Heart Circ Physiol. 2007:292. doi: 10.1152/ajpheart.01336.2006. [DOI] [PubMed] [Google Scholar]
- 11.Nash M.P., Mourad A., Clayton R.H. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–542. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- 12.Ten Tusscher K.H., Mourad A., Nash M.P. Organization of ventricular fibrillation in the human heart: experiments and models. Exp Physiol. 2009;94:553–562. doi: 10.1113/expphysiol.2008.044065. [DOI] [PubMed] [Google Scholar]
- 13.Clayton R.H., Nash M.P. Analysis of cardiac fibrillation using phase mapping. Card Electrophysiol Clin. 2015;7(1):49–58. doi: 10.1016/j.ccep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Revishvili A.S., Wissner E., Lebedev D.S. Validation of the mapping accuracy of a novel non-invasive epicardial and endocardial electrophysiology system. Europace. 2015 Feb 2 doi: 10.1093/europace/euu339. pii: euu339. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.