Abstract
In the present study, we demonstrate that leupaxin mRNA is overexpressed in prostate cancer (PCa) as compared with normal prostate tissue by using cDNA arrays and quantitative RT-PCR analyses. Moderate to strong expression of leupaxin protein was detected in approximately 22% of the PCa tissue sections analyzed, and leupaxin expression intensities were found to be significantly correlated with Gleason patterns/scores. In addition, different leupaxin expression levels were observed in PCa cell lines, and at the subcellular level, leupaxin was usually localized in focal adhesion sites. Furthermore, mutational analysis and transfection experiments of LNCaP cells using different green fluorescent protein-leupaxin constructs demonstrated that leupaxin contains functional nuclear export signals in its LD3 and LD4 motifs, thus shuttling between the cytoplasm and the nucleus. We could also demonstrate for the first time that leupaxin interacts with the androgen receptor in a ligand-dependent manner and serves as a transcriptional activator of this hormone receptor in PCa cells. Down-regulation of leupaxin expression using RNA interference in LNCaP cells resulted in a high rate of morphological changes, detachment, spontaneous apoptosis, and a reduction of prostate-specific antigen secretion. In contrast, knockdown of leupaxin expression in androgen-independent PC-3 and DU 145 cells induced a significant decrease of both the invasive capacity and motility. Our results therefore indicate that leupaxin could serve as a potential progression marker for a subset of PCa and may represent a novel coactivator of the androgen receptor. Leupaxin could function as a putative target for therapeutic interventions of a subset of advanced PCa.
PROSTATE CANCER (PCa) is the most frequent cancer in the industrialized countries and the second leading cause of cancer-related deaths in men (1). The cancer first develops as an androgen-dependent lesion in the prostate gland that can be successfully treated with surgical removal of the tumor or local radiation. Patients with locally advanced and metastatic diseases are treated by androgen deprivation therapy that is often combined with androgen receptor (AR) antagonists to achieve maximal androgen blockade. The androgen deprivation therapy initially stabilizes more than 80% of the patients; however, patients generally relapse within 18–24 months (2), showing a progression of the tumor to a hormone-refractory PCa (3).
Studies performed on tissue specimens of PCa patients have shown that the AR is expressed in most cancers of the prostate after androgen ablation therapy, indicating that loss of androgen dependence is not related to loss of expression of the AR (4). The mechanisms proposed to explain the development of hormone-refractory PCa can be separated into three general categories (5). The first category comprises the unmasking of pathways that facilitate proliferation and inhibit apoptosis, e.g. the up-regulated expression of the antiapoptotic Bcl-2 gene in late-stage clinical PCa samples or AR gene methylation in some hormone-refractory cancers (6, 7, 8). The second category involves the AR gene itself; i.e. amplification of the AR gene or point mutations were reported to occur in 0–5 and 10–20% of PCa specimens (3), respectively, and these AR modifications may cause resistance by altering the response of the receptor such that ligands other than testosterone or even AR antagonists behave as agonists (9, 10). The third category of androgen escape mechanisms includes the control of the AR function via phosphorylation by various kinase cascades. Increased MAPK or phosphoinositide 3-kinase/AKT signaling mediated by oncogenes causes ligand-independent activation of the AR, resulting in an increased recruitment of AR cofactors (coactivators and corepressors) and transcriptional activity of the AR (3, 11, 12, 13). Similarly, alterations in the balance between coactivators and corepressors can affect AR activation (14, 15).
AR coactivators may be involved in the development of androgen-insensitive tumors, increasing AR activity under conditions of low ligand concentrations, e.g. during androgen deprivation therapy, or altering the specificity of the AR and thus allowing anti-androgens and estrogens to bind and activate the AR. Interestingly, a recent study demonstrated that coactivators SRC-1, p300, Tip60, SRC-3 and c-Jun activated the AR in the absence of ligand and were overexpressed in hormone-refractory PCa or PCa cell lines (16).
The paxillin protein family belongs to the complex group of AR coactivators, and the members of this family consist of paxillin, AR-associated protein 55 (ARA55), and leupaxin (reviewed in Ref. 17). The most common characteristic of this protein family is its conserved domain structure; i.e. all three members contain either four (ARA55 and leupaxin) or five (paxillin) leucine-rich LD-motifs in the N-terminal region and four LIM domains in the C-terminal part of the protein. Because these domains are protein-protein binding interfaces, the members of the paxillin protein family are thought to be adaptor molecules. Furthermore, the LIM domains were shown to mediate the localization of paxillin and ARA55 to focal adhesions (18, 19), but both paxillin and ARA55 were also associated with the nucleus (20, 21). Paxillin was first characterized as a 68-kDa focal adhesion protein (22) with the principle function of integrating and disseminating signals from integrins and growth factor receptors to affect efficient cellular migration. It was recently shown that paxillin potentiates AR transactivation in PCa cell lines in a ligand-dependent manner (20). This result is consistent with an earlier finding that paxillin expression is up-regulated by androgen deprivation (23). The second member of the paxillin protein family, ARA55, which was first cloned as a TGFβ-inducible gene, was later characterized by Fujimoto et al. (24) as an AR coactivator. The latter study demonstrated that ARA55 can enhance AR transcriptional activity in the presence of the agonist dihydrotestosterone (DHT) or the antagonists E2 or hydroxyflutamide. Furthermore, it was shown that phosphorylation of ARA55 by proline-rich tyrosine kinase 2 (PYK2) decreases AR activity, because phosphorylated ARA55 cannot interact with the AR. Moreover, expression of PYK2 is reduced in advanced PCa (25), resulting in decreased ARA55 phosphorylation and increased AR/ARA55 interaction (26) and thus increasing AR-mediated transcription and prostate-specific antigen (PSA) secretion. The third member of the paxillin protein family, leupaxin, was originally identified as a cytoskeletal protein preferentially expressed in hematopoietic cells (27). On the one hand, leupaxin was shown to interact with some paxillin- and ARA55-binding proteins such as focal adhesion kinase (FAK), PYK2, protein tyrosine phosphatase-PEST, and p95 paxillin-kinase linker and to be an important adaptor protein in the formation of the adhesion zone in osteoclasts (28) as well. On the other hand, to date, there are no reports on the role of leupaxin as an interacting partner or coactivator of the AR and the involvement of leupaxin in PCa progression.
In the present study, we show that leupaxin is expressed in PCa cells and that leupaxin expression intensity is directly linked to PCa progression. In addition, our study highlights different roles for leupaxin in PCa cell survival, invasion, and motility as well as being a modulator of AR-mediated functions in PCa.
RESULTS
Up-Regulated Leupaxin Expression in PCa Cells
To identify genes that are differentially expressed in intraprostatic tumor tissues and capsule-penetrating tumor areas as compared with normal prostate tissue, RNA samples from one patient were isolated after laser capture microdissection (LCM). Subsequently, these RNA samples were amplified and used as probes to hybridize three identical Atlas Select human tumor arrays. After densitometric evaluation of the arrays, leupaxin was found to be up-regulated in both intraprostatic tumor tissue and capsular-penetrating prostate carcinoma as compared with matched normal prostate tissue (Fig. 1A). Subsequently, leupaxin expression was studied on the same RNA samples by quantitative RT-PCR. Leupaxin expression was up-regulated (>41-fold) in the intraprostatic tumor tissue sample as compared with normal prostate tissue, whereas 2.8-fold increased leupaxin expression was observed in the capsule-penetrating tumor as compared with central tumor tissue. In addition, leupaxin was found to be overexpressed in two of eight additional intraprostatic tumor tissue samples compared with normal prostate tissues (data not shown).
Fig. 1.
Leupaxin Expression in Prostate Carcinoma
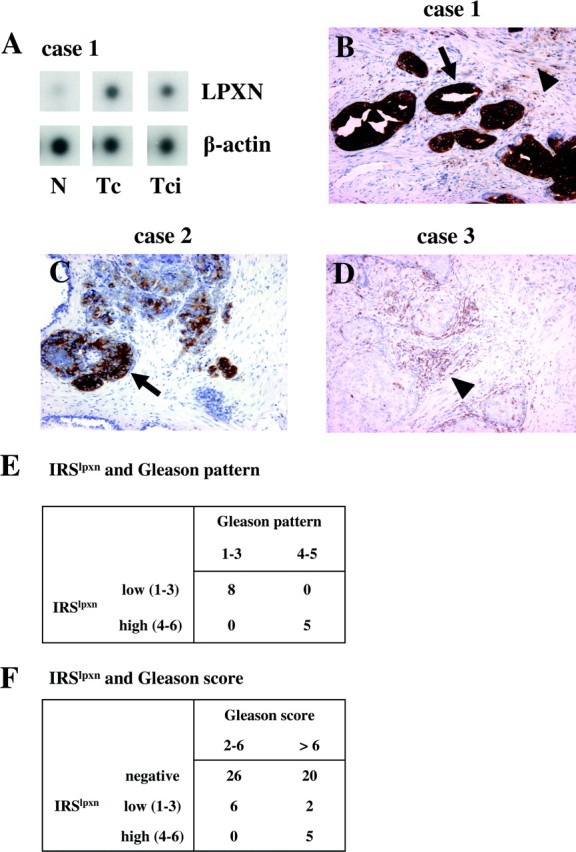
A, Laser capture microdissection (LCM)-derived RNA from normal (N), tumor central (Tc), and tumor-capsular invasive (Tci) prostate tissue (case 1) was labeled and hybridized to three identical Atlas Select Human Tumor array membranes. The signals were quantified using a Molecular Imager FX and normalized to the housekeeping gene β-actin spotted on the membrane. B–D, Expression of leupaxin in PCa sections by using immunohistochemistry. Sections of radical prostatectomies were stained with a leupaxin-specific antibody (clone 283G). Leupaxin is expressed in PCa cells (arrows) and infiltrating lymphocytes (arrowheads). The sections were counterstained with hemalum. Immunohistochemical staining of cases 1 and 2 correspond to cases 1 and 2 presented in supplemental Fig. 1. E, Leupaxin expression correlates with the Gleason pattern in leupaxin positive PCa specimens. F, Positive correlation between the Gleason score and leupaxin expression in PCa.
To investigate the expression of leupaxin in PCa specimens at the cellular level, standard tissue sections of 59 human prostate carcinomas comprising seven needle biopsies and 52 radical prostatectomy specimens were analyzed using a leupaxin-specific antibody. Epithelial leupaxin staining was observed in 13 cases (22%) exclusively in prostate carcinoma cells but not in normal glands, hyperplastic glands, or stromal cells (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Two different staining patterns for leupaxin expression were observed in PCa tissues, 1) a strong and diffuse staining for leupaxin in each gland and in each carcinoma cell of the prostate tumor (Fig. 1B) and 2) a low to intermediate but focal leupaxin expression in prostate carcinoma glands and cells (Fig. 1C), whereas strong leupaxin staining of lymphocytes and macrophages was always observed (Fig. 1D). Because leupaxin is often expressed focally, the needle biopsy specimens stained negative for leupaxin. We also compared the immunoreactive score for leupaxin (IRSlpxn) with the Gleason patterns of positively stained tumor areas. A high IRSlpxn (score 4–6) was found to be significantly associated with a Gleason pattern of 4 and 5 in comparison with a low IRSlpxn (score 1–3), which was associated with a Gleason pattern of 2 and 3 (P = 0.0008, Fisher’s exact test; Fig. 1E). Comparison of the IRSlpxn with the overall Gleason score of the individual prostate carcinoma confirmed these results (P = 0.02, χ2 test; Fig. 1F).
Expression and Subcellular Localization of Leupaxin in PCa Cell Lines
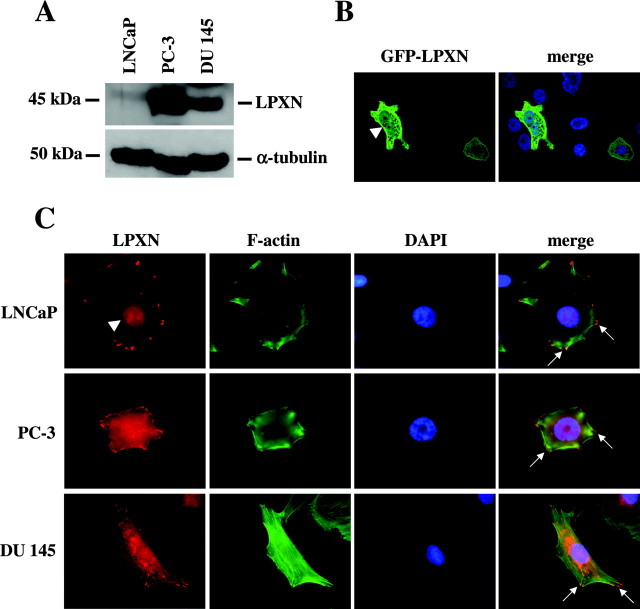
Leupaxin expression was also studied in LNCaP, PC-3, and DU 145 PCa cells. Western blot analyses clearly demonstrated strong leupaxin expression in invasive and androgen-independent PC-3 and DU 145 cells, respectively, as compared with a weaker leupaxin expression in noninvasive and androgen-dependent LNCaP cells (Fig. 2A). To examine leupaxin expression at the subcellular level, PC-3 cells were transfected with a green fluorescent protein (GFP)-leupaxin fusion construct demonstrating strong leupaxin staining in the cytoplasm in the majority of cells and weak leupaxin staining in the nucleus of 1–3% of transfected cells (Fig. 2B). Furthermore, LNCaP, PC-3, and DU 145 cells were grown on fibronectin (FN)-coated slides, fixed, and stained using a leupaxin-specific antibody. As can be seen in Fig. 2C and supplemental Fig. 2A, leupaxin was mainly localized in the cytoplasm and at focal adhesion sites in LNCaP cells, and this localization pattern was also observed in PC-3 and DU 145 cells (Fig. 2C). Again, about 1–3% of LNCaP, PC-3, and DU 145 cells showed an additional weak or moderate expression of leupaxin in the nucleus (Fig. 2C). Because PCa cells were grown on FN-coated slides for localization experiments, a possible influence of FN signaling on leupaxin expression was further studied. Protein extracts isolated from PCa cells grown on FN-coated slides and on noncoated slides were analyzed by Western blot, demonstrating that FN treatment did not alter leupaxin expression in PCa cells (supplemental Fig. 2B).
Fig. 2.
Expression of Leupaxin in Established PCa Cell Lines
A, Western blot analysis of whole-cell extracts from PCa cell lines using an anti-leupaxin antibody (clone 283C) detected a leupaxin-specific band at 45 kDa. Equal sample amounts were checked with a monoclonal anti-α-tubulin antibody. B, PC-3 cells were transfected with a GFP-leupaxin fusion construct, fixed after 36 h, and stained with DAPI (blue). The majority of transfected cells showed strong GFP-leupaxin staining in the cytoplasm, whereas in a small percentage of transfected cells, a weak nuclear expression of leupaxin (arrowhead) was observed. C, LNCaP, PC-3, and DU 145 cells were grown on FN-coated slides, fixed, and subjected to immunofluorescent staining of leupaxin (red) and F-actin (phalloidin-FITC, green). The nuclei were visualized using DAPI (blue). Arrows indicate localization of leupaxin to focal adhesion sites, and the arrowhead points to a weak nuclear staining of leupaxin in an LNCaP cell.
Leupaxin Contains Nuclear Export Signals (NES) in Its LD Motifs
Because both paxillin and ARA55 contain a NES within their LD motif regions (29, 30), leupaxin shuttling between the cytoplasm and nucleus in PCa cells was investigated by treatment of LNCaP and PC-3 cells with leptomycin B (LMB) dissolved in ethanol, which is known to inhibit the CRM-1-mediated nuclear export (31). LMB treatment of LNCaP and PC-3 cells transfected with GFP-LPXN fusion constructs, respectively, led to a nuclear accumulation of fusion proteins (Fig. 3A), indicating that leupaxin has the ability to enter the nucleus but is exported immediately. As a control for specificity, LNCaP and PC-3 cells were treated with the solvent (ethanol) alone showing GFP-LPXN staining exclusively in the cytoplasm (supplemental Fig. 2C). Furthermore, quantification of cellular localization of GFP-LPXN fusion proteins revealed that 98% of both transfected LNCaP and PC-3 cells showed nuclear localization of GFP-LPXN after LMB treatment. In contrast, ethanol treatment of both cell lines caused cytoplasmic localization of the fusion protein in almost 100% of transfected cells. In addition, transfection of LNCaP cells with two different GFP-fusion constructs harboring either only the four LD motifs (GFP-LPXN-LD) or the four LIM domains (GFP-LPXN-LIM) of leupaxin revealed that GFP-LPXN-LD was exclusively distributed in the cytoplasm, whereas GFP-LPXN-LIM accumulated in the nucleus (Fig. 3B). Computational analysis of the leupaxin amino acid sequence revealed putative NES sequences (Fig. 3C) that are homologous to the NES consensus sequence published by Nix and Beckerle (32). Our combined mutation and transfection studies using the corresponding GFP-LPXN fusion proteins demonstrated that the putative NES between LD2 and LD3 is not responsible for the nuclear export of leupaxin, whereas the LD4 motif of leupaxin represents the major NES and the LD3 motif of leupaxin also partially contributes to this nuclear export function (Fig. 3D).
Fig. 3.
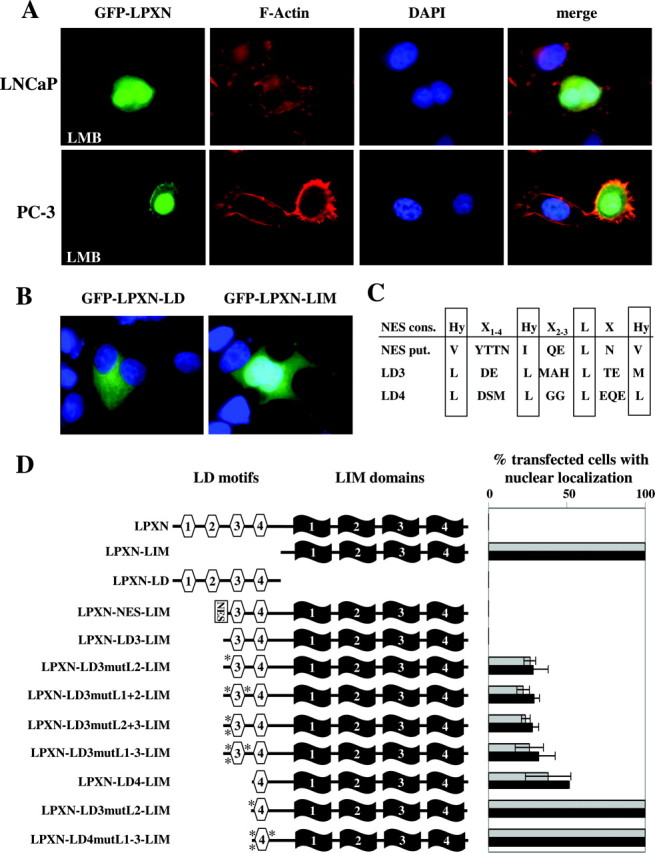
Leupaxin Contains NES
A, LNCaP or PC-3 cells were transfected with a GFP-LPXN fusion construct, incubated with LMB, fixed, and stained with phalloidin-tetramethylrhodamine isothiocyanate (red) and DAPI (blue). The GFP-LPXN fusion protein (green) accumulates in the nucleus after treatment with LMB. B, LNCaP cells were transfected with GFP-LPXN-LD and GFP-LPXN-LIM constructs, fixed and stained with DAPI. After transfection of LNCaP cells, the GFP-LPXN-LD fusion protein was exclusively distributed in the cytoplasm, whereas the GFP-LPXN-LIM fusion protein accumulated in the nucleus of transfected cells. C, Comparison of the NES consensus (NES cons.) sequence and putative NES sequences upstream of LD3 (NES put.) and in LD3 and LD4 motifs of the leupaxin sequence. D, LNCaP cells were grown on either uncoated (gray bars) or FN-coated (black bars) slides, transfected with different GFP-leupaxin constructs as indicated, fixed, and mounted in Vectashield DAPI. Transfection studies regarding the putative NES between LD2 and LD3 demonstrated that the corresponding fusion proteins GFP-LPXN-NES-LIM and GFP-LPXN-LD3-LIM did not show nuclear localization in LNCaP cells. To evaluate whether the LD3 motif in leupaxin is responsible for the nuclear export, the conserved leucine residues in the leupaxin LD3 motif were substituted by alanine residues in four different GFP-LPXN constructs (GFP-LPXN-LD3mut-LIM). Furthermore, a GFP-LPXN-LD4-LIM construct was generated lacking the entire LD3 motif. Approximately 40% of the transfected cells contained the mutated GFP-LPXN-LD3-LIM fusion proteins and the GFP-LPXN-LD4-LIM fusion protein in the nucleus, respectively. In addition, two different vectors (GFP-LPXN-LD4mutL2-LIM and GFP-LPXN-LD4mutL1–3-LIM) were constructed containing alanine residues instead of conserved leucines in the LD4 motif of leupaxin. Transfection of LNCaP cells with these constructs showed that the mutated fusion proteins accumulated in the nucleus of 100% of transfected cells, respectively. All transfected cells and transfected cells with nuclear distribution of the GFP-leupaxin constructs were counted by using the Olympus BX60 microscope and the analySIS software.
Leupaxin Interacts with the Human AR and Enhances Its Transcriptional Activity
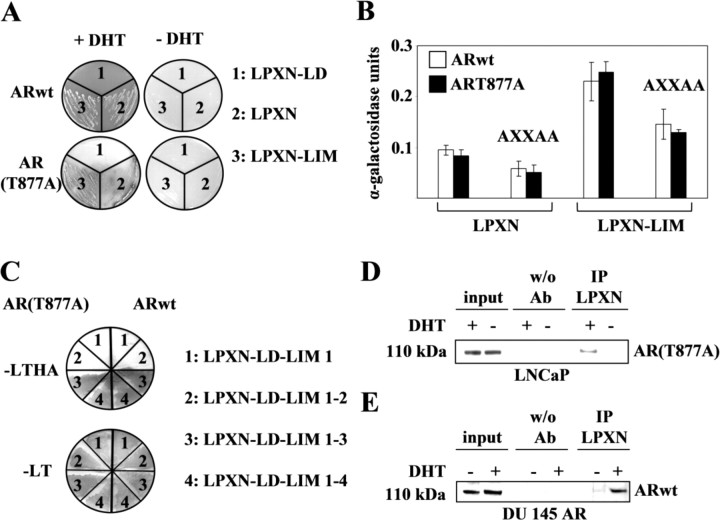
To show whether leupaxin interacts with the AR, we performed a direct yeast two-hybrid experiment using the full-length cDNA of leupaxin, a leupaxin cDNA containing only the four LIM domains, a leupaxin cDNA with only the LD motifs, and the ligand-binding domain (LBD) (amino acids 551–920) of the AR. Blue staining of the yeast colonies due to β-galactosidase activity could be observed only with full-length leupaxin and with the leupaxin construct containing all four LIM domains with both the wild-type AR and the mutant AR (T877A, generated from LNCaP cells) but only in the presence of the ligand DHT, whereas the LD motif region did not contribute to AR binding (Fig. 4A).
Fig. 4.
Interaction of Leupaxin with the AR
A, Leupaxin interacts with the AR in a direct yeast two-hybrid experiment in a ligand-dependent manner. Transformed yeast cells were plated on Trp− Leu− His− Adenine− medium containing 80 μg/ml X-Gal in the presence or absence of 100 nm DHT. B, The FXXLF motif of leupaxin contributes to the interaction of leupaxin and the AR. Transformed yeast cells were inoculated in liquid Trp− Leu− His− Adenine− medium and incubated for 48 h, and α-galactosidase activity was measured. The error bars indicate sd. C, LIM domain 3 of leupaxin is sufficient for the interaction with both wild-type (right) and mutant AR (left). Transformed yeast cells were plated on Trp− Leu− His− Adenine− (−LTHA) medium containing 80 μg/ml X-Gal in the presence of 100 nm DHT. As a control of transformation, yeast cells were plated on Trp− Leu− medium (−LT). D and E, Interaction of leupaxin and the AR in LNCaP and DU 145 cells. LNCaP cells (D) and DU 145 AR cells (E), stably expressing the wild-type AR, were transfected with a leupaxin construct, serum starved overnight, and stimulated with 100 nm DHT or ethanol for 2 h. Total cell lysates were immunoprecipitated (IP) with an anti-leupaxin antibody bound to protein A/G, and precipitated proteins were detected using an anti-AR antibody. As a negative control, IP was performed without the primary antibody.
To quantify leupaxin/AR binding capacity, a liquid yeast α-galactosidase assay was performed using the same leupaxin constructs as indicated above. This experiment demonstrated that the interaction of the LIM domain region of leupaxin with the AR is more efficient in vitro as compared with the binding capacity of full-length leupaxin and that the LD region of leupaxin alone was not sufficient for AR binding (Fig. 4B). Furthermore, computational sequence analysis revealed a conserved FXXLF motif at the C-terminal ends of both human and mouse leupaxin (amino acids 380–384, respectively). It was recently shown that the FXXLF motifs of AR coregulators, e.g. ARA55 and ARA70, were responsible for mediating AR-specific interactions (33). The substitution of the FXXLF motif by an AXXAA motif in two leupaxin constructs (LPXN-AXXAA and LPXN-LIM-AXXAA) led to a reduction of AR binding capacity of both mutant leupaxin proteins (Fig. 4B). Because mutation of the FXXLF motif resulted in only a reduction instead of an inhibition of the interaction between leupaxin and the AR, additional AR-interacting domains of leupaxin were investigated using the direct yeast two-hybrid system. Competent yeast cells were transfected with plasmids containing the four LD motifs of leupaxin and LIM domain 1 (LPXN-LD-LIM1), LIM domains 1 and 2 (LPXN-LD-LIM1–2), LIM domains 1, 2, and 3 (LPXN-LD-LIM1–3), or all four LIM domains (LPXN-LD-LIM1–4), respectively, together with either wild-type or mutant AR. The results demonstrated that LIM domains 1 and 2 of leupaxin were not capable of interacting with the AR, whereas LIM domain 3 alone and LIM domains 3 and 4 together were sufficient for binding to both the wild-type and the mutated AR (Fig. 4C).
To verify the results of leupaxin and AR interaction, an in vivo coimmunoprecipitation assay was performed by transfecting LNCaP cells with expression vectors containing full-length cDNAs of leupaxin and the AR. After immunoprecipitation of the cell lysate using a leupaxin-specific antibody and subsequent Western blot analysis with an anti-AR antibody, a specific band at 110 kDa for the AR was detected only in the presence of the ligand DHT (Fig. 4D). In addition, coimmunoprecipitation was repeated in stably transfected DU 145 AR cells expressing the wild-type AR (supplemental Fig. 5A), demonstrating that the interaction of leupaxin with both wild-type and mutant AR is independent of proteins exclusively expressed in LNCaP cells (Fig. 4E).
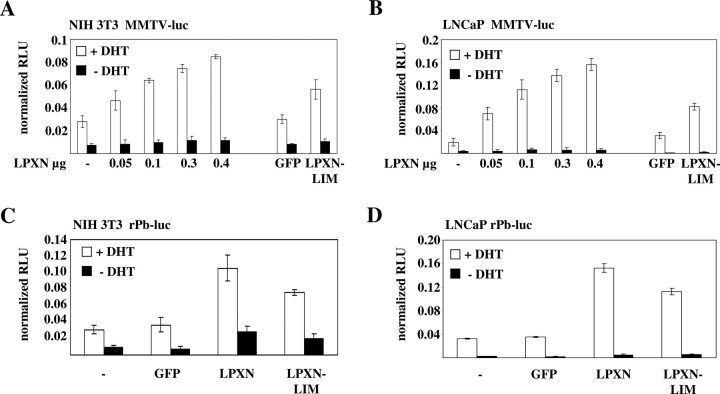
The biological relevance of this interaction was investigated using a reporter gene assay with the AR-responsive mouse mammary tumor virus (MMTV) promoter in front of a luciferase gene. The experiments were performed in murine NIH3T3 fibroblast cells (AR-negative) and in human LNCaP cells (AR-positive) in the presence or absence of DHT. By transfecting the cells with increasing amounts of a GFP-leupaxin construct either with (NIH3T3 cells) or without (LNCaP cells) an AR-expression vector, higher transcriptional activities of the AR were observed as compared with cells transfected with the AR construct alone (NIH3T3) and nontransfected (LNCaP) cells (Fig. 5, A and B). Furthermore, expression of truncated GFP-leupaxin fusion proteins (GFP-LPXN-LIM) containing only the four LIM domains could also increase the AR activity in NIH3T3 and LNCaP cells but to a lower extent (Fig. 5, A and B, right). The results from this assay were also confirmed in both cell lines by a second reporter construct, i.e. using the AR-responsive minimal promoter of the rat probasin (rPb) gene in front of the luciferase gene (Fig. 5, C and D).
Fig. 5.
Leupaxin Activates the Transcriptional Activity of the AR
A–D, AR coactivator activities of leupaxin were addressed by luciferase assays in two cell lines (NIH3T3 and LNCaP cells) using two different promoters (MMTV and rat probasin, rPb) in front of the luciferase reporter gene as described in Materials and Methods. As a control, cells were transfected with a GFP-expression vector to confirm that GFP itself did not contribute to increased AR transcriptional activities.
Down-Regulation of Leupaxin Expression in PCa Cells Leads to Distinct Effects in Different Cell Types
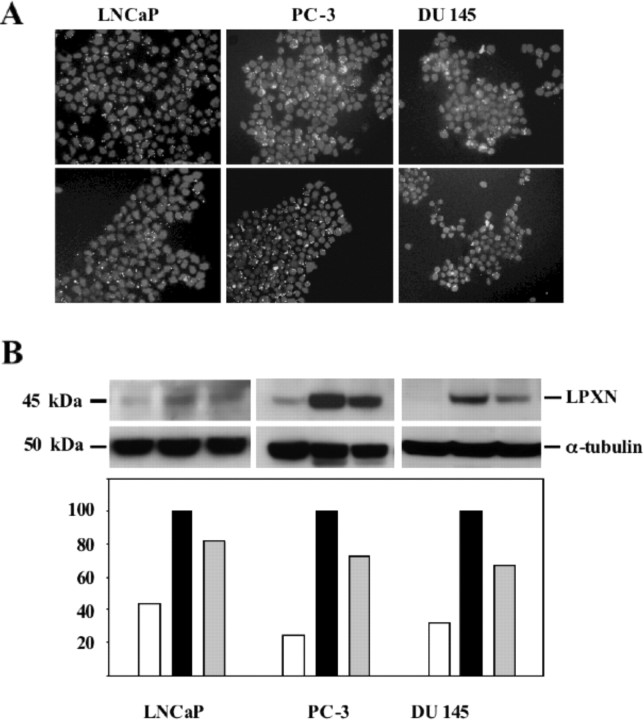
To further analyze the function of leupaxin in PCa cells, leupaxin expression was down-regulated using two different leupaxin-specific small interfering RNA (siRNA) oligonucleotides. First, transfection efficiencies of up to 80–90% in LNCaP, PC-3, and DU 145 cells were visualized using Cy3-labeled siRNA oligonucleotides (Fig. 6A). Second, knockdown of leupaxin expression (up to 70%) in PCa cells was confirmed as compared with luciferase siRNA transfected cells (control) using Western blot analysis (Fig. 6B).
Fig. 6.
Down-Regulation of Leupaxin Expression in PCa Cell Lines
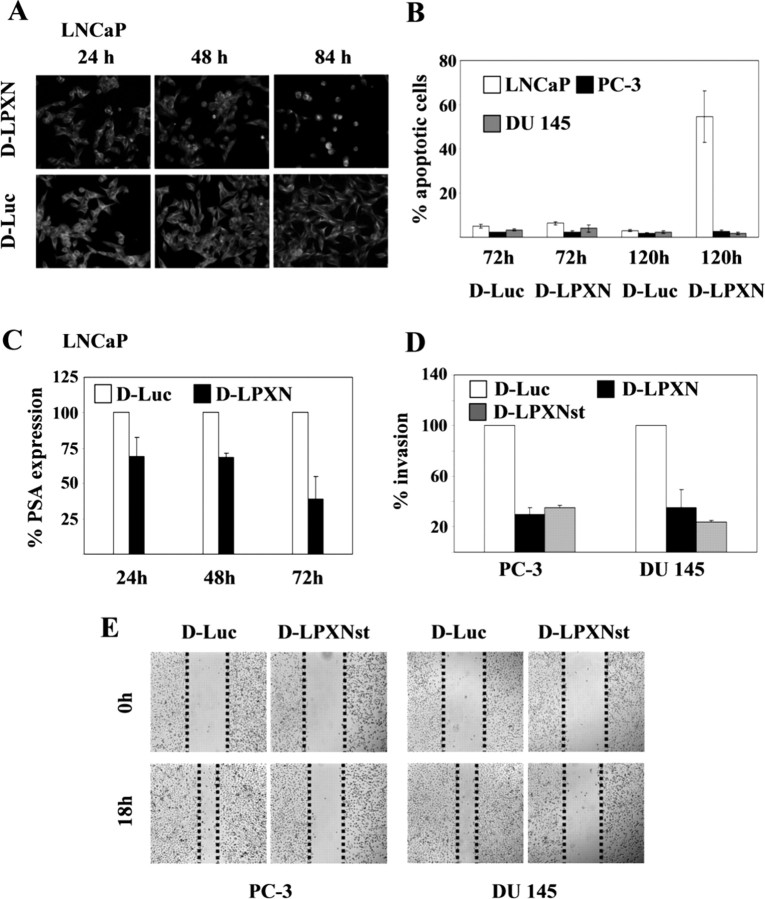
A, Transfection efficiency was examined using Cy3- labeled siRNA oligonucleotides against the leupaxin gene (D-LPXN) and against the luciferase gene, which served as a negative control (D-Luc). Note the white dots for siRNA duplex around the DAPI-stained nuclei. B, Western blot analysis of whole-cell extracts isolated from LNCaP, PC-3, and DU 145 cells transfected with either two different leupaxin-specific siRNA oligonucleotides (D-LPXN and D-LPXNst) or control siRNA oligonucleotides (D-Luc). After transfection, knockdown of leupaxin expression was checked by using an antileupaxin antibody (top panel) and an anti-α-tubulin antibody (middle panel) to ensure equal sample loading. The densitometric analysis (bottom panel) revealed a reduction of leupaxin expression of up to 60% in LNCaP cells, up to 80% in PC-3 cells, and up to 70% in DU 145 cells, respectively. The bar graphs in the bottom panel correspond to the lanes in the Western blot (middle panel).
Down-regulation of leupaxin expression in androgen-dependent LNCaP cells induced morphological changes; i.e. 48–72 h after transfection, LNCaP cells began to round up, and finally, cells detached from the bottom of the culture flask (Fig. 7A, upper panel). In contrast, control transfected LNCaP cells and androgen-independent PC-3 and DU 145 cells did not show any morphological modifications (Fig. 7A, lower panel, and data not shown). Furthermore, caspase 3 assays showed that over 50% of LNCaP cells treated with two different leupaxin-specific siRNA underwent apoptosis 120 h after transfection, whereas control transfected LNCaP cells and leupaxin siRNA transfected PC-3 and DU 145 cells remained viable (Fig. 7B and supplemental Fig. 3). In addition, it is worth noting that 30% of apoptotic LNCaP cells were already observed at 72 h after transfection with the D-LPXNst siRNA oligonucleotide, which showed a more pronounced knockdown of leupaxin expression as compared with the D-LPXN siRNA (supplemental Fig. 3). Next, to test the effect of leupaxin knockdown in LNCaP cells in the presence and absence of the AR, LNCaP cells were transfected with leupaxin-specific (D-LPXN) and luciferase-specific (D-Luc) siRNA oligonucleotides, respectively, in the presence of medium containing bicalutamide, stripped fetal calf serum (FCS), or normal culture medium. At 72 h after transfection, either inactivation of the AR or culturing the cells using stripped FCS together with leupaxin knockdown resulted in an earlier spontaneous apoptosis of LNCaP cells as compared with cells grown in normal culture medium (supplemental Fig. 4). Subsequently, an increased rate of apoptotic LNCaP cells (50–60%) was observed at 120 h after leupaxin knockdown for all culture conditions.
Fig. 7.
Down-Regulation of Leupaxin Expression Leads to Distinct Cellular Effects in Different PCa Cells
A, Androgen-dependent LNCaP cells with reduced leupaxin expression changed their morphology. The cells were grown on slides, transfected with leupaxin-specific (D-LPXN) and luciferase-specific (D-Luc) siRNA oligonucleotides, and incubated for the time periods as indicated. Subsequently, cells were fixed and stained with an anti-α-tubulin antibody to visualize the cell structure. Immunofluorescent images were acquired using ×100 magnification. B, Androgen-dependent LNCaP cells underwent spontaneous apoptosis 120 h after transfection with leupaxin-specific siRNA. LNCaP, PC-3, and DU 145 cells were transfected with leupaxin-specific (D-LPXN) and luciferase-specific (D-Luc) siRNA oligonucleotides. At 72 and 120 h after transfection, attached and floating cells were collected and cytocentrifuged on glass slides, and caspase 3 activities were detected as described in Materials and Methods. C, After leupaxin knockdown in LNCaP cells, subsequent inhibition of PSA secretion into the culture medium was detected at different time points (24, 48, and 72 h) using a PSA-specific ELISA. D, Androgen-independent and invasive PC-3 and DU 145 cells showed a reduction in their invasiveness in an in vitro Matrigel assay after down-regulation of leupaxin expression using leupaxin-specific siRNA (D-LPXN). Luciferase-siRNA-transfected cells were used as controls (D-Luc). E, Scratch assay analysis demonstrating a reduced direction migration of both PC-3 and DU 145 cells after transfection with siRNA against leupaxin (D-LPXNst) as compared with control-transfected cells (D-Luc).
In addition, PSA secretion of LNCaP cells was measured in conditioned media, demonstrating that down-regulated leupaxin expression reduced PSA secretion considerably as compared with control transfected cells (Fig. 7C). Moreover, proliferation of LNCaP cells was also affected after leupaxin down-regulation (48 and 72 h), presumably due to the beginning of apoptosis at these time points, whereas proliferation of PC-3 and DU 145 was not affected (data not shown).
To study the influence of down-regulated leupaxin expression on the invasiveness of PC-3 and DU 145 cells, an in vitro Matrigel assay was applied after cellular transfection that resulted in a reduction of the invasive capacity of PC-3 (up to 70%) and DU 145 (up to 75%) cells (Fig. 7D). A scratch assay analysis also demonstrated that knockdown of leupaxin expression in both PC-3 and DU 145 cells induced a reduced migration capability (Fig. 7E). In contrast, transduction of PC-3 and DU 145 cells with a TAT-LPXN-EGFP fusion protein leading to leupaxin overexpression resulted in an increase of both the invasiveness and migratory ability of these PCa cells, respectively (data not shown).
To further investigate the influence of the AR to the different effects observed in LNCaP vs. PC-3 and DU 145 cells, leupaxin expression was down-regulated in two different DU 145 AR cell clones stably expressing the wild-type AR (supplemental Fig. 5A). In contrast to LNCaP cells, DU 145 AR cell clones (C E and C I) did not show an increased apoptosis rate 120 h after transfection with leupaxin-specific siRNA (D-LPXN) as compared with parental DU 145 cells and control transfected cells (D-Luc; supplemental Fig. 5B). Furthermore, after knockdown of leupaxin expression a similar reduction in the migratory abilities of the DU 145 AR cell clones was observed as compared with control transfected and parental DU 145 cells without AR expression (supplemental Fig. 5C and Fig. 7E).
To additionally check the specificity of the leupaxin knockdown in LNCaP and PC-3 cells using the RNA interference (RNAi) technique, a siRNA-insensitive leupaxin cDNA was generated and subcloned into the EGFP-C1 vector to obtain the fusion construct EGFP-LPXNmut. LNCaP and PC-3 cells were stably transfected with EGFP-LPXNmut and EGFP-C1 (negative control) vectors, respectively, and expression of mutated leupaxin-EGFP fusion protein or EGFP in PCa cells was analyzed using fluorescence microscopy and Western blot analysis (supplemental Fig. 6, A and C). Then, LNCaP and PC-3 cells showing expression of EGFP-LPXNmut or EGFP (control) were transfected with siRNA against leupaxin (D-LPXN and D-LPXNst) or luciferase (control, D-Luc) and analyzed for complementation of apoptosis or motility and invasion. LNCaP cells with up-regulated expression of EGFP-LPXNmut showed almost no morphological changes, and apoptosis could be reduced to the normal level of control transfected LNCaP cells expressing EGFP (supplemental Fig. 6, A and B). Furthermore, reduction of motility and invasion after knockdown of leupaxin expression could be reversed in PC-3 cells with overexpression of EGFP-LPXNmut, respectively (supplemental Fig. 6, D and E).
DISCUSSION
One hypothesis put forward to explain the phenomenon that the AR continues to influence PCa growth despite androgen ablation therapy is based on the increased activity of the AR through an up-regulated expression of AR coactivators, which may be linked to a hypersensitive androgen signaling pathway (14, 34). In the present report, we show that leupaxin mRNA was overexpressed in intraprostatic tumor samples as well as in one capsule-penetrating prostate carcinoma as compared with matched normal prostate tissues. In addition, immunohistochemical staining of PCa specimens revealed that leupaxin is expressed in 22% of prostatic adenocarcinoma but not in normal prostate epithelium and in stromal cells. Two different staining patterns of leupaxin expression were detected in PCa glands. First, a high IRSlpxn together with diffuse cytoplasmic leupaxin staining of all PCa glands was observed in 30% of tissue specimens. Second, in the majority (70%) of leupaxin-positive tissue sections, low to intermediate cytoplasmic leupaxin expression with focal staining in discrete areas of the tumors could be demonstrated. This observation confirms the current concept that prostatic carcinoma is usually polyclonal and multifocal, resulting in phenotypic heterogeneity including variation in morphology and biomarker expression (35). In prostatectomy specimens, the different leupaxin staining intensities significantly correlated with Gleason patterns and Gleason scores, respectively. Finally, strong leupaxin staining was always noticed in infiltrating lymphocytes and macrophages, which is in agreement with recent studies where predominant leupaxin expression was reported in cells and tissues of hematopoietic origin (27, 36). Paxillin was also found to be overexpressed in invasive PC-3 and DU 145 cells as compared with LNCaP cells and together with FAK was shown to contribute to progression and invasion of human PCa (37). In contrast, for ARA55, it was demonstrated that it is expressed predominantly in stromal cells in human PCa specimens, but ARA55 expression was not detected in cells of epithelial origin (15, 38). In the present study, leupaxin was shown to be specifically expressed in LNCaP, PC-3, and DU 145 cells with the highest amounts in invasive PC-3 and DU 145 cells and the lowest expression level in noninvasive LNCaP cells. Taken together, our leupaxin expression analyses indicate that leupaxin expression is restricted to malignant epithelial cells and that up-regulated leupaxin expression could serve as a novel marker for progression of certain PCa.
At the subcellular level, leupaxin was mainly localized at focal adhesion sites as was also reported in previous studies for paxillin and ARA55 (18, 19). Both our transfection studies and the double-staining experiments with fluorescein isothiocyanate (FITC)-phalloidin to decorate actin filaments also confirmed the main localization site of leupaxin at the end of cellular stress fibers in PCa cells, whereas a small percentage (∼2%) of PCa cells showed an additional and weak nuclear distribution of leupaxin. Our results concerning leupaxin localization after LMB treatment of both LNCaP and PC-3 cells together with the data from recent studies demonstrated that LIM proteins of the zyxin family as well as paxillin protein family members shuttle between the cytoplasm and the nucleus in a CRM1/exportin 1-dependent manner (39). All these proteins contain a NES with the consensus sequence Hy-X1–4-Hy-X2–3-L-X-Hy (32), and in addition, for ARA55 it was shown that the LD3 motif is mainly responsible for nuclear protein export. However, our mutation and transfection experiments revealed that the leupaxin LD4 motif is the main NES and that the mutated LD3 motif led to only a moderate accumulation of GFP-leupaxin fusion protein in the nucleus. In summary, these results provide additional evidence that leupaxin, paxillin, and ARA55 could have different functions in cellular trafficking and signaling.
The molecular mechanisms for nuclear accumulation of the paxillin protein family members are yet unclear; only ARA55 was found to contain an oxidative-sensitive NES (30). The cytoplasmic-nuclear shuttling of paxillin proteins is in agreement with recent findings that paxillin and ARA55 are both known to interact with the protein tyrosine kinase PYK2, which translocates to the nucleus. Interestingly, PYK2 was also shown to be an interacting partner for leupaxin (27, 40). Furthermore, paxillin was found to be involved in the transport of mRNA to the leading edge of migrating cells by interaction with poly(A)-binding protein 1 (29). ARA55 participates in the transcriptional control of several genes such as c-fos and p21 by functioning as a potential coactivator of Sp1 through interaction with p300 and Smad3 (30, 41, 42, 43). Recent findings indicate that ARA55 is required for optimal glucocorticoid receptor-mediated gene expression possibly by providing a scaffold that organizes or stabilizes coactivator complexes at defined hormone-responsive promoters (44).
In addition, paxillin and ARA55 were described to interact with and transactivate nuclear hormone receptors such as the AR and the glucocorticoid receptor, but not the estrogen receptor-α, and ARA55 also exhibits coactivator functions for the progesterone receptor and the mineralocorticoid receptor (24, 20, 45). Thus, members of the paxillin protein family that localize predominantly within the cytoplasm, and particularly at cellular adhesion sites, have the capacity to accumulate within nuclei and exert direct effects on transcription.
In the present study, we could show that leupaxin binds via its LIM domains to the LBD of the AR in a hormone-dependent manner. The AR-LBD is proposed to bind preferentially to aromatic-rich motifs that are found within the N-terminal domain of the AR itself (FQNLF and WHTLF) and also in AR-specific coactivators such as ARA70 (33, 34, 46). Human leupaxin contains such a FXXLF motif and our mutational modification of this motif to AXXAA resulted only in an attenuation of the AR-leupaxin interaction. This observation is consistent with a previous report demonstrating that flanking regions of the FXXLF motif were necessary for binding of ARA55 to the AR-LBD (33). To identify additional AR-interacting domains of leupaxin, we could demonstrate that LIM domain 3 alone and LIM domains 3 and 4 together were capable of interacting with both the wild-type and the mutated AR. In contrast, LIM domains 1 and 2 were not sufficient to bind to the AR. Moreover, we also showed that leupaxin interacts with the wild-type AR in stably transfected DU 145 AR cells, indicating that this binding is independent of proteins exclusively expressed in LNCaP cells.
Using a reporter gene assay with two different AR-responsive promoters (MMTV and rPb), we could demonstrate that leupaxin acted as a transcriptional coactivator of the wild-type AR and the mutated AR (T877A) in a ligand-dependent manner. When only the LIM domains of leupaxin (GFP-LPXN-LIM) were used in these reporter gene assays, activation of the AR was also enhanced, but to a lesser extent. These results indicate that the transactivation domain of leupaxin is mainly located in the C-terminal part of the leupaxin protein. Recent studies demonstrated that the paxillin transactivation domain is also confined to the C-terminal region of the paxillin protein (20), whereas this activity resided solely within the N-terminal part of the ARA55 protein (45). In addition, binding of leupaxin and ARA55 to steroid receptors seem to be ligand dependent (24), whereas paxillin binding does not require ligand-bound steroid receptors (20). The detailed mechanisms of the AR activation are still unclear, but it is likely that leupaxin also plays a role in the regulation of transcription of nuclear hormone receptor-responsive genes and may therefore be involved in the progression of PCa.
This hypothesis is further supported by the results obtained from the leupaxin knockdown studies in LNCaP, PC-3, and DU 145 cells. Two different effects could be observed in PCa cells after down-regulation of leupaxin expression using the RNAi technique, presumably provoked by the presence or absence of the AR and the involvement of different signaling pathways in these cell lines. On the one hand, AR-positive, androgen-dependent, and noninvasive LNCaP cells changed their morphology, detached from the bottom, and underwent apoptosis. On the other hand, the same treatment of AR-negative, androgen-independent, and highly invasive PC-3 and DU 145 cells resulted in a 70% reduction of invasiveness and an impaired directional migration in a wound assay, whereas morphological changes or induction of apoptosis was not observed. In addition, the specificity of these phenotypic alterations after down-regulated leupaxin expression could be proven using a complementation assay; i.e. increased apoptosis and decreased motility and invasion of PCa cells could be reversed by introducing and expressing a siRNA-insensitive leupaxin cDNA. These different cellular effects could be explained by the fact that leupaxin represents a transcriptional coactivator of the AR and that leupaxin could also be integrated in the regulation of other signaling pathways. Data from recent reports demonstrated that down-regulation of AR protein expression resulted in an inhibition of proliferation and induction of apoptosis of androgen-dependent LNCaP cells, whereas androgen-independent PC-3 cells and PC-3 cells with a exogenously introduced AR were not affected (5, 47, 48, 49, 50). However, our results on the inhibition of AR activity in LNCaP cells using bicalutamide also indicate that leupaxin could control other signaling proteins, besides the AR, in this cell line.
In our study, the proliferation rate of LNCaP cells after leupaxin knockdown was significantly reduced, presumably due to the morphological changes resulting in detachment and apoptosis, whereas proliferation of PC-3 and DU 145 cells was not inhibited. Paxillin was found to be frequently involved in proliferative signals (51), but ARA55 expression coincided with growth inhibition and cellular senescence (19, 52). Furthermore, we could detect a strong reduction in the secretion of PSA after transfection of LNCaP cells with leupaxin-specific siRNA, highlighting the involvement of reduced AR activity in these phenotypical effects.
Because all paxillin protein family members are supposed to have (at least) dual functions within cellular signaling events (focal adhesion vs. gene regulation in the nucleus), it was not surprising that down-regulation of leupaxin protein expression in PC-3 and DU 145 cells caused a decrease of invasiveness and motility of these cells. In contrast, cell viability in these PCa cell lines was not affected. Our results using DU 145 cells stably expressing the wild-type AR (DU 145 AR) also showed that several signaling pathways are involved in different cellular events in these PCa cell lines. A leupaxin knockdown in these cells was not sufficient to induce apoptosis, whereas the inhibition of DU 145 AR cell motility was comparable to parental DU 145 cells after down-regulated leupaxin expression. A recent study reported that a knockdown of leupaxin in osteoclasts using antisense DNA oligonucleotides induced a decrease in the resorptive capacity of osteoclasts and inhibited migration of these cells (28), thus suggesting that leupaxin normally facilitates cell migration. Paxillin was also shown to stimulate cell migration in different cell types (reviewed in Ref. 17); e.g. a paxillin gene knockout in embryonic stem cells resulted in impaired cell spreading (53), and in highly metastatic human osteosarcoma HuO9 sublines, an attenuated motility after down-regulation of paxillin expression was observed (54). In contrast, a recent report from Yano and co-workers (55) provided evidence that an impaired FAK and paxillin signaling led to increased cell migration in both human fibroblasts and HeLa cells, indicating that these proteins can also induce motility retardation (56).
In conclusion, our analysis of clinical specimens and in vitro studies suggest that leupaxin could serve as an important indicator in diagnosis and prognosis of certain PCa and that disruption of leupaxin functions or its target genes and pathways may have potential therapeutic advantages in PCa treatment.
MATERIALS AND METHODS
Patient Material
Ethical approval was obtained from the ethical committee of the University of Göttingen for the use of human material in the present study. Radical prostatectomy and needle biopsy specimens of 59 patients were freshly obtained from the urological clinic of the university hospital. Radical prostatectomy specimens for laser microdissection and expression studies at the RNA level were treated as described previously (29). Adjacent prostate slices were fixed in formalin and embedded in paraffin for standard routine histological examination as well as immunohistochemistry. Staging was performed according to the International Union Against Cancer classification, and tumors were graded using the Gleason score.
Laser Microdissection, RNA Isolation and Amplification, Atlas Select Human Tumor Arrays
Laser microdissection of normal and prostate carcinoma tissues was performed as described previously (57). Total cellular RNA from the dissected normal prostate, from the tumor within the boundaries of the prostate gland, and from capsule-penetrating tumor areas was extracted using the RNeasy mini kit (QIAGEN GmbH, Hilden, Germany). RNA integrity and quantity were assessed using the Agilent Bioanalyzer 2100 with a RNA 6000 Pico LabChip Kit (Agilent Technologies, Waldbronn, Germany).
Total cellular RNA (50–100 ng) from each sample was amplified using the Super SMART PCR cDNA Synthesis Kit following the manufacturer’s instructions (BD Biosciences Clontech, Heidelberg, Germany). The 32P-labeled cDNA probes were prepared using the BD Atlas SMART Probe Amplification Kit and were hybridized side-by-side to three identical Atlas Select Human Tumor arrays (BD Biosciences Clontech) according to the user manuals. The arrays were scanned after a 3-d exposure by using a Molecular Imager FX and analyzed by using the Quantity One software (Bio-Rad, Hercules, CA).
Real-Time RT-PCR Analysis
Real-time RT-PCR analysis was performed as described previously (57). Primers used for quantitative RT-PCR were LPXN forward primer 5′-ACGCTCCACCCTTCAGGACA-3′, LPXN reverse primer, 5′-GACATTGAGCTCCTGGATATTGG-3′, LPXN probe 5′-FAM-ACACAAGTCCCTTGCCGGCG-TAMRA-3′, β-actin forward primer 5′-TCACCCACACTGTGCCCATCTACGA-3′, β-actin reverse primer GGTAACCGTTACTCGCCAAGGCGAC-3′, and β-actin probe 5′-Texas-Red-ATGCCCTCCCCCATGCCATCCTGCGT-BHQ-3′. Leupaxin mRNA expression was normalized to β-actin mRNA expression to compensate for different sample capacities.
Immunohistochemistry
Paraffin-embedded prostate tissue sections (5 μm) were deparaffinized and rehydrated. After antigen retrieval, sections were blocked with 3% hydrogen peroxide and 10% FCS/5% BSA in Tris-buffered saline. The mouse monoclonal anti-leupaxin (clone 283 G) antibody was kindly provided by ICOS Corp. (Bothell, WA) and was checked for specificity in Western blotting and immunocytochemistry (28). The primary antibody was applied at a concentration of 3.5 μg/ml for 2 h at room temperature. Sections were incubated with Envision-peroxidase (DAKO, Hamburg, Germany), and diaminobenzidine was used as chromogen. Finally, tissue sections were counterstained with hemalum. For negative controls, blocking solution was used in place of the primary antibody.
Semiquantitative Analysis of Leupaxin Immunoreactivity and Statistical Analysis
For quantification of the leupaxin immune signals in tissue sections, an additive immunoreactive score (IRS = SI + CN) was applied comprising the average signal intensity (SI) and the number of positive tumor cells (CN, supplemental Fig. 6). For comparative analyses of leupaxin immunoreactivity with the Gleason score and pattern, the χ2 test and Fisher’s exact test were applied.
Cell Culture and Transient Transfection
LNCaP, PC-3, and DU 145 cells were grown in RPMI 1640 medium (PAN-Systems, Nuremberg, Germany) containing 10% FCS. Mouse NIH3T3 cells were maintained in DMEM (PAN) with 10% FCS. Transient transfection experiments were performed using Roti-Fect (Carl Roth & Co., Karlsruhe, Germany) according to the manufacturer’s instructions.
Generation of DU 145 Cell Clones Expressing the Wild-Type AR
DU 145 cells were stably transfected with the construct pAR-IRES-EGFP (construct kindly provided by John T. Isaacs, John Hopkins University, Baltimore, MD), which contains the full-length open reading frame as well as regulating elements of the 5′ and 3′ untranslated regions of the wild-type AR (58). Transfections were performed using Roti-Fect (Carl Roth & Co.) according to the manufacturer’s instructions, and cell clones were selected in culture medium supplemented with 0.3 μg/ml G418 (Sigma, Taufkirchen, Germany).
Production of PCa Cells Expressing a Mutated Leupaxin-EGFP Fusion Protein
The complete open reading frame of human leupaxin (NM_004811, cDNA position 94–1254) was amplified from cDNA that was reverse transcribed from PC-3 cells. The whole leupaxin cDNA fragment was sequenced bidirectionally, cut with EcoRI, and subsequently cloned in frame into the EcoRI site of the pEGFP-C1 vector (CLONTECH). To prepare a siRNA-insensitive leupaxin cDNA, two PCR primers were synthesized, each covering one target sequence of siRNAs D-LPXN and LPXNst. The oligonucleotides contain base substitutions (lowercase letters) without changing the resulting leupaxin amino acid sequence: LPXNmut-Fw 5′-AATATTCgAAtCCtGCaCCaCTTCCCCTGGATCAGCATTCCAGAAAGG-3′ and LPXNmutST-Rev 5′-GTATACACcaattGGgCCGGCAAGGGACTTGTGTTATCCTG-3′. Additionally, both primers contained a leupaxin cDNA-specific internal restriction site (SspI and Bst1107I, respectively). PCR was performed using pEGFP-LPXN vector DNA as a template, and then the PCR product was cut with SspI and Bst1107I and ligated into the appropriate treated pEGFP-LPXN vector to produce the pEGFP-LPXNmut fusion construct.
To establish stable expressing PCa cell clones, the pEGFP-LPXNmut construct and the pEGFP-C1 vector (as negative control) were transfected into PC-3 and LNCaP cells, respectively, using FuGene (Roche, Mannheim, Germany) according to the manufacturer’s instructions. After 6 wk of selective culture in medium containing 0.8 and 0.2 μg/ml G418 (Calbiochem, Schwalbach, Germany), respectively, green fluorescent cell clones were collected and analyzed for EGFP-LPXNmut and EGFP expression by Western blotting or fluorescence microscopy.
Western Blot Analysis and Immunoprecipitation
Whole-cell lysates from parental and transfected LNCaP, PC-3, and DU 145 cells were prepared using lysis buffer as described previously (29), and 50 μg of cell lysates were boiled and denatured in sample buffer containing sodium dodecyl sulfate and dithiothreitol (Invitrogen, Karlsruhe, Germany) followed by gel electrophoresis using the NuPage 4–12% Bis-Tris precast gel in MES buffer (Invitrogen). Proteins were electrotransferred to a polyvinylidene difluoride membrane (Macherey-Nagel, Düren, Germany). The membrane was blocked in dry milk in PBS and then incubated with primary antibodies (1 μg/ml mouse monoclonal antileupaxin 283 C, kindly provided by ICOS Corp., Bothell, WA) (28), 1:2500 mouse monoclonal anti-α-tubulin from Sigma, and 1:2000 rabbit polyclonal anti-AR, Ab-2, NeoMarkers, Fremont, CA) at 4 C overnight. Proteins were visualized using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate or the ECL Plus kit (GE Healthcare, Freiburg, Germany). The in vivo coimmunoprecipitation assay was carried out using the Protein G Immunoprecipitation Kit (Sigma) according to the supplier’s instructions.
RNAi
Transfection of LNCaP, PC-3, and DU 145 cells was accomplished using Oligofectamine Reagent (Invitrogen) according to the supplier’s instructions with two different leupaxin gene-specific siRNA duplexes (LPXN sense 5′-UAUUCCAACCCAGCUCCUC-3′ and LPXN antisense 5′-GAGGAGCUGGGUUGGAAUA-3′ from Eurogentec, Seraing, Belgium, or LPXNst sense 5′-GGCGCAGCUCGUGUAUACUACCAAU-3′ and 5′-AUUGGUAGUAUACACGAGCUGCGCC-3′, stealth siRNA from Invitrogen) at a concentration of 80 nm in the transfection medium. Control cells were transfected with siRNA duplex oligonucleotides against the firefly luciferase gene (57). At different time points after transfection (24, 48, and 72 h), cells were collected and used in the following experiments. To analyze the transfection efficiency, duplex siRNA oligonucleotides were labeled with Cy3 using the Silencer siRNA Labeling Kit (Ambion Europe Ltd., Cambridgeshire, UK) as described previously (30). Transfected and untransfected cells were counted with an Olympus BX60 microscope (Olympus Optical Co. Ltd., Hamburg, Germany) and the analySIS software (Soft Imaging System GmbH, Münster, Germany).
Immunocytochemistry and Caspase 3 Assay
LNCaP, PC-3, and DU 145 cells were plated on culture slides coated with 10 μg/ml fibronectin (Sigma) and grown for 4–24 h under normal culture conditions. The cells were then fixed with 4% formaldehyde in PBS, permeabilized with 0.1% Triton X-100 in PBS, and blocked with 3% BSA in PBS for 30 min at room temperature. After an incubation step with primary antibodies (10 μg/ml mouse monoclonal anti-leupaxin, 1:100 rabbit polyclonal anti-AR, and 1:2000 mouse monoclonal anti-α-tubulin) overnight at 4 C, cells were washed with PBS and incubated for 2 h at room temperature with secondary antibodies (1:500 sheep antimouse-IgG-Cy3, 1:200 sheep antimouse-IgG-FITC, 1:200 sheep antirabbit-Cy3, and 1:200 goat antirabbit-FITC; Sigma). Subsequently, cells were washed in PBS and mounted using Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI). Immunofluorescent images were acquired using the Olympus BX60 microscope and the analySIS software. The caspase 3 assay and DAPI staining for quantification of apoptotic cells were performed as described previously (57).
Matrigel Invasion Assay and Scratch Assay
In vitro cell migration of PC-3 and DU 145 cells was determined in BioCoat Matrigel Invasion Chambers (BD Biosciences) as described previously (59). Leupaxin siRNA- and luciferase siRNA-transfected cells (2.5 × 104 cells, respectively) were suspended and incubated in invasion chambers for 22 h at 37 C. Invaded cells were stained with the Diff-Quik staining set (Dade Behring GmbH, Marburg, Germany) and counted from five different randomly chosen fields under a BX60 microscope using the analySIS software. Data are expressed as the percentage of cells with reduced leupaxin expression invaded through the Matrigel Matrix membrane in comparison with control transfected and parental cells. All experiments were assayed in triplicate.
To estimate the directional migration, PC-3 and DU 145 cells were transfected with siRNA against leupaxin and the luciferase gene (control) as described above. After incubation with the transfection medium for 16 h, cells were harvested and plated in 24-well plates at a density of 6 × 104 cells per well. After 24 h, the scratch was applied and the cells were photographed under an inverted microscope (Olympus IX81).
Direct Yeast Two-Hybrid Experiments
The yeast two-hybrid experiments were carried out by using the MATCHMAKER GAL4 two-hybrid system (Clontech). All procedures were performed according to the manufacturer’s protocols. The complete open reading frame of human leupaxin and truncated leupaxin sequences, i.e. LPXN-LD containing only the four LD motifs [nucleotides (nt) 94–528 of NM_004811] and LPXN-LIM containing the four LIM domains (nt 518-1251 of NM_004811) were cloned into the pGADT7 vector, respectively. The LBD of the AR (nt 2756–3875 of NM_00044) was cloned into the pGBKT7 vector. For assaying the protein-protein interactions, cotransformation of the constructs into the yeast host strain AH109 was performed. Cotransformants were selected in the presence or absence of 100 nm DHT (Sigma) on minimal synthetic dropout (SD) medium lacking the amino acids leucine, tryptophan, and histidine (SD-LTH). For high-stringency conditions, adenine was excluded and 80 mg/liter X-Gal (ICN, Costa Mesa, CA) was included into the SD medium. To quantify the protein-protein interactions, the liquid α-Gal Quantitative Assay was performed according to the Clontech protocol with minor modifications. The OD of each sample was recorded at 410 nm in a microplate reader (F800; Bio-Tek, Bad Friedrichshall, Germany). Each protein-protein interaction was measured in triplicate, and the whole experiment was performed independently three times.
Luciferase Assay and PSA Analysis
Cells (8 × 104) were plated in 12-well tissue culture plates, and after 24 h, cells were transfected with and expression vector cocktail containing 0.05 μg pCMV-β-Gal and 0.05–0.4 μg GFP-LPXN (as indicated) and with 0.2 μg MMTV-luc reporter plasmid (gift from R. Schuele, University of Freiburg, Germany), and NIH3T3 cells were additionally transfected with 0.2 μg pSV-AR0 vector. Total DNA concentrations in the transfection cocktails were adjusted using the plasmid pGL3-basic, and transfection efficiency was determined using the pCMV-β-Gal vector. The reporter plasmid rat probasin-luciferase (rPb-luc) was generated by PCR amplifying the minimal promoter region of the rat probasin gene (60). Subsequently, this DNA fragment was cloned in front of the luciferase gene of the pGL3-basic vector. At 36 h after transfection, cell lysates were prepared by adding lysis buffer, and 40 μl of the cell lysate was mixed with luciferase assay buffer following the user manual. Luciferase activities were measured in a microplate luminometer (LB953; Berthold, Pforzheim, Germany) by injecting 100 μl of a luciferin solution (P.J.K. GmbH, Kleinblittersdorf, Germany) per well. The luciferase activity was normalized against the α-galactosidase activity, which was measured by using the Galacto-Light kit (BD Bioscience) according to the manufacturer’s protocol.
For analysis of PSA secretion, LNCaP cells were transfected with RNAi oligonucleotides against leupaxin or with control oligonucleotides as described above. After indicated time points (24, 48, and 72 h), conditioned medium was collected and PSA secretion from LNCaP cells was measured with the Elecsys System 2010 (Roche).
Acknowledgments
We thank M. Kickstein, R. Kampe, and N. Putzer for technical assistance. We thank S. Beckemeyer for her excellent work with the TAT-constructs.
NURSA Molecule Pages:
Ligands: Dihydrotestosterone;
Nuclear Receptors: AR | GR.
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft (to P.B., BU 992/2-2 and to S.K., KA 2946/1-1), the Forschungsförderungsprogramm (Medical Faculty, University of Göttingen to P.B. and S.K.), the Horst-Müggenburg-Stiftung (to P.B., P.T., and B.H.), and the European Union: (E)UROESTROGEN(E)S contract QLK6-CT-200-00565 (to P.T.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: AR, Androgen receptor; ARA55, AR-associated protein 55; DAPI, 4′,6-diamidino-2-phenylindole; DHT, dihydrotestosterone; FCS, fetal calf serum; FITC, fluorescein isothiocyanate; FN, fibronectin; GFP, green fluorescent protein; IRSlpxn, immunoreactive score for leupaxin; LBD, ligand-binding domain; LMB, leptomycin B; MMTV, mouse mammary tumor virus; NES, nuclear export signal; nt, nucleotide; PCa, prostate cancer; PYK2, proline-rich tyrosine kinase 2; PSA, prostate-specific antigen; RNAi, RNA interference; SD, synthetic dropout; siRNA, small interfering RNA.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M 2001. Cancer Statistics. CA Cancer J Clin 51:15–36 [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg J, Blackledge G 2002. Looking to the future. Advances in the management of hormone-refractory prostate cancer. Eur Urol Suppl 1:44–53 [Google Scholar]
- 3.Edwards J, Bartlett MS 2005. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: modifications to the androgen receptor. BJU Int 95:1320–1326 [DOI] [PubMed] [Google Scholar]
- 4.Van der Kwast TH, Schalken J, Ruizeveld de Winter JA, Van Vroonhoven CC, Muller E, Boersma W 1993. Androgen receptors in endocrine therapy-resistant human prostate cancer. Int J Cancer 48:189–193 [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL 2004. Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- 6.Catz SD, Johnson JL 2003. BCL-2 in prostate cancer: a minireview. Apoptosis 8:29–37 [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF 2000. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res 60:3623–3630 [PubMed] [Google Scholar]
- 8.Sasaki M, Tanak Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R 2002. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Nat Cancer Inst 94:384–390 [DOI] [PubMed] [Google Scholar]
- 9.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kiuper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E 1992. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol 41:665–669 [DOI] [PubMed] [Google Scholar]
- 10.Matias PM, Carrondo MA, Coelho R, Thomaz M, Zhao XY, Wegg A, Crusius K, Egner U, Donner P 2002. Structural basis for the glucocorticoid response in a mutant human androgen receptor (ARccr) derived from an androgen-independent prostate cancer. J Med Chem 45:1439–1446 [DOI] [PubMed] [Google Scholar]
- 11.Craft A, Shostak Y, Carey M, Sawyers CL 1999. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med 5:280–285 [DOI] [PubMed] [Google Scholar]
- 12.Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christion RE, Settlage RE, Shabanowitz J, Hunt DF, Weber MJ 2002. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem 277:29304–29314 [DOI] [PubMed] [Google Scholar]
- 13.Uzgare AR, Kaplan PJ, Greenberg NM 2003. Differential expression and/or activation of P38MAPK, Erk1/2, and Jnk during the initiation and progression of prostate cancer. Prostate 55:128–139 [DOI] [PubMed] [Google Scholar]
- 14.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM 2001. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res 61:4315–4319 [PubMed] [Google Scholar]
- 15.Mestayer C, Blanchère M, Jaubert F, Dufour B, Mowszowicz I 2003. Expression of androgen receptor coactivators in normal and cancer prostate tissues and cultured cell lines. Prostate 56:192–200 [DOI] [PubMed] [Google Scholar]
- 16.Edwards J and Bartlett MS 2005. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: androgen-receptor cofactors and bypass pathways. BJU Int 95:1327–1335 [DOI] [PubMed] [Google Scholar]
- 17.Brown MC, Turner CE 2004. Paxillin: adapting to change. Physiol Rev 84:1315–1339 [DOI] [PubMed] [Google Scholar]
- 18.Brown MC, Perrotta JA, Turner CE 1996. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol 135:1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita H, Kamiguchi K, Cho D, Shibanuma M, Morimoto C, Tachibana K 1998. Interaction of Hic-5, a senescence-related protein with focal adhesion kinase. J Biol Chem 273:26516–26521 [DOI] [PubMed] [Google Scholar]
- 20.Kasai M, Guerrero-Santoro J, Friedmann R, Leman ES, Getzenberg RH, DeFranco DB 2003. The group 3 LIM domain protein paxillin potentiates androgen receptor transactivation in prostate cancer cell lines. Cancer Res 63:4927–4935 [PubMed] [Google Scholar]
- 21.Thomas SM, Hagel M, Turner CE 1999. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J Cell Sci 112:181–190 [DOI] [PubMed] [Google Scholar]
- 22.Turner, CE, Glenney Jr JR, Burridge K 1990. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol 111:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremblay L, Hauck W, Nguyen LT, Allard P, Landry F, Chapdelaine A, Chevalier S 1996. Regulation and activation of focal adhesion kinase and paxillin during adhesion, proliferation, and differentiation of prostatic epithelial cells in vitro and in vivo Mol Endocrinol 10:1010–1020 [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto N, YehS, Kang H, Inui S, Chang H, Mizokami A, Chang C 1999. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J Biol Chem 274:8316–8321 [DOI] [PubMed] [Google Scholar]
- 25.Stanzione R, Picascia A, Chieffi P, Imbimbo C, Palmieri A, Mirone V, Staibano S, Franco R, De Rosa G, Schlessinger J, Tramontano D 2001. Variations of proline-rich kinase Pyk2 expression correlate with prostate cancer progression. Lab Invest 81:51–59 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Yang Y, Guo X, Sampson ER, Hsu C, Tsai M, Yeh S, Wu G, Guo Y, Chang C 2002. Suppression of androgen receptor transactivation by PYK2 via interaction and phosphorylation of the ARA55 coregulator. J Biol Chem 277:15426–15431 [DOI] [PubMed] [Google Scholar]
- 27.Lipsky BP, Beals CR, Staunton DE 1998. Leupaxin is a novel LIM domain protein that forms a complex with PYK2. J Biol Chem 273:11709–11713 [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Lee BS, Khadeer MA, Tang Z, Chellaiah M, Abu-Amer Y, Goldknopf J, Hruska KA 2003. Leupaxin is a critical adapter protein in the adhesion zone of the osteoclast. J Bone Miner Res 8:669–685 [DOI] [PubMed] [Google Scholar]
- 29.Woods AJ, Roberts MS, Choudhary J, Barry ST, Mazaki Y, Sabe H, Morley SJ, Critchley DR, Norman JC 2002. Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells. J Biol Chem 277:6428–6437 [DOI] [PubMed] [Google Scholar]
- 30.Shibanuma M, Kim-Kaneyama JR, Ishino K, Sakamoto N, Hishiki T, Yamaguchi K, Mori K, Mashimo J, Nose K 2003. Hic-5 communicates between focal adhesions and the nucleus through oxidant-sensitive nuclear export signal. Mol Biol Cell 14:1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T 1994. Leptomycin B targets a regulatory cascade of crm 1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem 269:6320–6324 [PubMed] [Google Scholar]
- 32.Nix DA, Beckerle MC 1997. Nuclear-cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J Cell Biol 138:1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He B, Minges JT, Lee LW, Wilson EM 2002. The FXXLF motif mediates androgen receptor-specific interactions with coregulators. J Biol Chem 277:10226–10235 [DOI] [PubMed] [Google Scholar]
- 34.Zhou ZX, He B, Hall SH, Wilson EM, French FS 2002. Domain interactions between coregulator ARA70 and the androgen receptor (AR). Mol Endocrinol 16:287–300 [DOI] [PubMed] [Google Scholar]
- 35.Alers JC, Krijtenburg PJ, Vissers CJ, Bosman FT, van der Kwast TH, van Dekken H 1995. Cytogenetic heterogeneity and histologic tumor growth patterns in prostatic cancer. Cytometry 21:84. [DOI] [PubMed] [Google Scholar]
- 36.Yuminamochi T, Yatomi Y, Osada M, Ohmori T, Ishii Y, Nakazawa K, Hosogaya S, Ozaki Y 2003. Expression of the LIM proteins paxillin and Hic-5 in human tissues. J Histochem Cytochem 51:513–521 [DOI] [PubMed] [Google Scholar]
- 37.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S 1996. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer 68:164–171 [DOI] [PubMed] [Google Scholar]
- 38.Li P, Yu X, Ge K, Melamed J, Roeder RG, Wang Z 2002. Heterogenous expression and functions of androgen receptor co-factors in primary prostate cancer. Am J Pathol 161:1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Gilmore TD 2003. Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochim Biophys Acta 1593:115–120 [DOI] [PubMed] [Google Scholar]
- 40.Aoto H, Sasaki H, Ishino M, Sasaki T 2002. Nuclear translocation of cell adhesion kinase β/proline-rich tyrosine kinase 2. Cell Struct Funct 27:47–61 [DOI] [PubMed] [Google Scholar]
- 41.Kim-Kaneyama J, Shibanuma M, Nose K 2002. Transcriptional activation of the c-fos gene by a LIM protein, Hic-5. Biochem Biophys Res Commun 299:360–365 [DOI] [PubMed] [Google Scholar]
- 42.Shibanuma M, Kim-Kaneyama JR, Sato S, Nose K 2004. A LIM protein, Hic-5, functions as a potential coactivator for Sp1. J Cell Biochem 91:633–645 [DOI] [PubMed] [Google Scholar]
- 43.Shibanuma M, Mochizuki E, Maniwa R, Mashimo J, Nishiya N, Imai S, Takano T, Oshimura M, Nose K 1997. Induction of senescence-like phenotypes by forced expression of hic-5, which encodes a novel LIM motif protein, in immortalized human fibroblasts. Mol Cell Biol 17:1224–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heitzer MD, DeFranco DB 2006. Mechanism of action of Hic-5/ARA55, a LIM domain-containing nuclear receptor coactivator. Mol Endocrinol 20:56–64 [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Guerrero J, Hong H, DeFranco DB, Stallcup MR 2000. Interaction of the τ2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol Biol Cell 11:2007–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He B, Lee LW, Minges JT, Wilson EM 2002. Dependence of selective gene activation on the androgen receptor NH2- and COOH-terminal interaction. J Biol Chem 277:25631–25639 [DOI] [PubMed] [Google Scholar]
- 47.Wright ME, Tsai M, Aebersold R 2003. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol 17:1726–1737 [DOI] [PubMed] [Google Scholar]
- 48.Liao X, Tang S, Thrasher JB, Griebling TL, Li B 2005. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther 4:505–515 [DOI] [PubMed] [Google Scholar]
- 49.Yang Q, Fung K, Day WV, Kropp BP, Lin H 2005. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int 5:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haag P, Bektic J, Bartsch G, Klocker H, Eder IE 2005. Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol 96:251–258 [DOI] [PubMed] [Google Scholar]
- 51.Turner CE 2000. Paxillin and focal adhesion signaling. Nat Cell Biol 2:E231–E236 [DOI] [PubMed]
- 52.Nishiya N, Tachibana K, Shibanuma M, Mashimo J, Nose K 2001. Hic-5 reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol Cell Biol 21:5332–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wade R, Bohl J, Vande Pol S 2002. Paxillin null embryonic stem cells are impaired in cell spreading and tyrosine phosphorylation of focal adhesion kinase. Oncogene 21:96–107 [DOI] [PubMed] [Google Scholar]
- 54.Azuma K, Tanaka M, Uekita T, Inoue S, Yokota J, Ouchi Y, Sakai R 2005. Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene 24:4754–4764 [DOI] [PubMed] [Google Scholar]
- 55.Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H 2004. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol 166:283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaller MD 2004. FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J Cell Biol 166:157–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grzmil M, Thelen P, Hemmerlein B, Schweyer S, Voigt S, Mury D, Burfeind P 2003. Bax inhibitor-1 is overexpressed in prostate cancer and its specific down-regulation by RNA interference leads to cell death in human prostate carcinoma cells. Am J Pathol 163:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Litvinov IV, Antony L, Isaacs JT 2004. Molecular characterization of an improved vector for evaluation of the tumor suppressor versus oncogene abilities of the androgen receptor. Prostate 61:299–304 [DOI] [PubMed] [Google Scholar]
- 59.Grzmil M, Voigt S, Thelen P, Hemmerlein B, Helmke K, Burfeind P 2004. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int J Oncol 24:97–105 [PubMed] [Google Scholar]
- 60.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ 1995. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 92:3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]